Activation of TLRs Triggers GLP-1 Secretion in Mice
Abstract
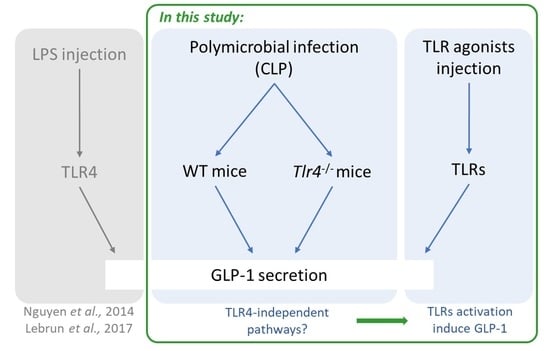
1. Introduction
2. Results
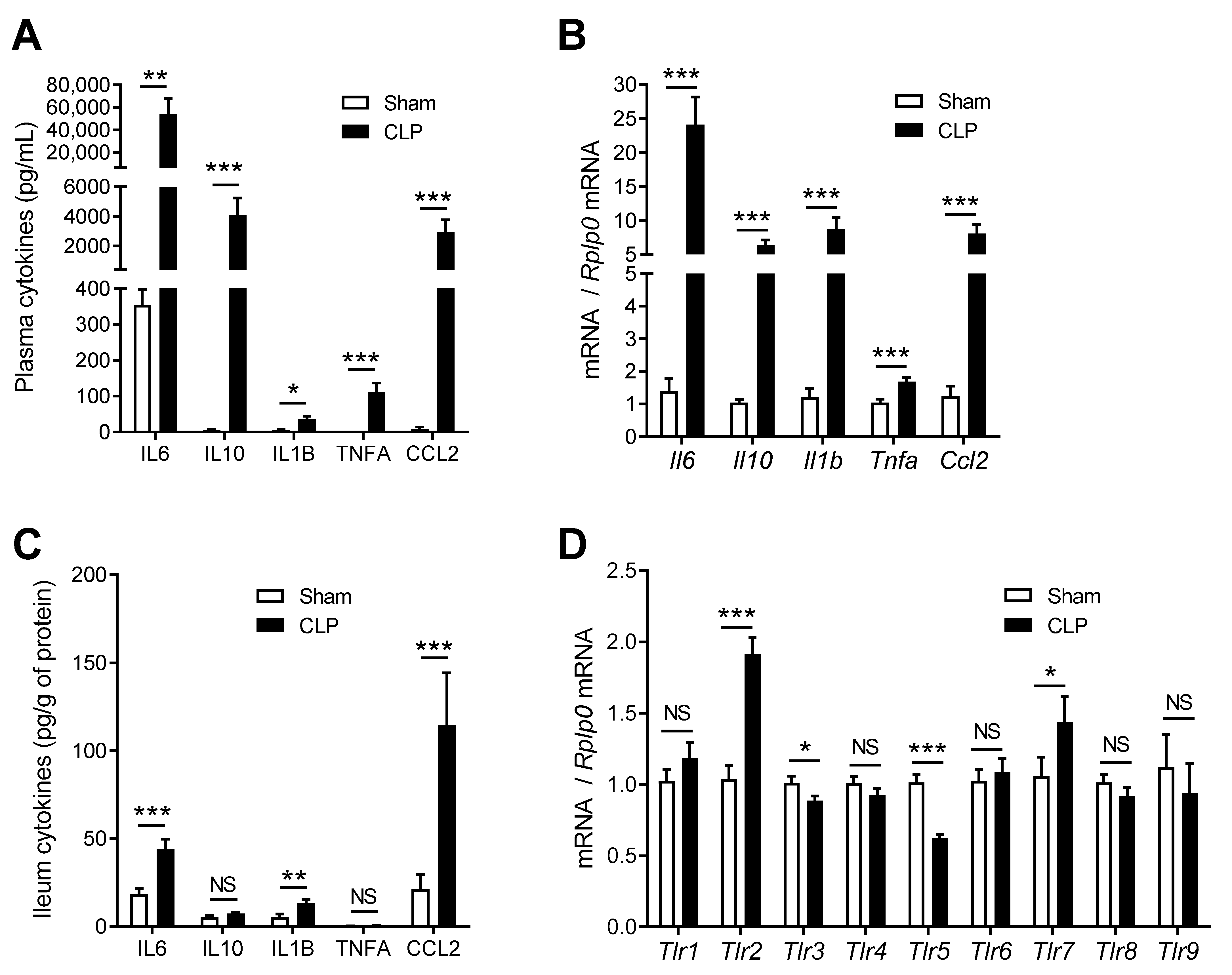
2.1. CLP Induces Systemic Inflammation and Modulates Inflammatory Gene Expression in the Gut
2.2. TLR Agonists Increase Cytokines and Expression of TLRs
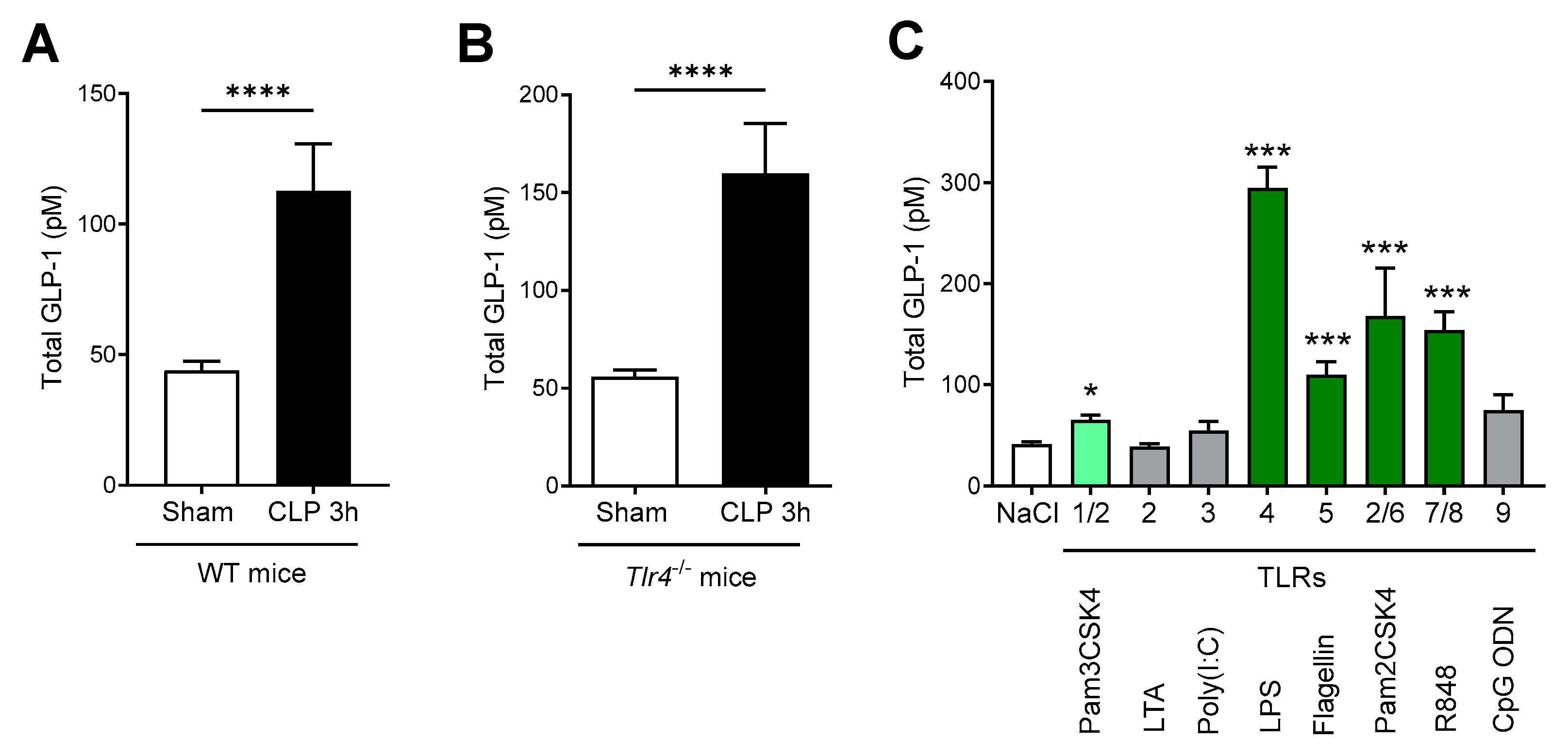
2.3. GLP-1 Secretion Is Mediated through Multiple TLRs
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Samplings
4.2. Cecal Ligation Puncture (CLP) Treatment
4.3. Drugs Administration in Mice
4.4. Real-Time Quantitative PCR
4.5. Plasma and Tissues Biochemical Analyses
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human Gut-Associated Lymphoid Tissues (GALT); Diversity, Structure, and Function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.T.; Fukata, M.; Arditi, M. TLR Signaling in the Gut in Health and Disease. J. Immunol. 2005, 174, 4453–4460. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Habener, J.F.; Holst, J.J. Discovery, Characterization, and Clinical Development of the Glucagon-like Peptides. J. Clin. Investig. 2017, 127, 4217–4227. [Google Scholar] [CrossRef]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic Effects of GLP-1 and Analogs on Cell Signaling, Metabolism, and Function. Front. Endocrinol. (Lausanne) 2018, 9, 672. [Google Scholar] [CrossRef]
- Iorga, R.A.; Bacalbasa, N.; Carsote, M.; Bratu, O.G.; Stanescu, A.M.A.; Bungau, S.; Pantis, C.; Diaconu, C.C. Metabolic and Cardiovascular Benefits of GLP-1 Agonists, besides the Hypoglycemic Effect (Review). Exp. Ther. Med. 2020, 20, 2396–2400. [Google Scholar] [CrossRef] [PubMed]
- Vandemark, C.; Nguyen, J.; Zhao, Z.-Q. Cardiovascular Protection with a Long-Acting GLP-1 Receptor Agonist Liraglutide: An Experimental Update. Molecules 2023, 28, 1369. [Google Scholar] [CrossRef]
- Merza, N.; Akram, M.; Mengal, A.; Mustafa Rashid, A.; Mahboob, A.; Faryad, M.; Fatima, Z.; Ahmed, M.; Ansari, S.A. The Safety and Efficacy of GLP-1 Receptor Agonists in Heart Failure Patients: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2023, 48, 101602. [Google Scholar] [CrossRef]
- Perl, S.H.; Bloch, O.; Zelnic-Yuval, D.; Love, I.; Mendel-Cohen, L.; Flor, H.; Rapoport, M.J. Sepsis-Induced Activation of Endogenous GLP-1 System Is Enhanced in Type 2 Diabetes. Diabetes/Metab. Res. Rev. 2018, 34, e2982. [Google Scholar] [CrossRef]
- Braun, J.-P.; Buhner, S.; Kastrup, M.; Dietz, E.; Langer, K.; Dohmen, P.; Lochs, H.; Spies, C. Barrier Function of the Gut and Multiple Organ Dysfunction after Cardiac Surgery. J. Int. Med. Res. 2007, 35, 72–83. [Google Scholar] [CrossRef]
- Nguyen, M.; Tavernier, A.; Gautier, T.; Aho, S.; Morgant, M.C.; Bouhemad, B.; Guinot, P.-G.; Grober, J. Glucagon-like Peptide-1 Is Associated with Poor Clinical Outcome, Lipopolysaccharide Translocation and Inflammation in Patients Undergoing Cardiac Surgery with Cardiopulmonary Bypass. Cytokine 2020, 133, 155182. [Google Scholar] [CrossRef] [PubMed]
- Yusta, B.; Baggio, L.L.; Koehler, J.; Holland, D.; Cao, X.; Pinnell, L.J.; Johnson-Henry, K.C.; Yeung, W.; Surette, M.G.; Bang, K.W.A.; et al. GLP-1R Agonists Modulate Enteric Immune Responses Through the Intestinal Intraepithelial Lymphocyte GLP-1R. Diabetes 2015, 64, 2537–2549. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Wang, Z. Liraglutide Attenuates Intestinal Ischemia/Reperfusion Injury via NF-ΚB and PI3K/Akt Pathways in Mice. Life Sci. 2022, 309, 121045. [Google Scholar] [CrossRef] [PubMed]
- Nozu, T.; Miyagishi, S.; Kumei, S.; Nozu, R.; Takakusaki, K.; Okumura, T. Glucagon-like Peptide-1 Analog, Liraglutide, Improves Visceral Sensation and Gut Permeability in Rats. J. Gastroenterol. Hepatol. 2018, 33, 232–239. [Google Scholar] [CrossRef]
- Brubaker, P.L. The Molecular Determinants of Glucagon-like Peptide Secretion by the Intestinal L Cell. Endocrinology 2022, 163, bqac159. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Parker, H.E.; Wallis, K.; le Roux, C.W.; Wong, K.Y.; Reimann, F.; Gribble, F.M. Molecular Mechanisms Underlying Bile Acid-Stimulated Glucagon-like Peptide-1 Secretion. Br. J. Pharm. 2012, 165, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Knauf, C. A Newly Identified Protein from Akkermansia Muciniphila Stimulates GLP-1 Secretion. Cell Metab. 2021, 33, 1073–1075. [Google Scholar] [CrossRef]
- Tomaro-Duchesneau, C.; LeValley, S.L.; Roeth, D.; Sun, L.; Horrigan, F.T.; Kalkum, M.; Hyser, J.M.; Britton, R.A. Discovery of a Bacterial Peptide as a Modulator of GLP-1 and Metabolic Disease. Sci. Rep. 2020, 10, 4922. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Mandard, S.; Dray, C.; Deckert, V.; Valet, P.; Besnard, P.; Drucker, D.J.; Lagrost, L.; Grober, J. Lipopolysaccharides-Mediated Increase in Glucose-Stimulated Insulin Secretion: Involvement of the Glucagon-like Peptide 1 (GLP1) Pathway. Diabetes 2014, 63, 471. [Google Scholar] [CrossRef]
- Kahles, F.; Meyer, C.; Möllmann, J.; Diebold, S.; Findeisen, H.M.; Lebherz, C.; Trautwein, C.; Koch, A.; Tacke, F.; Marx, N.; et al. GLP-1 Secretion Is Increased by Inflammatory Stimuli in an IL-6–Dependent Manner, Leading to Hyperinsulinemia and Blood Glucose Lowering. Diabetes 2014, 63, 3221–3229. [Google Scholar] [CrossRef]
- Lebrun, L.J.; Lenaerts, K.; Kiers, D.; Pais de Barros, J.-P.; Le Guern, N.; Plesnik, J.; Thomas, C.; Bourgeois, T.; Dejong, C.H.C.; Kox, M.; et al. Enteroendocrine L Cells Sense LPS after Gut Barrier Injury to Enhance GLP-1 Secretion. Cell Rep. 2017, 21, 1160–1168. [Google Scholar] [CrossRef]
- Worthington, J.J.; Reimann, F.; Gribble, F.M. Enteroendocrine Cells-Sensory Sentinels of the Intestinal Environment and Orchestrators of Mucosal Immunity. Mucosal Immunol. 2018, 11, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Aravanis, C.V.; Kapelouzou, A.; Vagios, S.; Tsilimigras, D.I.; Katsimpoulas, M.; Moris, D.; Demesticha, T.D.; Schizas, D.; Kostakis, A.; Machairas, A.; et al. Toll-Like Receptors -2, -3, -4 and -7 Expression Patterns in the Liver of a CLP-Induced Sepsis Mouse Model. J. Investig. Surg 2020, 33, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Krivan, S.; Kapelouzou, A.; Vagios, S.; Tsilimigras, D.I.; Katsimpoulas, M.; Moris, D.; Aravanis, C.V.; Demesticha, T.D.; Schizas, D.; Mavroidis, M.; et al. Increased Expression of Toll-like Receptors 2, 3, 4 and 7 MRNA in the Kidney and Intestine of a Septic Mouse Model. Sci. Rep. 2019, 9, 4010. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulos, A.; Kapelouzou, A.; Tsilimigras, D.I.; Katsimpoulas, M.; Schizas, D.; Aravanis, C.; Balafas, E.; Mavroidis, M.; Pavlakis, K.; Machairas, A.; et al. Expression of Toll-like Receptors (TLRs) in the Lungs of an Experimental Sepsis Mouse Model. PLoS ONE 2017, 12, e0188050. [Google Scholar] [CrossRef] [PubMed]
- Bogunovic, M.; Davé, S.H.; Tilstra, J.S.; Chang, D.T.W.; Harpaz, N.; Xiong, H.; Mayer, L.F.; Plevy, S.E. Enteroendocrine Cells Express Functional Toll-like Receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1770–G1783. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Function and Mechanisms of Enteroendocrine Cells and Gut Hormones in Metabolism. Nat. Rev. Endocrinol. 2019, 15, 226–237. [Google Scholar] [CrossRef]
- Harrison, E.; Lal, S.; McLaughlin, J.T. Enteroendocrine Cells in Gastrointestinal Pathophysiology. Curr. Opin. Pharmacol. 2013, 13, 941–945. [Google Scholar] [CrossRef]
- Genton, L.; Kudsk, K.A. Interactions between the Enteric Nervous System and the Immune System: Role of Neuropeptides and Nutrition. Am. J. Surg. 2003, 186, 253–258. [Google Scholar] [CrossRef]
- Lee, J.H.; Patel, K.; Tae, H.J.; Lustig, A.; Kim, J.W.; Mattson, M.P.; Taub, D.D. Ghrelin Augments Murine T-Cell Proliferation by Activation of the Phosphatidylinositol-3-Kinase, Extracellular Signal-Regulated Kinase and Protein Kinase C Signaling Pathways. FEBS Lett. 2014, 588, 4708–4719. [Google Scholar] [CrossRef]
- Reardon, C.; Duncan, G.S.; Brüstle, A.; Brenner, D.; Tusche, M.W.; Olofsson, P.S.; Rosas-Ballina, M.; Tracey, K.J.; Mak, T.W. Lymphocyte-Derived ACh Regulates Local Innate but Not Adaptive Immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 1410–1415. [Google Scholar] [CrossRef]
- Palazzo, M.; Balsari, A.; Rossini, A.; Selleri, S.; Calcaterra, C.; Gariboldi, S.; Zanobbio, L.; Arnaboldi, F.; Shirai, Y.F.; Serrao, G.; et al. Activation of Enteroendocrine Cells via TLRs Induces Hormone, Chemokine, and Defensin Secretion. J. Immunol. 2007, 178, 4296–4303. [Google Scholar] [CrossRef]
- Drucker, D.J.; Yusta, B. Physiology and Pharmacology of the Enteroendocrine Hormone Glucagon-like Peptide-2. Annu. Rev. Physiol 2014, 76, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Hunt, J.E.; Holst, J.J.; Jeppesen, P.B.; Kissow, H. GLP-1 and Intestinal Diseases. Biomedicines 2021, 9, 383. [Google Scholar] [CrossRef]
- Keller, J.; Binnewies, U.; Rösch, M.; Juul Holst, J.; Beglinger, C.; Andresen, V.; Layer, P. Gastric Emptying and Disease Activity in Inflammatory Bowel Disease. Eur. J. Clin. Investig. 2015, 45, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Lucotti, P.; Lovati, E.; Lenti, M.V.; Valvo, B.; Sprio, E.; Aronico, N.; Giuffrida, P.; Dell’Aera, D.; Pasini, A.; Ubezio, C.; et al. Abnormal Post-Prandial Glucagon-like Peptide Release in Patients with Crohn’s Disease. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101533. [Google Scholar] [CrossRef]
- Brakenridge, S.C.; Moore, F.A.; Mercier, N.R.; Cox, M.; Wu, Q.; Moldawer, L.L.; Mohr, A.M.; Efron, P.A.; Smith, R.S. Persistently Elevated Glucagon-Like Peptide 1 Levels Among Critically-Ill Surgical Patients After Sepsis and Development of Chronic Critical Illness and Dismal Long-Term Outcomes. J. Am. Coll Surg. 2019, 229, 58–67.e1. [Google Scholar] [CrossRef] [PubMed]
- Lebherz, C.; Schlieper, G.; Möllmann, J.; Kahles, F.; Schwarz, M.; Brünsing, J.; Dimkovic, N.; Koch, A.; Trautwein, C.; Flöge, J.; et al. GLP-1 Levels Predict Mortality in Patients with Critical Illness as Well as End-Stage Renal Disease. Am. J. Med. 2017, 130, 833–841.e3. [Google Scholar] [CrossRef]
- Zanotti-Cavazzoni, S.L.; Guglielmi, M.; Parrillo, J.E.; Walker, T.; Dellinger, R.P.; Hollenberg, S.M. Fluid Resuscitation Influences Cardiovascular Performance and Mortality in a Murine Model of Sepsis. Intensive Care Med. 2009, 35, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, S.F.; Triantos, C.; Thomopoulos, K.; Fligou, F.; Maroulis, I.; Marangos, M.; Gogos, C.A. Gut-Origin Sepsis in the Critically Ill Patient: Pathophysiology and Treatment. Infection 2018, 46, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.L.; Ma, C.; Rosenberger, C.M.; Bergstrom, K.S.B.; Valdez, Y.; Huang, J.T.; Khan, M.A.; Vallance, B.A. Toll-like Receptor 2 Plays a Critical Role in Maintaining Mucosal Integrity during Citrobacter Rodentium-Induced Colitis. Cell Microbiol. 2008, 10, 388–403. [Google Scholar] [CrossRef]
- Kamdar, K.; Johnson, A.M.F.; Chac, D.; Myers, K.; Kulur, V.; Truevillian, K.; DePaolo, R.W. Innate Recognition of the Microbiota by Toll-like Receptor-1 Promotes Epithelial Homeostasis and Prevents Chronic Inflammation. J. Immunol. 2018, 201, 230–242. [Google Scholar] [CrossRef]
- Cario, E.; Gerken, G.; Podolsky, D.K. Toll-like Receptor 2 Controls Mucosal Inflammation by Regulating Epithelial Barrier Function. Gastroenterology 2007, 132, 1359–1374. [Google Scholar] [CrossRef]
- Dheer, R.; Santaolalla, R.; Davies, J.M.; Lang, J.K.; Phillips, M.C.; Pastorini, C.; Vazquez-Pertejo, M.T.; Abreu, M.T. Intestinal Epithelial Toll-Like Receptor 4 Signaling Affects Epithelial Function and Colonic Microbiota and Promotes a Risk for Transmissible Colitis. Infect. Immun. 2016, 84, 798–810. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide Causes an Increase in Intestinal Tight Junction Permeability in Vitro and in Vivo by Inducing Enterocyte Membrane Expression and Localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef]
- Nighot, M.; Al-Sadi, R.; Guo, S.; Rawat, M.; Nighot, P.; Watterson, M.D.; Ma, T.Y. Lipopolysaccharide-Induced Increase in Intestinal Epithelial Tight Permeability Is Mediated by Toll-Like Receptor 4/Myeloid Differentiation Primary Response 88 (MyD88) Activation of Myosin Light Chain Kinase Expression. Am. J. Pathol. 2017, 187, 2698–2710. [Google Scholar] [CrossRef]
- Tatum, P.M.; Harmon, C.M.; Lorenz, R.G.; Dimmitt, R.A. Toll-like Receptor 4 Is Protective against Neonatal Murine Ischemia-Reperfusion Intestinal Injury. J. Pediatr. Surg. 2010, 45, 1246–1255. [Google Scholar] [CrossRef]
- Chen, L.-W.; Chang, W.-J.; Chen, P.-H.; Liu, W.-C.; Hsu, C.-M. TLR Ligand Decreases Mesenteric Ischemia and Reperfusion Injury-Induced Gut Damage through TNF-α Signaling. Shock 2008, 30, 563. [Google Scholar] [CrossRef]
- Kitajima, S.; Takuma, S.; Morimoto, M. Changes in Colonic Mucosal Permeability in Mouse Colitis Induced with Dextran Sulfate Sodium. Exp. Anim. 1999, 48, 137–143. [Google Scholar] [CrossRef]
- Fukata, M.; Michelsen, K.S.; Eri, R.; Thomas, L.S.; Hu, B.; Lukasek, K.; Nast, C.C.; Lechago, J.; Xu, R.; Naiki, Y.; et al. Toll-like Receptor-4 Is Required for Intestinal Response to Epithelial Injury and Limiting Bacterial Translocation in a Murine Model of Acute Colitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 288, G1055–G1065. [Google Scholar] [CrossRef]
- Rose, W.A.; Sakamoto, K.; Leifer, C.A. TLR9 Is Important for Protection against Intestinal Damage and for Intestinal Repair. Sci. Rep. 2012, 2, 574. [Google Scholar] [CrossRef]
- Chassaing, B.; Ley, R.E.; Gewirtz, A.T. Intestinal Epithelial Cell Toll-like Receptor 5 Regulates the Intestinal Microbiota to Prevent Low-Grade Inflammation and Metabolic Syndrome in Mice. Gastroenterology 2014, 147, 1363–1377.e17. [Google Scholar] [CrossRef]
- Del Olmo-Garcia, M.I.; Merino-Torres, J.F. GLP-1 Receptor Agonists and Cardiovascular Disease in Patients with Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 4020492. [Google Scholar] [CrossRef]
- Bang-Berthelsen, C.H.; Holm, T.L.; Pyke, C.; Simonsen, L.; Søkilde, R.; Pociot, F.; Heller, R.S.; Folkersen, L.; Kvist, P.H.; Jackerott, M.; et al. GLP-1 Induces Barrier Protective Expression in Brunner’s Glands and Regulates Colonic Inflammation. Inflamm. Bowel Dis. 2016, 22, 2078–2097. [Google Scholar] [CrossRef]
- Zatorski, H.; Sałaga, M.; Fichna, J. Role of Glucagon-like Peptides in Inflammatory Bowel Diseases—Current Knowledge and Future Perspectives. Naunyn-Schmiedeberg’s Arch. Pharm. 2019, 392, 1321–1330. [Google Scholar] [CrossRef]
- Lourie, J. A Novel Use of Liraglutide: Induction of Partial Remission in Ulcerative Colitis and Ankylosing Spondylitis. Clin Med Rev Case Rep. 2019, 6, 281. [Google Scholar] [CrossRef]
- Villumsen, M.; Schelde, A.B.; Jimenez-Solem, E.; Jess, T.; Allin, K.H. GLP-1 Based Therapies and Disease Course of Inflammatory Bowel Disease. eClinicalMedicine 2021, 37, 100979. [Google Scholar] [CrossRef]
- Helmstädter, J.; Keppeler, K.; Aust, F.; Küster, L.; Frenis, K.; Filippou, K.; Vujacic-Mirski, K.; Tsohataridis, S.; Kalinovic, S.; Kröller-Schön, S.; et al. GLP-1 Analog Liraglutide Improves Vascular Function in Polymicrobial Sepsis by Reduction of Oxidative Stress and Inflammation. Antioxidants 2021, 10, 1175. [Google Scholar] [CrossRef]
- Hirasawa, H.; Oda, S.; Nakamura, M. Blood Glucose Control in Patients with Severe Sepsis and Septic Shock. World J. Gastroenterol. 2009, 15, 4132–4136. [Google Scholar] [CrossRef]
- Jafar, N.; Edriss, H.; Nugent, K. The Effect of Short-Term Hyperglycemia on the Innate Immune System. Am. J. Med. Sci. 2016, 351, 201–211. [Google Scholar] [CrossRef]
- Leonidou, L.; Mouzaki, A.; Michalaki, M.; DeLastic, A.L.; Kyriazopoulou, V.; Bassaris, H.P.; Gogos, C.A. Cytokine Production and Hospital Mortality in Patients with Sepsis-Induced Stress Hyperglycemia. J. Infect. 2007, 55, 340–346. [Google Scholar] [CrossRef]
- Yang, F.; Zeng, F.; Luo, X.; Lei, Y.; Li, J.; Lu, S.; Huang, X.; Lan, Y.; Liu, R. GLP-1 Receptor: A New Target for Sepsis. Front. Pharm. 2021, 12, 706908. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, R.; Li, S.; Kang, X.; Ren, Y.; Sun, E.; Wang, C.; He, J.; Zhan, J. Preliminary Evaluation of Potential Properties of Three Probiotics and Their Combination with Prebiotics on GLP-1 Secretion and Type 2 Diabetes Alleviation. J. Food Qual. 2022, 2022, e8586843. [Google Scholar] [CrossRef]
- Pegah, A.; Abbasi-Oshaghi, E.; Khodadadi, I.; Mirzaei, F.; Tayebinai, H. Probiotic and Resveratrol Normalize GLP-1 Levels and Oxidative Stress in the Intestine of Diabetic Rats. Metab. Open 2021, 10, 100093. [Google Scholar] [CrossRef]
- Wei, S.-H.; Chen, Y.-P.; Chen, M.-J. Selecting Probiotics with the Abilities of Enhancing GLP-1 to Mitigate the Progression of Type 1 Diabetes in Vitro and in Vivo. J. Funct. Foods 2015, 18, 473–486. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.-H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial Metabolic Effects of a Probiotic via Butyrate-Induced GLP-1 Hormone Secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef]
- Williams, L.; Alshehri, A.; Robichaud, B.; Cudmore, A.; Gagnon, J. The Role of the Bacterial Muramyl Dipeptide in the Regulation of GLP-1 and Glycemia. Int. J. Mol. Sci. 2020, 21, 5252. [Google Scholar] [CrossRef]
- Klein, A.; Deckert, V.; Schneider, M.; Dutrillaux, F.; Hammann, A.; Athias, A.; Le Guern, N.; Pais de Barros, J.-P.; Desrumaux, C.; Masson, D.; et al. Alpha-Tocopherol Modulates Phosphatidylserine Externalization in Erythrocytes: Relevance in Phospholipid Transfer Protein-Deficient Mice. Arter. Thromb. Vasc. Biol. 2006, 26, 2160–2167. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebrun, L.J.; Dusuel, A.; Xolin, M.; Le Guern, N.; Grober, J. Activation of TLRs Triggers GLP-1 Secretion in Mice. Int. J. Mol. Sci. 2023, 24, 5333. https://doi.org/10.3390/ijms24065333
Lebrun LJ, Dusuel A, Xolin M, Le Guern N, Grober J. Activation of TLRs Triggers GLP-1 Secretion in Mice. International Journal of Molecular Sciences. 2023; 24(6):5333. https://doi.org/10.3390/ijms24065333
Chicago/Turabian StyleLebrun, Lorène J., Alois Dusuel, Marion Xolin, Naig Le Guern, and Jacques Grober. 2023. "Activation of TLRs Triggers GLP-1 Secretion in Mice" International Journal of Molecular Sciences 24, no. 6: 5333. https://doi.org/10.3390/ijms24065333
APA StyleLebrun, L. J., Dusuel, A., Xolin, M., Le Guern, N., & Grober, J. (2023). Activation of TLRs Triggers GLP-1 Secretion in Mice. International Journal of Molecular Sciences, 24(6), 5333. https://doi.org/10.3390/ijms24065333