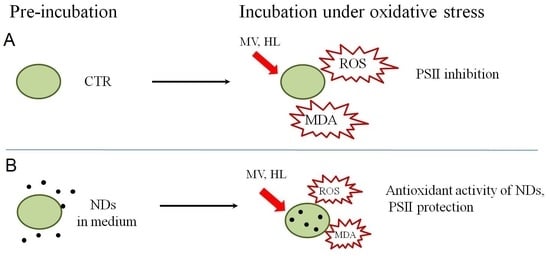
Nanodiamond Particles Reduce Oxidative Stress Induced by Methyl Viologen and High Light in the Green Alga Chlamydomonas reinhardtii
Abstract
1. Introduction
2. Results
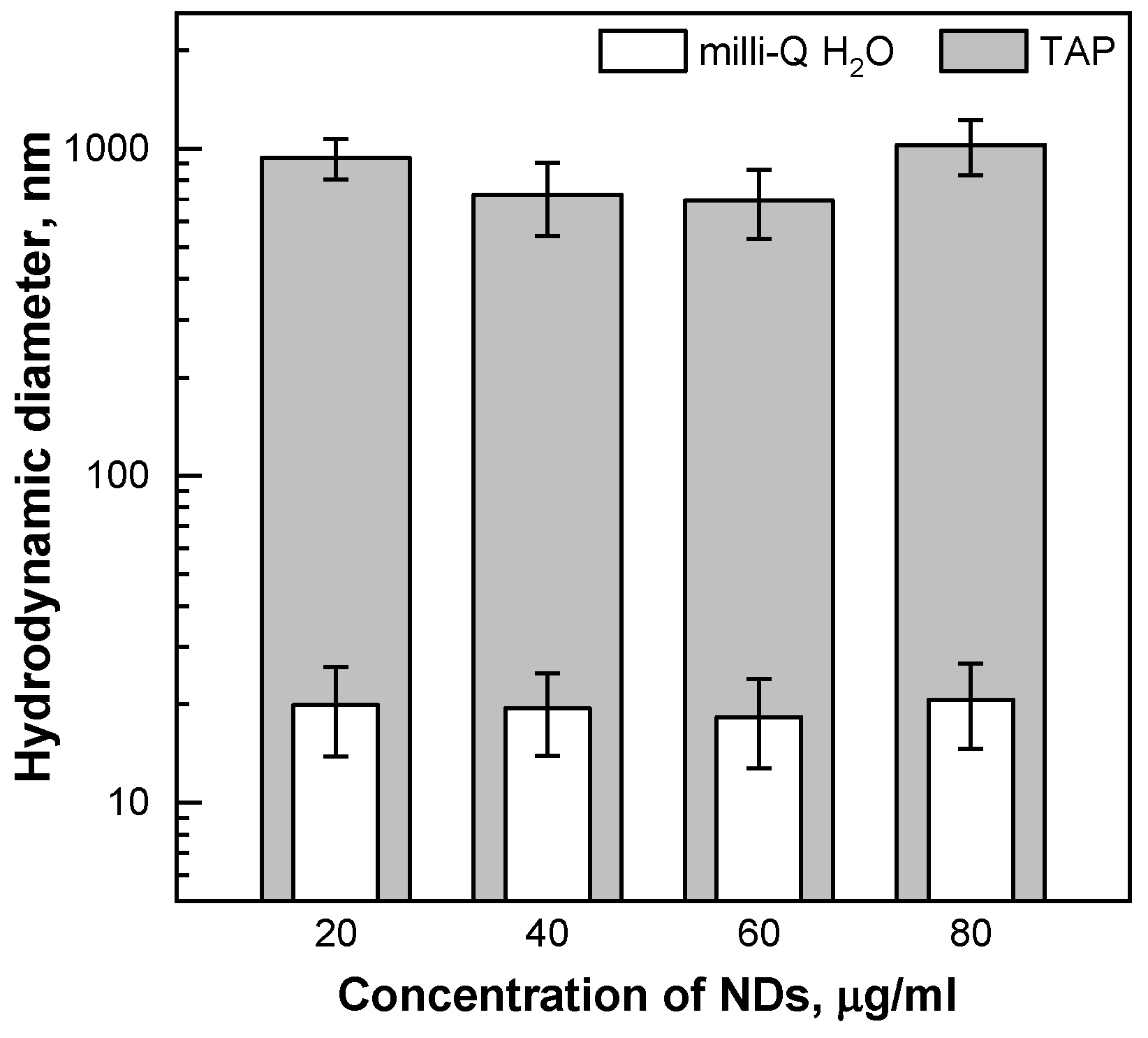
2.1. Characterisation of the Nanomaterial
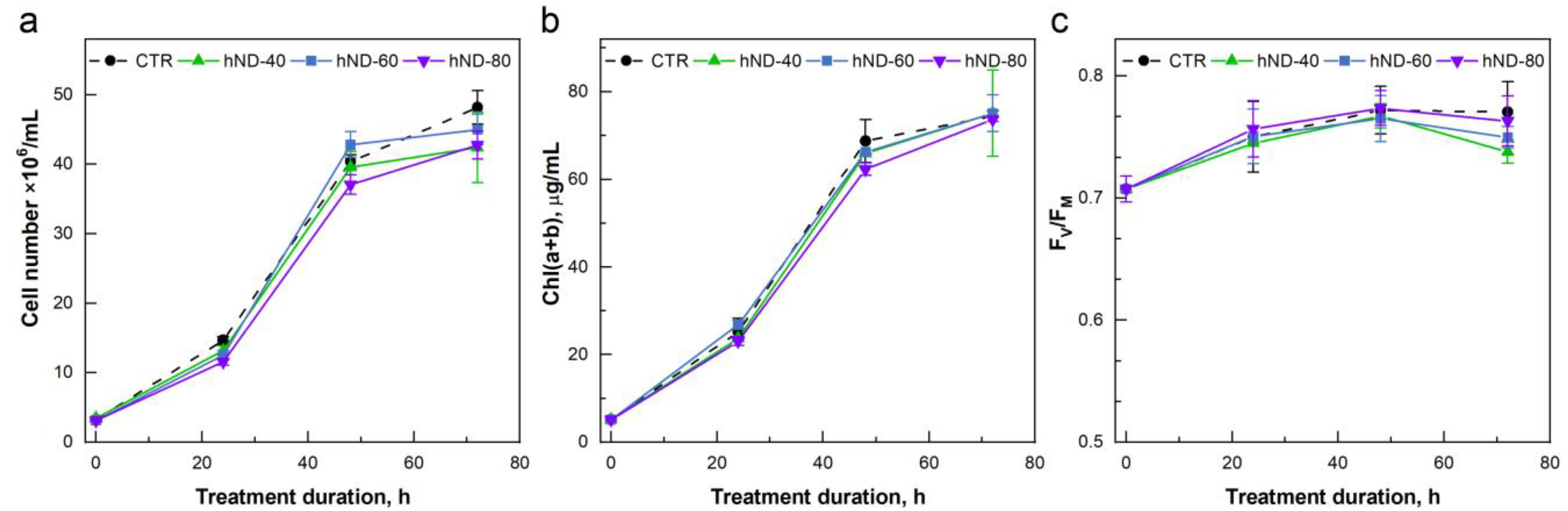
2.2. hND Phytotoxicity in C. reinhardtii Cells
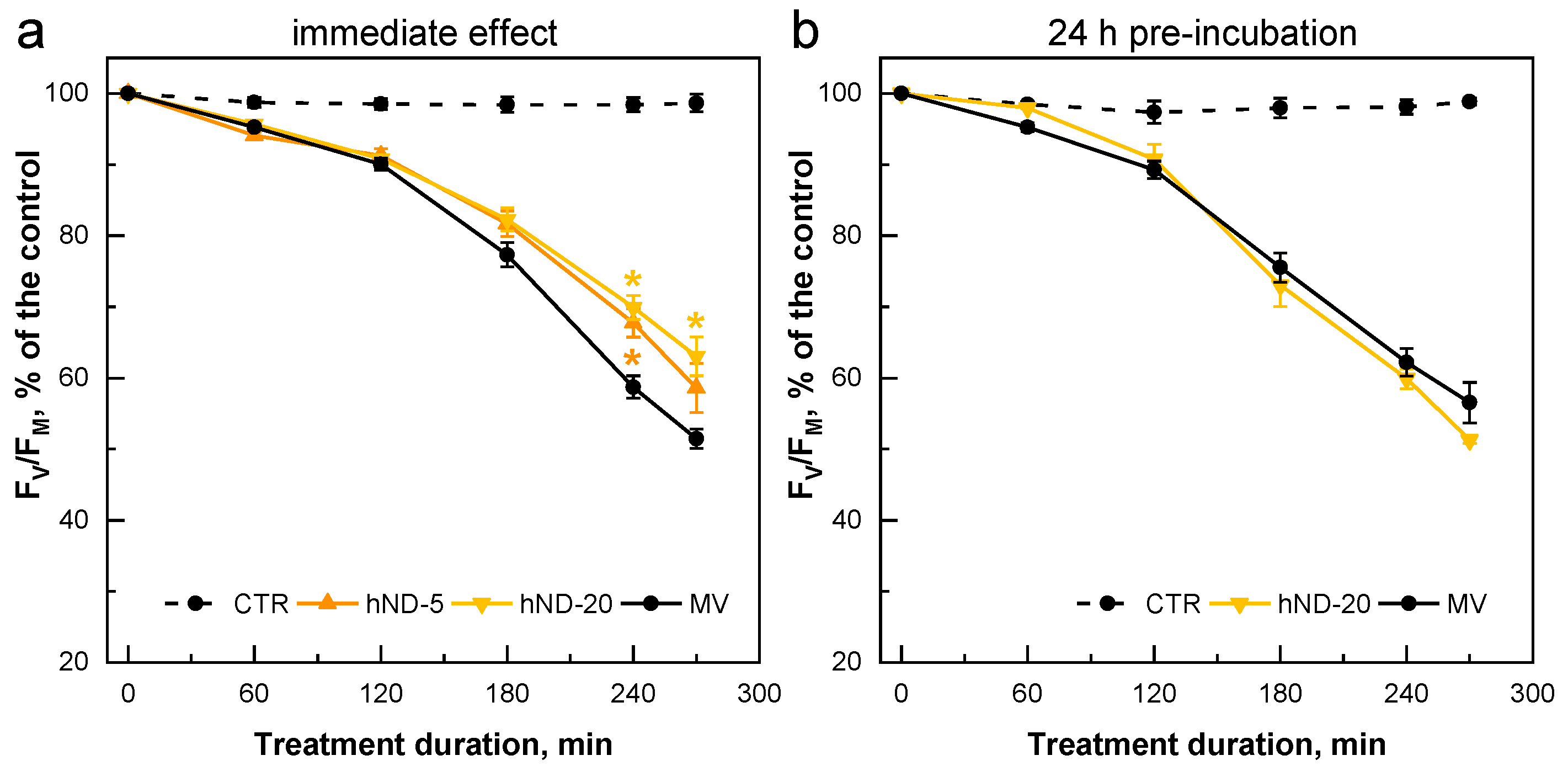
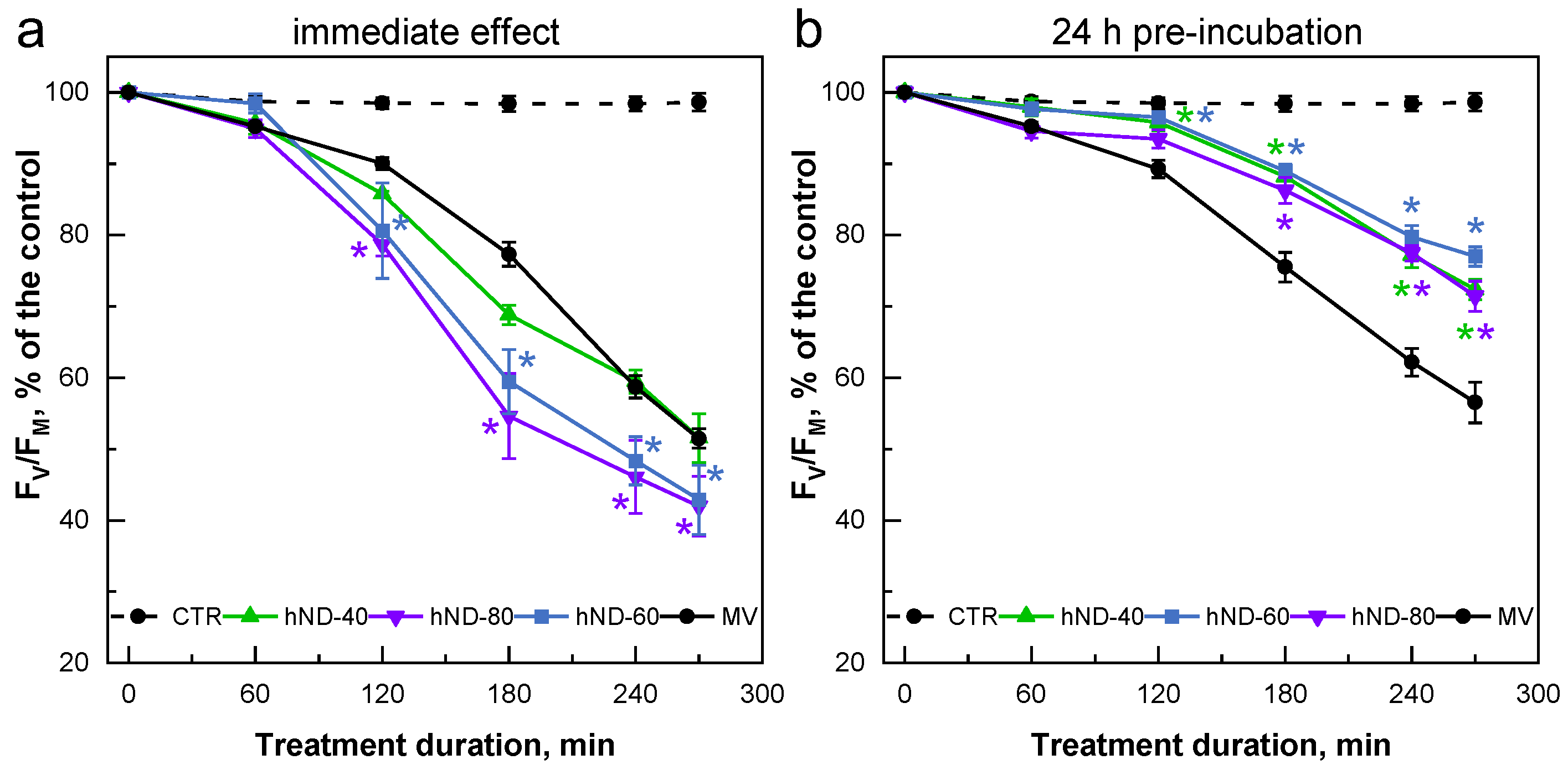
2.3. hND-Mediated Protection of C. reinhardtii Cells against Oxidative Stress Induced by MV
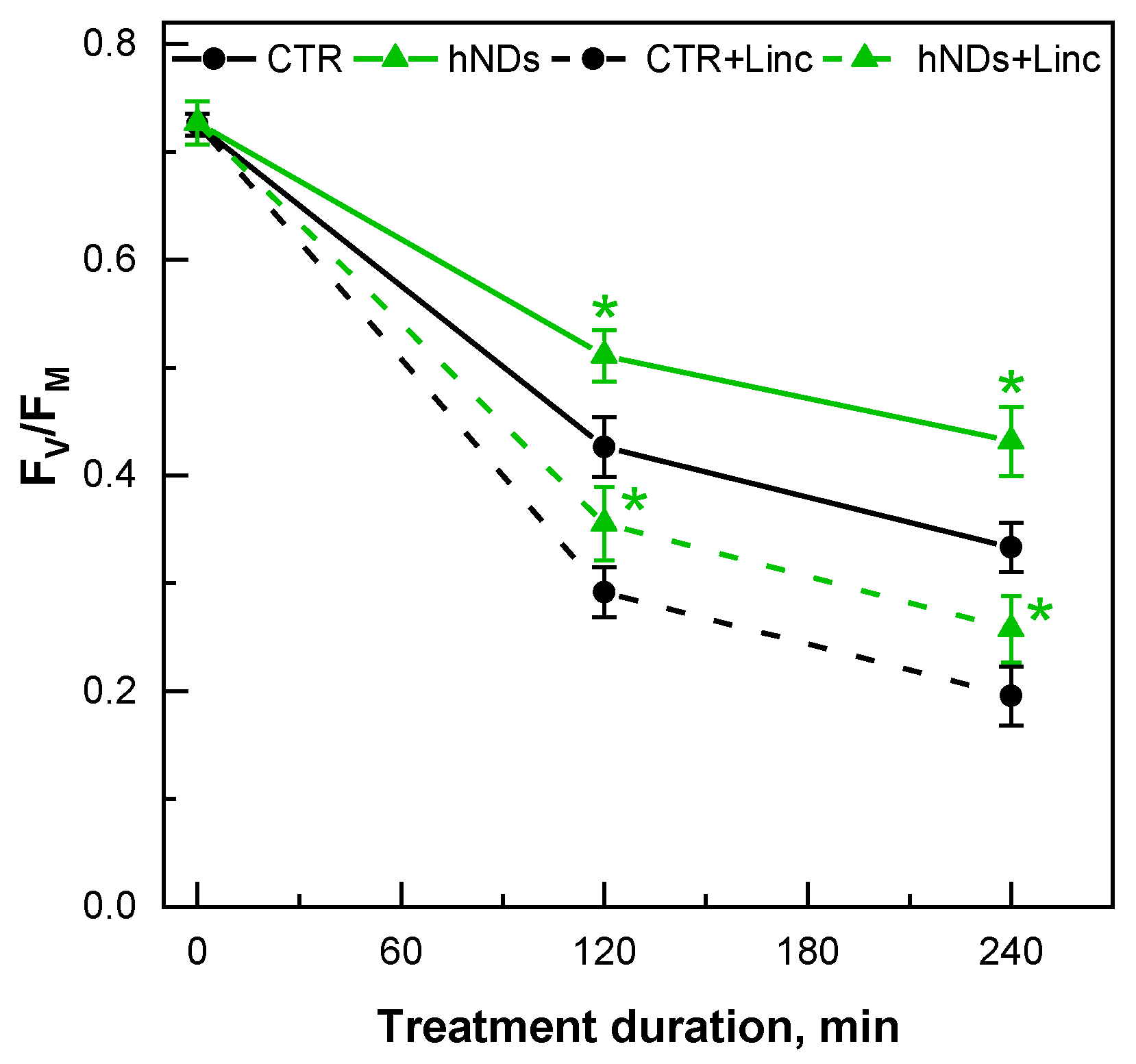
2.4. hND-Mediated Protection of C. reinhardtii Cells under High Light Conditions
3. Discussion
4. Materials and Methods
4.1. Properties and Characterisation of the Nanomaterial
4.2. Algal Strain and Growth Conditions
4.3. Chl a Fluorescence Measurements
4.4. Assessment of hND Phytotoxicity
4.5. Assessment of hND Antioxidant Capacity in C. reinhardtii Cultures
4.5.1. MV-Induced Oxidative Stress
4.5.2. HL-Induced Oxidative Stress
4.6. Oxygen Evolution and Dark Respiration Measurements
4.7. Lipid Peroxidation
4.8. Ferric Reducing Antioxidant Power (FRAP) Assay
4.9. Detection of Singlet Oxygen Production
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The Properties and Applications of Nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Schrand, A.M.; Dai, L.; Schlager, J.J.; Hussain, S.M.; Osawa, E. Differential Biocompatibility of Carbon Nanotubes and Nanodiamonds. Diam. Relat. Mater. 2007, 16, 2118–2123. [Google Scholar] [CrossRef]
- Schrand, A.M.; Hens, S.A.C.; Shenderova, O.A. Nanodiamond Particles: Properties and Perspectives for Bioapplications. Crit. Rev. Solid State Mater. Sci. 2009, 34, 18–74. [Google Scholar] [CrossRef]
- Santacruz-Gomez, K.; Sarabia-Sainz, A.; Acosta-Elias, M.; Sarabia-Sainz, M.; Janetanakit, W.; Khosla, N.; Melendrez, R.; Montero, M.P.; Lal, R. Antioxidant Activity of Hydrated Carboxylated Nanodiamonds and Its Influence on Water γ-Radiolysis. Nanotechnology 2018, 29, 125707. [Google Scholar] [CrossRef]
- Gismondi, A.; Reina, G.; Orlanducci, S.; Mizzoni, F.; Gay, S.; Terranova, M.L.; Canini, A. Biomaterials Nanodiamonds Coupled with Plant Bioactive Metabolites: A Nanotech Approach for Cancer Therapy. Biomaterials 2015, 38, 22–35. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Mochalin, V.N. Biomedical Applications of Nanodiamond (Review). Nanotechnology 2017, 28, 252001. [Google Scholar] [CrossRef]
- Krüger, A.; Kataoka, F.; Ozawa, M.; Fujino, T.; Suzuki, Y.; Aleksenskii, A.E.; Vul’, A.Y.; Osawa, E. Unusually Tight Aggregation in Detonation Nanodiamond: Identification and Disintegration. Carbon N. Y. 2005, 43, 1722–1730. [Google Scholar] [CrossRef]
- Buonasera, K.; Pezzotti, G.; Scognamiglio, V.; Tibuzzi, A.; Giardi, M.T. New Platform of Biosensors for Prescreening of Pesticide Residues to Support Laboratory Analyses. J. Agric. Food Chem. 2010, 58, 5982–5990. [Google Scholar] [CrossRef]
- Janssen, P.J.D.; Lambreva, M.D.; Plumeré, N.; Bartolucci, C.; Antonacci, A.; Buonasera, K.; Frese, R.N.; Scognamiglio, V.; Rea, G.; Plumeré, N.; et al. Photosynthesis at the Forefront of a Sustainable Life. Front. Chem. 2014, 2, 36. [Google Scholar] [CrossRef]
- Rea, G.; Antonacci, A.; Lambreva, M.D.; Mattoo, A.K. Features of Cues and Processes during Chloroplast-Mediated Retrograde Signaling in the Alga Chlamydomonas. Plant Sci. 2018, 272, 193–206. [Google Scholar] [CrossRef]
- Lambreva, M.D.; Lavecchia, T.; Tyystjärvi, E.; Antal, T.K.; Orlanducci, S.; Margonelli, A.; Rea, G. Potential of Carbon Nanotubes in Algal Biotechnology. Photosynth. Res. 2015, 125, 451–471. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.C.; Davis, R. The Potentials and Challenges of Algae Based Biofuels: A Review of the Techno-Economic, Life Cycle, and Resource Assessment Modeling. Bioresour. Technol. 2015, 184, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Doshi, A.; Pascoe, S.; Coglan, L.; Rainey, T.J. Economic and Policy Issues in the Production of Algae-Based Biofuels: A Review. Renew. Sustain. Energy Rev. 2016, 64, 329–337. [Google Scholar] [CrossRef]
- Khalid, M. Nanotechnology and Chemical Engineering as a Tool to Bioprocess Microalgae for Its Applications in Therapeutics and Bioresource Management. Crit. Rev. Biotechnol. 2020, 40, 46–63. [Google Scholar] [CrossRef]
- Antal, T.K.; Volgusheva, A.A.; Kukarskikh, G.P.; Lukashev, E.P.; Bulychev, A.A.; Margonelli, A.; Orlanducci, S.; Leo, G.; Cerri, L.; Tyystjärvi, E.; et al. Single-Walled Carbon Nanotubes Protect Photosynthetic Reactions in Chlamydomonas reinhardtii against Photoinhibition. Plant Physiol. Biochem. 2022, 192, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Schrand, A.M.; Huang, H.; Carlson, C.; Schlager, J.J.; Osawa, E.; Hussain, S.M.; Dai, L. Are Diamond Nanoparticles Cytotoxic? J. Phys. Chem. B 2007, 111, 2–7. [Google Scholar] [CrossRef]
- Solarska-Sciuk, K.; Gajewska, A.; Glińska, S.; Michlewska, S.; Balcerzak, Ł.; Jamrozik, A.; Skolimowski, J.; Burda, K.; Bartosz, G. Effect of Functionalized and Non-Functionalized Nanodiamond on the Morphology and Activities of Antioxidant Enzymes of Lung Epithelial Cells (A549). Chem. Biol. Interact. 2014, 222, 135–147. [Google Scholar] [CrossRef]
- Kaluç, N.; Thomas, P.B. A Carboxylated Nanodiamond Reduces Oxidative Stress and Shows No Sign of Toxicity in Yeast. Fuller. Nanotub. Carbon Nanostructures 2022, 30, 487–494. [Google Scholar] [CrossRef]
- Domínguez, G.A.; Torelli, M.D.; Buchman, J.T.; Haynes, C.L.; Hamers, R.J.; Klaper, R.D. Size Dependent Oxidative Stress Response of the Gut of Daphnia magna to Functionalized Nanodiamond Particles. Environ. Res. 2018, 167, 267–275. [Google Scholar] [CrossRef]
- Xing, Y.; Xiong, W.; Zhu, L.; O’Sawa, E.; Hussin, S.; Dai, L. DNA Damage in Embryonic Stem Cells Caused by Nanodiamonds. ACS Nano 2011, 5, 2376–2384. [Google Scholar] [CrossRef]
- Wehling, J.; Dringen, R.; Zare, R.N.; Maas, M.; Rezwan, K. Bactericidal Activity of Partially Oxidized Nanodiamonds. ACS Nano 2014, 8, 6475–6483. [Google Scholar] [CrossRef]
- Ong, S.Y.; Van Harmelen, R.J.J.; Norouzi, N.; Offens, F.; Venema, I.M.; Habibi Najafi, M.B.; Schirhagl, R. Interaction of Nanodiamonds with Bacteria. Nanoscale 2018, 10, 17117–17124. [Google Scholar] [CrossRef]
- Holt, K.B.; Ziegler, C.; Caruana, D.J.; Zang, J.; Millán-Barrios, E.J.; Hu, J.; Foord, J.S. Redox Properties of Undoped 5 Nm Diamond Nanoparticles. Phys. Chem. Chem. Phys. 2007, 10, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Xu Xu, R.H.J.; Yang, N.; Karim, A.A.; Loh, X.J.; Zhang, K. UV Protection and Antioxidant Activity of Nanodiamonds and Fullerenes for Sunscreen Formulations. ACS Appl. Nano Mater. 2019, 2, 7604–7616. [Google Scholar] [CrossRef]
- Adach, K.; Fijalkowski, M.; Skolimowski, J. Antioxidant Effect of Hydroxylated Diamond Nanoparticles Measured in Soybean Oil. Fuller. Nanotub. Carbon Nanostructures 2015, 23, 1024–1032. [Google Scholar] [CrossRef]
- Szczypiński, M.M. Carbon-Modified Plastic Materials for Food Packaging, Technical University of Liberec. 2021. Available online: https://dspace.tul.cz/handle/15240/160675 (accessed on 15 January 2023).
- Mitura, K.; Kornacka, J.; Kopczyńska, E.; Kalisz, J.; Czerwińska, E.; Affeltowicz, M.; Kaczorowski, W.; Kolesińska, B.; Frączyk, J.; Bakalova, T.; et al. Active Carbon-Based Nanomaterials in Food Packaging. Coatings 2021, 11, 161. [Google Scholar] [CrossRef]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The Emerging Role of Nanotechnology in Skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, X.; Chu, Y.; Ren, N.; Ho, S.H. An Overlooked Effect Induced by Surface Modification: Different Molecular Response of: Chlorella pyrenoidosa to Graphitized and Oxidized Nanodiamonds. Environ. Sci. Nano 2020, 7, 2302–2312. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Chou, W.C.; Ho, S.H. Phytotoxic Effect and Molecular Mechanism Induced by Nanodiamonds towards Aquatic Chlorella pyrenoidosa by Integrating Regular and Transcriptomic Analyses. Chemosphere 2021, 270, 129473. [Google Scholar] [CrossRef]
- Laporta, M.; Pegoraro, M.; Zanderighi, L. Perfluorosulfonated Membrane (Nafion): FT-IR Study of the State of Water with Increasing Humidity. Phys. Chem. Chem. Phys. 1999, 1, 4619–4628. [Google Scholar] [CrossRef]
- Girard, H.A.; Petit, T.; Perruchas, S.; Gacoin, T.; Gesset, C.; Arnault, J.C.; Bergonzo, P. Surface Properties of Hydrogenated Nanodiamonds: A Chemical Investigation. Phys. Chem. Chem. Phys. 2011, 13, 11517–11523. [Google Scholar] [CrossRef]
- Petit, T.; Puskar, L. FTIR Spectroscopy of Nanodiamonds: Methods and Interpretation. Diam. Relat. Mater. 2018, 89, 52–66. [Google Scholar] [CrossRef]
- Mezzi, A.; Kaciulis, S. Surface Investigation of Carbon Films: From Diamond to Graphite. Surf. Interface Anal. 2010, 42, 1082–1084. [Google Scholar] [CrossRef]
- Desai, C.; Chen, K.; Mitra, S. Aggregation Behavior of Nanodiamonds and Their Functionalized Analogs in an Aqueous Environment. Environ. Sci. Process. Impacts 2014, 16, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Lascano, R.; Munoz, N.; Robert, G.; Rodriguez, M.; Melchiorre, M.; Trippi, V.; Quero, G. Paraquat: An Oxidative Stress Inducer. In Herbicides-Properties, Synthesis and Control of Weeds; IntechOpen: London, UK, 2012; pp. 135–148. [Google Scholar] [CrossRef]
- Trebst, A. Measurement of Hill Reactions and Photoreduction. Methods Enzymol. 1972, 24, 146–165. [Google Scholar] [CrossRef]
- Tyystjärvi, E. Photoinhibition of Photosystem II. In International Review of Cell and Molecular Biology; Jeon, K., Ed.; Academic Press, Elsevier: Amsterdam, The Netherlands, 2013; pp. 243–303. ISBN 9780124052109. [Google Scholar]
- Rubio, C.P.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric Assays for Total Antioxidant Capacity (TAC) in Dog Serum: An Update. BMC Vet. Res. 2016, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Volgusheva, A.; Todorenko, D.; Baizhumanov, A.; Chivkunova, O.; Solovchenko, A.; Antal, T. Cadmium- and Chromium-Induced Damage and Acclimation Mechanisms in Scenedesmus Quadricauda and Chlorella sorokiniana. J. Appl. Phycol. 2022, 34, 1435–1446. [Google Scholar] [CrossRef]
- Mattila, H.; Mishra, S.; Tyystjärvi, T.; Tyystjärvi, E. Singlet Oxygen Production by Photosystem II Is Caused by Misses of the Oxygen Evolving Complex. New Phytol. 2023, 237, 113–125. [Google Scholar] [CrossRef]
- Hakala, M.; Tuominen, I.; Keränen, M.; Tyystjärvi, T.; Tyystjärvi, E. Evidence for the Role of the Oxygen-Evolving Manganese Complex in Photoinhibition of Photosystem II. Biochim. Biophys. Acta-Bioenerg. 2005, 1706, 68–80. [Google Scholar] [CrossRef]
- Kodru, S.; Ur Rehman, A.; Vass, I. Chloramphenicol Enhances Photosystem II Photodamage in Intact Cells of the Cyanobacterium Synechocystis PCC 6803. Photosynth. Res. 2020, 145, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yu, W.; Zhang, L.; Liu, J. Chlororespiration Protects the Photosynthetic Apparatus against Photoinhibition by Alleviating Inhibition of Photodamaged-PSII Repair in Haematococcus pluvialis at the Green Motile Stage. Algal Res. 2021, 54, 102140. [Google Scholar] [CrossRef]
- Lee, M.Y.; Yu, J.W. Antioxidant Effect of Functional Nanodiamond and a Use. Therefore. Patent WO/2011/115314, 22 September 2011. [Google Scholar]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. A New Paradigm for the Action of Reactive Oxygen Species in the Photoinhibition of Photosystem II. Biochim. Biophys. Acta 2006, 1757, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Orlanducci, S.; Fulgenzi, G.; Margonelli, A.; Rea, G.; Antal, T.K.; Lambreva, M.D. Mapping Single Walled Carbon Nanotubes in Photosynthetic Algae by Single-Cell Confocal Raman Microscopy. Materials 2020, 13, 5121. [Google Scholar] [CrossRef]
- Bolli, E.; Kaciulis, S.; Mezzi, A. ESCA as a Tool for Exploration of Metals’ Surface. Coatings 2020, 10, 1182. [Google Scholar] [CrossRef]
- Prekodravac, J.R.; Budimir, M.D.; Kleut, D.N.; Vasiljević, B.R.; Rajić, V.B.; Ciasca, G.; Todorović Marković, B.M. Surface Functionality as a Key Parameter for the Conductivity of Microwave Synthesized CQDs Thin Films. Diam. Relat. Mater. 2022, 129, 109366. [Google Scholar] [CrossRef]
- Harris, E.H. The Chlamydomonas Sourcebook: Introduction to Chlamydomonas and Its Laboratory Use; Elsevier Science: Amsterdam, The Netherlands, 1989; Volume 1, ISBN 9780123268808. [Google Scholar]
- Lambreva, M.D.; Giardi, M.T.; Rambaldi, I.; Antonacci, A.; Pastorelli, S.; Bertalan, I.; Husu, I.; Johanningmeier, U.; Rea, G. A Powerful Molecular Engineering Tool Provided Efficient Chlamydomonas Mutants as Bio-Sensing Elements for Herbicides Detection. PLoS ONE 2013, 8, 61851. [Google Scholar] [CrossRef]
- Sipka, G.; Magyar, M.; Mezzetti, A.; Akhtar, P.; Zhu, Q.; Xiao, Y.; Han, G.; Santabarbara, S.; Shen, J.-R.; Lambrev, P.H.; et al. Light-Adapted Charge-Separated State of Photosystem II: Structural and Functional Dynamics of the Closed Reaction Center. Plant Cell 2021, 33, 1286–1302. [Google Scholar] [CrossRef]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll Fluorescence as a Nonintrusive Indicator for Rapid Assessment of In Vivo Photosynthesis. In Ecophysiology of Photosynthesis; Schulze, E.-D., Caldwell, M.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 49–70. ISBN 978-3-642-79354-7. [Google Scholar]
- Haraguchi, H.; Ishikawa, H.; Kubo, I. Antioxidative Action of Diterpenoids from Podocarpus nagi. Planta Med. 1997, 63, 213–215. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of Aluminium on Lipid Peroxidation, Superoxide Dismutase, Catalase, and Peroxidase Activities in Root Tips of Soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Dai, Q.; Yan, B.; Huang, S.; Liu, X.; Peng, S.; Miranda, M.L.L.; Chavez, A.Q.; Vergara, B.S.; Olszyk, D.M. Response of Oxidative Stress Defense Systems in Rice (Oryza sativa) Leaves with Supplemental UV-B Radiation. Physiol. Plant. 1997, 101, 301–308. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.U.; Cser, K.; Sass, L.; Vass, I. Characterization of Singlet Oxygen Production and Its Involvement in Photodamage of Photosystem II in the Cyanobacterium Synechocystis PCC 6803 by Histidine-Mediated Chemical Trapping. Biochim. Biophys. Acta-Bioenerg. 2013, 1827, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Sedoud, A.; López-Igual, R.; Rehman, A.U.; Wilson, A.; Perreau, F.; Boulay, C.; Vass, I.; Krieger-Liszkay, A.; Kirilovsky, D. The Cyanobacterial Photoactive Orange Carotenoid Protein Is an Excellent Singlet Oxygen Quencher. Plant Cell 2014, 26, 1781–1791. [Google Scholar] [CrossRef]

| Sample | MDA µmol/g DW | FRAP mmol Fe(II)eq/g DW | ||
|---|---|---|---|---|
| CTR | NDs | CTR | NDs | |
| T0 | 1.8 ± 0.2 | 1.7 ± 0.1 | 0.20 ± 0.01 | 0.20 ± 0.02 |
| T3 | 3.1 ± 0.3 * | 2.6 ± 0.3 * | 0.36 ± 0.04 * | 0.31 ± 0.04 * |
| Sample | O2 Evolution Rate µmol O2/mg Chl/h | Respiration Rate µmol O2/mg Chl/h | ||
|---|---|---|---|---|
| CTR | NDs | CTR | NDs | |
| T0 | 176 ± 8 | 168 ± 14 | 18 ± 0.7 | 18 ± 1.1 |
| T2 | 101 ± 15 * | 120 ± 11 * | 40 ± 1.1 *,b | 35 ± 1.5 *,a |
| Sample | MDA µmol/g DW | FRAP mmol Fe(II)eq/g DW | 1O2 Production Rate µmol 1O2/mg Chl/h | |||
|---|---|---|---|---|---|---|
| CTR | NDs | CTR | NDs | CTR | NDs | |
| T0 | 1.8 ± 0.2 | 1.7 ± 0.1 | 0.20 ± 0.01 | 0.20 ± 0.02 | 16 ± 3 | 12 ± 2 |
| T2 | 2.3 ± 0.4 | 2.0 ± 0.3 | 0.24 ± 0.01 | 0.23 ± 0.03 | 26 ± 3 * | 20 ± 4 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antal, T.K.; Volgusheva, A.A.; Baizhumanov, A.A.; Kukarskikh, G.P.; Mezzi, A.; Caschera, D.; Ciasca, G.; Lambreva, M.D. Nanodiamond Particles Reduce Oxidative Stress Induced by Methyl Viologen and High Light in the Green Alga Chlamydomonas reinhardtii. Int. J. Mol. Sci. 2023, 24, 5615. https://doi.org/10.3390/ijms24065615
Antal TK, Volgusheva AA, Baizhumanov AA, Kukarskikh GP, Mezzi A, Caschera D, Ciasca G, Lambreva MD. Nanodiamond Particles Reduce Oxidative Stress Induced by Methyl Viologen and High Light in the Green Alga Chlamydomonas reinhardtii. International Journal of Molecular Sciences. 2023; 24(6):5615. https://doi.org/10.3390/ijms24065615
Chicago/Turabian StyleAntal, Taras K., Alena A. Volgusheva, Adil A. Baizhumanov, Galina P. Kukarskikh, Alessio Mezzi, Daniela Caschera, Gabriele Ciasca, and Maya D. Lambreva. 2023. "Nanodiamond Particles Reduce Oxidative Stress Induced by Methyl Viologen and High Light in the Green Alga Chlamydomonas reinhardtii" International Journal of Molecular Sciences 24, no. 6: 5615. https://doi.org/10.3390/ijms24065615
APA StyleAntal, T. K., Volgusheva, A. A., Baizhumanov, A. A., Kukarskikh, G. P., Mezzi, A., Caschera, D., Ciasca, G., & Lambreva, M. D. (2023). Nanodiamond Particles Reduce Oxidative Stress Induced by Methyl Viologen and High Light in the Green Alga Chlamydomonas reinhardtii. International Journal of Molecular Sciences, 24(6), 5615. https://doi.org/10.3390/ijms24065615