CCP1, a Regulator of Tubulin Post-Translational Modifications, Potentially Plays an Essential Role in Cerebellar Development
Abstract
1. Introduction
2. Results
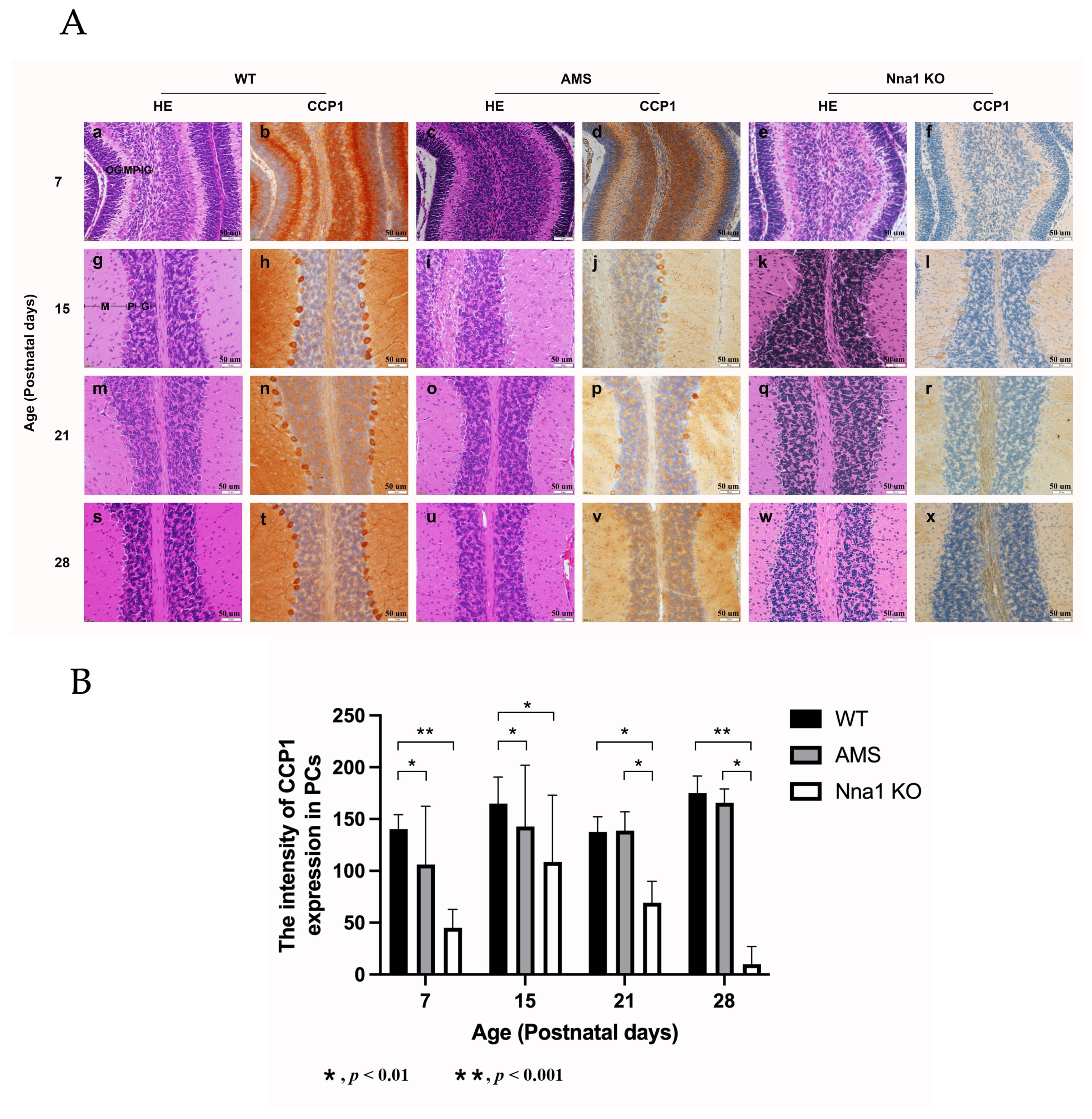
2.1. Ams Mutations and Deletions of CCP1 Alleles Lead to Morphological Changes during the Development of the Mouse Cerebellum
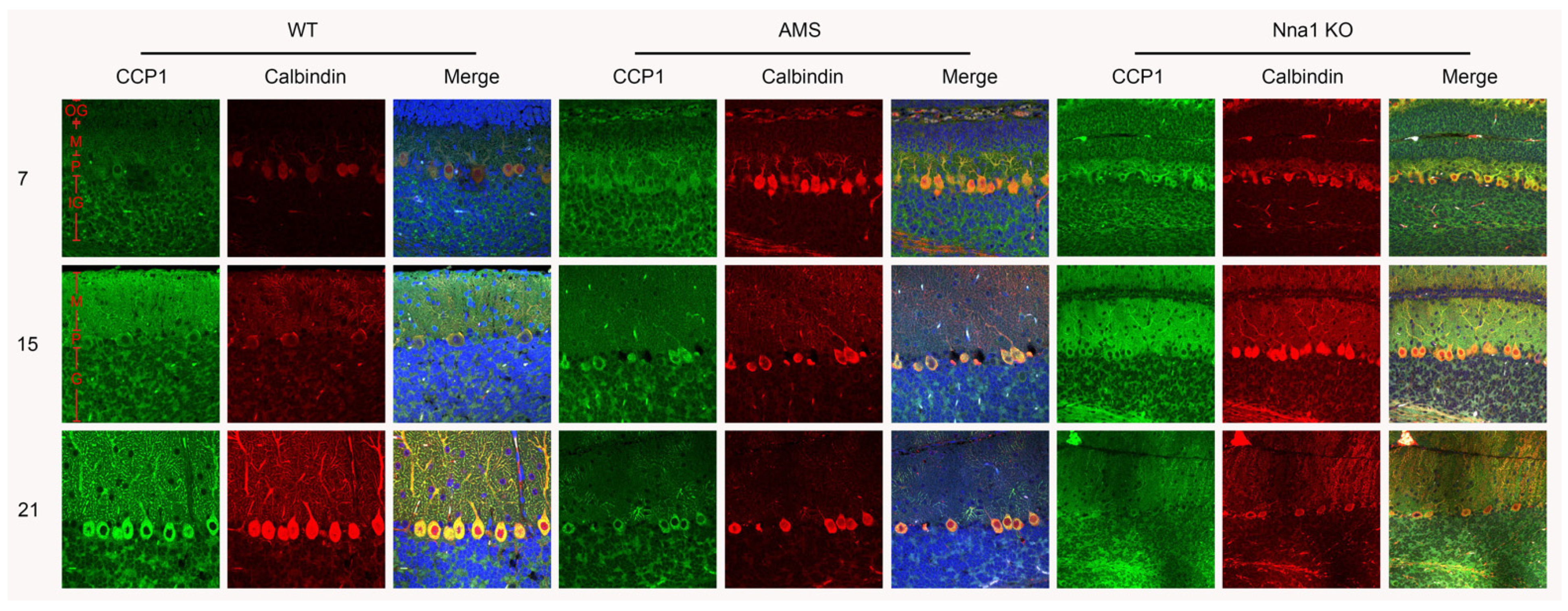
2.2. CCP1 Protein Expression during Cerebellar Development in the Two Mutant Mouse Strains
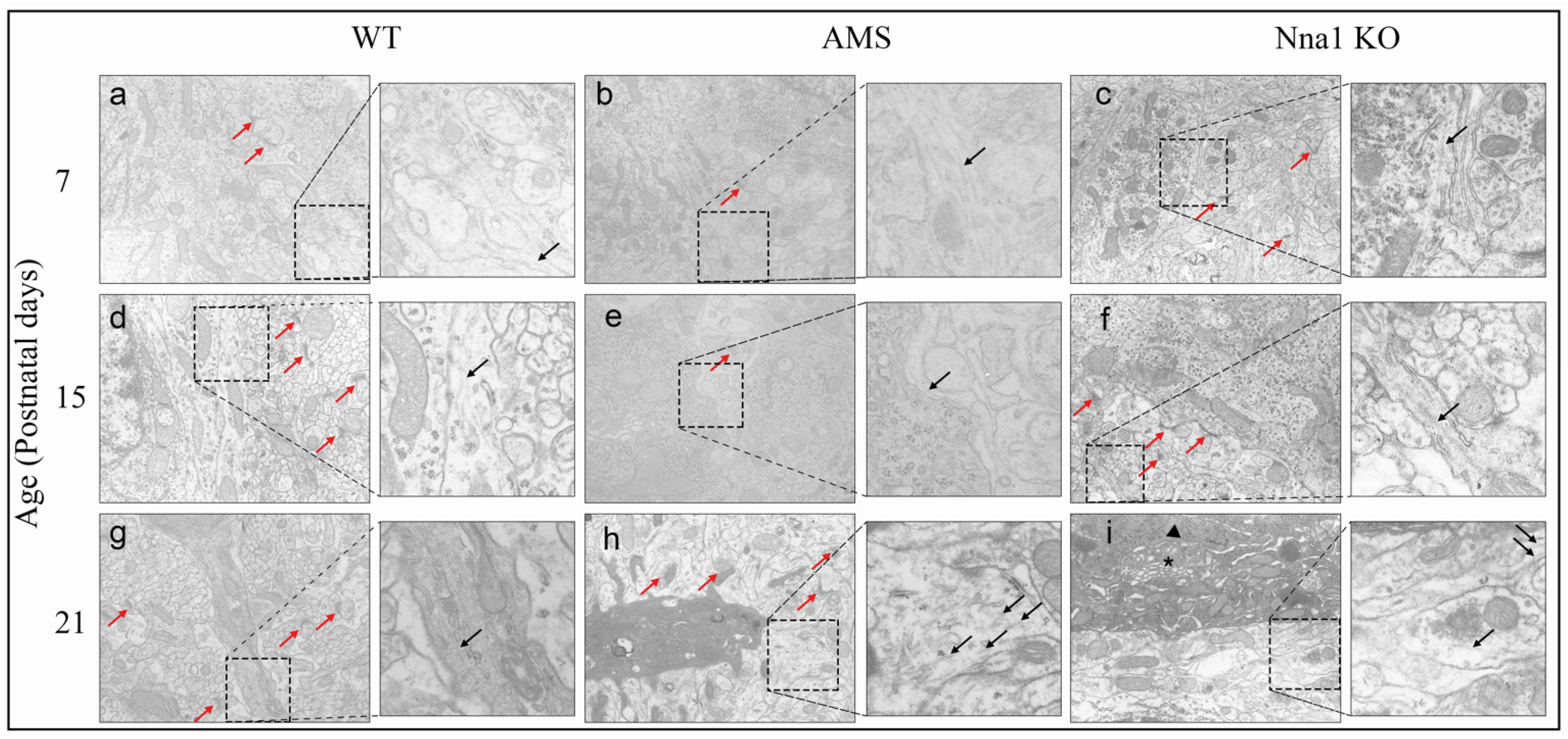
2.3. Ultrastructural Alterations on PCs Caused by CCP1 Gene Mutation and Knockout
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Mouse Genotyping
4.3. Tissue Preparation and Morphological Analysis
4.4. Western Blotting
4.5. Transmission Electron Microscope
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandez-Gonzalez, A.; La Spada, A.R. Purkinje cell degeneration (pcd) phenotypes caused by mutations in the axotomy-induced gene, Nna1. Science 2002, 295, 1904–1906. [Google Scholar] [CrossRef] [PubMed]
- Triarhou, L.C. Rate of neuronal fallout in a transsynaptic cerebellar model. Brain Res. Bull. 1998, 47, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Shashi, V.; Magiera, M.M. Loss of tubulin deglutamylase CCP1 causes infantile-onset neurodegeneration. EMBO J. 2018, 37, e100540. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.M.; Yano, S. Alteration of Neural Stem Cell Functions in Ataxia and Male Sterility Mice: A Possible Role of beta-Tubulin Glutamylation in Neurodegeneration. Cells 2021, 10, 155. [Google Scholar] [CrossRef]
- Harada, T.; Pineda, L.L. Ataxia and male sterility (AMS) mouse. A new genetic variant exhibiting degeneration and loss of cerebellar Purkinje cells and spermatic cells. Pathol. Int. 2003, 53, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Araki, A.; Maruyama, R. Analysis of the light-sensitivity of the photoreceptor cells of the ataxia and male sterility (AMS) mouse, an Nna1 mutant. Pathol. Int. 2012, 62, 719–727. [Google Scholar] [CrossRef]
- Leto, K.; Arancillo, M. Consensus Paper: Cerebellar Development. Cerebellum 2016, 15, 789–828. [Google Scholar] [CrossRef]
- Kaneko, M.; Yamaguchi, K. Remodeling of monoplanar Purkinje cell dendrites during cerebellar circuit formation. PLoS ONE 2011, 6, e20108. [Google Scholar] [CrossRef]
- Li, J.; Snyder, E.Y. Nna1 gene deficiency triggers Purkinje neuron death by tubulin hyperglutamylation and ER dysfunction. JCI Insight 2020, 5, e136078. [Google Scholar] [CrossRef]
- Ramadan, Y.H.; Gu, A. CCP1, a Tubulin Deglutamylase, Increases Survival of Rodent Spinal Cord Neurons following Glutamate-Induced Excitotoxicity. eNeuro 2021, 8, 0431. [Google Scholar] [CrossRef]
- Audebert, S.; Desbruyeres, E. Reversible polyglutamylation of alpha- and beta-tubulin and microtubule dynamics in mouse brain neurons. Mol. Biol. Cell. 1993, 4, 615–626. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, J.; Rogowski, K. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol. Cell. 2007, 26, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, M.T.; Saunders, H.A. Microtubule control of functional architecture in neurons. Curr. Opin. Neurobiol. 2019, 57, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kapitein, L.C.; Yau, K.W.; Hoogenraad, C.C. Chapter 7—Microtubule Dynamics in Dendritic Spines. In Microtubules: In Vivo; Methods in Cell Biology Book Series; Elsevier Inc.: Amsterdam, The Netherlands, 2010; Volume 97, pp. 111–132. [Google Scholar]
- Bodakuntla, S.; Janke, C. Tubulin polyglutamylation, a regulator of microtubule functions, can cause neurodegeneration. Neurosci. Lett. 2021, 746, 135656. [Google Scholar] [CrossRef]
- Rogowski, K.; van Dijk, J. A family of protein-deglutamylating enzymes associated with neurodegeneration. Cell 2010, 143, 564–578. [Google Scholar] [CrossRef]
- Wu, H.Y.; Wei, P. Role of Cytosolic Carboxypeptidase 5 in Neuronal Survival and Spermatogenesis. Sci. Rep. 2017, 7, 41428. [Google Scholar] [CrossRef]
- Zhou, L.; Hossain, M.I. Deletion of exons encoding carboxypeptidase domain of Nna1 results in Purkinje cell degeneration (pcd) phenotype. J. Neurochem. 2018, 147, 557–572. [Google Scholar] [CrossRef]
- Munoz-Castaneda, R.; Diaz, D. Cytoskeleton stability is essential for the integrity of the cerebellum and its motor- and affective-related behaviors. Sci. Rep. 2018, 8, 3072. [Google Scholar] [CrossRef]
- Wang, R.; Lin, L. Identification of 2-PMPA as a novel inhibitor of cytosolic carboxypeptidases. Biochem. Biophys. Res. Commun. 2020, 533, 1393–1399. [Google Scholar] [CrossRef]
- Alexander, J.E.; Hunt, D.F. Characterization of posttranslational modifications in neuron-specific class III beta-tubulin by mass spectrometry. Proc. Natl. Acad. Sci. USA 1991, 88, 4685–4689. [Google Scholar] [CrossRef]
- Wolff, A.; de Nechaud, B. Distribution of glutamylated alpha and beta-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur. J. Cell Biol. 1992, 59, 425–432. [Google Scholar] [PubMed]
- Karakaya, M.; Paketci, C. Biallelic variant in AGTPBP1 causes infantile lower motor neuron degeneration and cerebellar atrophy. Am. J. Med. Genet. A 2019, 179, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Sheffer, R.; Gur, M. Biallelic variants in AGTPBP1, involved in tubulin deglutamylation, are associated with cerebellar degeneration and motor neuropathy. Eur. J. Hum. Genet. 2019, 27, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Parris, J. The carboxypeptidase-like substrate-binding site in Nna1 is essential for the rescue of the Purkinje cell degeneration (pcd) phenotype. Mol. Cell. Neurosci. 2006, 33, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez de la Vega, M.; Sevilla, R.G. Nna1-like proteins are active metallocarboxypeptidases of a new and diverse M14 subfamily. FASEB J. 2007, 21, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, E.; Biswas, R. A novel subfamily of mouse cytosolic carboxypeptidases. FASEB J. 2007, 21, 836–850. [Google Scholar] [CrossRef] [PubMed]
- Murakami, D.; Takamori, S. Periostin Expression in Non-Small Cell Lung Cancer: Clinical Significance. Kurume Med. J. 2018, 64, 13–20. [Google Scholar] [CrossRef]
- Hayashi, T.; Morishita, E. Expression of annexin II in human atherosclerotic abdominal aortic aneurysms. Thromb. Res. 2008, 123, 274–280. [Google Scholar] [CrossRef]
- Blosa, M.; Bursch, C. Reorganization of Synaptic Connections and Perineuronal Nets in the Deep Cerebellar Nuclei of Purkinje Cell Degeneration Mutant Mice. Neural Plast. 2016, 2016, 2828536. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Z. A modified quantitative EMSA and its application in the study of RNA--protein interactions. J. Biochem. Biophys. Methods 2004, 60, 85–96. [Google Scholar] [CrossRef]

| Name | Forward | Reverse |
|---|---|---|
| Nna1 exon17 | 5′-AATCGGCACAATCCTC-3′ | 5′-TGAACTGACAGATATGTTCATAG-3′ |
| Nna1-LOX | 5′-TTACAGACTCCTGCACCTGG-3′ | 5′-GAGTCTGACGCATTACCCAC-3′ |
| B2 | 5′-CTTGTACAAAGTGGCCCGAC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, B.; Araki, A.; Zhou, L.; Takebayashi, H.; Harada, T.; Kadota, K. CCP1, a Regulator of Tubulin Post-Translational Modifications, Potentially Plays an Essential Role in Cerebellar Development. Int. J. Mol. Sci. 2023, 24, 5335. https://doi.org/10.3390/ijms24065335
Pang B, Araki A, Zhou L, Takebayashi H, Harada T, Kadota K. CCP1, a Regulator of Tubulin Post-Translational Modifications, Potentially Plays an Essential Role in Cerebellar Development. International Journal of Molecular Sciences. 2023; 24(6):5335. https://doi.org/10.3390/ijms24065335
Chicago/Turabian StylePang, Bo, Asuka Araki, Li Zhou, Hirohide Takebayashi, Takayuki Harada, and Kyuichi Kadota. 2023. "CCP1, a Regulator of Tubulin Post-Translational Modifications, Potentially Plays an Essential Role in Cerebellar Development" International Journal of Molecular Sciences 24, no. 6: 5335. https://doi.org/10.3390/ijms24065335
APA StylePang, B., Araki, A., Zhou, L., Takebayashi, H., Harada, T., & Kadota, K. (2023). CCP1, a Regulator of Tubulin Post-Translational Modifications, Potentially Plays an Essential Role in Cerebellar Development. International Journal of Molecular Sciences, 24(6), 5335. https://doi.org/10.3390/ijms24065335





