Muscle Lipid Oxidation Is Not Affected by Obstructive Sleep Apnea in Diabetes and Healthy Subjects
Abstract
1. Introduction
2. Results
2.1. Anthropometric, Biochemical, and Sleep Characteristics
2.2. Oxygen Consumption Rate and Substrate Utilization in Skeletal Muscle Biopsy
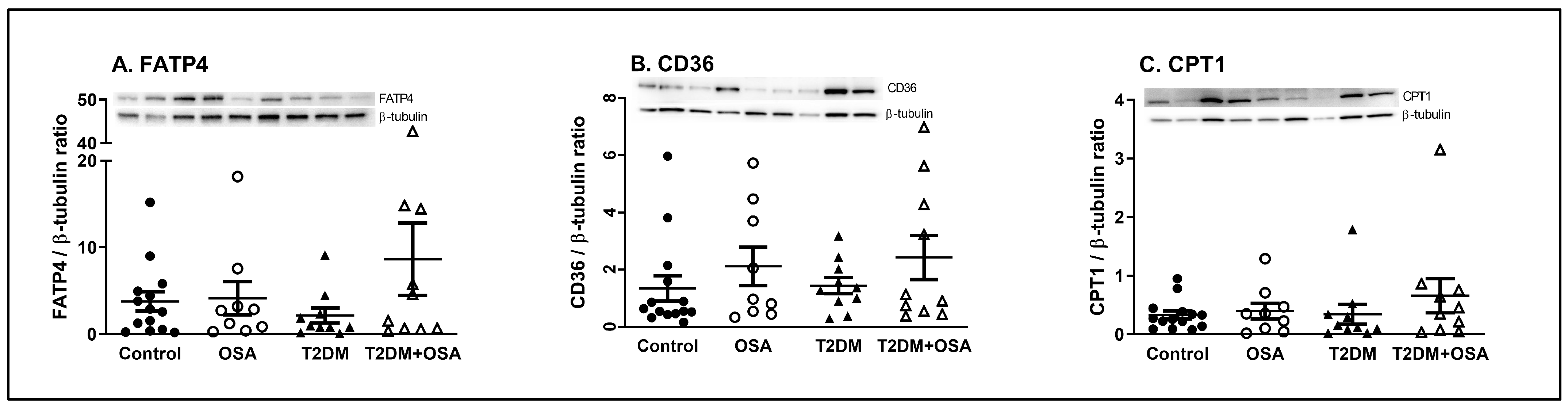
2.3. Protein Expression
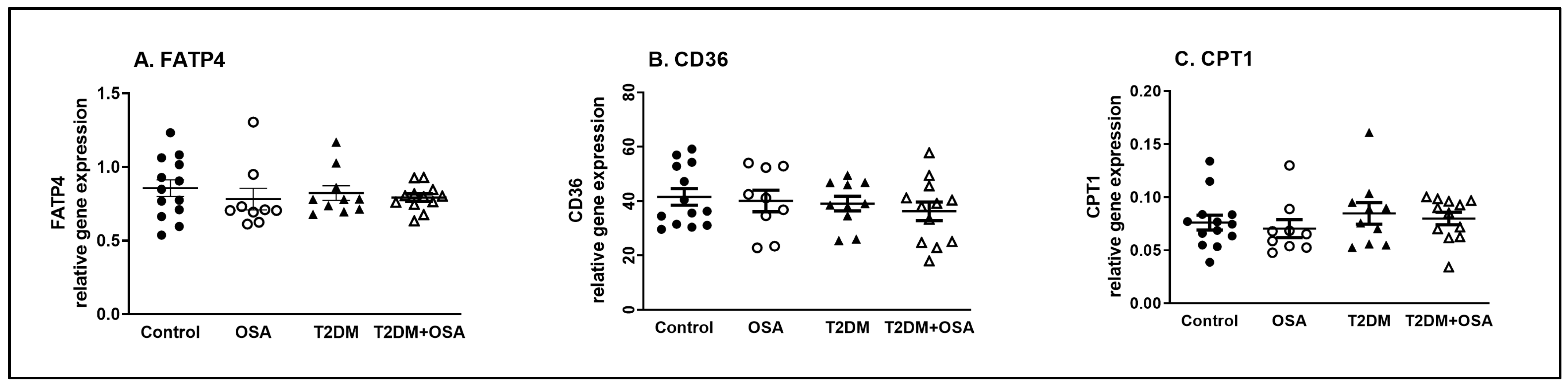
2.4. Gene Expression
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Sleep Study
4.3. Biochemical Analysis, Clinical Investigations, and Muscle Biopsy
4.4. Lipid Oxidation Determination Using High-Resolution Respirometry
4.5. Protein and Gene Expression Quantification
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aronsohn, R.S.; Whitmore, H.; Van Cauter, E.; Tasali, E. Impact of Untreated Obstructive Sleep Apnea on Glucose Control in Type 2 Diabetes. Am. J. Respir. Crit. Care Med. 2010, 181, 507–513. [Google Scholar] [CrossRef]
- Westlake Katerina, P.J. Screening for Obstructive Sleep Apnea in Type 2 Diabetes Patients—Questionnaires Are Not Good Enough. Front. Endocrinol. 2016, 7, 124. [Google Scholar] [CrossRef]
- Punjabi, N.M. Disorders of Glucose Metabolism in Sleep Apnea. J. Appl. Physiol. 2005, 99, 1998–2007. [Google Scholar] [CrossRef]
- Punjabi, N.M.; Caffo, B.S.; Goodwin, J.L.; Gottlieb, D.J.; Newman, A.B.; O’Connor, G.T.; Rapoport, D.M.; Redline, S.; Resnick, H.E.; Robbins, J.A.; et al. Sleep-Disordered Breathing and Mortality: A Prospective Cohort Study. PLoS Med. 2009, 6, e1000132. [Google Scholar] [CrossRef]
- Neubauer, J.A. Invited Review: Physiological and Pathophysiological Responses to Intermittent Hypoxia. J. Appl. Physiol. (1985) 2001, 90, 1593–1599. [Google Scholar] [CrossRef]
- Tasali, E.; Mokhlesi, B.; Van Cauter, E. Obstructive Sleep Apnea and Type 2 Diabetes. Chest 2008, 133, 496–506. [Google Scholar] [CrossRef]
- Aurora, R.N.; Punjabi, N.M. Obstructive Sleep Apnoea and Type 2 Diabetes Mellitus: A Bidirectional Association. Lancet Respir. Med. 2013, 1, 329–338. [Google Scholar] [CrossRef]
- Briancon-Marjollet, A.; Weiszenstein, M.; Henri, M.; Thomas, A.; Godin-Ribuot, D.; Polak, J.; Briançon-Marjollet, A.; Weiszenstein, M.; Henri, M.; Thomas, A.; et al. The Impact of Sleep Disorders on Glucose Metabolism: Endocrine and Molecular Mechanisms. Diabetol. Metab. Syndr. 2015, 7, 25. [Google Scholar] [CrossRef]
- Pavlacky, J.; Polak, J. Technical Feasibility and Physiological Relevance of Hypoxic Cell Culture Models. Front. Endocrinol. 2020, 11, 57. [Google Scholar] [CrossRef]
- Gu, C.J.; Yi, H.H.; Feng, J.; Zhang, Z.G.; Zhou, J.; Zhou, L.N.; Zhou, J.P.; Li, M.; Li, Q.Y. Intermittent Hypoxia Disrupts Glucose Homeostasis in Liver Cells in an Insulin-Dependent and Independent Manner. Cell. Physiol. Biochem. 2018, 47, 1042–1050. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, J.; Qiao, Y.; Sun, X.; Mao, T.; Lei, S.; Zheng, Q.; Liu, Y. Intermittent Hypoxia Composite Abnormal Glucose Metabolism-Mediated Atherosclerosis In Vitro and In Vivo: The Role of SREBP-1. Oxid. Med. Cell. Longev. 2019, 2019, 4862760. [Google Scholar] [CrossRef]
- Musutova, M.; Weiszenstein, M.; Koc, M.; Polak, J. Intermittent Hypoxia Stimulates Lipolysis, But Inhibits Differentiation and De Novo Lipogenesis in 3T3-L1 Cells. Metab. Syndr. Relat. Disord. 2020, 18, 146–153. [Google Scholar] [CrossRef]
- Polak, J.; Shimoda, L.A.; Drager, L.F.; Undem, C.; McHugh, H.; Polotsky, V.Y.; Punjabi, N.M. Intermittent Hypoxia Impairs Glucose Homeostasis in C57BL6/J Mice: Partial Improvement with Cessation of the Exposure. Sleep 2013, 36, 1483–1490. [Google Scholar] [CrossRef]
- Polotsky, V.Y.; Li, J.; Punjabi, N.M.; Rubin, A.E.; Smith, P.L.; Schwartz, A.R.; O’Donnell, C.P. Intermittent Hypoxia Increases Insulin Resistance in Genetically Obese Mice. J. Physiol. 2003, 552, 253–264. [Google Scholar] [CrossRef]
- Iiyori, N.; Alonso, L.C.; Li, J.; Sanders, M.H.; Garcia-Ocana, A.; O’Doherty, R.M.; Polotsky, V.Y.; O’Donnell, C.P. Intermittent Hypoxia Causes Insulin Resistance in Lean Mice Independent of Autonomic Activity. Am. J. Respir. Crit. Care Med. 2007, 175, 851–857. [Google Scholar] [CrossRef]
- Xu, J.; Long, Y.-S.; Gozal, D.; Epstein, P.N. Beta-Cell Death and Proliferation after Intermittent Hypoxia: Role of Oxidative Stress. Free Radic. Biol. Med. 2009, 46, 783–790. [Google Scholar] [CrossRef]
- Yokoe, T.; Alonso, L.C.; Romano, L.C.; Rosa, T.C.; O’Doherty, R.M.; Garcia-Ocana, A.; Minoguchi, K.; O’Donnell, C.P. Intermittent Hypoxia Reverses the Diurnal Glucose Rhythm and Causes Pancreatic β-Cell Replication in Mice. J. Physiol. 2008, 586, 899–911. [Google Scholar] [CrossRef]
- Shin, M.-K.; Yao, Q.; Jun, J.C.; Bevans-Fonti, S.; Yoo, D.-Y.; Han, W.; Mesarwi, O.; Richardson, R.; Fu, Y.-Y.; Pasricha, P.J.; et al. Carotid Body Denervation Prevents Fasting Hyperglycemia during Chronic Intermittent Hypoxia. J. Appl. Physiol. 2014, 117, 765–776. [Google Scholar] [CrossRef]
- Weiszenstein, M.; Shimoda, L.A.; Koc, M.; Seda, O.; Polak, J. Inhibition of Lipolysis Ameliorates Diabetic Phenotype in a Mouse Model of Obstructive Sleep Apnea. Am. J. Respir. Cell. Mol. Biol. 2016, 55, 299–307. [Google Scholar] [CrossRef]
- Mishima, T.; Miner, J.H.; Morizane, M.; Stahl, A.; Sadovsky, Y. The Expression and Function of Fatty Acid Transport Protein-2 and -4 in the Murine Placenta. PLoS ONE 2011, 6, e25865. [Google Scholar] [CrossRef]
- Rafacho, A.; Gonçalves-Neto, L.M.; Ferreira, F.B.D.; Protzek, A.O.P.; Boschero, A.C.; Nunes, E.A.; Zoccal, D.B. Glucose Homoeostasis in Rats Exposed to Acute Intermittent Hypoxia. Acta Physiol. 2013, 209, 77–89. [Google Scholar] [CrossRef]
- Louis, M.; Punjabi, N.M. Effects of Acute Intermittent Hypoxia on Glucose Metabolism in Awake Healthy Volunteers. J. Appl. Physiol. 2009, 106, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, L.P.; Joyner, M.J.; Curry, T.B.; Laurenti, M.C.; Man, C.D.; Cobelli, C.; Vella, A.; Limberg, J.K. Three Hours of Intermittent Hypoxia Increases Circulating Glucose Levels in Healthy Adults. Physiol. Rep. 2017, 5, e13106. [Google Scholar] [CrossRef]
- Kent, B.D.; McNicholas, W.T.; Ryan, S. Insulin Resistance, Glucose Intolerance and Diabetes Mellitus in Obstructive Sleep Apnoea. J. Thorac. Dis. 2015, 7, 1343–1357. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Peng, Y.-J.; Nanduri, J. Hypoxia-Inducible Factors and Obstructive Sleep Apnea. J. Clin. Investig. 2020, 130, 5042–5051. [Google Scholar] [CrossRef]
- Chopra, S.; Rathore, A.; Younas, H.; Pham, L.V.; Gu, C.; Beselman, A.; Kim, I.-Y.; Wolfe, R.R.; Perin, J.; Polotsky, V.Y.; et al. Obstructive Sleep Apnea Dynamically Increases Nocturnal Plasma Free Fatty Acids, Glucose, and Cortisol During Sleep. J. Clin. Endocrinol. Metab. 2017, 102, 3172–3181. [Google Scholar] [CrossRef]
- Plihalova, A.; Bartakova, H.; Vasakova, M.; Gulati, S.; deGlisezinski, I.; Stich, V.; Polak, J. The Effect of Hypoxia and Re-Oxygenation on Adipose Tissue Lipolysis in COPD Patients. Eur. Respir. J. 2016, 48, 1218–1220. [Google Scholar] [CrossRef]
- Kelley, D.E.; Goodpaster, B.; Wing, R.R.; Simoneau, J.A. Skeletal Muscle Fatty Acid Metabolism in Association with Insulin Resistance, Obesity, and Weight Loss. Am. J. Physiol. 1999, 277, E1130–E1141. [Google Scholar] [CrossRef]
- Kelley, D.E.; Mandarino, L.J. Fuel Selection in Human Skeletal Muscle in Insulin Resistance: A Reexamination. Diabetes 2000, 49, 677–683. [Google Scholar] [CrossRef]
- Boden, G.; Chen, X.; Capulong, E.; Mozzoli, M. Effects of Free Fatty Acids on Gluconeogenesis and Autoregulation of Glucose Production in Type 2 Diabetes 1. Diabetes 2001, 50, 810–816. [Google Scholar] [CrossRef]
- Samuel, V.T.; Petersen, K.F.; Shulman, G.I. Lipid-Induced Insulin Resistance: Unravelling the Mechanism. Lancet 2010, 375, 2267–2277. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Park, E.; Mori, Y.; Haber, C.A.; Han, P.; Uchida, T.; Stavar, L.; Oprescu, A.I.; Koulajian, K.; Ivovic, A.; et al. FFA-Induced Hepatic Insulin Resistance in Vivo Is Mediated by PKCδ, NADPH Oxidase, and Oxidative Stress. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E34–E46. [Google Scholar] [CrossRef]
- Cnop, M.; Welsh, N.; Jonas, J.-C.; Jörns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of Pancreatic Beta-Cell Death in Type 1 and Type 2 Diabetes: Many Differences, Few Similarities. Diabetes 2005, 54 Suppl 2, S97–S107. [Google Scholar] [CrossRef]
- Acosta-Montaño, P.; García-González, V. Effects of Dietary Fatty Acids in Pancreatic Beta Cell Metabolism, Implications in Homeostasis. Nutrients 2018, 10, 393. [Google Scholar] [CrossRef]
- Mensink, M.; Blaak, E.E.; van Baak, M.A.; Wagenmakers, A.J.; Saris, W.H. Plasma Free Fatty Acid Uptake and Oxidation Are Already Diminished in Subjects at High Risk for Developing Type 2 Diabetes. Diabetes 2001, 50, 2548–2554. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, I.S.; Blindauer, C.A.; Stewart, A.J. Changes in Plasma Free Fatty Acids Associated with Type-2 Diabetes. Nutrients 2019, 11, 2022. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine Transport and Fatty Acid Oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Trinh, M.D.; Plihalova, A.; Gojda, J.; Westlake, K.; Spicka, J.; Lattova, Z.; Pretl, M.; Polak, J. Obstructive Sleep Apnoea Increases Lipolysis and Deteriorates Glucose Homeostasis in Patients with Type 2 Diabetes Mellitus. Sci. Rep. 2021, 11, 3567. [Google Scholar] [CrossRef]
- Boden, G. Fatty Acid-Induced Inflammation and Insulin Resistance in Skeletal Muscle and Liver. Curr. Diab. Rep. 2006, 6, 177–181. [Google Scholar] [CrossRef]
- Delarue, J.; Magnan, C. Free Fatty Acids and Insulin Resistance. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 142–148. [Google Scholar] [CrossRef]
- Boden, G. Obesity and Free Fatty Acids. Endocrinol. Metab. Clin. North. Am. 2008, 37, 635–636ix. [Google Scholar] [CrossRef]
- Barcelo, A.; Pierola, J.; de la Pena, M.; Esquinas, C.; Fuster, A.; Sanchez-de-la-Torre, M.; Carrera, M.; Alonso-Fernandez, A.; Ladaria, A.; Bosch, M.; et al. Free Fatty Acids and the Metabolic Syndrome in Patients with Obstructive Sleep Apnoea. Eur. Respir. J. 2011, 37, 1418–1423. [Google Scholar] [CrossRef]
- Jun, J.C.; Drager, L.F.; Najjar, S.S.; Gottlieb, S.S.; Brown, C.D.; Smith, P.L.; Schwartz, A.R.; Polotsky, V.Y. Effects of Sleep Apnea on Nocturnal Free Fatty Acids in Subjects with Heart Failure. Sleep 2011, 34, 1207–1213. [Google Scholar] [CrossRef]
- Bonen, A.; Luiken, J.J.F.P.; Liu, S.; Dyck, D.J.; Kiens, B.; Kristiansen, S.; Turcotte, L.P.; van der Vusse, G.J.; Glatz, J.F.C. Palmitate Transport and Fatty Acid Transporters in Red and White Muscles. Am. J. Physiol.-Endocrinol. Metab. 1998, 275, E471–E478. [Google Scholar] [CrossRef]
- Habets, D.D.J.; Coumans, W.A.; Voshol, P.J.; den Boer, M.A.M.; Febbraio, M.; Bonen, A.; Glatz, J.F.C.; Luiken, J.J.F.P. AMPK-Mediated Increase in Myocardial Long-Chain Fatty Acid Uptake Critically Depends on Sarcolemmal CD36. Biochem. Biophys. Res. Commun. 2007, 355, 204–210. [Google Scholar] [CrossRef]
- Holloway, G.P.; Luiken, J.J.F.P.; Glatz, J.F.C.; Spriet, L.L.; Bonen, A. Contribution of FAT/CD36 to the Regulation of Skeletal Muscle Fatty Acid Oxidation: An Overview. Acta Physiol. 2008, 194, 293–309. [Google Scholar] [CrossRef]
- Chabowski, A.; Chatham, J.C.; Tandon, N.N.; Calles-Escandon, J.; Glatz, J.F.C.; Luiken, J.J.F.P.; Bonen, A. Fatty Acid Transport and FAT/CD36 Are Increased in Red but Not in White Skeletal Muscle of ZDF Rats. Am. J. Physiol.-Endocrinol. Metab. 2006, 291, E675–E682. [Google Scholar] [CrossRef]
- Bonen, A.; Parolin, M.L.; Steinberg, G.R.; Calles-Escandon, J.; Tandon, N.N.; Glatz, J.F.; Luiken, J.J.; Heigenhauser, G.J.; Dyck, D.J. Triacylglycerol Accumulation in Human Obesity and Type 2 Diabetes Is Associated with Increased Rates of Skeletal Muscle Fatty Acid Transport and Increased Sarcolemmal FAT/CD36. FASEB J. 2004, 18, 1144–1146. [Google Scholar] [CrossRef]
- Ritov, V.B.; Menshikova, E.V.; He, J.; Ferrell, R.E.; Goodpaster, B.H.; Kelley, D.E. Deficiency of Subsarcolemmal Mitochondria in Obesity and Type 2 Diabetes. Diabetes 2005, 54, 8–14. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Hickner, R.C.; Cortright, R.L.; Dohm, G.L.; Houmard, J.A. Lipid Oxidation Is Reduced in Obese Human Skeletal Muscle. Am. J. Physiol.-Endocrinol. Metab. 2000, 279, E1039–E1044. [Google Scholar] [CrossRef]
- Bruce, C.R.; Hoy, A.J.; Turner, N.; Watt, M.J.; Allen, T.L.; Carpenter, K.; Cooney, G.J.; Febbraio, M.A.; Kraegen, E.W. Overexpression of Carnitine Palmitoyltransferase-1 in Skeletal Muscle Is Sufficient to Enhance Fatty Acid Oxidation and Improve High-Fat Diet–Induced Insulin Resistance. Diabetes 2009, 58, 550–558. [Google Scholar] [CrossRef]
- Bonen, A.; Luiken, J.J.F.P.; Glatz, J.F.C. Regulation of Fatty Acid Transport and Membrane Transporters in Health and Disease. Mol. Cell. Biochem. 2002, 239, 181–192. [Google Scholar] [CrossRef]
- Park, S.S.; Seo, Y.-K. Excess Accumulation of Lipid Impairs Insulin Sensitivity in Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 1949. [Google Scholar] [CrossRef]
- Gimeno, R.E. Fatty Acid Transport Proteins. Curr. Opin. Lipidol. 2007, 18, 271–276. [Google Scholar] [CrossRef]
- Musutova, M.; Elkalaf, M.; Klubickova, N.; Koc, M.; Povysil, S.; Rambousek, J.; Volckaert, B.; Duska, F.; Trinh, M.D.; Kalous, M.; et al. The Effect of Hypoxia and Metformin on Fatty Acid Uptake, Storage, and Oxidation in L6 Differentiated Myotubes. Front. Endocrinol. 2018, 9, 616. [Google Scholar] [CrossRef]
- Reinke, C.; Bevans-Fonti, S.; Drager, L.F.; Shin, M.K.; Polotsky, V.Y. Effects of Different Acute Hypoxic Regimens on Tissue Oxygen Profiles and Metabolic Outcomes. J. Appl. Physiol. (1985) 2011, 111, 881–890. [Google Scholar] [CrossRef]
- Horscroft, J.A.; Murray, A.J. Skeletal Muscle Energy Metabolism in Environmental Hypoxia: Climbing towards Consensus. Extrem. Physiol. Med. 2014, 3, 19. [Google Scholar] [CrossRef]
- Grocott, M.P.W.; Martin, D.S.; Levett, D.Z.H.; McMorrow, R.; Windsor, J.; Montgomery, H.E. Arterial Blood Gases and Oxygen Content in Climbers on Mount Everest. New Engl. J. Med. 2009, 360, 140–149. [Google Scholar] [CrossRef]
- Vacek, L.; Dvorak, A.; Bechynska, K.; Kosek, V.; Elkalaf, M.; Trinh, M.D.; Fiserova, I.; Pospisilova, K.; Slovakova, L.; Vitek, L.; et al. Hypoxia Induces Saturated Fatty Acids Accumulation and Reduces Unsaturated Fatty Acids Independently of Reverse Tricarboxylic Acid Cycle in L6 Myotubes. Front. Endocrinol. 2022, 13, 663625. [Google Scholar] [CrossRef]
- Mylonis, I.; Simos, G.; Paraskeva, E. Hypoxia-Inducible Factors and the Regulation of Lipid Metabolism. Cells 2019, 8, 214. [Google Scholar] [CrossRef]
- Koenig, A.M.; Koehler, U.; Hildebrandt, O.; Schwarzbach, H.; Hannemann, L.; Boneberg, R.; Heverhagen, J.T.; Mahnken, A.H.; Keller, M.; Kann, P.H.; et al. The Effect of Obstructive Sleep Apnea and Continuous Positive Airway Pressure Therapy on Skeletal Muscle Lipid Content in Obese and Nonobese Men. J. Endocr. Soc. 2021, 5, bvab082. [Google Scholar] [CrossRef]
- Heiling, V.J.; Miles, J.M.; Jensen, M.D. How Valid Are Isotopic Measurements of Fatty Acid Oxidation? Am. J. Physiol. 1991, 261, E572–E577. [Google Scholar] [CrossRef]
- Stefanovski, D.; Boston, R.C.; Punjabi, N.M. Sleep-Disordered Breathing and Free Fatty Acid Metabolism. Chest 2020, 158, 2155–2164. [Google Scholar] [CrossRef]
- De Jonge, L.; Zhao, X.; Mattingly, M.S.; Zuber, S.M.; Piaggi, P.; Csako, G.; Cizza, G. Poor Sleep Quality and Sleep Apnea Are Associated with Higher Resting Energy Expenditure in Obese Individuals with Short Sleep Duration. J. Clin. Endocrinol. Metab. 2012, 97, 2881–2889. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Sun, M.; Chen, B. Adherence to CPAP in Patients with Obstructive Sleep Apnea in a Chinese Population. Respir. Care 2012, 57, 238–243. [Google Scholar] [CrossRef]
- Westlake, K.; Dostalova, V.; Plihalova, A.; Pretl, M.; Polak, J. The Clinical Impact of Systematic Screening for Obstructive Sleep Apnea in a Type 2 Diabetes Population—Adherence to the Screening-Diagnostic Process and the Acceptance and Adherence to the CPAP Therapy Compared to Regular Sleep Clinic Patients. Front. Endocrinol. 2018, 9, 714. [Google Scholar] [CrossRef]
- Rotenberg, B.W.; Murariu, D.; Pang, K.P. Trends in CPAP Adherence over Twenty Years of Data Collection: A Flattened Curve. J. Otolaryngol.-Head Neck Surg. 2016, 45, 43. [Google Scholar] [CrossRef]
- Jacques, M.; Kuang, J.; Bishop, D.J.; Yan, X.; Alvarez-Romero, J.; Munson, F.; Garnham, A.; Papadimitriou, I.; Voisin, S.; Eynon, N. Mitochondrial Respiration Variability and Simulations in Human Skeletal Muscle: The Gene SMART Study. FASEB J. 2020, 34, 2978–2986. [Google Scholar] [CrossRef]
- Hughes, M.C.; Ramos, S.V.; Turnbull, P.C.; Nejatbakhsh, A.; Baechler, B.L.; Tahmasebi, H.; Laham, R.; Gurd, B.J.; Quadrilatero, J.; Kane, D.A.; et al. Mitochondrial Bioenergetics and Fiber Type Assessments in Microbiopsy vs. Bergstrom Percutaneous Sampling of Human Skeletal Muscle. Front. Physiol. 2015, 6, 360. [Google Scholar] [CrossRef]
- Doerrier, C.; Garcia-Souza, L.F.; Krumschnabel, G.; Wohlfarter, Y.; Mészáros, A.T.; Gnaiger, E. High-Resolution FluoRespirometry and OXPHOS Protocols for Human Cells, Permeabilized Fibers from Small Biopsies of Muscle, and Isolated Mitochondria. In Mitochondrial Bioenergetics: Methods and Protocols; Springer: New York, NY, USA, 2018; pp. 31–70. [Google Scholar]
- Samovski, D.; Jacome-Sosa, M.; Abumrad, N.A. Fatty Acid Transport and Signaling: Mechanisms and Physiological Implications. Annu. Rev. Physiol. 2023, 85, 317–337. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Carneiro-Freire, N.; Seco-Filgueira, M.; Fernández-Fernández, C.; Mouriño-Bayolo, D. Mitochondrial β-Oxidation of Saturated Fatty Acids in Humans. Mitochondrion 2019, 46, 73–90. [Google Scholar] [CrossRef]
- Zinchuk, A.V.; Gentry, M.J.; Concato, J.; Yaggi, H.K. Phenotypes in Obstructive Sleep Apnea: A Definition, Examples and Evolution of Approaches. Sleep Med. Rev. 2017, 35, 113–123. [Google Scholar] [CrossRef]
- Authors/Task Force Members; Rydén, L.; Grant, P.J.; Anker, S.D.; Berne, C.; Cosentino, F.; Danchin, N.; Deaton, C.; Escaned, J.; Hammes, H.-P.; et al. ESC Guidelines on Diabetes, Pre-Diabetes, and Cardiovascular Diseases Developed in Collaboration with the EASD. Eur. Heart J. 2013, 34, 3035–3087. [Google Scholar] [CrossRef]
- Boston, R.C.; Stefanovski, D.; Moate, P.J.; Sumner, A.E.; Watanabe, R.M.; Bergman, R.N. MINMOD Millennium: A Computer Program to Calculate Glucose Effectiveness and Insulin Sensitivity from the Frequently Sampled Intravenous Glucose Tolerance Test. Diabetes Technol. Ther. 2003, 5, 1003–1015. [Google Scholar] [CrossRef]
- Hayot, M. Skeletal Muscle Microbiopsy: A Validation Study of a Minimally Invasive Technique. Eur. Respir. J. 2005, 25, 431–440. [Google Scholar] [CrossRef]
- Pecinová, A.; Drahota, Z.; Nůsková, H.; Pecina, P.; Houštěk, J. Evaluation of Basic Mitochondrial Functions Using Rat Tissue Homogenates. Mitochondrion 2011, 11, 722–728. [Google Scholar] [CrossRef]
- Ziak, J.; Krajcova, A.; Jiroutkova, K.; Nemcova, V.; Dzupa, V.; Duska, F. Assessing the Function of Mitochondria in Cytosolic Context in Human Skeletal Muscle: Adopting High-Resolution Respirometry to Homogenate of Needle Biopsy Tissue Samples. Mitochondrion 2015, 21, 106–112. [Google Scholar] [CrossRef]
- Larsen, S.; Kraunsøe, R.; Gram, M.; Gnaiger, E.; Helge, J.W.; Dela, F. The Best Approach: Homogenization or Manual Permeabilization of Human Skeletal Muscle Fibers for Respirometry? Anal. Biochem. 2014, 446, 64–68. [Google Scholar] [CrossRef]

| Control (n = 14) | OSA (n = 9) | T2DM (n = 10) | T2DM + OSA (n = 11) | |
|---|---|---|---|---|
| Men/Women | 3/9 | 6/3 | 3/7 | 6/5 |
| Age (years) | 62.2 ± 1.3 | 63.4 ± 1.9 | 64.0 ± 2.1 | 63.4 ± 1.9 |
| BMI (kg/m2) | 33.5 ± 1.1 | 33.8 ± 1.0 | 32.7 ± 0.8 | 35.2 ± 1.0 |
| Fat (%) | 30.9 ± 2.6 | 28.1 ± 2.9 | 30.9 ± 2.6 | 30.6 ± 2.5 |
| AHI | 4.3 ± 0.7 | 47.2 ± 4.0 * | 5.9 ± 0.9 | 47.8 ± 4.9 *# |
| ODI | 4.3 ± 0.5 | 39.0 ± 3.4 * | 6.8 ± 1.2 | 46.3 ± 5.1 *# |
| T90 (%) | 1.8 ± 0.7 | 24.2 ± 7.6 * | 10.1 ± 4.1 | 34.6 ± 9.2 *# |
| T85 (%) | 0.2 ± 0.1 | 4.6 ± 2.3 * | 0.4 ± 0.3 | 10.1 ± 4.3 *# |
| HBA1C (mmol/mol) | 36.2 ± 0.1 | 38.4 ± 1.2 | 52.7 ± 3.5 *† | 51.7 ± 3.4 *† |
| Glucose (mmol/L) | 99.6 ± 1.0 | 102.7 ± 1.4 | 138.3 ± 6.1 *† | 134.9 ± 4.7 *† |
| Insulin (mU/L) | 10.3 ± 0.6 | 10.1 ± 0.3 | 14.4 ± 0.8 | 17.6 ± 0.6 * |
| Disposition index | 1077.1 ± 88.5 | 652.1 ± 112.9 * | 355.2 ± 109.5 | 174.2 ± 33.4 *† |
| AIRg (mU/L/min) | 708.6 ± 63.1 | 507.4 ± 88.2 | 371.6 ± 112.0 | 152.0 ± 29.3 *† |
| IR (mM/mU/L2) | 2.4 ± 0.1 | 2.4 ± 0.3 | 4.4 ± 0.3† | 5.5 ± 0.5 *† |
| HOMA-IR | 2.6 ± 0.2 | 2.6 ± 0.3 | 4.9 ± 0.3 *† | 5.9 ± 0.6 *† |
| FFA (µmol/L) | 530 ± 40 | 530 ± 50 | 500 ± 30 | 560 ± 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lattova, Z.; Slovakova, L.; Plihalova, A.; Gojda, J.; Elkalaf, M.; Westlake, K.; Polak, J. Muscle Lipid Oxidation Is Not Affected by Obstructive Sleep Apnea in Diabetes and Healthy Subjects. Int. J. Mol. Sci. 2023, 24, 5308. https://doi.org/10.3390/ijms24065308
Lattova Z, Slovakova L, Plihalova A, Gojda J, Elkalaf M, Westlake K, Polak J. Muscle Lipid Oxidation Is Not Affected by Obstructive Sleep Apnea in Diabetes and Healthy Subjects. International Journal of Molecular Sciences. 2023; 24(6):5308. https://doi.org/10.3390/ijms24065308
Chicago/Turabian StyleLattova, Zuzana, Lucie Slovakova, Andrea Plihalova, Jan Gojda, Moustafa Elkalaf, Katerina Westlake, and Jan Polak. 2023. "Muscle Lipid Oxidation Is Not Affected by Obstructive Sleep Apnea in Diabetes and Healthy Subjects" International Journal of Molecular Sciences 24, no. 6: 5308. https://doi.org/10.3390/ijms24065308
APA StyleLattova, Z., Slovakova, L., Plihalova, A., Gojda, J., Elkalaf, M., Westlake, K., & Polak, J. (2023). Muscle Lipid Oxidation Is Not Affected by Obstructive Sleep Apnea in Diabetes and Healthy Subjects. International Journal of Molecular Sciences, 24(6), 5308. https://doi.org/10.3390/ijms24065308






