Integrated ATAC-Seq and RNA-Seq Data Analysis to Reveal OsbZIP14 Function in Rice in Response to Heat Stress
Abstract
1. Introduction
2. Results
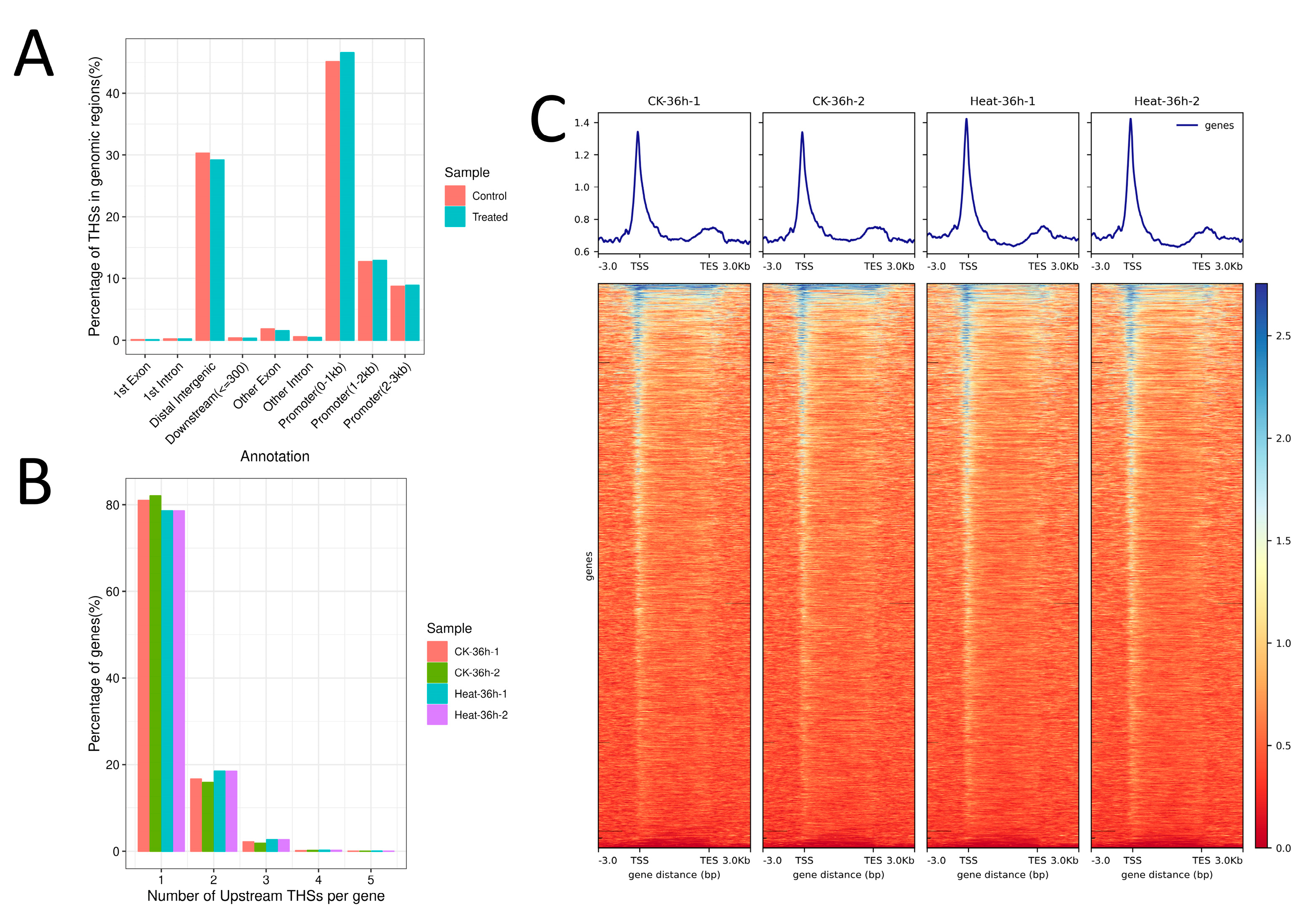
2.1. Analysis of the Chromatin Accessibility in Rice under Heat Stress
2.2. Analysis of the Enriched Motif in ATAC Peaks in Rice under Heat Stress
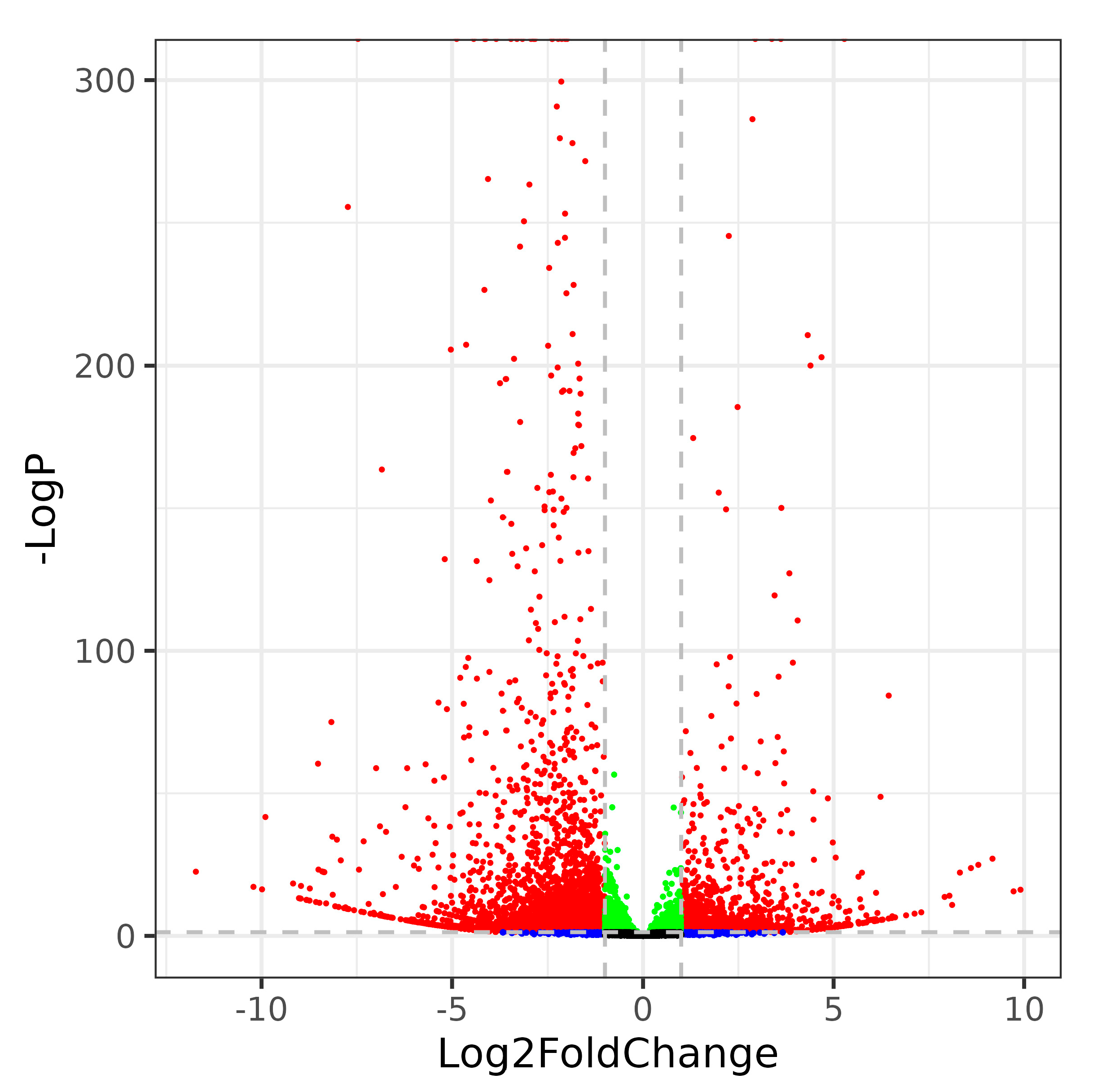
2.3. Identification of DEGs in Rice under Heat Stress
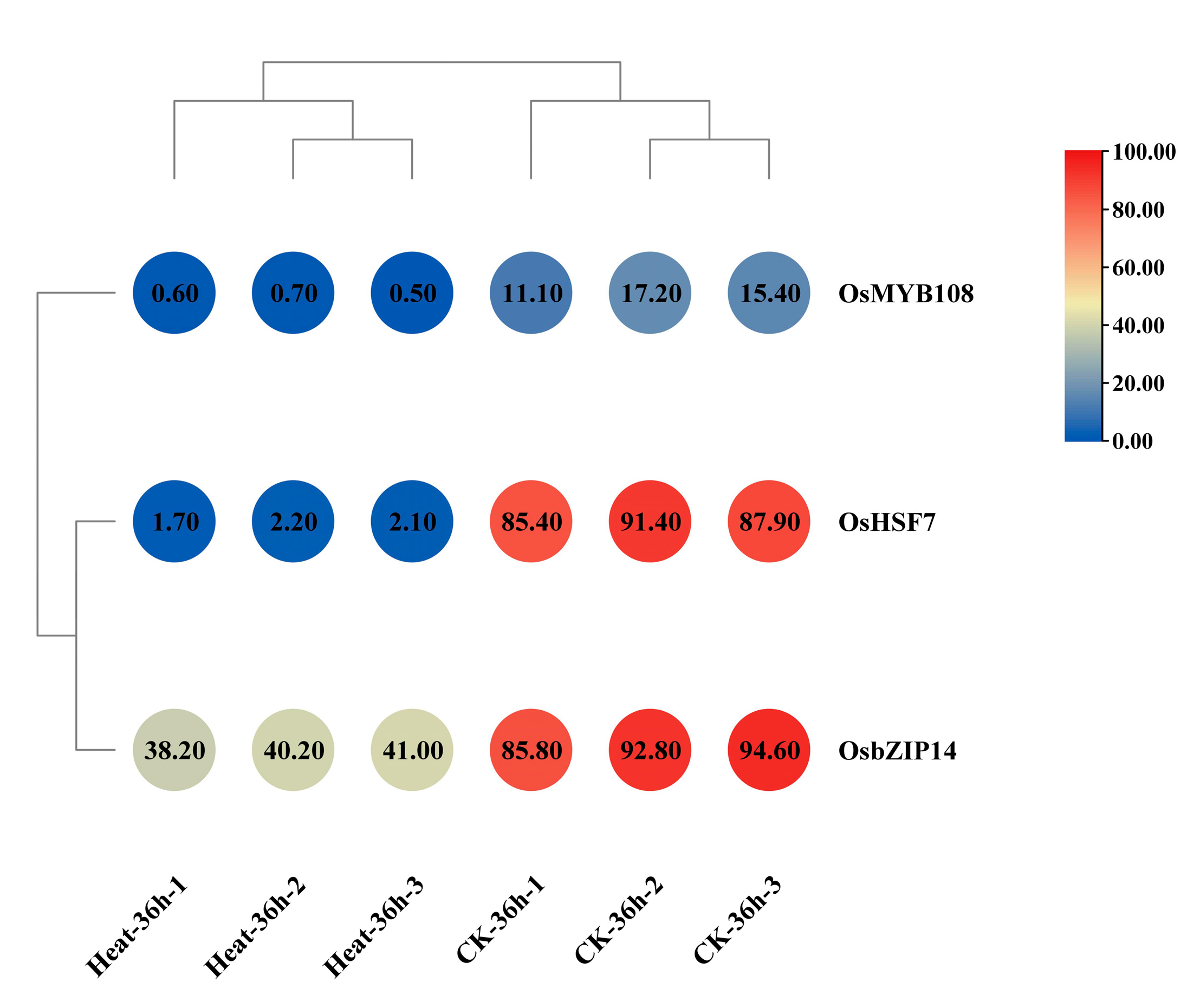
2.4. Verification of the Gene Expression of the Key TF Genes in Rice under Heat Stress
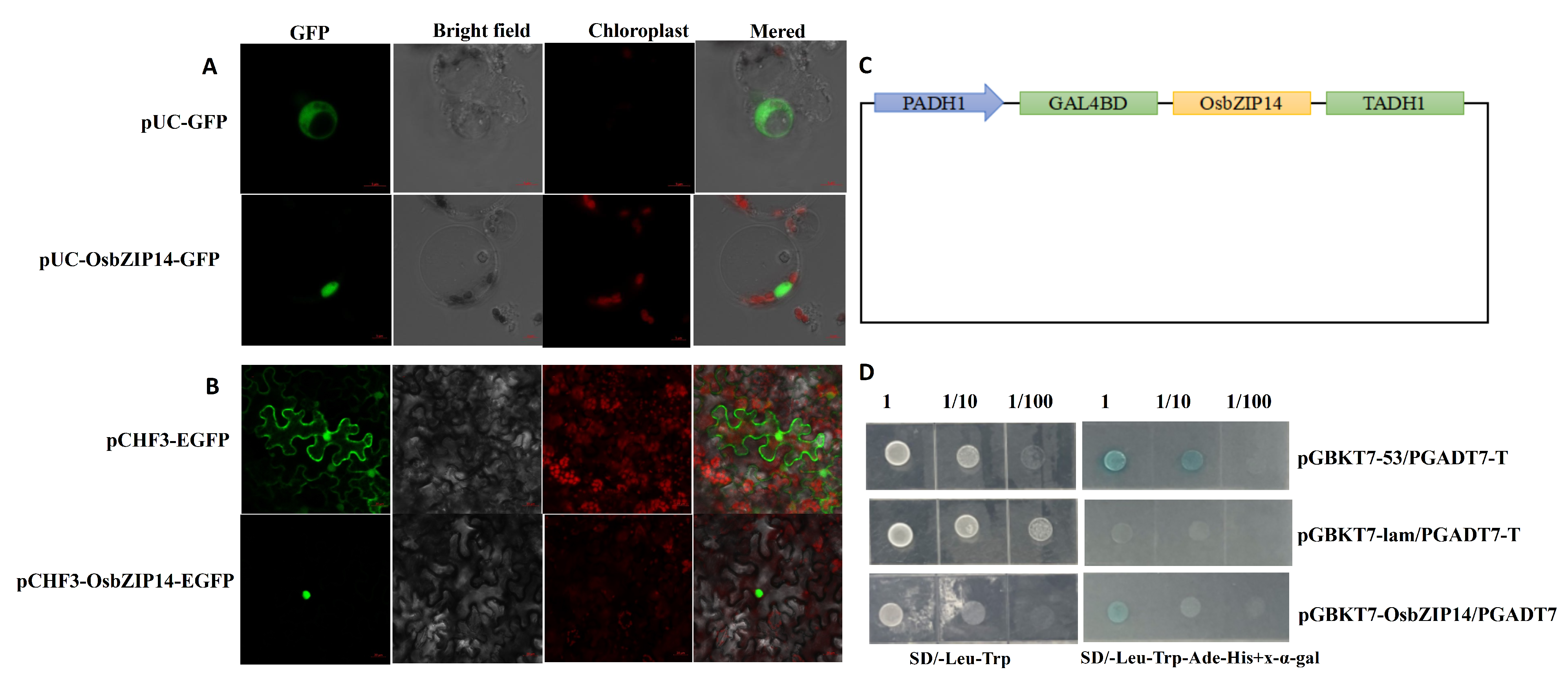
2.5. Sub-Cellular Localization and Transcriptional Activity of OsbZIP14
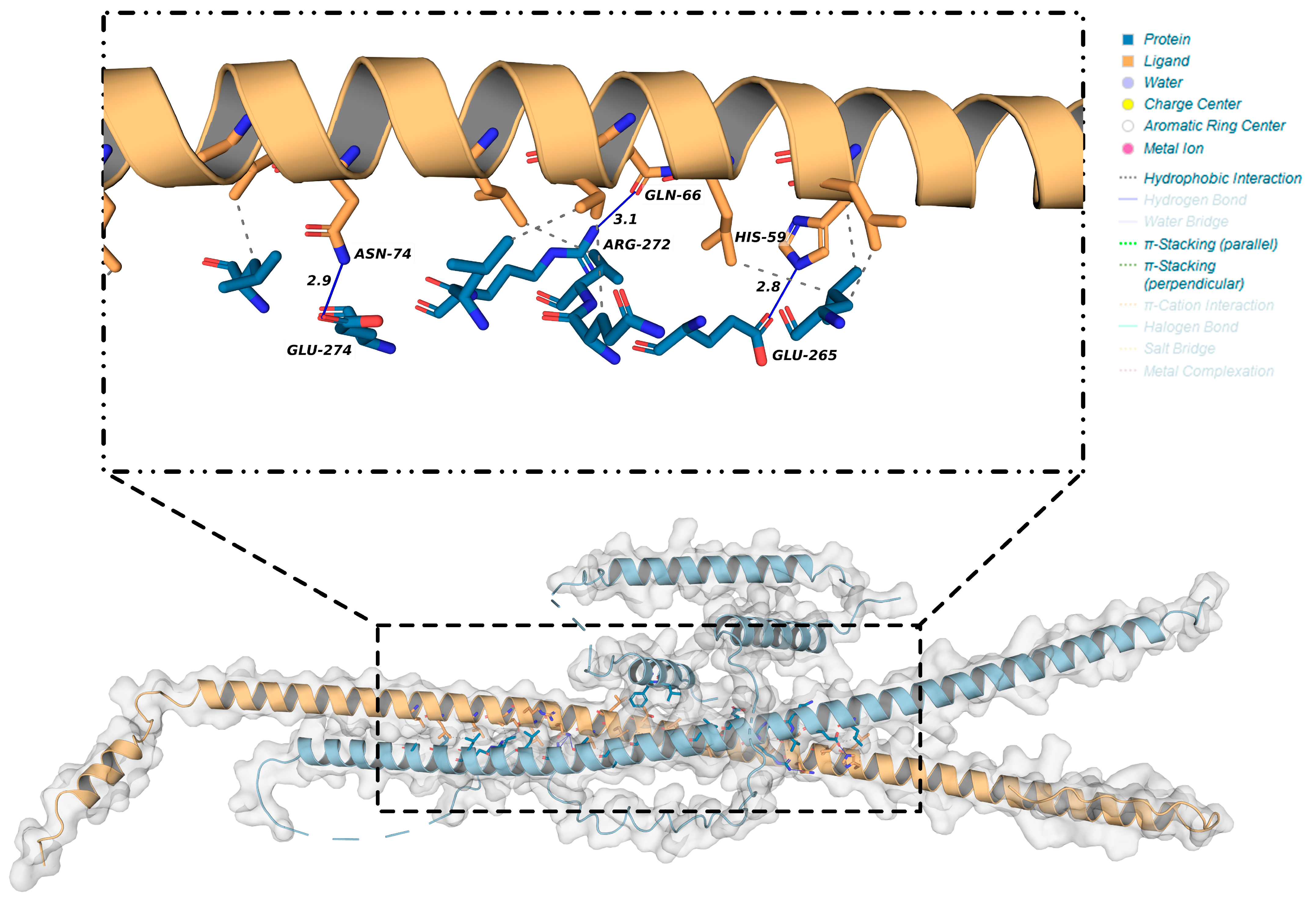
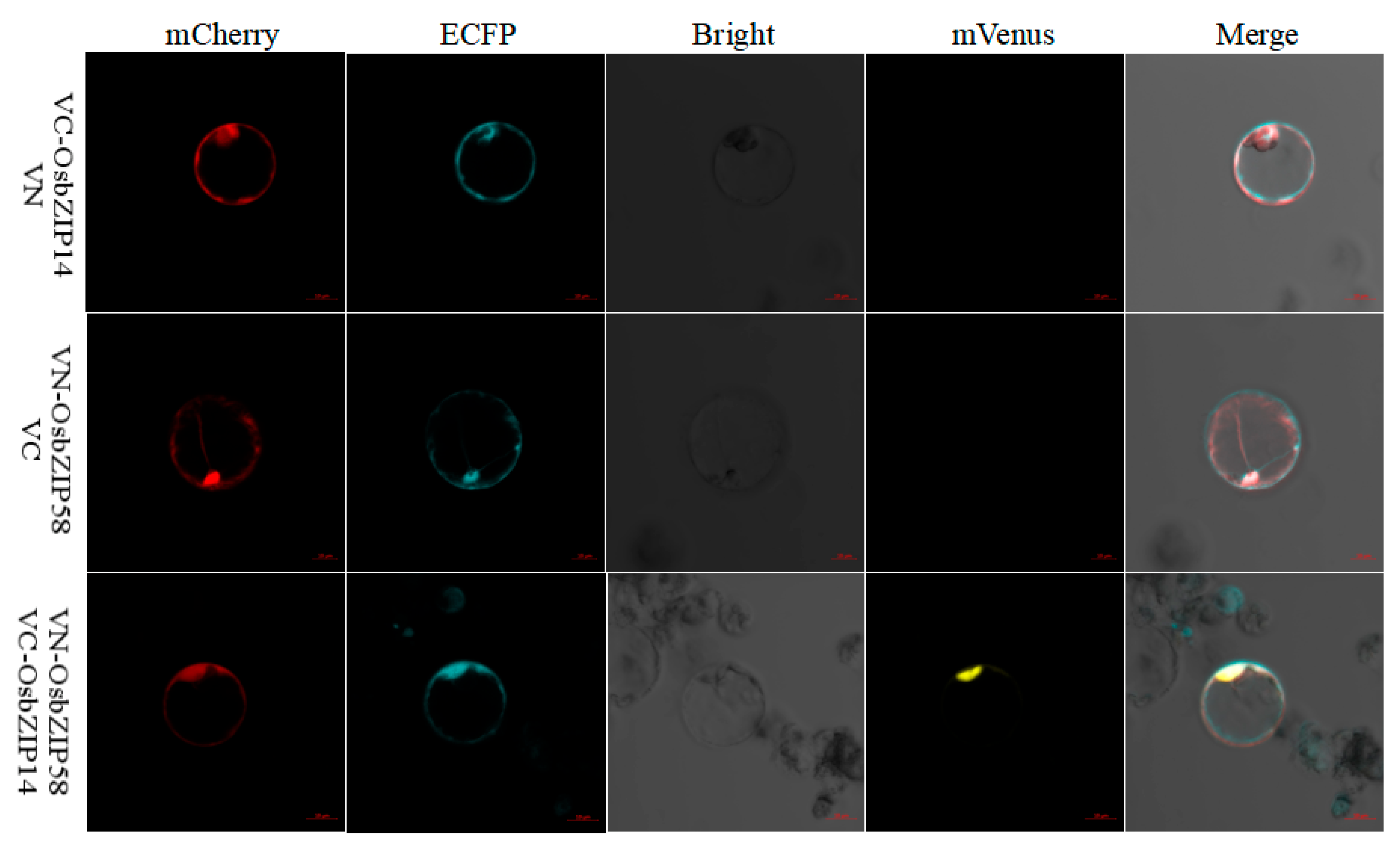
2.6. Interacting Proteins of OsbZIP14
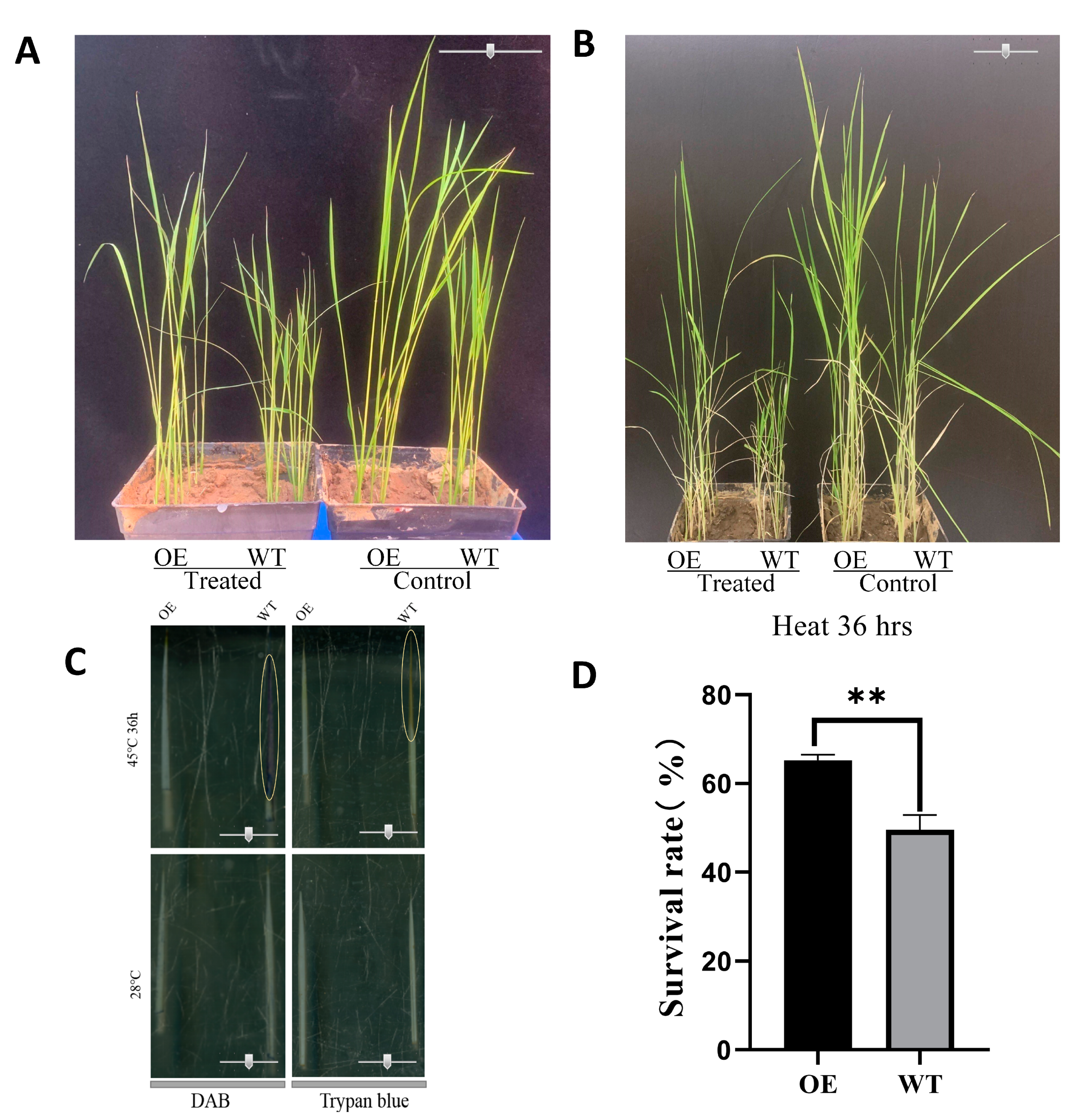
2.7. Validation of the Functions of OsbZIP14 Gene in Rice
3. Discussion
4. Materials and Methods
4.1. Data Collection Procedures
4.2. RNA-seq Data Analysis
4.3. ATAC-seq Data Analysis
4.4. Experimental Design and Procedures
4.5. RT-qPCR Analysis
4.6. Vector Construction and Genetic Transformation
4.7. Subcellular Localization Analysis
4.8. In-Silico Protein-Protein Interaction Analysis
4.9. Yeast Two-Hybrid (Y2H)
4.10. BiFC Assay
4.11. DAB and Trypan Blue Staining
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sreenivasulu, N.; Butardo, V.M.; Misra, G.; Cuevas, R.P.; Anacleto, R.; Kishor, P.B.K. Designing Climate-Resilient Rice with Ideal Grain Quality Suited for High-Temperature Stress. J. Exp. Bot. 2015, 66, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2014 Synthesis Report Summary for Policymakers; IPCC: Geneva, Switzerland, 2014; pp. 1–31. [Google Scholar]
- Song, Y.; Wang, C.; Linderholm, H.W.; Fu, Y.; Cai, W.; Xu, J.; Zhuang, L.; Wu, M.; Shi, Y.; Wang, G.; et al. The Negative Impact of Increasing Temperatures on Rice Yields in Southern China. Sci. Total Environ. 2022, 820, 153262. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Ling, X.; Huang, J.; Peng, S. Meta-Analysis and Dose-Response Analysis of High Temperature Effects on Rice Yield and Quality. Environ. Exp. Bot. 2017, 141, 1–9. [Google Scholar] [CrossRef]
- Cheng, F.; Zhong, L.; Zhao, N.; Liu, Y.; Zhang, G. Temperature Induced Changes in the Starch Components and Biosynthetic Enzymes of Two Rice Varieties. Plant Growth Regul. 2005, 46, 87–95. [Google Scholar] [CrossRef]
- Yamakawa, H.; Hirose, T.; Kuroda, M.; Yamaguchi, T. Comprehensive Expression Profiling of Rice Grain Filling-Related Genes under High Temperature Using DNA Microarray. Plant Physiol. 2007, 144, 258–277. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Hu, M.; Wang, Z.; Hua, H.; Yin, C.; Zeng, H. Different Effects of Night versus Day High Temperature on Rice Quality and Accumulation Profiling of Rice Grain Proteins during Grain Filling. Plant Cell Rep. 2011, 30, 1641–1659. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, L.; Dai, J.S.; Zhang, C.Q.; Li, J.; Gu, M.H.; Liu, Q.Q.; Zhu, Y. Major QTLs Reduce the Deleterious Effects of High Temperature on Rice Amylose Content by Increasing Splicing Efficiency of Wx Pre-mRNA. Theor. Appl. Genet. 2013, 127, 273–282. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Feng, M.; Zhu, Y. Suppression of OsMADS7 in Rice Endosperm Stabilizes Amylose Content under High Temperature Stress. Plant Biotechnol. J. 2018, 16, 18–26. [Google Scholar] [CrossRef]
- Ray, S.; Dansana, P.K.; Bhaskar, A.; Giri, J.; Kapoor, S.; Khurana, J.P.; Tyagi, A.K. Emerging Trends in Functional Genomics for Stress Tolerance in Crop Plants. In Plant Stress Biology: From Genomics to Systems Biology; John Wiley & Sons: New York, NY, USA, 2010; pp. 37–63. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, H.; Kong, L.; Gao, G.; Luo, J. PlantTFDB 3.0: A Portal for the Functional and Evolutionary Study of Plant Transcription Factors. Nucleic Acids Res. 2014, 42, D1182–D1187. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Shao, H.; Tang, X. Recent Advances in Utilizing Transcription Factors to Improve Plant Abiotic Stress Tolerance by Transgenic Technology. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Gujjar, R.S.; Akhtar, M.; Singh, M. Transcription Factors in Abiotic Stress Tolerance. Ind. J. Plant Physiol. 2014, 19, 306–316. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, Z.; Jiang, H.; Wang, Z.; Wu, F.; Xiong, Y.; Yao, J. A Heat Stress Responsive NAC Transcription Factor Heterodimer Plays Key Roles in Rice Grain Filling. J. Exp. Bot. 2021, 72, 2947–2964. [Google Scholar] [CrossRef]
- Li, Z.; Tang, J.; Srivastava, R.; Bassham, D.C.; Howell, S.H. The Transcription Factor BZIP60 Links the Unfolded Protein Response to the Heat Stress Response in Maize. Plant Cell 2020, 32, 3559–3575. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, S.; Wu, J.; Sun, X.; Ma, A. Heat Stress in Macrofungi: Effects and Response Mechanisms. Appl. Microbiol. Biotechnol. 2021, 105, 7567–7576. [Google Scholar] [CrossRef]
- Wang, C.T.; Ru, J.N.; Liu, Y.W.; Li, M.; Zhao, D.; Yang, J.F.; Fu, J.D.; Xu, Z.S. Maize Wrky Transcription Factor Zmwrky106 Confers Drought and Heat Tolerance in Transgenic Plants. Int. J. Mol. Sci. 2018, 19, 3046. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2010, 10, 57–63. [Google Scholar] [CrossRef]
- Anderson, J.T.; Mitchell-Olds, T. Ecological Genetics and Genomics of Plant Defences: Evidence and Approaches. Funct. Ecol. 2011, 25, 312–324. [Google Scholar] [CrossRef]
- Li, M.W.; Qi, X.; Ni, M.; Lam, H.M. Silicon Era of Carbon-Based Life: Application of Genomics and Bioinformatics in Crop Stress Research. Int. J. Mol. Sci. 2013, 14, 11444–11483. [Google Scholar] [CrossRef]
- Chappell, L.; Russell, A.J.C.; Voet, T. Single-Cell (Multi) Omics Technologies. Annu. Rev. Genom. Hum. Genet. 2018, 19, 15–41. [Google Scholar] [CrossRef]
- Buenrostro, J.D.; Giresi, P.G.; Zaba, L.C.; Chang, H.Y.; Greenleaf, W.J. Transposition of Native Chromatin for Fast and Sensitive Epigenomic Profiling of Open Chromatin, DNA-Binding Proteins and Nucleosome Position. Nat. Methods 2013, 10, 1213–1218. [Google Scholar] [CrossRef]
- Lu, Z.; Hofmeister, B.T.; Vollmers, C.; DuBois, R.M.; Schmitz, R.J. Combining ATAC-Seq with Nuclei Sorting for Discovery of Cis-Regulatory Regions in Plant Genomes. Nucleic Acids Res. 2017, 45, e41. [Google Scholar] [CrossRef] [PubMed]
- Sijacic, P.; Bajic, M.; McKinney, E.C.; Meagher, R.B.; Deal, R.B. Changes in Chromatin Accessibility between Arabidopsis Stem Cells and Mesophyll Cells Illuminate Cell Type-Specific Transcription Factor Networks. Plant J. 2018, 94, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, O.; Hafemeister, C.; Plessis, A.; Holloway-Phillips, M.M.; Pham, G.M.; Nicotra, A.B.; Gregorio, G.B.; Krishna Jagadish, S.V.; Septiningsih, E.M.; Bonneau, R.; et al. EGRINs (Environmental Gene Regulatory Influence Networks) in Rice That Function in the Response to Water Deficit, High Temperature, and Agricultural Environments. Plant Cell 2016, 28, 2365–2384. [Google Scholar] [CrossRef] [PubMed]
- Bajic, M.; Maher, K.A.; Deal, R.B. Plant Chromatin Dynamics. Methods Mol. Biol. 2018, 1675, 183–201. [Google Scholar] [CrossRef]
- Concia, L.; Veluchamy, A.; Ramirez-Prado, J.S.; Martin-Ramirez, A.; Huang, Y.; Perez, M.; Domenichini, S.; Rodriguez Granados, N.Y.; Kim, S.; Blein, T.; et al. Wheat Chromatin Architecture Is Organized in Genome Territories and Transcription Factories. Genome Biol. 2020, 21, 104. [Google Scholar] [CrossRef]
- Ren, C.; Li, H.; Wang, Z.; Dai, Z.; Lecourieux, F.; Kuang, Y.; Xin, H.; Li, S.; Liang, Z. Characterization of Chromatin Accessibility and Gene Expression upon Cold Stress Reveals That the RAV1 Transcription Factor Functions in Cold Response in Vitis Amurensis. Plant Cell Physiol. 2021, 62, 1615–1629. [Google Scholar] [CrossRef]
- Wang, S.; He, J.; Deng, M.; Wang, C.; Wang, R.; Yan, J.; Luo, M.; Ma, F.; Guan, Q.; Xu, J. Integrating ATAC-Seq and RNA-Seq Reveals the Dynamics of Chromatin Accessibility and Gene Expression in Apple Response to Drought. Int. J. Mol. Sci. 2022, 23, 11191. [Google Scholar] [CrossRef]
- Liu, J.G.; Qin, Q.L.; Zhang, Z.; Peng, R.H.; Xiong, A.S.; Chen, J.M.; Yao, Q.H. OsHSF7 Gene in Rice, Oryza Sativa L., Encodes a Transcription Factor That Functions as a High Temperature Receptive and Responsive Factor. BMB Rep. 2009, 42, 16–21. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhou, Y.; Liu, Z.; Zhang, L.; Song, G.; Guo, Z.; Wang, W.; Qu, X.; Zhu, Y.; Yang, D. An Alternatively Spliced Heat Shock Transcription Factor, OsHSFA2dI, Functions in the Heat Stress-Induced Unfolded Protein Response in Rice. Plant Biol. 2015, 17, 419–429. [Google Scholar] [CrossRef]
- Yang, A.; Dai, X.; Zhang, W.H. A R2R3-Type MYB Gene, OsMYB2, Is Involved in Salt, Cold, and Dehydration Tolerance in Rice. J. Exp. Bot. 2012, 63, 2541–2556. [Google Scholar] [CrossRef]
- Nijhawan, A.; Jain, M.; Tyagi, A.K.; Khurana, J.P. Genomic Survey and Gene Expression Analysis of the Basic Leucine Zipper Transcription Factor Family in Rice. Plant Physiol. 2008, 146, 333–350. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Ou, S.; Wang, W.; Liu, L.; Wu, Y.; Chu, C.; Wang, X. OsbZIP71, a BZIP Transcription Factor, Confers Salinity and Drought Tolerance in Rice. Plant Mol. Biol. 2014, 84, 19–36. [Google Scholar] [CrossRef]
- Gao, S.-Q.; Chen, M.; Xu, Z.-S.; Zhao, C.-P.; Li, L.; Xu, H.; Tang, Y.; Zhao, X.; Ma, Y.-Z. The Soybean GmbZIP1 Transcription Factor Enhances Multiple Abiotic Stress Tolerances in Transgenic Plants. Plant Mol. Biol. 2011, 75, 537–553. [Google Scholar] [CrossRef]
- Zhao, Y.; Chan, Z.; Gao, J.; Xing, L.; Cao, M.; Yu, C.; Hu, Y.; You, J.; Shi, H.; Zhu, Y.; et al. ABA Receptor PYL9 Promotes Drought Resistance and Leaf Senescence. Proc. Natl. Acad. Sci. USA 2016, 113, 1949–1954. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Y.; Zhu, Y.; Tang, J.; Hu, B.; Liu, L.; Ou, S.; Wu, H.; Sun, X.; Chu, J.; et al. OsNAP Connects Abscisic Acid and Leaf Senescence by Fine-Tuning Abscisic Acid Biosynthesis and Directly Targeting Senescence-Associated Genes in Rice. Proc. Natl. Acad. Sci. USA 2014, 111, 10013–10018. [Google Scholar] [CrossRef]
- He, Y.; Li, L.; Zhang, Z.; Wu, J.L. Identification and Comparative Analysis of Premature Senescence Leaf Mutants in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2018, 19, 2339. [Google Scholar] [CrossRef]
- Sathe, A.P.; Su, X.; Chen, Z.; Chen, T.; Wei, X.; Tang, S.; Zhang, X.B.; Wu, J.L. Identification and Characterization of a Spotted-Leaf Mutant Spl40 with Enhanced Bacterial Blight Resistance in Rice. Rice 2019, 12, 68. [Google Scholar] [CrossRef]
- Yan, Q.; Wu, F.; Ma, T.; Zong, X.; Ma, Q.; Li, J.; Zhao, Y.; Wang, Y.; Zhang, J. Comprehensive Analysis of BZIP Transcription Factors Uncovers Their Roles during Dimorphic Floret Differentiation and Stress Response in Cleistogenes Songorica. BMC Genom. 2019, 20, 760. [Google Scholar] [CrossRef]
- Para, A.; Li, Y.; Marshall-Coloń, A.; Varala, K.; Francoeur, N.J.; Moran, T.M.; Edwards, M.B.; Hackley, C.; Bargmann, B.O.R.; Birnbaum, K.D.; et al. Hit-and-Run Transcriptional Control by BZIP1 Mediates Rapid Nutrient Signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 10371–10376. [Google Scholar] [CrossRef]
- Yang, W.; Xu, P.; Zhang, J.; Zhang, S.; Li, Z.; Yang, K.; Chang, X.; Li, Y. OsbZIP60-Mediated Unfolded Protein Response Regulates Grain Chalkiness in Rice. J. Genet. Genom. 2022, 49, 414–426. [Google Scholar] [CrossRef]
- Yan, Y.; Tao, H.; He, J.; Huang, S.Y. The HDOCK Server for Integrated Protein–Protein Docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein-Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A. PDBsum New Things. Nucleic Acids Res. 2009, 37, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.E.; Kamran Haider, M. Hydrogen Bonds in Proteins: Role and Strength. eLS 2010. [Google Scholar] [CrossRef]
- Jiang, L.; Lai, L. CH···O Hydrogen Bonds at Protein-Protein Interfaces. J. Biol. Chem. 2002, 277, 37732–37740. [Google Scholar] [CrossRef]
- Donald, J.E.; Kulp, D.W.; DeGrado, W.F. Salt Bridges: Geometrically Specific, Designable Interactions. Proteins 2011, 79, 898–915. [Google Scholar] [CrossRef]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef]
- Yamamoto, M.P.; Onodera, Y.; Touno, S.M.; Takaiwa, F. Synergism between RPBF Dof and RISBZ1 BZIP Activators in the Regulation of Rice Seed Expression Genes. Plant Physiol. 2006, 141, 1694–1707. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Zhang, H.; Wang, L.; Zhu, Z.; Gao, J.; Li, C.; Zhu, Y. High Temperature Inhibits the Accumulation of Storage Materials by Inducing Alternative Splicing of OsbZIP58 during Filling Stage in Rice. Plant Cell Environ. 2020, 43, 1879–1896. [Google Scholar] [CrossRef]
- Daccord, N.; Celton, J.M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; Van De Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R.; et al. High-Quality de Novo Assembly of the Apple Genome and Methylome Dynamics of Early Fruit Development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wang, C.; Shen, L.; Fu, Y.; Yan, C.; Wang, K. A Simple CRISPR/Cas9 System for Multiplex Genome Editing in Rice. J. Genet. Genom. 2015, 42, 703–706. [Google Scholar] [CrossRef]
- He, F.; Zhang, F.; Sun, W.; Ning, Y.; Wang, G.L. A Versatile Vector Toolkit for Functional Analysis of Rice Genes. Rice 2018, 11, 27. [Google Scholar] [CrossRef]



| Enriched Motif | TF (Arabidopsis) | TF (Rice) | Gene ID |
|---|---|---|---|
 | HSFA1E | OsHSF7 | Os03g0161900 |
 | MYB108 | OsMYB2 | Os12g0175400 |
 | HSF3 | OsHsfB2b | Os08g0546800 |
 | MYB63 | OsMYB30 | Os02g0624300 |
 | bZIP2 | OsbZIP14 | Os02g0132500 |
| Enriched Motif | TF (Arabidopsis) | TF (Rice) | Gene ID | Log2FC (Nip) | FDR | p-Value |
|---|---|---|---|---|---|---|
 | HSFA1E | OsHSF7 | Os03g0161900 | −5.48 | 0.0000 | 1.00 × 10−18 |
 | MYB108 | OsMYB2 | Os12g0175400 | −4.65 | 0.0000 | 1.00 × 10−6 |
 | bZIP2 | OsbZIP14 | Os02g0132500 | −1.20 | 0.0000 | 1.00 × 10−7 |
| Rank | Model1 | Model2 | Model3 | Model4 | Model5 | Model6 | Model7 | Model8 | Model9 | Model10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Docking Score | −441.33 | −436.81 | −378.22 | −350.78 | −345.74 | −345.02 | −336.86 | −330.27 | −326.05 | −324.79 |
| Confidence Score | 0.9971 | 0.9968 | 0.9897 | 0.9823 | 0.9804 | 0.9802 | 0.9767 | 0.9735 | 0.9713 | 0.9706 |
| Ligand rmsd (Å) | 120.03 | 122.27 | 24.74 | 119.18 | 26.21 | 25.07 | 38.82 | 50.8 | 22.74 | 119.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, F.; Zheng, Y.; Lin, Y.; Woldegiorgis, S.T.; Xu, S.; Feng, C.; Huang, G.; Shen, H.; Xu, Y.; Kabore, M.A.F.; et al. Integrated ATAC-Seq and RNA-Seq Data Analysis to Reveal OsbZIP14 Function in Rice in Response to Heat Stress. Int. J. Mol. Sci. 2023, 24, 5619. https://doi.org/10.3390/ijms24065619
Qiu F, Zheng Y, Lin Y, Woldegiorgis ST, Xu S, Feng C, Huang G, Shen H, Xu Y, Kabore MAF, et al. Integrated ATAC-Seq and RNA-Seq Data Analysis to Reveal OsbZIP14 Function in Rice in Response to Heat Stress. International Journal of Molecular Sciences. 2023; 24(6):5619. https://doi.org/10.3390/ijms24065619
Chicago/Turabian StyleQiu, Fuxiang, Yingjie Zheng, Yao Lin, Samuel Tareke Woldegiorgis, Shichang Xu, Changqing Feng, Guanpeng Huang, Huiling Shen, Yinying Xu, Manegdebwaoga Arthur Fabrice Kabore, and et al. 2023. "Integrated ATAC-Seq and RNA-Seq Data Analysis to Reveal OsbZIP14 Function in Rice in Response to Heat Stress" International Journal of Molecular Sciences 24, no. 6: 5619. https://doi.org/10.3390/ijms24065619
APA StyleQiu, F., Zheng, Y., Lin, Y., Woldegiorgis, S. T., Xu, S., Feng, C., Huang, G., Shen, H., Xu, Y., Kabore, M. A. F., Ai, Y., Liu, W., & He, H. (2023). Integrated ATAC-Seq and RNA-Seq Data Analysis to Reveal OsbZIP14 Function in Rice in Response to Heat Stress. International Journal of Molecular Sciences, 24(6), 5619. https://doi.org/10.3390/ijms24065619








