Altered Lung Heat Shock Protein-70 Expression and Severity of Sepsis-Induced Acute Lung Injury in a Chronic Kidney Disease Rat Model
Abstract
1. Introduction
1.1. Sepsis-Induced Acute Lung Injury
1.2. Role of Heat Shock Protein in Acute Lung Injury
1.3. Chronic Kidney Disease (CKD) and Lung Disorder
1.4. Objective of the Study
2. Results
2.1. Clinical Parameters
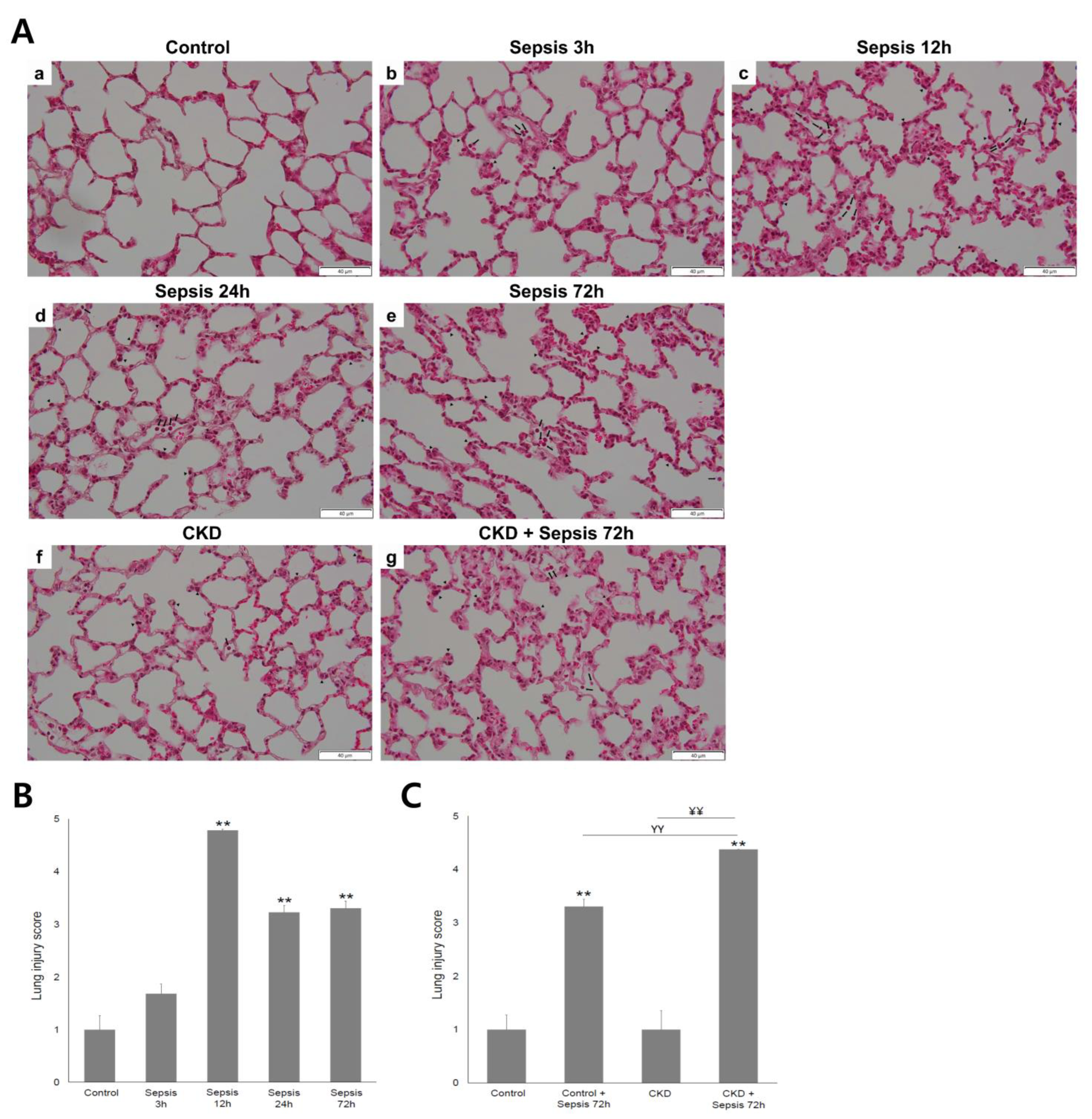
2.2. Severity of Acute Lung Injury
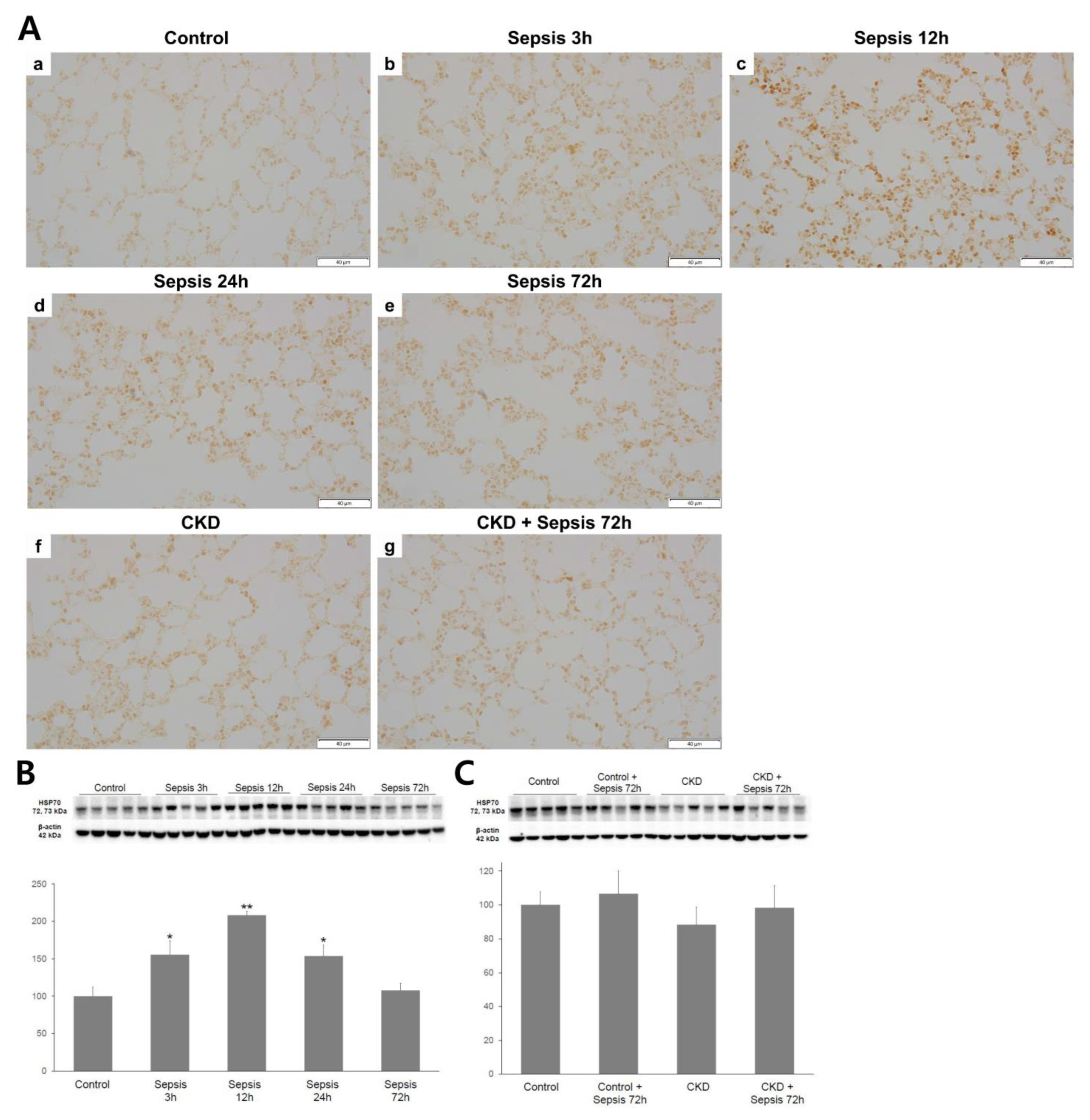
2.3. Lung HSP-70 Expression
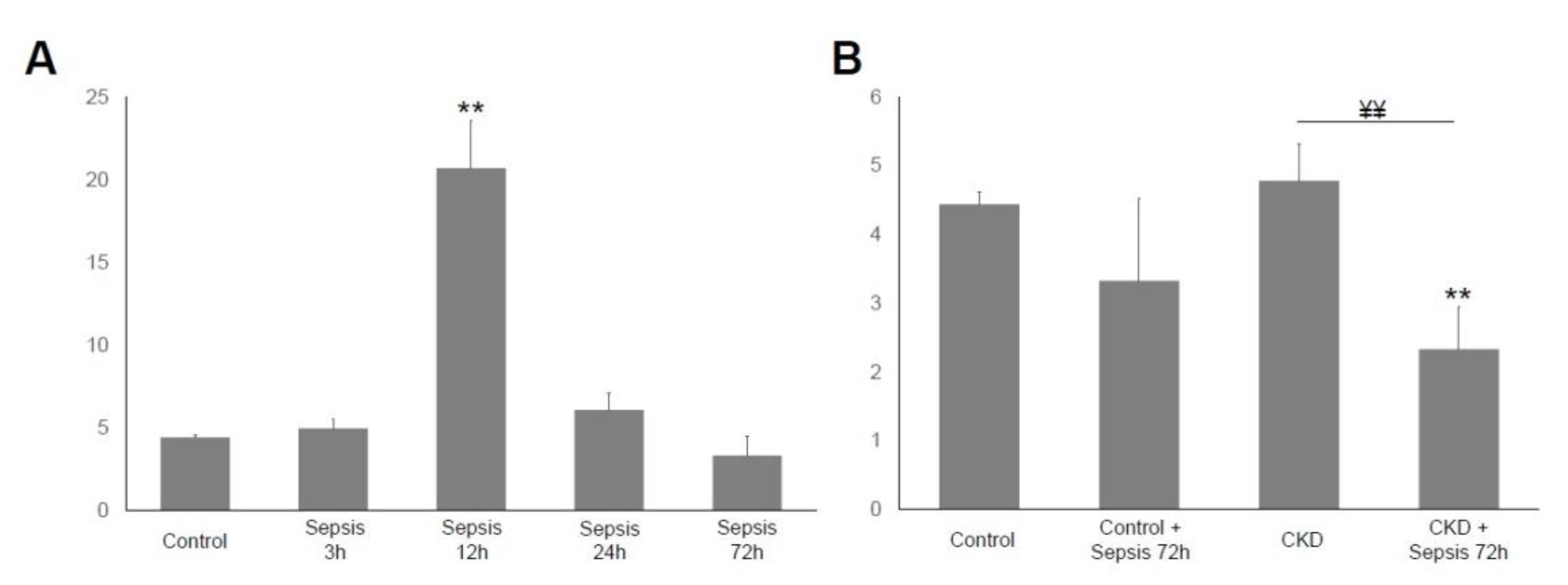
2.4. Serum IL-6 Immunoassay
3. Discussion
4. Materials and Methods
4.1. Animals
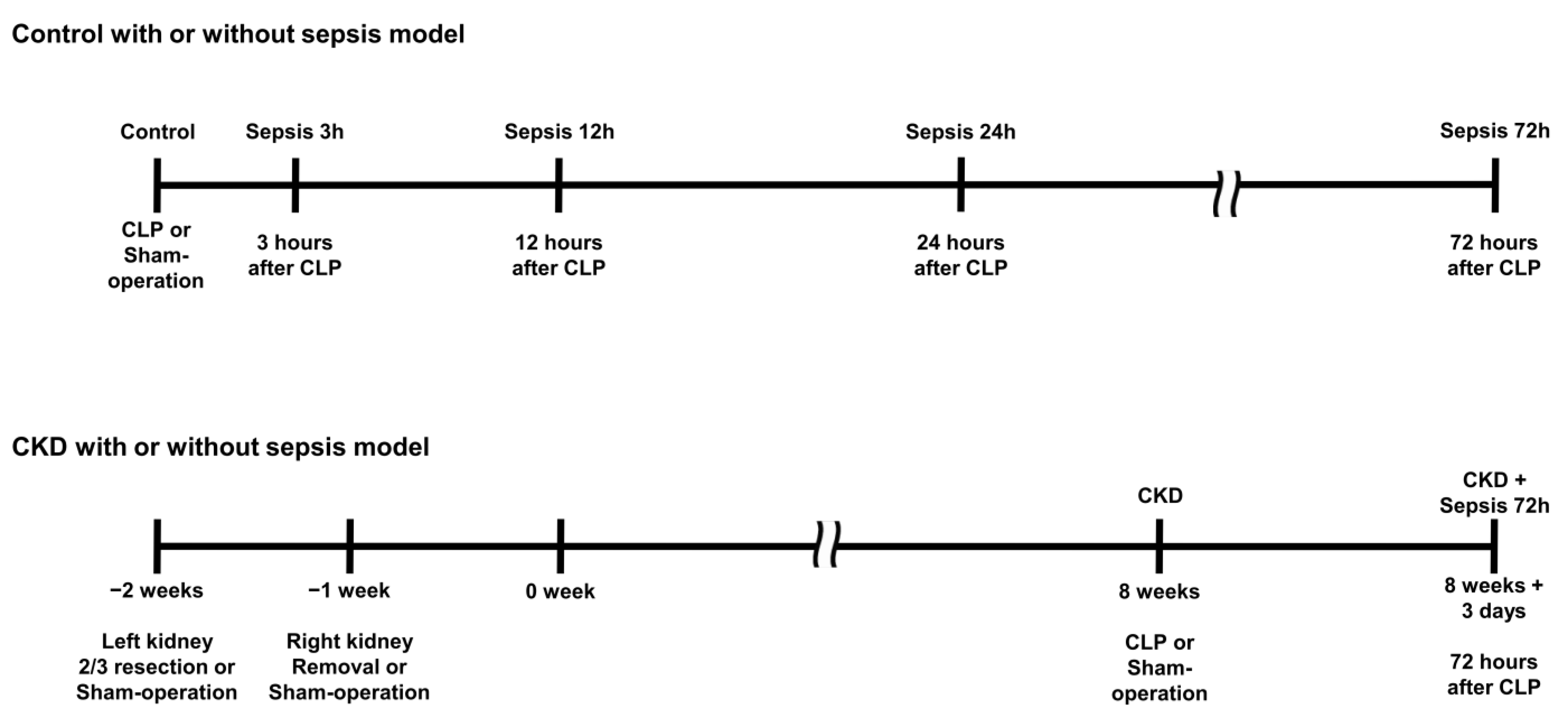
4.2. Sepsis and Chronic Kidney Disease Model
4.3. Blood and Urine Test
4.4. Histologic Analysis and Immunohistochemistry
4.5. Western Blotting
4.6. Interleukin-6 Immunoassay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dushianthan, A.; Grocott, M.P.W.; Postle, A.D.; Cusack, R. Acute respiratory distress syndrome and acute lung injury. Postgrad. Med. J. 2011, 87, 612–622. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.P.; Bernard, G.R. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet 2007, 369, 1553–1564. [Google Scholar] [CrossRef]
- Gajic, O.; Dabbagh, O.; Park, P.K.; Adesanya, A.; Chang, S.Y.; Hou, P.; Anderson, H., III; Hoth, J.J.; Mikkelsen, M.E.; Gentile, N.T.; et al. Early identification of patients at risk of acute lung injury: Evaluation of lung injury prediction score in a multicenter cohort study. Am. J. Respir. Crit. Care Med. 2011, 183, 462–470. [Google Scholar] [CrossRef]
- Mikkelsen, M.E.; Shah, C.V.; Meyer, N.J.; Gaieski, D.F.; Lyon, S.; Miltiades, A.N.; Goyal, M.; Fuchs, B.D.; Bellamy, S.L.; Christie, J.D. The epidemiology of acute respiratory distress syndrome in patients presenting to the emergency department with severe sepsis. Shock 2013, 40, 375. [Google Scholar] [CrossRef]
- Blondonnet, R.; Constantin, J.-M.; Sapin, V.; Jabaudon, M. A pathophysiologic approach to biomarkers in acute respiratory distress syndrome. Dis. Markers 2016, 2016, 3501373. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.R.; Shanley, T. (Eds.) Molecular Biology of Acute Lung Injury; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Wheeler, D.S.; Wong, H.R. Heat shock response and acute lung injury. Free Radic. Biol. Med. 2007, 42, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Mokdad, A.H.; Xian, H.; Yan, Y.; Li, T.; Maddukuri, G.; Tsai, C.-Y.; Floyd, T.; Al-Aly, Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef]
- Bello, A.K.; Alrukhaimi, M.; Ashuntantang, G.E.; Basnet, S.; Rotter, R.C.; Douthat, W.G.; Kazancioglu, R.; Köttgen, A.; Nangaku, M.; Powe, N.R.; et al. Complications of chronic kidney disease: Current state, knowledge gaps, and strategy for action. Kidney Int. Suppl. 2017, 7, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Maizel, J.; Deransy, R.; Dehedin, B.; Secq, E.; Zogheib, E.; Lewandowski, E.; Tribouilloy, C.; Massy, Z.A.; Choukroun, G.; Slama, M. Impact of non-dialysis chronic kidney disease on survival in patients with septic shock. BMC Nephrol. 2013, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Domenech, P.; Perez, T.; Saldarini, A.; Uad, P.; Musso, C.G. Kidney–lung pathophysiological crosstalk: Its charateristics and importance. Int. Urol. Nephrol. 2017, 49, 1211–1215. [Google Scholar] [CrossRef]
- Nemmar, A.; Karaca, T.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Ali, B.H. Lung oxidative stress, DNA damage, apoptosis, and fibrosis in adenine-induced chronic kidney disease in mice. Front. Physiol. 2017, 8, 896. [Google Scholar] [CrossRef]
- Mukai, H.; Ming, P.; Lindholm, B.; Heimbürger, O.; Barany, P.; Stenvinkel, P.; Qureshi, A.R. Lung dysfunction and mortality in patients with chronic kidney disease. Kidney Blood Press. Res. 2018, 43, 522–535. [Google Scholar] [CrossRef]
- Musiał, K.; Zwolińska, D. Heat shock proteins in chronic kidney disease. Pediatr. Nephrol. 2011, 26, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Marzec, L.; Zdrojewski, Z.; Liberek, T.; Bryl, E.; Chmielewski, M.; Witkowski, J.M.; Rutkowski, B. Expression of Hsp72 protein in chronic kidney disease patients. Scand. J. Urol. Nephrol. 2009, 43, 400–408. [Google Scholar] [CrossRef]
- Musial, K.; Szprynger, K.; Szczepańska, M.; Zwolińska, D. The heat shock protein profile in children with chronic kidney disease. Perit. Dial. Int. 2010, 30, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.P.; Hsu, M.E.; Chiou, Y.Y.; Hsu, H.Y.; Tsai, H.C.; Peng, Y.J.; Lu, C.Y.; Pan, C.Y.; Yu, W.C.; Chen, C.H.; et al. Comparative proteomic analysis of rat aorta in a subtotal nephrectomy model. Proteomics 2010, 10, 2429–2443. [Google Scholar] [CrossRef]
- Bao, Y.-W.; Yuan, Y.; Chen, J.-H.; Lin, W.-Q. Kidney disease models: Tools to identify mechanisms and potential therapeutic targets. Zool. Res. 2018, 39, 72. [Google Scholar]
- Gong, W.; Mao, S.; Yu, J.; Song, J.; Jia, Z.; Huang, S.; Zhang, A. NLRP3 deletion protects against renal fibrosis and attenuates mitochondrial abnormality in mouse with 5/6 nephrectomy. Am. J. Physiol. Renal Physiol. 2016, 310, F1081–F1088. [Google Scholar] [CrossRef] [PubMed]
- Neuhofer, W.; Lugmayr, K.; Fraek, M.-L.; Beck, F.-X. Regulated overexpression of heat shock protein 72 protects Madin-Darby canine kidney cells from the detrimental effects of high urea concentrations. J. Am. Soc. Nephrol. 2011, 12, 2565–2571. [Google Scholar] [CrossRef]
- Bender, T.; Riesenhuber, A.; Endemann, M.; Herkner, K.; Witowski, J.; Jörres, A.; Aufricht, C. Correlation between HSP-72 expression and IL-8 secretion in human mesothelial cells. Inter. J. Artif. Organs 2007, 30, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Neuhofer, W.; Fraek, M.L.; Ouyang, N.; Beck, F.X. Differential expression of heat shock protein 27 and 70 in renal papillary collecting duct and interstitial cells–implications for urea resistance. J. Physiol. 2005, 564, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.; Cantley, L.; Spokes, K.; Epstein, F.H. Effect of water diuresis and water restriction on expression of HSPs-27, -60 and-70 in rat kidney. Kidney Int. 1996, 50, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Bruemmer-Smith, S.; Stüber, F.; Schroeder, S. Protective functions of intracellular heat-shock protein (HSP) 70-expresion in patients with severe sepsis. Intensive Care Med. 2001, 27, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- McConnell, K.W.; Fox, A.C.; Clark, A.T.; Chang, N.-Y.N.; Dominguez, J.A.; Farris, A.B.; Buchman, T.G.; Hunt, C.R.; Coopersmith, C.M. The role of heat shock protein 70 in mediating age-dependent mortality in sepsis. J. Immunol. 2011, 186, 3718–3725. [Google Scholar] [CrossRef]
- Lin, H.-J.; Wang, C.-T.; Niu, K.-C.; Gao, C.; Li, Z.; Lin, M.-T.; Chang, C.-P. Hypobaric hypoxia preconditioning attenuates acute lung injury during high-altitude exposure in rats via up-regulating heat-shock protein 70. Clin. Sci. 2011, 121, 223–231. [Google Scholar] [CrossRef]
- Singleton, K.D.; Wischmeyer, P.E. Glutamine’s protection against sepsis and lung injury is dependent on heat shock protein 70 expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1839–R1845. [Google Scholar] [CrossRef]
- Park, W.Y.; Goodman, R.B.; Steinberg, K.P.; Ruzinski, J.T.; Radella, F.; Park, D.R.; Pugin, J.; Skerrett, S.J.; Hudson, L.D.; Martin, T.R. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2001, 164, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Van Heeckeren, A.M.; Tscheikuna, J.; Walenga, R.W.; Konstan, M.W.; Davis, P.B.; Erokwu, B.; Haxhiu, M.A.; Ferkol, T.W. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am. J. Respir. Crit. Care Med. 2000, 161, 271–279. [Google Scholar] [CrossRef]
- Doerschug, K.C.; Powers, L.S.; Monick, M.M.; Thorne, P.S.; Hunninghake, G.W. Antibiotics delay but do not prevent bacteremia and lung injury in murine sepsis. Crit. Care Med. 2004, 32, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wilmanski, J.; Wang, C.; Qiu, G.; Tahamont, M. Lung compartmentalization of inflammatory cells in sepsis. Inflammation 2000, 24, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Tomashefski, J.F., Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin. Chest Med. 2000, 21, 435–466. [Google Scholar] [CrossRef] [PubMed]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M. Acute Lung Injury in Animal Study group. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, W.J.; Choudhry, M.; Schwacha, M.G.; Kerby, J.D.; Rue, L.W., III; Bland, K.I.; Chaudry, I.H. Cecal ligation and puncture. Shock 2005, 4, 52–57. [Google Scholar] [CrossRef]
- Deitch, E.A. Rodent models of intra-abdominal infection. Shock 2005, 24, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Rittirsch, D.; Huber-Lang, M.S.; Flierl, M.A.; Ward, P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009, 4, 31–36. [Google Scholar] [CrossRef]
- Ruiz, S.; Vardon-Bounes, F.; Merlet-Dupuy, V.; Conil, J.-M.; Buléon, M.; Fourcade, O.; Tack, I.; Minville, V. Sepsis modeling in mice: Ligation length is a major severity factor in cecal ligation and puncture. Intensive Care Med. Exp. 2016, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Maddock, A.L.; Westenfelder, C. Urea induces the heat shock response in human neuroblastoma cells. J. Am. Soc. Nephrol. 1996, 7, 275–282. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, S.J.; Kwon, S.K.; Kim, H.-Y.; Jeon, H. Renal Tubular Glucagon-Like Peptide-1 Receptor Expression Is Increased in Early Sepsis but Reduced in Chronic Kidney Disease and Sepsis-Induced Kidney Injury. Int. J. Mol. Sci. 2019, 20, 6024. [Google Scholar] [CrossRef] [PubMed]
- Wen, H. Sepsis induced by cecal ligation and puncture. In Mouse Models of Innate Immunity; Humana Press: New York, NY, USA, 2013; pp. 117–124. [Google Scholar]
- Aeffner, F.; Bolon, B.; Davis, I.C. Mouse models of acute respiratory distress syndrome: A review of analytical a proaches, pathologic features, and common measurements. Toxicol. Pathol. 2015, 43, 1074–1092. [Google Scholar] [CrossRef] [PubMed]
| Control | Control with Sepsis at 72 h | CKD | CKD with Sepsis at 72 h | |
|---|---|---|---|---|
| Body weight (g) | 520.0 ± 21.3 | 451.2 ± 51.0 * | 425.5 ± 52.4 ** | 415.2 ± 33.3 ** |
| Urine volume for 24 h (mL) | 11.4 ± 1.3 | 11.6 ± 5.2 | 30.5 ± 5.2 **, ΥΥ | 23.4 ± 8.8 **, Υ |
| Urine pH | 7.43 ± 0.55 | 9.16 ± 0.12 ** | 7.56 ± 0.28 ΥΥ | 8.67 ± 0.27 **, ΥΥ, ¥¥ |
| Serum Cr (mg/dL) | 0.40 ± 0.03 | 0.54 ± 0.28 | 0.90 ± 0.26 ** | 0.90 ± 0.17 **, Υ |
| BUN (mg/dL) | 22.9 ± 3.9 | 25.85 ± 10.7 | 53.6 ± 14.4 **, Υ | 37.4 ± 4.5 **, ¥ |
| Serum albumin (mg/dL) | 2.3 ± 0.2 | 1.78 ± 02 ** | 2.1 ± 0.2 Υ | 1.9 ± 0.2 * |
| ClCr (mL/min/100 g) | 0.37 ± 0.06 | 0.32 ± 0.20 | 0.15 ± 0.04 ** | 0.21 ± 0.03 **, ¥¥ |
| Plasma CRP | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.-Y.; Kim, S.-J.; Woo, C.-G.; Kwon, S.-K.; Choe, K.-H.; Kim, E.-G.; Shin, Y.-M. Altered Lung Heat Shock Protein-70 Expression and Severity of Sepsis-Induced Acute Lung Injury in a Chronic Kidney Disease Rat Model. Int. J. Mol. Sci. 2023, 24, 5641. https://doi.org/10.3390/ijms24065641
Cho J-Y, Kim S-J, Woo C-G, Kwon S-K, Choe K-H, Kim E-G, Shin Y-M. Altered Lung Heat Shock Protein-70 Expression and Severity of Sepsis-Induced Acute Lung Injury in a Chronic Kidney Disease Rat Model. International Journal of Molecular Sciences. 2023; 24(6):5641. https://doi.org/10.3390/ijms24065641
Chicago/Turabian StyleCho, Jun-Yeun, Seung-Jung Kim, Chang-Gok Woo, Soon-Kil Kwon, Kang-Hyeon Choe, Eung-Gook Kim, and Yoon-Mi Shin. 2023. "Altered Lung Heat Shock Protein-70 Expression and Severity of Sepsis-Induced Acute Lung Injury in a Chronic Kidney Disease Rat Model" International Journal of Molecular Sciences 24, no. 6: 5641. https://doi.org/10.3390/ijms24065641
APA StyleCho, J.-Y., Kim, S.-J., Woo, C.-G., Kwon, S.-K., Choe, K.-H., Kim, E.-G., & Shin, Y.-M. (2023). Altered Lung Heat Shock Protein-70 Expression and Severity of Sepsis-Induced Acute Lung Injury in a Chronic Kidney Disease Rat Model. International Journal of Molecular Sciences, 24(6), 5641. https://doi.org/10.3390/ijms24065641







