Associations of Biopterins and ADMA with Vascular Function in Peripheral Microcirculation from Patients with Chronic Kidney Disease
Abstract
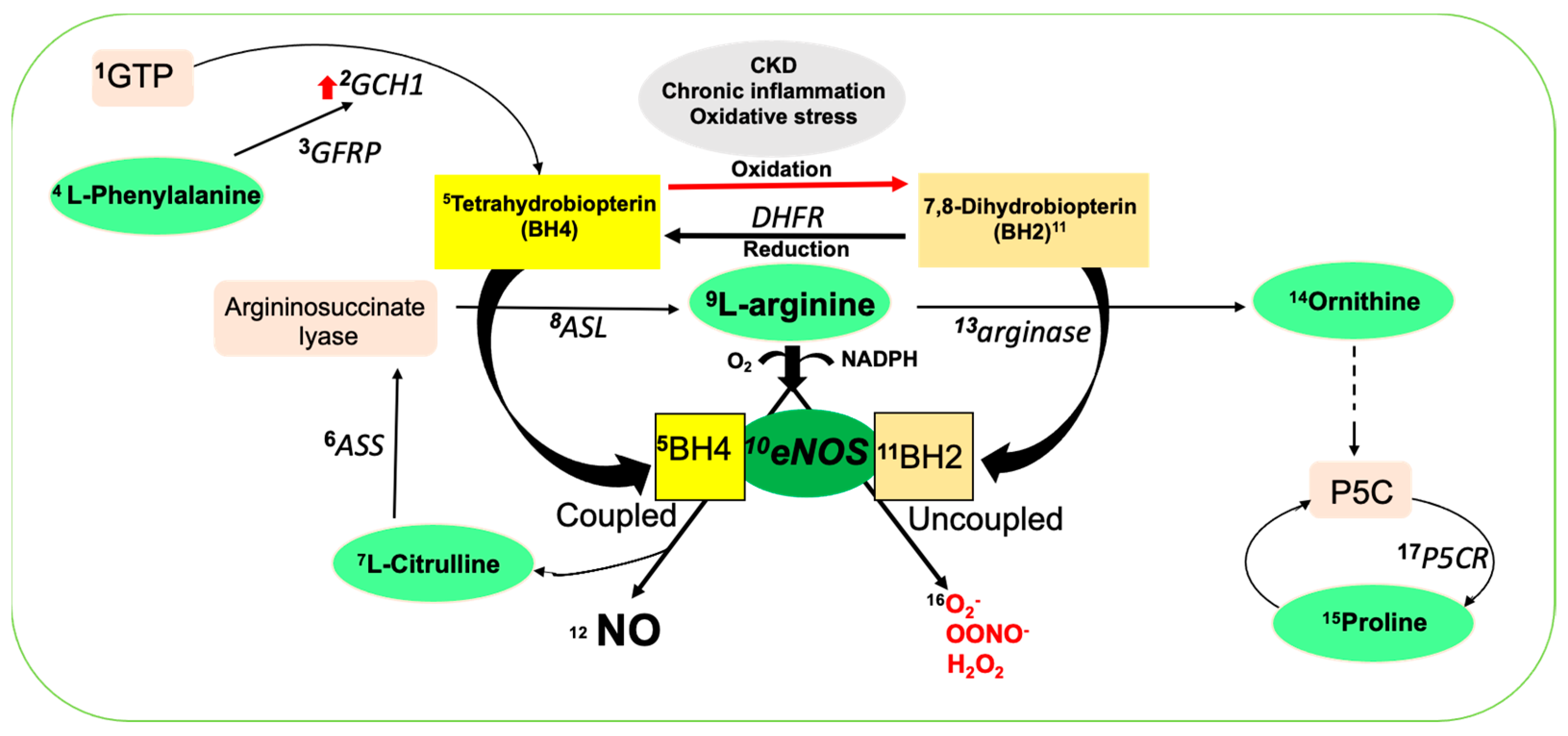
1. Introduction
2. Results
2.1. Study Population
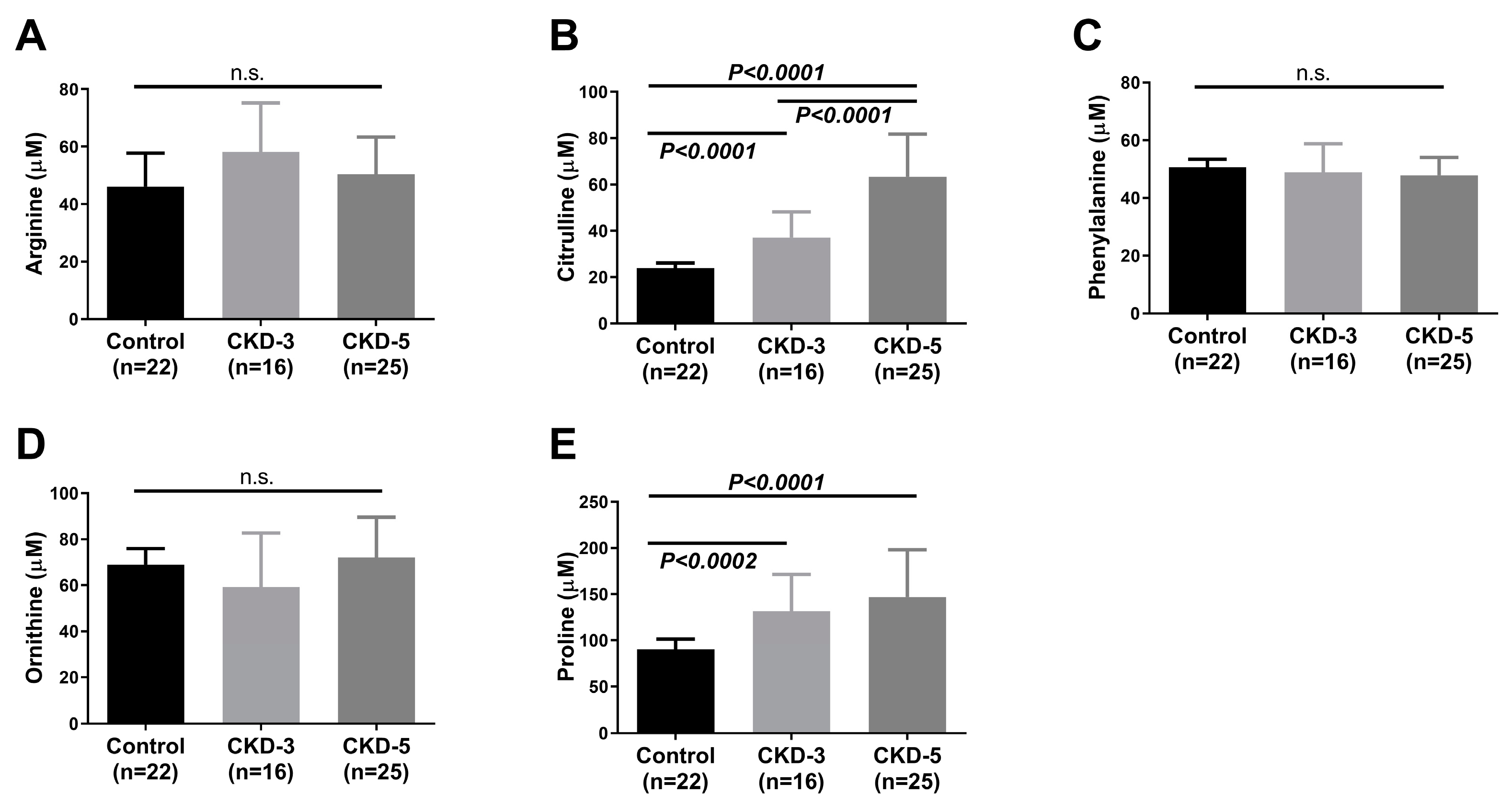
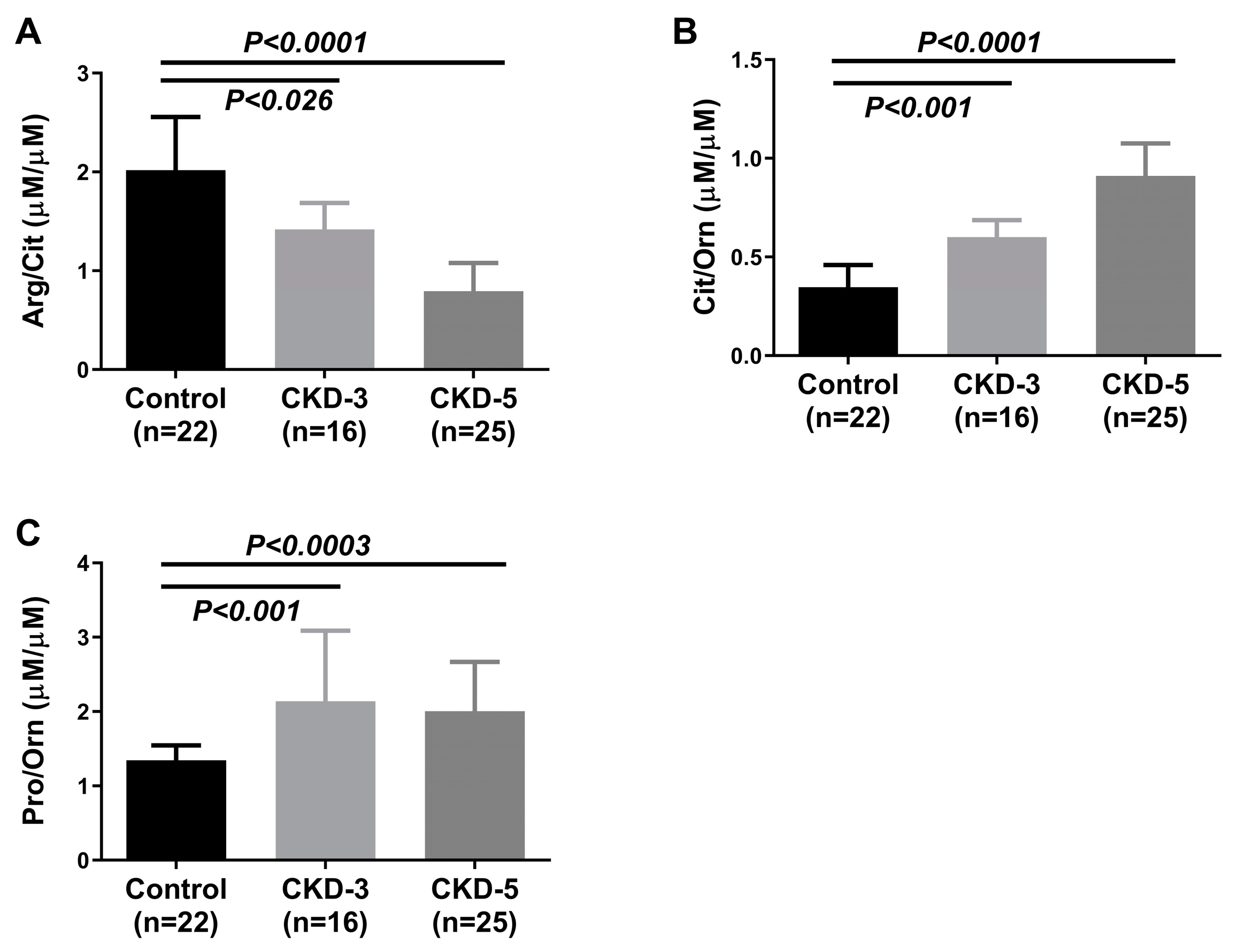
2.2. Plasma Amino Acids and Their Metabolites
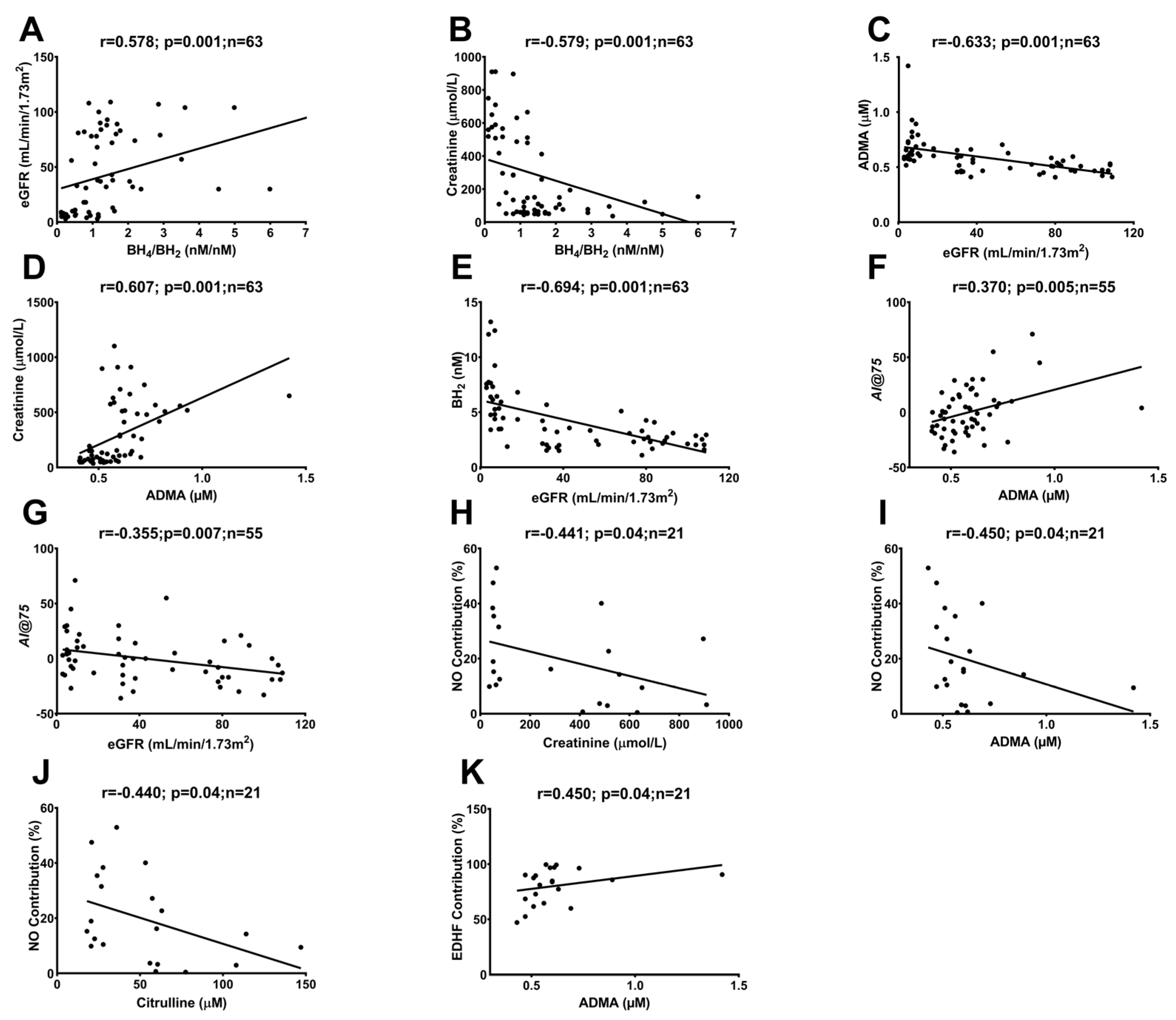
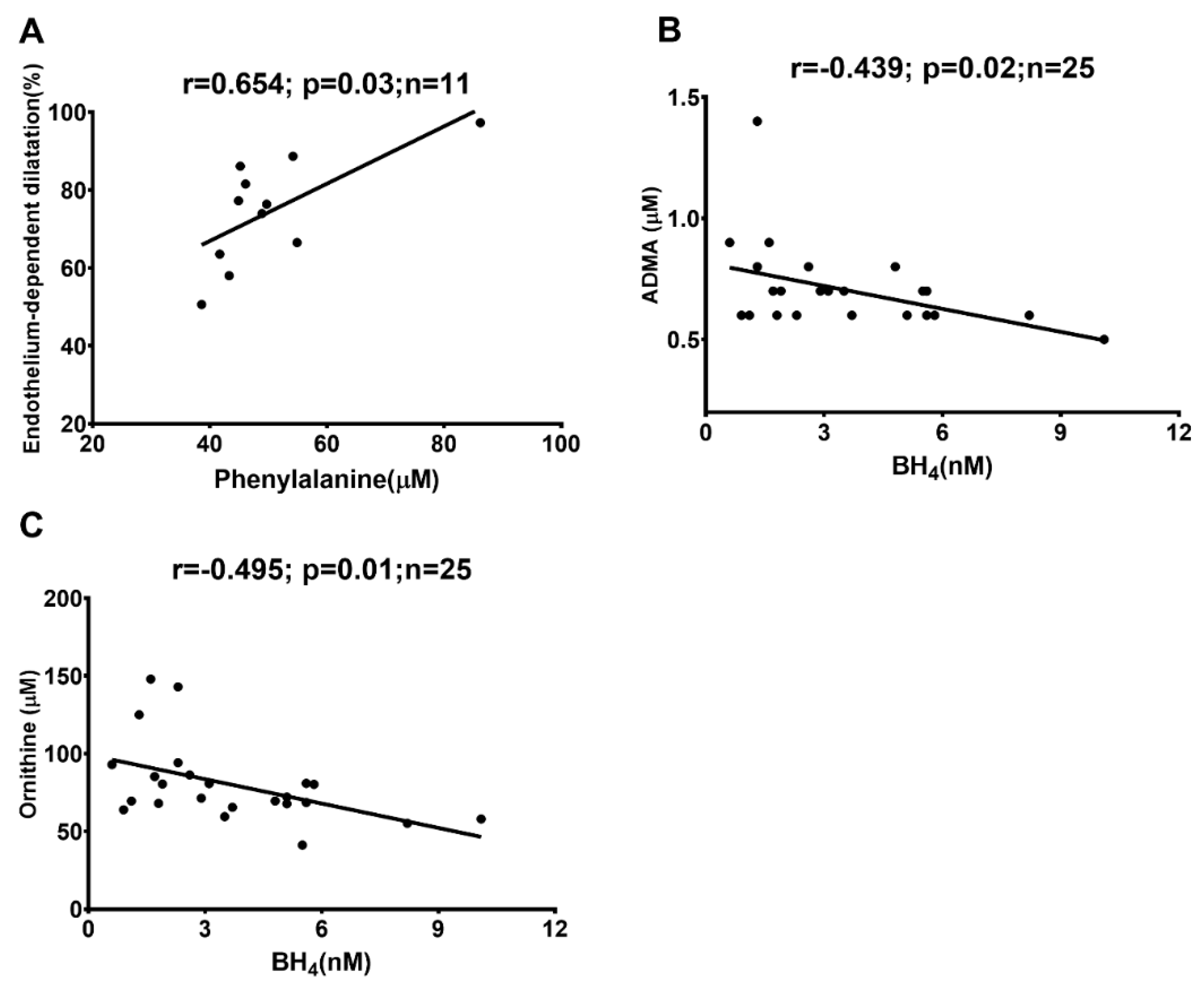
2.3. Correlations between In Vivo and Ex Vivo Measurements and AA Metabolites
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Collection of Blood Samples for the Measurements of Amino Acids and Biopterines
4.3. Analysis of Amino Acids in Plasma
4.4. Analysis of BH2 and BH4 in Plasma
4.5. In Vivo Endothelial Function and Stiffness
4.6. Ex Vivo Vascular Function and Structure
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. (2011) 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Gu, L.; Liu, P.; Cao, J.; Zhang, S. The Critical Role of Tetrahydrobiopterin (BH4) Metabolism in Modulating Radiosensitivity: BH4/NOS Axis as an Angel or a Devil. Front. Oncol. 2021, 11, 720632. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Sucher, R.; Gehwolf, P.; Oberhuber, R.; Hermann, M.; Margreiter, C.; Werner, E.R.; Obrist, P.; Schneeberger, S.; Ollinger, R.; Margreiter, R.; et al. Tetrahydrobiopterin protects the kidney from ischemia-reperfusion injury. Kidney Int. 2010, 77, 681–689. [Google Scholar] [CrossRef]
- Goligorsky, M.S. Pathogenesis of endothelial cell dysfunction in chronic kidney disease: A retrospective and what the future may hold. Kidney Res. Clin. Pract. 2015, 34, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Luksha, N.; Luksha, L.; Carrero, J.J.; Hammarqvist, F.; Stenvinkel, P.; Kublickiene, K. Impaired resistance artery function in patients with end-stage renal disease. Clin. Sci. 2011, 120, 525–536. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Shang, R.; Chen, Y. Asymmetric dimethylarginine (ADMA) as an important risk factor for the increased cardiovascular diseases and heart failure in chronic kidney disease. Nitric Oxide 2018, 78, 113–120. [Google Scholar] [CrossRef]
- Bendall, J.K.; Douglas, G.; McNeill, E.; Channon, K.M.; Crabtree, M.J. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid. Redox Signal. 2014, 20, 3040–3077. [Google Scholar] [CrossRef]
- Li, M.; Wu, Y.; Ye, L. The Role of Amino Acids in Endothelial Biology and Function. Cells 2022, 11, 1372. [Google Scholar] [CrossRef]
- Kakoki, M.; Kim, H.S.; Edgell, C.J.; Maeda, N.; Smithies, O.; Mattson, D.L. Amino acids as modulators of endothelium-derived nitric oxide. Am. J. Physiol. Renal. Physiol. 2006, 291, F297–F304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, C.; Yang, X.; Zhang, C. L-phenylalanine attenuates high salt-induced hypertension in Dahl SS rats through activation of GCH1-BH4. PLoS ONE 2021, 16, e0250126. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rezvan, A.; Salerno, J.C.; Husain, A.; Kwon, K.; Jo, H.; Harrison, D.G.; Chen, W. GTP cyclohydrolase I phosphorylation and interaction with GTP cyclohydrolase feedback regulatory protein provide novel regulation of endothelial tetrahydrobiopterin and nitric oxide. Circ. Res. 2010, 106, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Saunders-Pullman, R.; Blau, N.; Hyland, K.; Zschocke, J.; Nygaard, T.; Raymond, D.; Shanker, V.; Mohrmann, K.; Arnold, L.; Tabbal, S.; et al. Phenylalanine loading as a diagnostic test for DRD: Interpreting the utility of the test. Mol. Genet. Metab. 2004, 83, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-dependent regulation of collagen metabolism. Cell. Mol. Life Sci. 2020, 77, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Phang, J.M. Proline Metabolism in Cell Regulation and Cancer Biology: Recent Advances and Hypotheses. Antioxid. Redox Signal. 2019, 30, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, P.O.; Pumper, G.M.; Higano, S.T.; Holmes, D.R.; Kuvin, J.T.; Lerman, A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004, 44, 2137–2141. [Google Scholar] [CrossRef]
- Young, A.; Garcia, M.; Sullivan, S.M.; Liu, C.; Moazzami, K.; Ko, Y.A.; Shah, A.J.; Kim, J.H.; Pearce, B.; Uphoff, I.; et al. Impaired Peripheral Microvascular Function and Risk of Major Adverse Cardiovascular Events in Patients with Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1801–1809. [Google Scholar] [CrossRef]
- Werner, E.R.; Werner-Felmayer, G.; Wachter, H.; Mayer, B. Biosynthesis of nitric oxide: Dependence on pteridine metabolism. Rev. Physiol. Biochem. Pharmacol. 1996, 127, 97–135. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef]
- Crabtree, M.J.; Tatham, A.L.; Hale, A.B.; Alp, N.J.; Channon, K.M. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: Relative importance of the de novo biopterin synthesis versus salvage pathways. J. Biol. Chem. 2009, 284, 28128–28136. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Tajima, M.; Yoshida, H.; Nakayama, M.; Tokutome, G.; Sakagami, H.; Hosoya, T. Plasma pteridine concentrations in patients with chronic renal failure. Nephrol. Dial. Transplant. 2002, 17, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Reddy, Y.S.; Kiranmayi, V.S.; Bitla, A.R.; Krishna, G.S.; Rao, P.V.; Sivakumar, V. Nitric oxide status in patients with chronic kidney disease. Indian. J. Nephrol. 2015, 25, 287–291. [Google Scholar] [CrossRef]
- Singh, R.R.; Easton, L.K.; Booth, L.C.; Schlaich, M.P.; Head, G.A.; Moritz, K.M.; Denton, K.M. Renal Nitric Oxide Deficiency and Chronic Kidney Disease in Young Sheep Born with a Solitary Functioning Kidney. Sci. Rep. 2016, 6, 26777. [Google Scholar] [CrossRef] [PubMed]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef]
- Galvan, D.L.; Green, N.H.; Danesh, F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017, 92, 1051–1057. [Google Scholar] [CrossRef]
- Eiselt, J.; Rajdl, D.; Racek, J.; Vostrý, M.; Rulcová, K.; Wirth, J. Asymmetric dimethylarginine and progression of chronic kidney disease: A one-year follow-up study. Kidney Blood Press. Res. 2014, 39, 50–57. [Google Scholar] [CrossRef]
- Oliva-Damaso, E.; Oliva-Damaso, N.; Rodriguez-Esparragon, F.; Payan, J.; Baamonde-Laborda, E.; Gonzalez-Cabrera, F.; Santana-Estupiñan, R.; Rodriguez-Perez, J.C. Asymmetric (ADMA) and Symmetric (SDMA) Dimethylarginines in Chronic Kidney Disease: A Clinical Approach. Int. J. Mol. Sci. 2019, 20, 3668. [Google Scholar] [CrossRef]
- Tripepi, G.; Mattace Raso, F.; Sijbrands, E.; Seck, M.S.; Maas, R.; Boger, R.; Witteman, J.; Rapisarda, F.; Malatino, L.; Mallamaci, F.; et al. Inflammation and asymmetric dimethylarginine for predicting death and cardiovascular events in ESRD patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 1714–1721. [Google Scholar] [CrossRef]
- Al Banchaabouchi, M.; Marescau, B.; D’Hooge, R.; Engelborghs, S.; De Deyn, P.P. Consequences of renal mass reduction on amino acid and biogenic amine levels in nephrectomized mice. Amino Acids 2000, 18, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Lundin, U.; Gayrard, N.; Mischak, H.; Aparicio, M.; Mourad, G.; Daurès, J.P.; Weinberger, K.M.; Argilés, A. Plasma and urinary amino acid metabolomic profiling in patients with different levels of kidney function. Clin. J. Am. Soc. Nephrol. 2014, 9, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Mallamaci, F.; Maas, R.; Benedetto, F.A.; Tripepi, G.; Malatino, L.S.; Cataliotti, A.; Bellanuova, I.; Böger, R.; Investigators, C. Left ventricular hypertrophy, cardiac remodeling and asymmetric dimethylarginine (ADMA) in hemodialysis patients. Kidney Int. 2002, 62, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, F.; Tripepi, G.; Maas, R.; Malatino, L.; Böger, R.; Zoccali, C. Analysis of the relationship between norepinephrine and asymmetric dimethyl arginine levels among patients with end-stage renal disease. J. Am. Soc. Nephrol. 2004, 15, 435–441. [Google Scholar] [CrossRef]
- Calver, A.; Collier, J.; Leone, A.; Moncada, S.; Vallance, P. Effect of local intra-arterial asymmetric dimethylarginine (ADMA) on the forearm arteriolar bed of healthy volunteers. J. Hum. Hypertens. 1993, 7, 193–194. [Google Scholar]
- Zoccali, C.; Benedetto, F.A.; Maas, R.; Mallamaci, F.; Tripepi, G.; Salvatore Malatino, L.; Böger, R. Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. J. Am. Soc. Nephrol. 2002, 13, 490–496. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Donnerstag, F.; Gasper, S.; Menne, J.; Kielstein, A.; Martens-Lobenhoffer, J.; Scalera, F.; Cooke, J.P.; Fliser, D.; Bode-Böger, S.M. ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke 2006, 37, 2024–2029. [Google Scholar] [CrossRef]
- Ghanaeian, A.; Soheilifard, R. Mechanical elasticity of proline-rich and hydroxyproline-rich collagen-like triple-helices studied using steered molecular dynamics. J. Mech. Behav. Biomed. Mater. 2018, 86, 105–112. [Google Scholar] [CrossRef]
- Heikal, L.; Starr, A.; Hussein, D.; Prieto-Lloret, J.; Aaronson, P.; Dailey, L.A.; Nandi, M. l-Phenylalanine Restores Vascular Function in Spontaneously Hypertensive Rats Through Activation of the GCH1-GFRP Complex. JACC Basic Transl. Sci. 2018, 3, 366–377. [Google Scholar] [CrossRef]
- Crabtree, M.J.; Channon, K.M. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide 2011, 25, 81–88. [Google Scholar] [CrossRef]
- Dong, J.Y.; Qin, L.Q.; Zhang, Z.; Zhao, Y.; Wang, J.; Arigoni, F.; Zhang, W. Effect of oral L-arginine supplementation on blood pressure: A meta-analysis of randomized, double-blind, placebo-controlled trials. Am. Heart J. 2011, 162, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, A.; Fischer, S.M.; Dillon, K.N.; Kang, Y.; Martinez, M.A.; Figueroa, A. Effects of L-Citrulline Supplementation on Endothelial Function and Blood Pressure in Hypertensive Postmenopausal Women. Nutrients 2022, 14, 4396. [Google Scholar] [CrossRef] [PubMed]
- van Ginkel, W.G.; van Reemst, H.E.; Kienstra, N.S.; Daly, A.; Rodenburg, I.L.; MacDonald, A.; Burgerhof, J.G.M.; de Blaauw, P.; van de Krogt, J.; Santra, S.; et al. The Effect of Various Doses of Phenylalanine Supplementation on Blood Phenylalanine and Tyrosine Concentrations in Tyrosinemia Type 1 Patients. Nutrients 2019, 11, 2816. [Google Scholar] [CrossRef] [PubMed]
- Fekkes, D.; Voskuilen-Kooijman, A. Quantitation of total biopterin and tetrahydrobiopterin in plasma. Clin. Biochem. 2007, 40, 411–413. [Google Scholar] [CrossRef]
- Yuan, T.F.; Huang, H.Q.; Gao, L.; Wang, S.T.; Li, Y. A novel and reliable method for tetrahydrobiopterin quantification: Benzoyl chloride derivatization coupled with liquid chromatography-tandem mass spectrometry analysis. Free Radic. Biol. Med. 2018, 118, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Luksha, L.; Agewall, S.; Kublickiene, K. Endothelium-derived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis 2009, 202, 330–344. [Google Scholar] [CrossRef]

| CKD-5 (n = 25) | CKD-3 (n = 16) | Control (n = 22) | p-Values | ||
|---|---|---|---|---|---|
| CKD-5 vs. Control | CKD-3 vs. Control | ||||
| Demography | |||||
| Age (years) | 48 (36–54) | 44 (31–61) | 42 (36–55) | 0.99 | 0.87 |
| Males (%) | 18 (72%) | 12 (75%) | 6 (27%) | 0.001 | 0.001 |
| Body mass index (kg/m2) | 25 (23–28) | 24 (21–28) | 26 (21–28) | 0.95 | 0.54 |
| eGFR(mL/min/1.73 m2) | 7 (5–9) | 35 (31–42) | 100 (85–108) | 0.001 | 0.001 |
| SBP (mmHg) | 146 (129–165) | 137 (125–144) | 120 (109–127) | 0.001 | 0.02 |
| DBP (mmHg) | 93 (86–104) | 78 (70–83) | 77 (69–82) | 0.001 | 0.16 |
| Metabolic markers | |||||
| Creatinine (µM/L) | 706 (573–903) | 166 (141–211) | 73 (67–81) | 0.001 | 0.001 |
| Total cholesterol (mM/L) | 4.4 (3.4–5.3) | 4.8 (4.5–6.1) | 4.7 (4.4–5.2) | 0.36 | 0.68 |
| HDL cholesterol (mM/L) | 1.2 (1.0–1.7) | 1.4 (1.2–1.8) | 1.6 (1.2–1.9) | 0.05 | 0.60 |
| Triglycerides (mM/L) | 1.5 (0.9–1.8) | 1.3 (1.1–2.0) | 0.92 (0.7–1.3) | 0.005 | 0.01 |
| HbA1c, IFCC units (mM/M) | 36 (33–38) | 36 (32–37) | 34 (32–36) | 0.39 | 0.84 |
| Inflammation | |||||
| hsCRP (mg/L) | 0.8 (0.4–1.8) | 0.9 (0.4–2.0) | 0.57 (0.3–2.1) | 0.67 | 0.16 |
| Albumin (g/L) | 36 (32–39) | 32 (26–37) | 40 (36–41) | 0.005 | 0.001 |
| Calcification & bone markers | |||||
| Calcium (mM/L) | 2.3 (2.1–2.4) | 2.2 (2.1–2.3) | 2.3 (2.2–2.4) | 0.45 | 0.64 |
| Phosphate (mM/L) | 1.8 (1.4–2.3) | 1.2 (1.1–1.3) | 0.9 (0.9–1.1) | 0.001 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arefin, S.; Löfgren, L.; Stenvinkel, P.; Granqvist, A.B.; Kublickiene, K. Associations of Biopterins and ADMA with Vascular Function in Peripheral Microcirculation from Patients with Chronic Kidney Disease. Int. J. Mol. Sci. 2023, 24, 5582. https://doi.org/10.3390/ijms24065582
Arefin S, Löfgren L, Stenvinkel P, Granqvist AB, Kublickiene K. Associations of Biopterins and ADMA with Vascular Function in Peripheral Microcirculation from Patients with Chronic Kidney Disease. International Journal of Molecular Sciences. 2023; 24(6):5582. https://doi.org/10.3390/ijms24065582
Chicago/Turabian StyleArefin, Samsul, Lars Löfgren, Peter Stenvinkel, Anna B. Granqvist, and Karolina Kublickiene. 2023. "Associations of Biopterins and ADMA with Vascular Function in Peripheral Microcirculation from Patients with Chronic Kidney Disease" International Journal of Molecular Sciences 24, no. 6: 5582. https://doi.org/10.3390/ijms24065582
APA StyleArefin, S., Löfgren, L., Stenvinkel, P., Granqvist, A. B., & Kublickiene, K. (2023). Associations of Biopterins and ADMA with Vascular Function in Peripheral Microcirculation from Patients with Chronic Kidney Disease. International Journal of Molecular Sciences, 24(6), 5582. https://doi.org/10.3390/ijms24065582






