Effect of Ovocystatin on Amyloid β 1-42 Aggregation—In Vitro Studies
Abstract
1. Introduction
2. Results
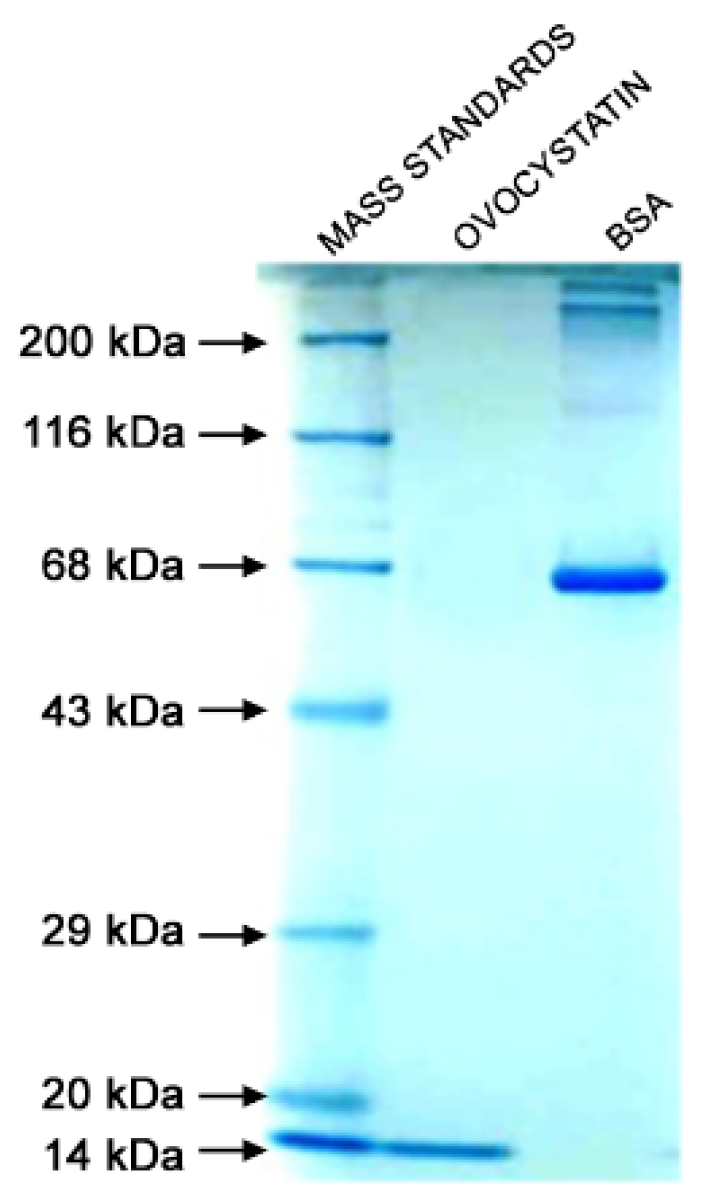
2.1. Isolation and Purification of Ovocystatin
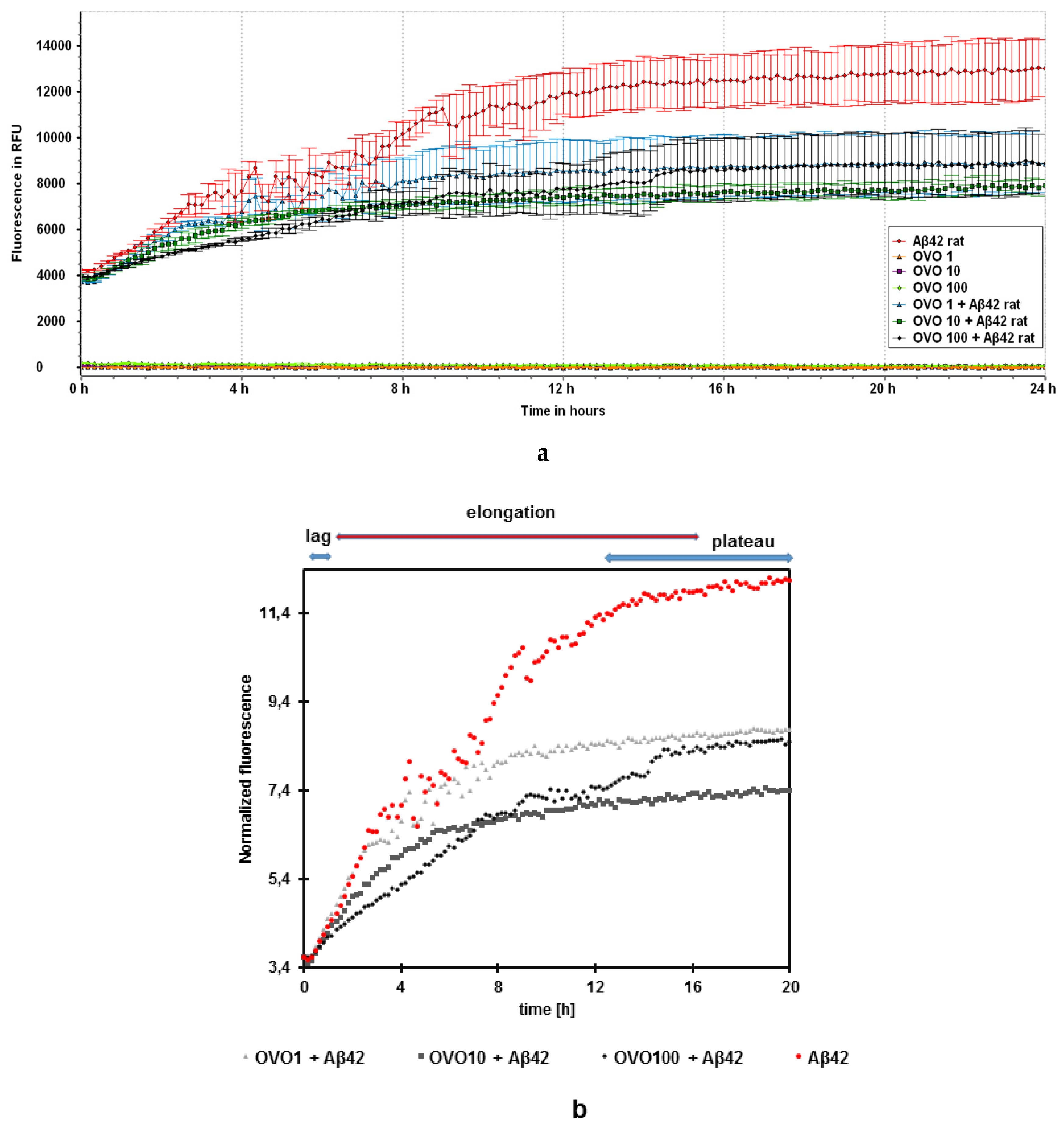
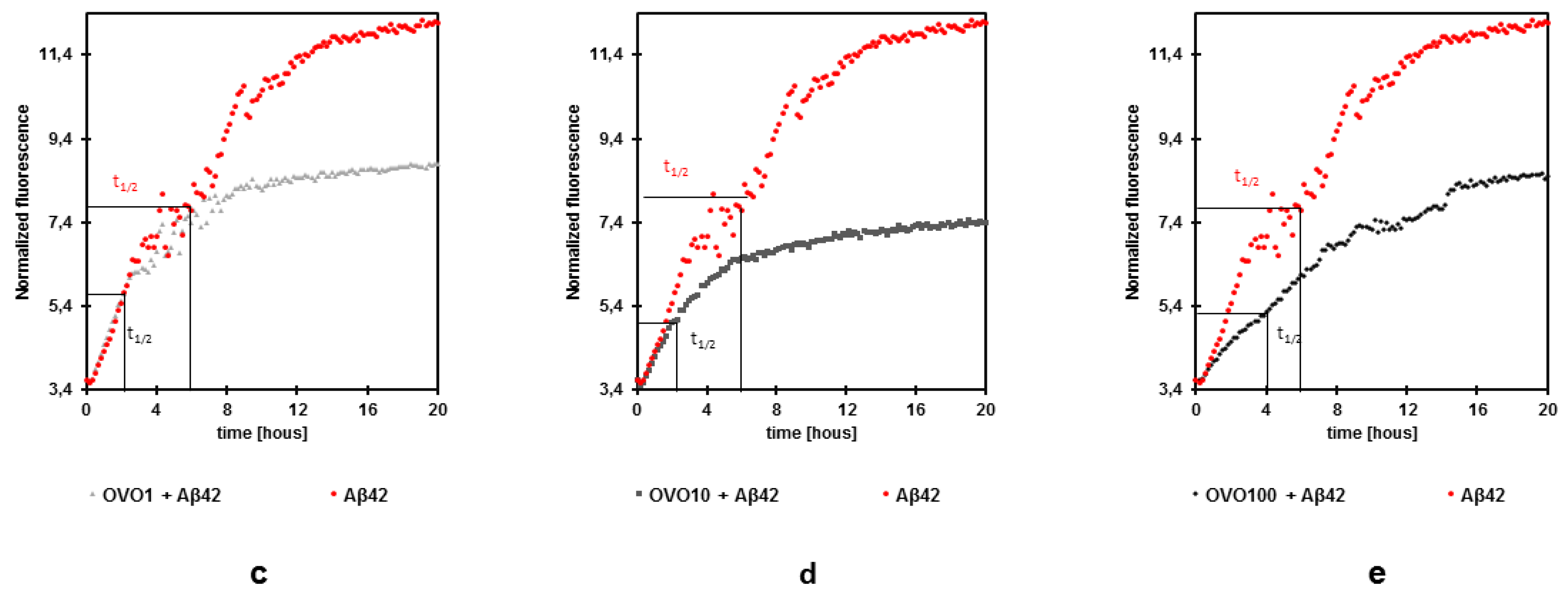
2.2. Effects of Ovocystatin against Aβ42 Fibrils Formation by ThT Assay
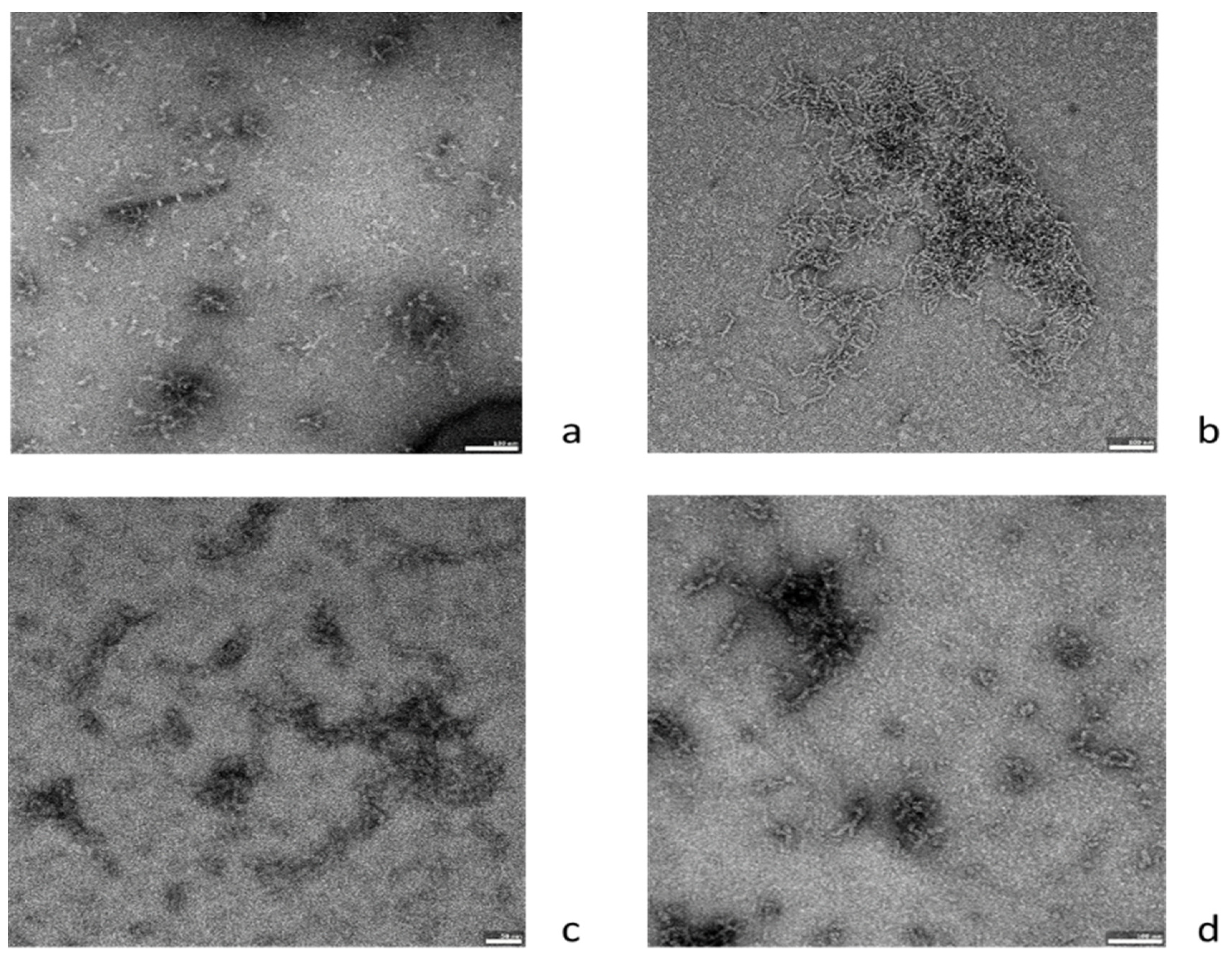
2.3. Effects of Ovocystatin on Aβ42 Fibril Morphology
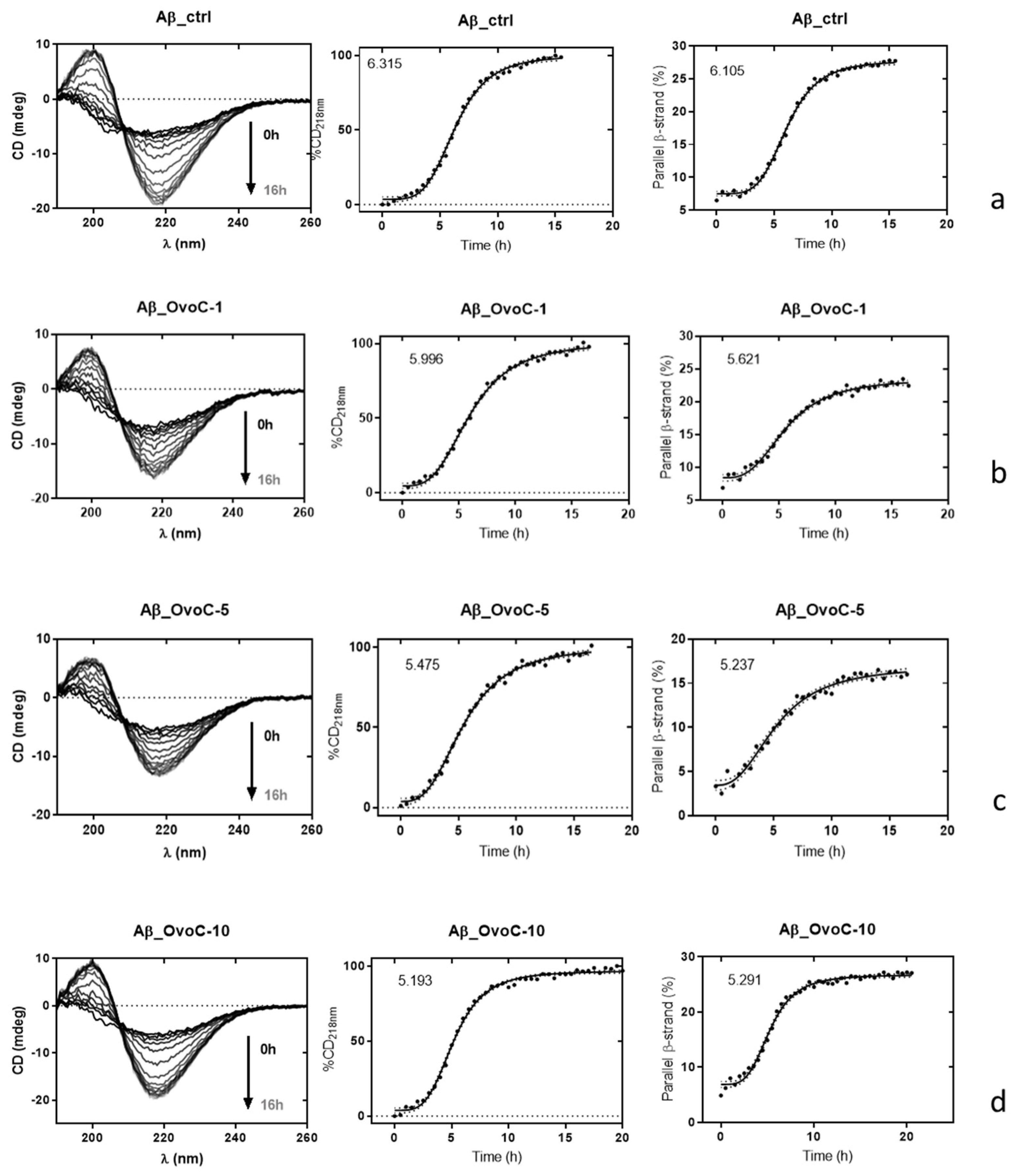
2.4. Effects of Ovocystatin on Aβ42 Secondary Structure during Fibrillation
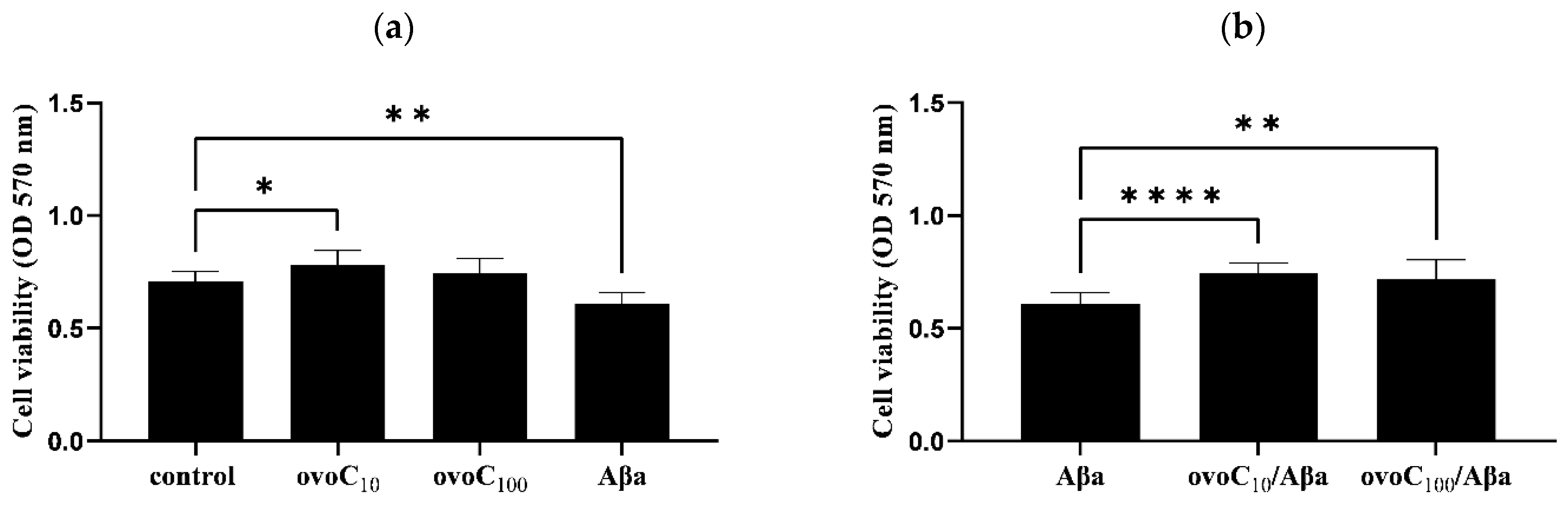
2.5. Ovocystatin Increased PC12 Cells Viability
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Ovocystatin Isolation
4.3. Amyloid β42 Preparation for Thioflavin T (ThT) Assay and Transmission Electron Microscopy (TEM)
4.4. ThT Assay of Aβ42 Fibril Formation
4.5. TEM
4.6. Circular Dichroism Spectroscopy (CD)
4.6.1. Aβ42 Preparation for CD Measurement
4.6.2. CD
4.7. Cell Viability Determination
4.7.1. Cell Culture
4.7.2. Amyloid Preparation for Cell Treatment
4.7.3. MTT Reduction Assay
4.8. Data Analysis and Graphical Visualization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belkacemi, A.; Ramassamy, C. Time Sequence of Oxidative Stress in the Brain from Transgenic Mouse Models of Alzheimer’s Disease Related to the Amyloid-β Cascade. Free Radic. Biol. Med. 2012, 52, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Arain, H.A.; Stecker, M.M.; Siegart, N.M.; Kasselman, L.J. Amyloid Toxicity in Alzheimer’s Disease. Rev. Neurosci. 2018, 29, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Eckert, G.P.; Renner, K.; Eckert, S.H.; Eckmann, J.; Hagl, S.; Abdel-Kader, R.M.; Kurz, C.; Leuner, K.; Muller, W.E. Mitochondrial Dysfunction—A Pharmacological Target in Alzheimer’s Disease. Mol. Neurobiol. 2012, 46, 136–150. [Google Scholar] [CrossRef]
- Tapia-Arancibia, L.; Aliaga, E.; Silhol, M.; Arancibia, S. New Insights into Brain BDNF Function in Normal Aging and Alzheimer Disease. Brain Res. Rev. 2008, 59, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Giza, J.; Proenca, C.C.; Jing, D.; Elliott, M.; Dincheva, I.; Shmelkov, S.V.; Kim, J.; Schreiner, R.; Huang, S.-H.; et al. Slitrk5 Mediates BDNF-Dependent TrkB Receptor Trafficking and Signaling. Dev. Cell 2015, 33, 690–702. [Google Scholar] [CrossRef]
- Krstic, D.; Knuesel, I. Deciphering the Mechanism Underlying Late-Onset Alzheimer Disease. Nat. Rev. Neurol. 2013, 9, 25–34. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s Disease. Nat. Rev. Dis. Prim. 2015, 1, 15056. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s Disease: Genotypes, Phenotype, and Treatments. Science 1997, 275, 630–631. [Google Scholar] [CrossRef]
- Finckh, U.; Kuschel, C.; Anagnostouli, M.; Patsouris, E.; Pantes, G.V.; Gatzonis, S.; Kapaki, E.; Davaki, P.; Lamszus, K.; Stavrou, D.; et al. Novel Mutations and Repeated Findings of Mutations in Familial Alzheimer Disease. Neurogenetics 2005, 6, 85–89. [Google Scholar] [CrossRef]
- Dá Mesquita, S.; Ferreira, A.C.; Sousa, J.C.; Correia-Neves, M.; Sousa, N.; Marques, F. Insights on the Pathophysiology of Alzheimer’s Disease: The Crosstalk between Amyloid Pathology, Neuroinflammation and the Peripheral Immune System. Neurosci. Biobehav. Rev. 2016, 68, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Karran, E.; De Strooper, B. The Amyloid Cascade Hypothesis: Are We Poised for Success or Failure? J. Neurochem. 2016, 139, 237–252. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.I.; Sharoar, M.G.; Ryu, E.-K.; Park, I.-S. Limited Activation of the Intrinsic Apoptotic Pathway Plays a Main Role in Amyloid-β-Induced Apoptosis without Eliciting the Activation of the Extrinsic Apoptotic Pathway. Int. J. Mol. Med. 2017, 40, 1971–1982. [Google Scholar] [CrossRef]
- Levy, E. Cystatin C: A Potential Target for Alzheimer’s Treatment. Expert. Rev. Neurother. 2008, 8, 687–689. [Google Scholar] [CrossRef]
- Gauthier, S.; Kaur, G.; Mi, W.; Tizon, B.; Levy, E. Protective Mechanisms by Cystatin C in Neurodegenerative Diseases. Front. Biosci. 2011, 3, 541–554. [Google Scholar]
- Sastre, M.; Calero, M.; Pawlik, M.; Mathews, P.M.; Kumar, A.; Danilov, V.; Schmidt, S.D.; Nixon, R.A.; Frangione, B.; Levy, E. Binding of Cystatin C to Alzheimer’s Amyloid Beta Inhibits in Vitro Amyloid Fibril Formation. Neurobiol. Aging 2004, 25, 1033–1043. [Google Scholar] [CrossRef]
- Selenica, M.L.; Wang, X.; Ostergaard-Pedersen, L.; Westlind-Danielsson, A.; Grubb, A. Cystatin C Reduces the in Vitro Formation of Soluble A-Beta1-42 Oligomers and Protofibrils. Scand. J. Clin. Lab. Investig. 2007, 67, 179–190. [Google Scholar] [CrossRef]
- Mi, W.; Pawlik, M.; Sastre, M.; Jung, S.S.; Radvinsky, D.S.; Klein, A.M.; Sommer, J.; Schmidt, S.D.; Nixon, R.A.; Mathews, P.M.; et al. Cystatin C Inhibits Amyloid-Beta Deposition in Alzheimer’s Disease Mouse Models. Nat. Genet. 2007, 39, 1440–1442. [Google Scholar] [CrossRef]
- Kaur, G.; Levy, E. Cystatin C in Alzheimer’s Disease. Front. Mol. Neurosci. 2012, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Stańczykiewicz, B.; Jakubik-Witkowska, M.; Polanowski, A.; Trziszka, T.; Rymaszewska, J. An Animal Model of the Procognitive Properties of Cysteine Protease Inhibitor and Immunomodulatory Peptides Based on Colostrum. Adv. Clin. Exp. Med. 2017, 26, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Stańczykiewicz, B.; Rutkowska, M.; Lemieszewska, M.; Jakubik-Witkowska, M.; Gburek, J.; Gołąb, K.; Juszczyńska, K.; Trziszka, T.; Rymaszewska, J. Potential Protective Effect of Ovocystatin on Aging-Related Cognitive Impairment in Rats Wpływ Ovocystatyny Na Funkcje Poznawcze Szczurów. Postep. Hig. Med. Dosw. 2017, 71, 1202–1208. [Google Scholar] [CrossRef]
- Stańczykiewicz, B.; Jakubik-Witkowska, M.; Rutkowska, M.; Polanowski, A.; Gburek, J.; Gołąb, K.; Juszczyńska, K.; Trziszka, T.; Rymaszewska, J. Beneficial Effect of Ovocystatin on the Cognitive Decline in APP/PS1 Transgenic Mice. Adv. Med. Sci. 2019, 64, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Stanczykiewicz, B.; Gburek, J.; Rutkowska, M.; Lemieszewska, M.; Gołąb, K.; Juszczyńska, K.; Piotrowska, A.; Trziszka, T.; Dzięgiel, P.; Podhorska-Okołów, M.; et al. Ovocystatin Induced Changes in Expression of Alzheimer’s Disease Relevant Proteins in APP/PS1 Transgenic Mice. J. Clin. Med. 2022, 11, 2372. [Google Scholar] [CrossRef]
- Stoka, V.; Turk, V.; Turk, B. Lysosomal Cathepsins and Their Regulation in Aging and Neurodegeneration. Ageing Res. Rev. 2016, 32, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, A.; Bano, B. Journey of Cystatins from Being Mere Thiol Protease Inhibitors to at Heart of Many Pathological Conditions. Int. J. Biol. Macromol. 2017, 102, 674–693. [Google Scholar] [CrossRef]
- Engh, R.A.; Dieckmann, T.; Bode, W.; Auerswald, E.A.; Turk, V.; Huber, R.; Oschkinat, H. Conformational Variability of Chicken Cystatin: Comparison of Structures Determined by X-ray Diffraction and NMR Spectroscopy. J. Mol. Biol. 1993, 234, 1060–1069. [Google Scholar] [CrossRef]
- Khurana, R.; Coleman, C.; Ionescu-Zanetti, C.; Carter, S.A.; Krishna, V.; Grover, R.K.; Roy, R.; Singh, S. Mechanism of Thioflavin T Binding to Amyloid Fibrils. J. Struct. Biol. 2005, 151, 229–238. [Google Scholar] [CrossRef]
- Paul, A.; Frenkel-Pinter, M.; Escobar Alvarez, D.; Milordini, G.; Gazit, E.; Zacco, E.; Segal, D. Tryptophan-Galactosylamine Conjugates Inhibit and Disaggregate Amyloid Fibrils of Aβ42 and HIAPP Peptides While Reducing Their Toxicity. Commun. Biol. 2020, 3, 484. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Lin, T.Y.; Chang, D.; Guo, Z. Thioflavin T as an Amyloid Dye: Fibril Quantification, Optimal Concentration and Effect on Aggregation. R. Soc. Open Sci. 2017, 4, 160696. [Google Scholar] [CrossRef]
- McDade, E.; Bateman, R.J. Stop Alzheimer’s before It Starts. Nature 2017, 547, 153–155. [Google Scholar] [CrossRef]
- Almeida, Z.L.; Brito, R.M.M. Structure and Aggregation Mechanisms in Amyloids. Molecules 2020, 25, 1195. [Google Scholar] [CrossRef]
- Perlenfein, T.J.; Mehlhoff, J.D.; Murphy, R.M. Insights into the mechanism of cystatin C oligomer and amyloid formation and its interaction with β-amyloid. J. Biol. Chem. 2017, 282, 11485. [Google Scholar] [CrossRef] [PubMed]
- Szpak, M.; Trziszka, T.; Polanowski, A.; Gburek, J.; Gołąb, K.; Juszczyńska, K.; Janik, P.; Malicki, A.; Szyplik, K. Evaluation of the Antibacterial Activity of Cystatin against Selected Strains of Escherichia coli. Folia Biol. 2014, 62, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Malicka-Blaszkiewicz, M.; Filipczak, N.; Gołąb, K.; Juszczyńska, K.; Sebzda, T.; Gburek, J. Ovocystatin Affects Actin Cytoskeleton Organization and Induces Proapoptotic Activity. Acta Biochim. Pol. 2014, 61, 753–758. [Google Scholar] [PubMed]
- Pellarin, R.; Caflisch, A. Interpreting the Aggregation Kinetics of Amyloid Peptides. J. Mol. Biol. 2006, 360, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.; Park, G.; Guo, Z. Key Residues for the Formation of Aβ42 Amyloid Fibrils. ACS Omega 2018, 3, 8401–8407. [Google Scholar] [CrossRef] [PubMed]
- Linse, S. Mechanism of Amyloid Protein Aggregation and the Role of Inhibitors. Pure Appl. Chem. 2019, 91, 211–229. [Google Scholar] [CrossRef]
- Zabłocka, A.; Janusz, M.; Rybka, K.; Wirkus-Romanowska, I.; Kupryszewski, G.; Lisowski, J. Cytokine-Inducing Activity of a Proline-Rich Polypeptide Complex (PRP) from Ovine Colostrum and Its Active Nonapeptide Fragment Analogs. Eur. Cytokine Netw. 2001, 12, 462–467. [Google Scholar]
- Owen, S.C.; Doak, A.K.; Ganesh, A.N.; Nedyalkova, L.; McLaughlin, C.K.; Shoichet, B.K.; Shoichet, M.S. Colloidal Drug Formulations Can Explain “Bell-Shaped” Concentration–Response Curves. ACS Chem. Biol. 2014, 9, 777–784. [Google Scholar] [CrossRef]
- Ni, W.; Zhang, X.; Wang, B.; Chen, Y.; Han, H.; Fan, Y.; Zhou, Y.; Tai, G. Antitumor Activities and Immunomodulatory Effects of Ginseng Neutral Polysaccharides in Combination with 5-Fluorouracil. J. Med. Food 2010, 13, 270–277. [Google Scholar] [CrossRef]
- Wang, W.; Wang, F.; Yang, Y.-J.; Hu, Z.-L.; Long, L.-H.; Fu, H.; Xie, N.; Chen, J.-G. The Flavonoid Baicalein Promotes NMDA Receptor-Dependent Long-Term Potentiation and Enhances Memory. Br. J. Pharmacol. 2011, 162, 1364–1379. [Google Scholar] [CrossRef] [PubMed]
- Clerici, M.; Cogliati, M.; Rizzardini, G.; Colombo, F.; Fossati, S.; Rhodes, J.; Bray, D.; Piconi, S. In Vitro Immunomodulatory Properties of Tucaresol in HIV Infection. Clin. Immunol. 2000, 97, 211–220. [Google Scholar] [CrossRef]
- Mathews, P.M.; Levy, E. Cystatin C in Aging and in Alzheimer’s Disease. Ageing Res. Rev. 2016, 32, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Perlenfein, T.J.; Murphy, R.M. A Mechanistic Model to Predict Effects of Cathepsin B and Cystatin C on β-Amyloid Aggregation and Degradation. J. Biol. Chem. 2017, 292, 21071–21082. [Google Scholar] [CrossRef] [PubMed]
- Ladiwala, A.R.A.; Lin, J.C.; Bale, S.S.; Marcelino-Cruz, A.M.; Bhattacharya, M.; Dordick, J.S.; Tessier, P.M. Resveratrol Selectively Remodels Soluble Oligomers and Fibrils of Amyloid Abeta into Off-Pathway Conformers. J. Biol. Chem. 2010, 285, 24228–24237. [Google Scholar] [CrossRef] [PubMed]
- Berhanu, W.M.; Masunov, A.E. Natural Polyphenols as Inhibitors of Amyloid Aggregation. Molecular Dynamics Study of GNNQQNY Heptapeptide Decamer. Biophys. Chem. 2010, 149, 12–21. [Google Scholar] [CrossRef]
- Nie, Q.; Du, X.; Geng, M. Small Molecule Inhibitors of Amyloid β Peptide Aggregation as a Potential Therapeutic Strategy for Alzheimer’s Disease. Acta Pharmacol. Sin. 2011, 32, 545–551. [Google Scholar] [CrossRef]
- Spodzieja, M.; Kalejta, K.; Kołodziejczyk, A.S.; Maszota-Zieleniak, M.; Rodziewicz-Motowidło, S.; Żmudzińska, W.; Czaplewska, P. Characteristics of C-Terminal, β-Amyloid Peptide Binding Fragment of Neuroprotective Protease Inhibitor, Cystatin C. J. Mol. Recognit. 2017, 30, e2581. [Google Scholar] [CrossRef] [PubMed]
- Juszczyk, P.; Paraschiv, G.; Szymanska, A.; Kolodziejczyk, A.S.; Rodziewicz-Motowidlo, S.; Grzonka, Z.; Przybylski, M. Binding Epitopes and Interaction Structure of the Neuroprotective Protease Inhibitor Cystatin C with Beta-Amyloid Revealed by Proteolytic Excision Mass Spectrometry and Molecular Docking Simulation. J. Med. Chem. 2009, 52, 2420–2428. [Google Scholar] [CrossRef]
- Pagano, K.; Tomaselli, S.; Molinari, H.; Ragona, L. Natural Compounds as Inhibitors of Aβ Peptide Aggregation: Chemical Requirements and Molecular Mechanisms. Front. Neurosci. 2020, 14, 619667. [Google Scholar] [CrossRef] [PubMed]
- Alghazwi, M.; Charoensiddhi, S.; Smid, S.; Zhang, W. Impact of Ecklonia Radiata Extracts on the Neuroprotective Activities against Amyloid Beta (Aβ1-42) Toxicity and Aggregation. J. Funct. Foods 2020, 68, 103893. [Google Scholar] [CrossRef]
- Alghazwi, M.; Smid, S.; Musgrave, I.; Zhang, W. In Vitro Studies of the Neuroprotective Activities of Astaxanthin and Fucoxanthin against Amyloid Beta (Aβ1-42) Toxicity and Aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin Inhibits Formation of Amyloid Beta Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid in Vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.H.; Paudel, P.; Jung, H.A.; Choi, J.S. Identifying Phlorofucofuroeckol-A as a Dual Inhibitor of Amyloid-Β25-35 Self-Aggregation and Insulin Glycation: Elucidation of the Molecular Mechanism of Action. Mar. Drugs 2019, 17, 600. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, A.; Brown, M.A.; Kembhavi, A.A.; Nicklin, M.J.; Sayers, C.A.; Sunter, D.C.; Barrett, A.J. Cystatin, a Protein Inhibitor of Cysteine Proteinases. Improved Purification from Egg White, Characterization, and Detection in Chicken Serum. Biochem. J. 1983, 211, 129–138. [Google Scholar] [CrossRef]
- Golab, K.; Gburek, J.; Juszczyńska, K.; Trziszka, T.; Polanowski, A. Stabilization of Monomeric Chicken Egg White Cystatin. Przem. Chem. 2012, 91, 741–744. [Google Scholar]
- Barrett, A.J. A New Assay for Cathepsin B1 and Other Thiol Proteinases. Anal. Biochem. 1972, 47, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Bulyáki, É.; Kun, J.; Moussong, É.; Lee, Y.-H.; Goto, Y.; Réfrégiers, M.; Kardos, J. BeStSel: A Web Server for Accurate Protein Secondary Structure Prediction and Fold Recognition from the Circular Dichroism Spectra. Nucleic Acids Res. 2018, 46, W315–W322. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stańczykiewicz, B.; Goszczyński, T.M.; Migdał, P.; Piksa, M.; Pawlik, K.; Gburek, J.; Gołąb, K.; Konopska, B.; Zabłocka, A. Effect of Ovocystatin on Amyloid β 1-42 Aggregation—In Vitro Studies. Int. J. Mol. Sci. 2023, 24, 5433. https://doi.org/10.3390/ijms24065433
Stańczykiewicz B, Goszczyński TM, Migdał P, Piksa M, Pawlik K, Gburek J, Gołąb K, Konopska B, Zabłocka A. Effect of Ovocystatin on Amyloid β 1-42 Aggregation—In Vitro Studies. International Journal of Molecular Sciences. 2023; 24(6):5433. https://doi.org/10.3390/ijms24065433
Chicago/Turabian StyleStańczykiewicz, Bartłomiej, Tomasz M. Goszczyński, Paweł Migdał, Marta Piksa, Krzysztof Pawlik, Jakub Gburek, Krzysztof Gołąb, Bogusława Konopska, and Agnieszka Zabłocka. 2023. "Effect of Ovocystatin on Amyloid β 1-42 Aggregation—In Vitro Studies" International Journal of Molecular Sciences 24, no. 6: 5433. https://doi.org/10.3390/ijms24065433
APA StyleStańczykiewicz, B., Goszczyński, T. M., Migdał, P., Piksa, M., Pawlik, K., Gburek, J., Gołąb, K., Konopska, B., & Zabłocka, A. (2023). Effect of Ovocystatin on Amyloid β 1-42 Aggregation—In Vitro Studies. International Journal of Molecular Sciences, 24(6), 5433. https://doi.org/10.3390/ijms24065433




