Combining Metabolomics and Transcriptomics to Reveal the Regulatory Mechanism of Taproot Enlargement in Panax ginseng
Abstract
:1. Introduction
2. Results
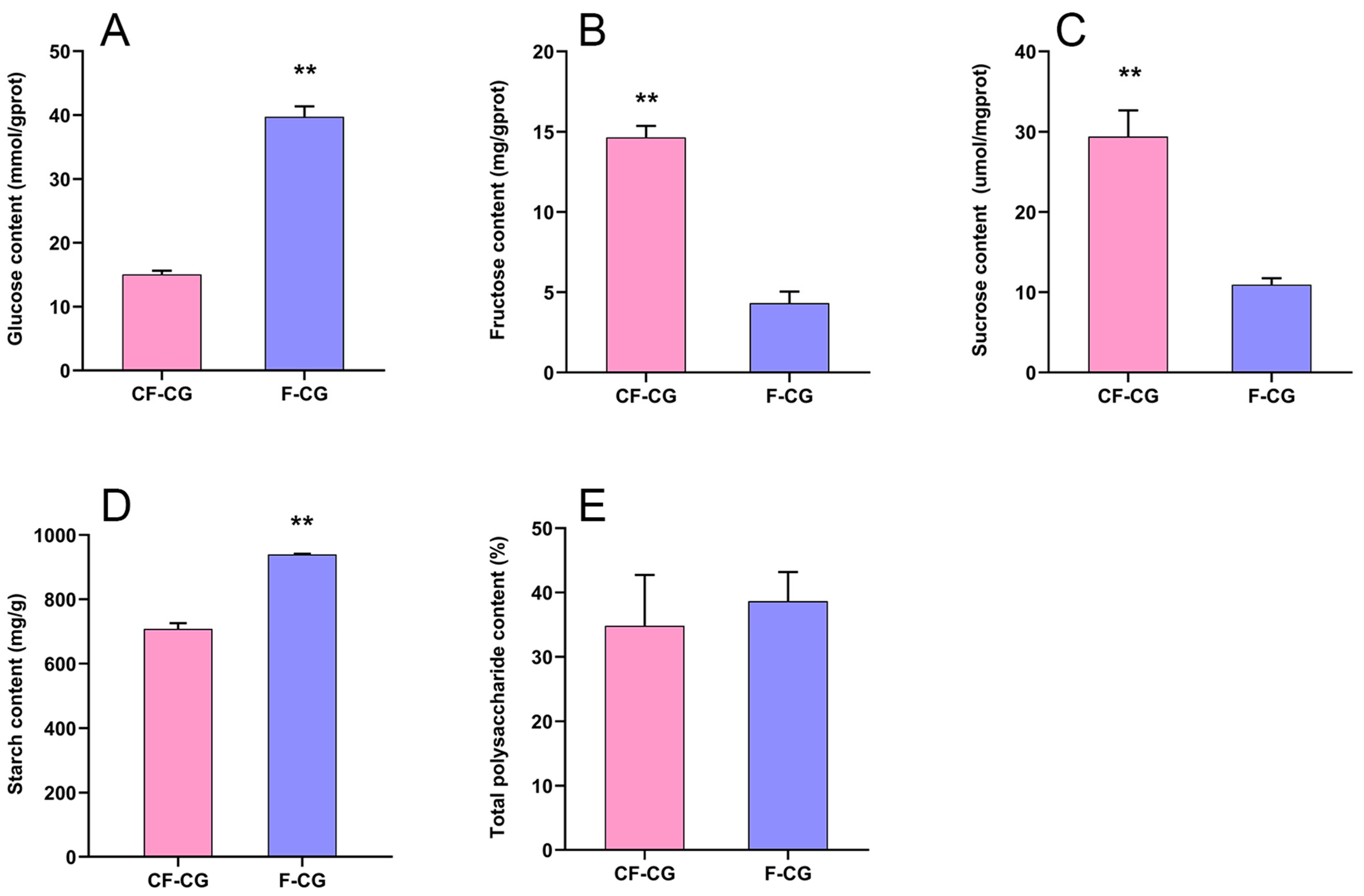
2.1. Growth and Development Indicators and Sugar Content
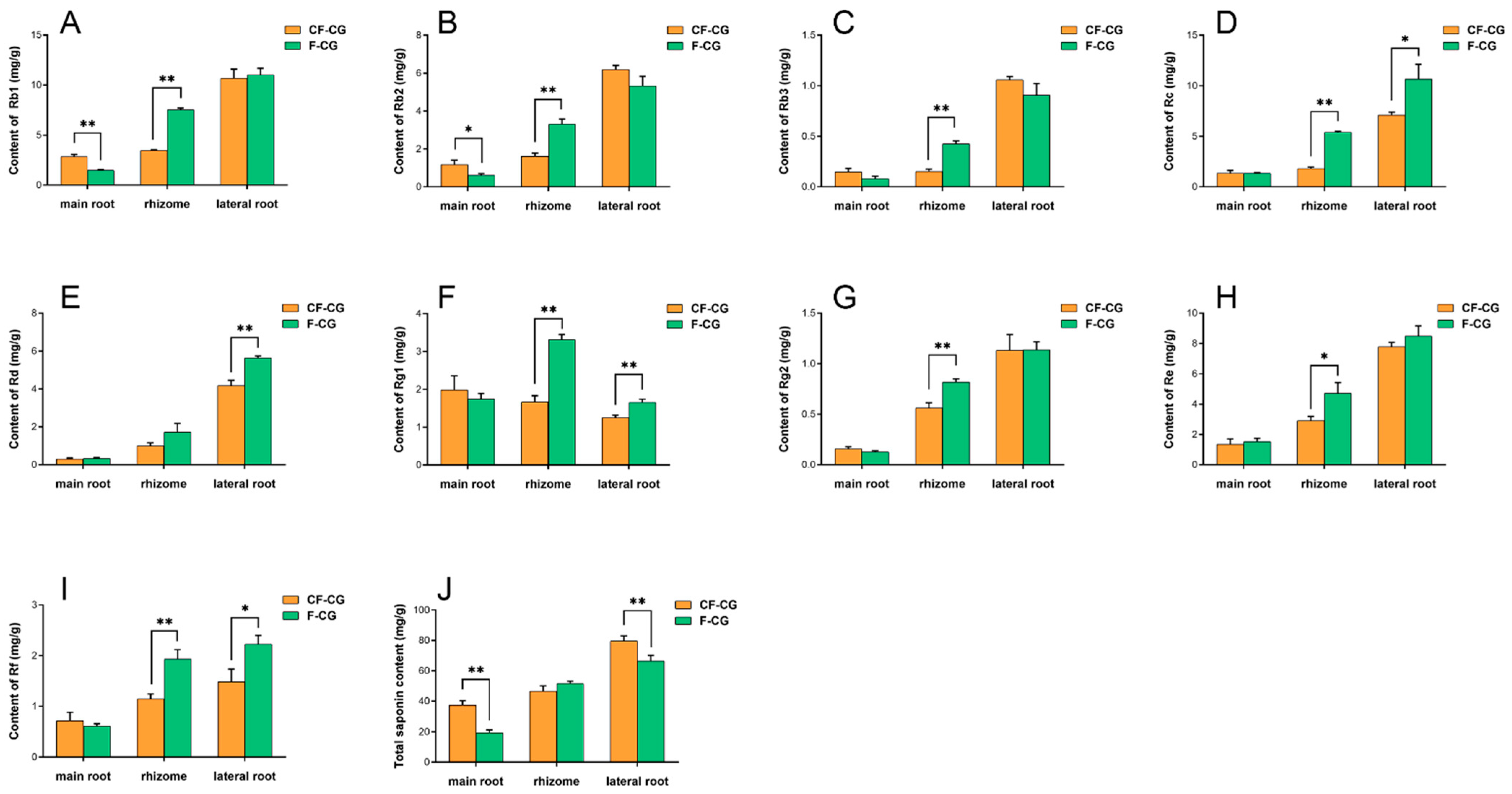
2.2. Ginsenoside Content
2.3. Metabolome Data Analysis
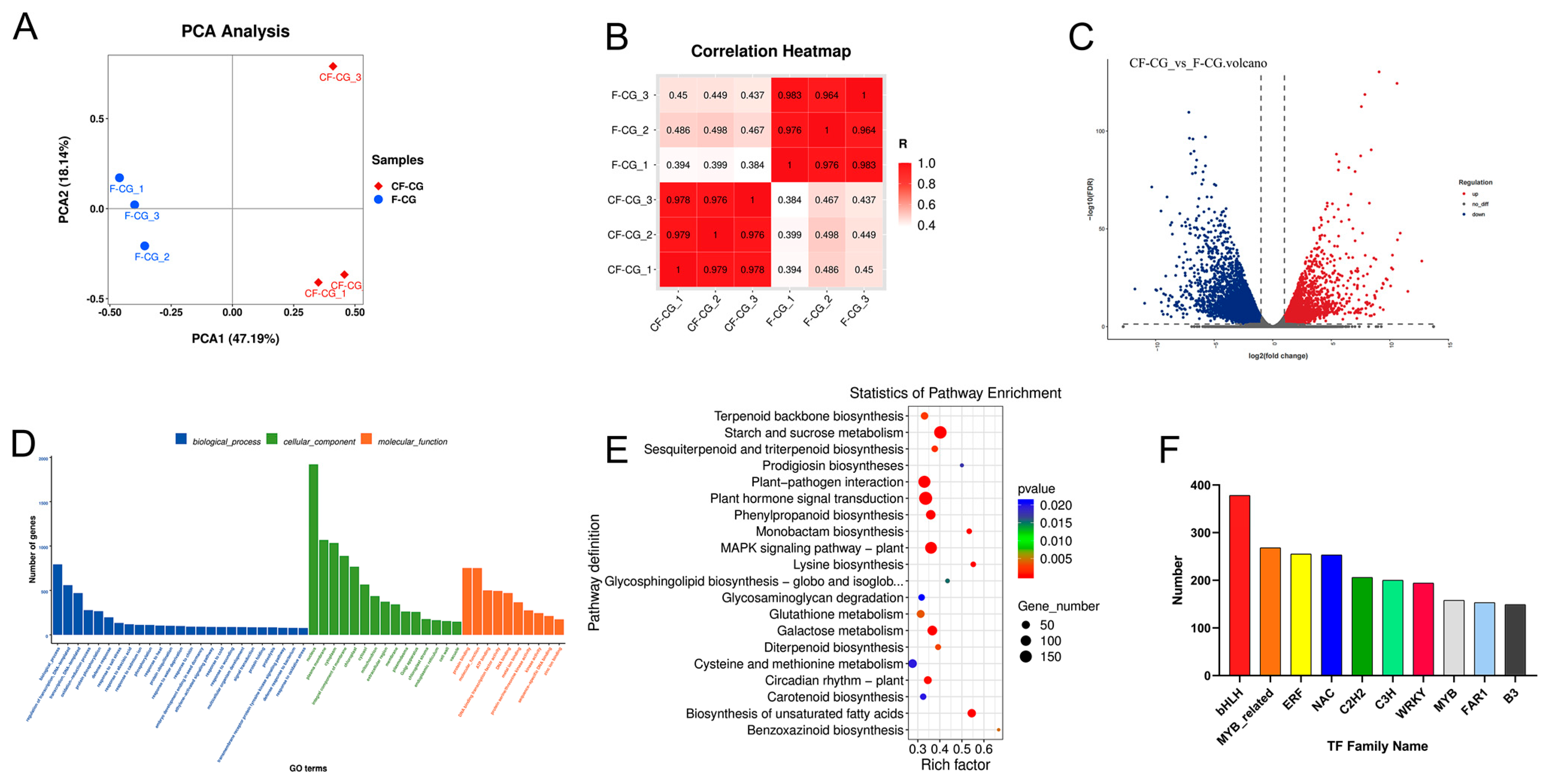
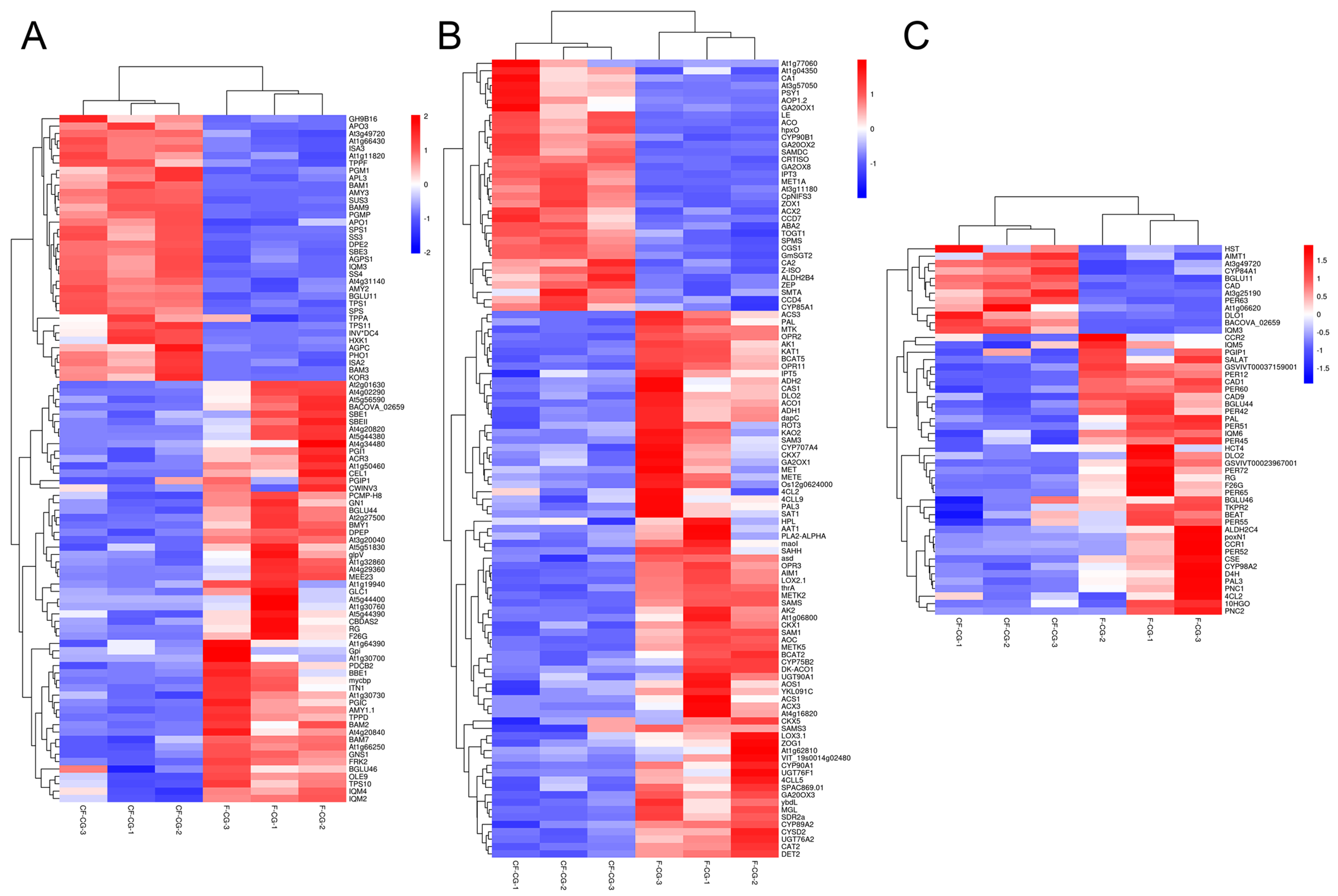
2.4. Transcriptome Data Analysis
2.5. qRT-PCR Validation of RNA-seq Data
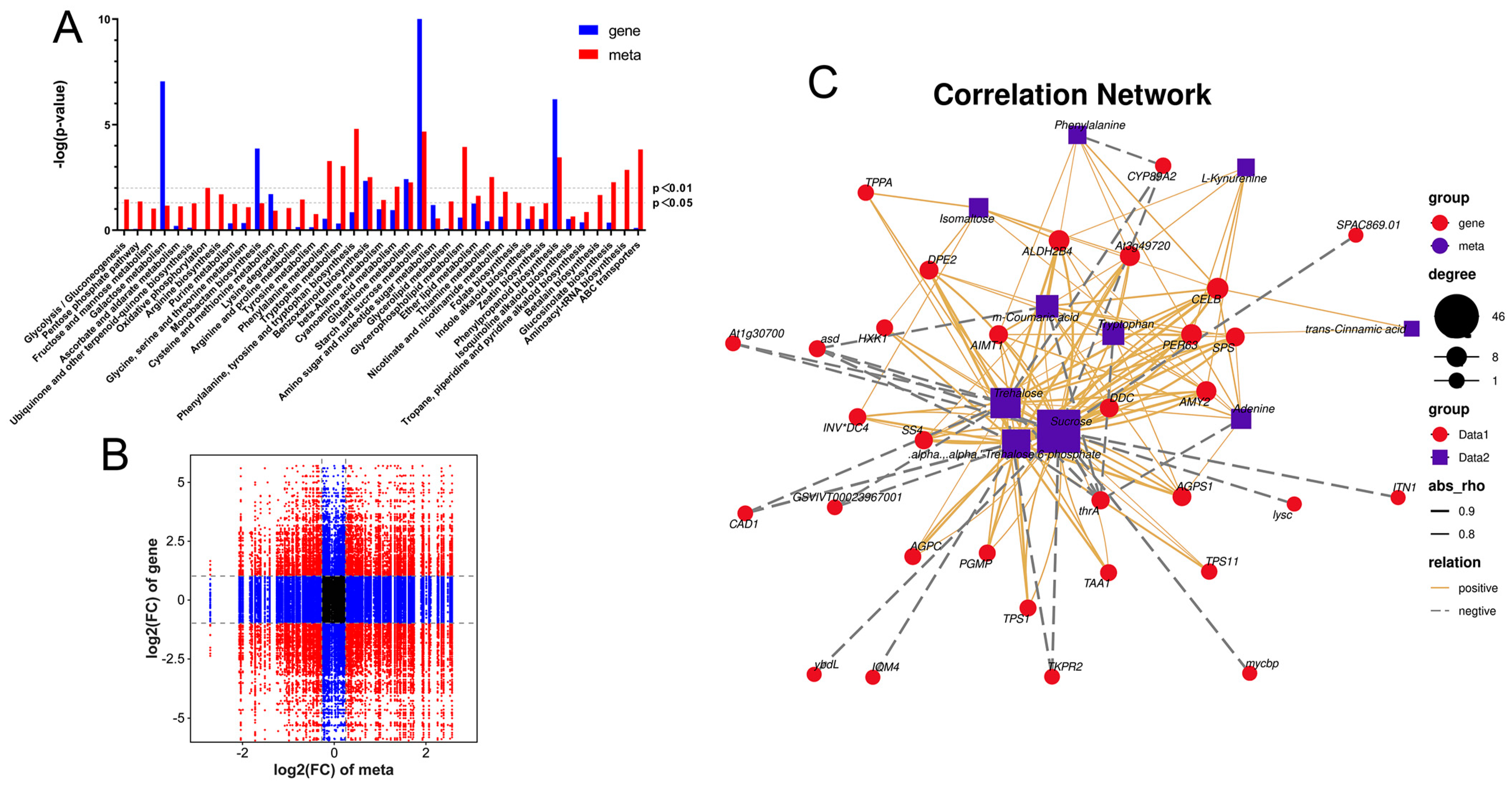
2.6. Combined Analysis of Transcriptome and Metabolome
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Determination of Carbohydrate Contents in Ginseng
4.3. Determination of Monomeric Saponins and Total Saponins in Ginseng
4.4. Metabolome Analysis
4.5. Transcriptome Analysis
4.6. qRT-PCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mancuso, C.; Santangelo, R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem. Toxicol. 2017, 107, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Yi, L.W.; Zhao, L.; Zhou, Y.Z.; Guo, F.; Huo, Y.S.; Zhao, D.Q.; Xu, F.; Wang, X.; Cai, S.Q. 177 Saponins, Including 11 New Compounds in Wild Ginseng Tentatively Identified via HPLC-IT-TOF-MSn, and Differences among Wild Ginseng, Ginseng under Forest, and Cultivated Ginseng. Molecules 2021, 26, 3371. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Y.; Zeng, J.Z.; Wong, A.S.T. Chemical structures and pharmacological profiles of ginseng saponins. Molecules 2019, 24, 2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Ren, C.; Zhang, Y.; Wu, X. Ginseng: An nonnegligible natural remedy for healthy aging. Aging Dis. 2017, 8, 708–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, G.D.; Chiu, C.H.; Hsu, Y.J.; Hou, C.W.; Chen, Y.M.; Huang, C.C. Changbai mountain ginseng (Panax ginseng C.A. Mey) extract supplementation improves exercise performance and energy utilization and decreases fatigue-associated param eters in mice. Molecules 2017, 22, 237. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, J.H.; Baek, S.H.; Ko, J.H.; Nam, D.; Ahn, K.S. Korean red ginseng extract enhances the anticancer effects of sorafenib through abrogation of CREB and c-jun activation in renal cell carcinoma. Phytother Res. 2017, 31, 1078–1089. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Yang, W.; Zhao, C.; Zhang, L.; Zhang, J.; Qin, Y.; Xu, H.; Huang, L. Characterization of the components and pharmacological effects of mountain-cultivated ginseng and garden ginseng based on the integrative pharmacology strategy. Front. Pharmacol. 2021, 12, 659954. [Google Scholar] [CrossRef]
- Li, M.R.; Shi, F.X.; Zhou, Y.X.; Li, Y.L.; Wang, X.F.; Zhang, C.; Wang, X.T.; Liu, B.; Xiao, H.X.; Li, L.F. Genetic and epigenetic diversities shed light on domestication of cultivated ginseng (Panax ginseng). Mol. Plant 2015, 8, 1612–1622. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Xu, L.; Dou, D.; Huang, L. The distinct of chemical profiles of mountainous forest cultivated ginseng and garden ginseng based on ginsenosides and oligosaccharides. J. Food Compos. Anal. 2021, 104, 104165. [Google Scholar] [CrossRef]
- Szakiel, A.; Paczkowski, C.; Henry, M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2010, 10, 471–491. [Google Scholar] [CrossRef]
- Geem, K.R.; Kim, J.; Bae, W.; Jee, M.G.; Yu, J.; Jang, I.; Lee, D.Y.; Hong, C.P.; Shim, D.; Ryu, H. Nitrate enhances the secondary growth of storage roots in Panax ginseng. J. Ginseng Res. 2022. In Press. [Google Scholar] [CrossRef]
- Mitsui, Y.; Shimomura, M.; Komatsu, K.; Namiki, N.; Shibata-Hatta, M.; Imai, M.; Katayose, Y.; Mukai, Y.; Kanamori, H.; Kurita, K.; et al. The radish genome and comprehensive gene expression profile of tuberous root formation and development. Sci Rep. 2015, 5, 10835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Chen, Y.; Zhang, Q.; Yu, P.; Li, Q.; Qi, W.; Chen, C. Transcriptomic and Metabolomic Differences Between Two Saposhnikovia divaricata (Turcz.) Schischk Phenotypes With Single-and Double-Headed Roots. Front. Bioeng. Biotechnol. 2021, 9, 764093. [Google Scholar] [CrossRef] [PubMed]
- Kondhare, K.R.; Patil, A.B.; Giri, A.P. Auxin: An emerging regulator of tuber and storage root development. Plant Sci. 2021, 306, 110854. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Luo, H.; Wang, A.; Zhou, Y.; Huang, W.; Zhu, P.; He, L. Phytohormone profiling during tuber development of Chinese yam by ultra-high performance liquid chromatography–triple quadrupole tandem mass spectrometry. J. Plant Growth Regul. 2017, 36, 362–373. [Google Scholar] [CrossRef]
- Suzuki, A.; Suriyagoda, L.; Shigeyama, T.; Tominaga, A.; Sasaki, M.; Hiratsuka, Y.; Yoshinaga, A.; Arima, S.; Agarie, S.; Sakai, T.; et al. Lotus japonicus nodulation is photomorphogenetically controlled by sensing the red/far red (R/FR) ratio through jasmonic acid (JA) signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 16837–16842. [Google Scholar] [CrossRef] [Green Version]
- Borzenkova, R.A.; Borovkova, M.P. Developmental patterns of phytohormone content in the cortex and pith of potato tubers as related to their growth and starch content. Russ. J. Plant Physiol. 2003, 50, 119–124. [Google Scholar] [CrossRef]
- Dong, T.; Zhu, M.; Yu, J.; Han, R.; Tang, C.; Xu, T.; Liu, J.; Li, Z. RNA-Seq and iTRAQ reveal multiple pathways involved in storage root formation and development in sweet potato (Ipomoea batatas L.). BMC Plant Biol. 2019, 19, 136. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.P.; Kim, J.; Lee, J.; Yoo, S.I.; Bae, W.; Geem, K.R.; Yu, J.; Jang, I.; Jo, I.H.; Cho, H.; et al. Gibberellin signaling promotes the secondary growth of storage roots in Panax ginseng. Int. J. Mol. Sci. 2021, 22, 8694. [Google Scholar] [CrossRef]
- Wang, G.-L.; Que, F.; Xu, Z.; Wang, F.; Xiong, A. Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot. BMC Plant. Biol. 2015, 15, 290. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Yang, R.; Bartels, D.; Dong, T.; Duan, H. Roles of Abscisic Acid and Gibberellins in Stem/Root Tuber Development. Int. J. Mol. Sci. 2022, 23, 4955. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, T.; Zhang, J.; Li, C.; Xu, Y.; Zheng, H.; Zhou, J.; Zha, L.; Jiang, C.; Jin, Y.; et al. Ginsenosides regulate adventitious root formation in Panax ginseng via a CLE45–WOX11 regulatory module. J. Exp. Bot. 2020, 71, 6396–6407. [Google Scholar] [CrossRef]
- Rodríguez-García, A.; Sola-Landa, A.; Barreiro, C. RNA-Seq-Based Comparative Transcriptomics: RNA Preparation and Bioinformatics[M]//Microbial Steroids; Humana Press: New York, NY, USA, 2017; pp. 59–72. [Google Scholar]
- Firon, N.; LaBonte, D.; Villordon, A.; Kfir, Y.; Solis, J.; Lapis, E.; Perlman, T.S.; Doron-Faigenboim, A.; Hetzroni, A.; Althan, L.; et al. Transcriptional profiling of sweetpotato (Ipomoea batatas) roots indicates down-regulation of lignin biosynthesis and up-regulation of starch biosynthesis at an early stage of storage root formation. BMC Genom. 2013, 14, 460. [Google Scholar] [CrossRef] [Green Version]
- Noh, S.A.; Lee, H.S.; Huh, E.J.; Huh, G.H.; Paek, K.H.; Shin, J.S.; Bae, J.M. SRD1 is involved in the auxin-mediated initial thickening growth of storage root by enhancing proliferation of metaxylem and cambium cells in sweetpotato (Ipomoea batatas). J. Exp. Bot. 2010, 61, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, H.; Hao, X.; Wu, Y.; Bian, X.; Yin, M.; Zhang, Y.; Fan, W.; Dai, H.; Yuan, L.; et al. Dynamic network biomarker analysis discovers IbNAC083 in the initiation and regulation of sweet potato root tuberization. Plant J. 2021, 108, 793–813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qu, S.; Guo, Z.; Zhang, Z. Biology function of bZIP transcription factors in plants. Acta Bot. Boreali-Occident. Sin. 2011, 31, 1066–1075. [Google Scholar]
- Liu, Y.; Zhang, L.; Zhou, J.; Cao, M. Research progress of the bHLH transcription factors involved in genic male sterility in plants. Yi Chuan = Hered. 2015, 37, 1194–1203. [Google Scholar]
- Yu, R.; Wang, J.; Xu, L.; Wang, Y.; Wang, R.; Zhu, X.; Sun, X.; Luo, X.; Xie, Y.; Everlyne, M.; et al. Transcriptome profiling of taproot reveals complex regulatory networks during taproot thickening in radish (Raphanus sativus L.). Front. Plant Sci. 2016, 7, 1210. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Zemach, H.; Shabtai, S.; Aloni, R.; Yang, J.; Zhang, P.; Sergeeva, L.; Ligterink, W.; Firon, N. Proximal and distal parts of sweetpotato adventitious roots display differences in root architecture, lignin, and starch metabolism and their developmental fates. Front. Plant Sci. 2021, 11, 609923. [Google Scholar] [CrossRef]
- Baroja-Fernández, E.; Muñoz, F.; Montero, M.; Etxeberria, E.; Sesma, M.T.; Ovecka, M.; Bahaji, A.; Ezquer, I.; Li, J.; Prat, S.; et al. Enhancing sucrose synthase activity in transgenic potato (Solanum tuberosum L.) tubers results in increased levels of starch, ADPglucose and UDPglucose and total yield. Plant Cell Physiol. 2009, 50, 1651–1662. [Google Scholar] [CrossRef]
- Jung, J.K.H.; McCouch, S. Getting to the roots of it: Genetic and hormonal control of root architecture. Front. Plant Sci. 2013, 4, 186. [Google Scholar] [CrossRef] [Green Version]
- Schaller, G.E.; Street, I.H.; Kieber, J.J. Cytokinin and the cell cycle. Curr. Opin. Plant Biol. 2014, 21, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhu, Y.; Gao, C.; She, W.; Lin, W.; Chen, Y.; Han, N.; Bian, H.; Zhu, M.; Wang, J. Tissue-specific expression of SMALL AUXIN UP RNA41 differentially regulates cell expansion and root meristem patterning in Arabidopsis. Plant Cell Physiol. 2013, 54, 609–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.B.; He, C.; Ma, Y.; Herde, M.; Ding, Z. Jasmonic acid enhances Al-induced root growth inhibition. Plant Physiol. 2017, 173, 1420–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Li, C.; Su, R.; Tan, C.; Lai, B. Transcriptome Profiling Reveals Candidate Genes Involved in Stem Swelling of Tumorous Stem Mustard. Hortic. Plant J. 2020, 6, 158–166. [Google Scholar] [CrossRef]
- Zhu, W.; Jiao, D.; Zhang, J.; Xue, C.; Chen, M.; Yang, Q. Genome-wide identification and analysis of BES1/BZR1 transcription factor family in potato (Solanum tuberosum. L). Plant Growth Regul. 2020, 92, 375–387. [Google Scholar] [CrossRef]
- Dhonukshe, P.; Weits, D.A.; Cruz-Ramirez, A.; Deinum, E.E.; Tindemans, S.H.; Kakar, K.; Prasad, K.; Mähönen, A.P.; Ambrose, C.; Sasabe, M.; et al. RETRACTED: A PLETHORA-Auxin Transcription Module Controls Cell Division Plane Rotation through MAP65 and CLASP. Curr. Biol. 2012, 149, 383–396. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.L.; Que, F.; Xu, Z.S.; Wang, F.; Xiong, A.S. Exogenous gibberellin enhances secondary xylem development and lignification in carrot taproot. Protoplasma 2017, 254, 839–848. [Google Scholar] [CrossRef]
- Singh, V.; Sergeeva, L.; Ligterink, W.; Aloni, R.; Zemach, H.; Doron-Faigenboim, A.; Yang, J.; Zhang, P.; Shabtai, S.; Firon, N. Gibberellin promotes sweetpotato root vascular lignification and reduces storage-root formation. Front. Plant Sci. 2019, 10, 1320. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, I.; Kuboi, T.; Fujiwara, T.; Hara, M. Overexpression of an extraplastidic β-amylase which accumulates in the radish taproot influences the starch content of Arabidopsis thaliana. Plant Biotechnol. 2012, 29, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Matilla, A.J.; Carrillo-Barral, N.; Rodríguez-Gacio, M.C. An update on the role of NCED and CYP707A ABA metabolism genes in seed dormancy induction and the response to after-ripening and nitrate. J. Plant Growth Regul. 2015, 34, 274–293. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Z.; Du, P.; Xiao, W.; Tan, Q.; Chen, X.; Li, L.; Gao, D. Expression of ABA metabolism-related genes suggests similarities and differences between seed dormancy and bud dormancy of peach (Prunus persica). Front. Plant Sci. 2016, 6, 1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Zhang, D.; Yang, D.C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv. 2015, 33, 717–735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Lei, F.; Fang, S.; Jia, M.; Zhang, L. Effects of ginsenosides on the growth and activity of antioxidant enzymes in American ginseng seedlings. J. Med. Plants Res. 2011, 5, 3217–3223. [Google Scholar]
- Zhong, R.; Ye, Z.H. Transcriptional regulation of lignin biosynthesis. Plant Signal. Behav. 2009, 4, 1028–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hara, L.E.; Paul, M.J.; Wingler, A. How do sugars regulate plant growth and development? New insight into the role of trehalose-6-phosphate. Mol. Plant 2013, 6, 261–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meitzel, T.; Radchuk, R.; McAdam, E.L.; Thormählen, I.; Feil, R.; Munz, E.; Hilo, A.; Geigenberger, P.; Ross, J.J.; Lunn, J.E.; et al. Trehalose 6-phosphate promotes seed filling by activating auxin biosynthesis. New Phytol. 2021, 229, 1553–1565. [Google Scholar] [CrossRef]



| Sample | Raw_Reads | Raw_Bases | Valid_Reads | Valid_Bases | Valid% | Q20% | Q30% | GC% |
|---|---|---|---|---|---|---|---|---|
| CF-CG_1 | 58,387,112 | 8.76G | 57,132,936 | 7.99G | 97.85 | 98.06 | 93.91 | 43.49 |
| CF-CG_2 | 57,994,892 | 8.70G | 56,941,662 | 7.96G | 98.18 | 97.97 | 93.69 | 43.46 |
| CF-CG_3 | 34,002,510 | 5.10G | 33,173,734 | 4.64G | 97.56 | 98.01 | 93.54 | 43.59 |
| F-CG_1 | 58,074,704 | 8.71G | 57,342,028 | 8.02G | 98.74 | 97.96 | 93.61 | 43.64 |
| F-CG_2 | 58,443,348 | 8.77G | 57,543,876 | 8.04G | 98.46 | 97.97 | 93.65 | 43.48 |
| F-CG_3 | 54,182,540 | 8.13G | 53,095,988 | 7.42G | 97.99 | 97.97 | 93.68 | 44.06 |
| Index | All | GC% | Min Length | Median Length | Max Length | Total Assembled Bases | N50 |
|---|---|---|---|---|---|---|---|
| Transcript | 241,332 | 39.46 | 201 | 679.00 | 15,897 | 229,477,537 | 1430 |
| Gene | 65,913 | 39.42 | 201 | 512 | 15,897 | 57,009,225 | 1476 |
| DB | All | GO | KEGG | Pfam | Swissprot | eggNOG | NR |
|---|---|---|---|---|---|---|---|
| Num | 65,913 | 30,113 | 23,104 | 25,738 | 24,622 | 33,498 | 34,141 |
| Ratio (%) | 100 | 45.69 | 35.05 | 39.05 | 37.36 | 50.82 | 51.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Sun, Y.; Di, P.; Han, M.; Yang, L. Combining Metabolomics and Transcriptomics to Reveal the Regulatory Mechanism of Taproot Enlargement in Panax ginseng. Int. J. Mol. Sci. 2023, 24, 5590. https://doi.org/10.3390/ijms24065590
Zhang M, Sun Y, Di P, Han M, Yang L. Combining Metabolomics and Transcriptomics to Reveal the Regulatory Mechanism of Taproot Enlargement in Panax ginseng. International Journal of Molecular Sciences. 2023; 24(6):5590. https://doi.org/10.3390/ijms24065590
Chicago/Turabian StyleZhang, Meng, Yingxin Sun, Ping Di, Mei Han, and Limin Yang. 2023. "Combining Metabolomics and Transcriptomics to Reveal the Regulatory Mechanism of Taproot Enlargement in Panax ginseng" International Journal of Molecular Sciences 24, no. 6: 5590. https://doi.org/10.3390/ijms24065590
APA StyleZhang, M., Sun, Y., Di, P., Han, M., & Yang, L. (2023). Combining Metabolomics and Transcriptomics to Reveal the Regulatory Mechanism of Taproot Enlargement in Panax ginseng. International Journal of Molecular Sciences, 24(6), 5590. https://doi.org/10.3390/ijms24065590





