Cathepsin H Knockdown Reverses Radioresistance of Hepatocellular Carcinoma via Metabolic Switch Followed by Apoptosis
Abstract
1. Introduction
2. Results
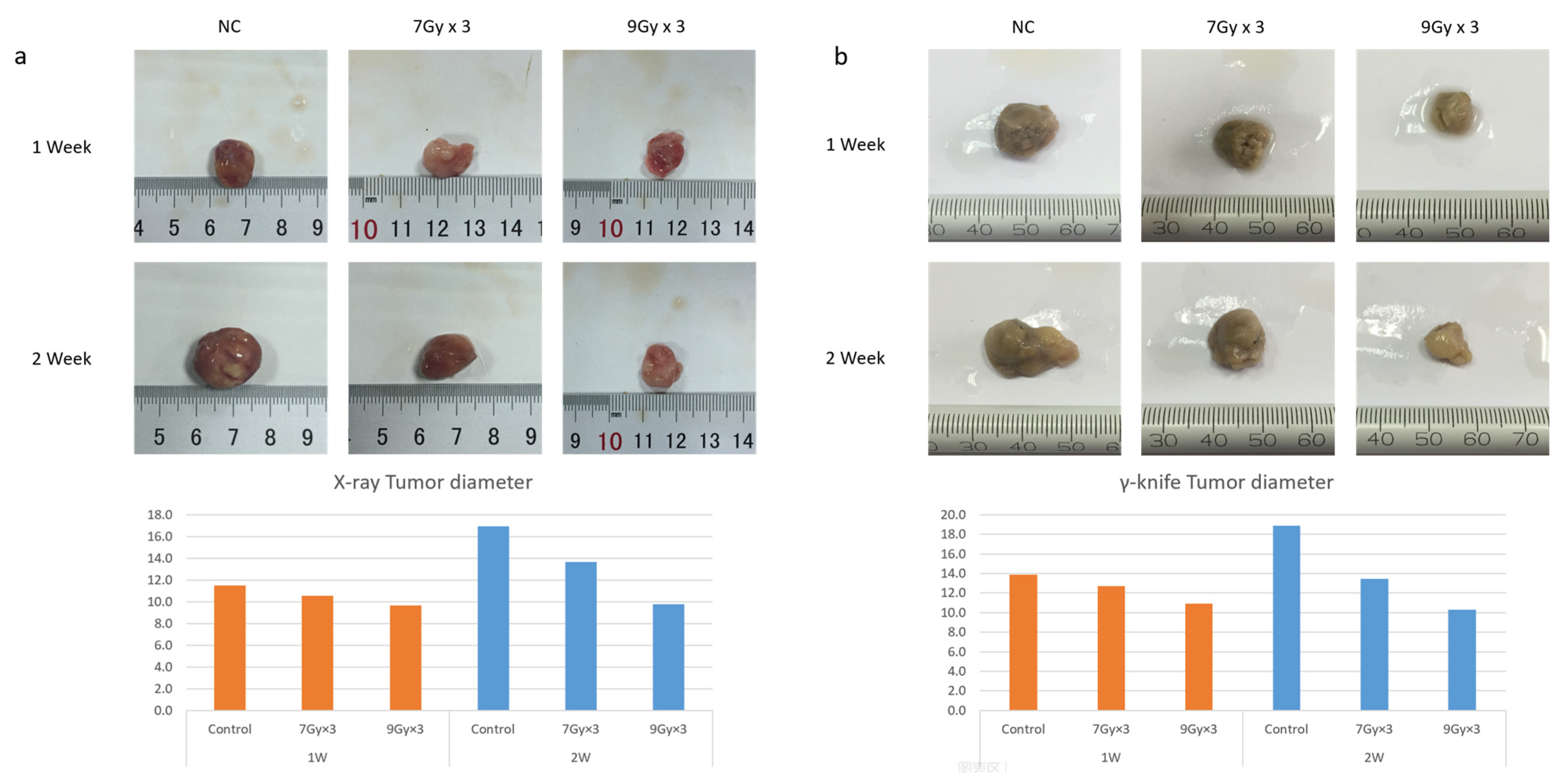
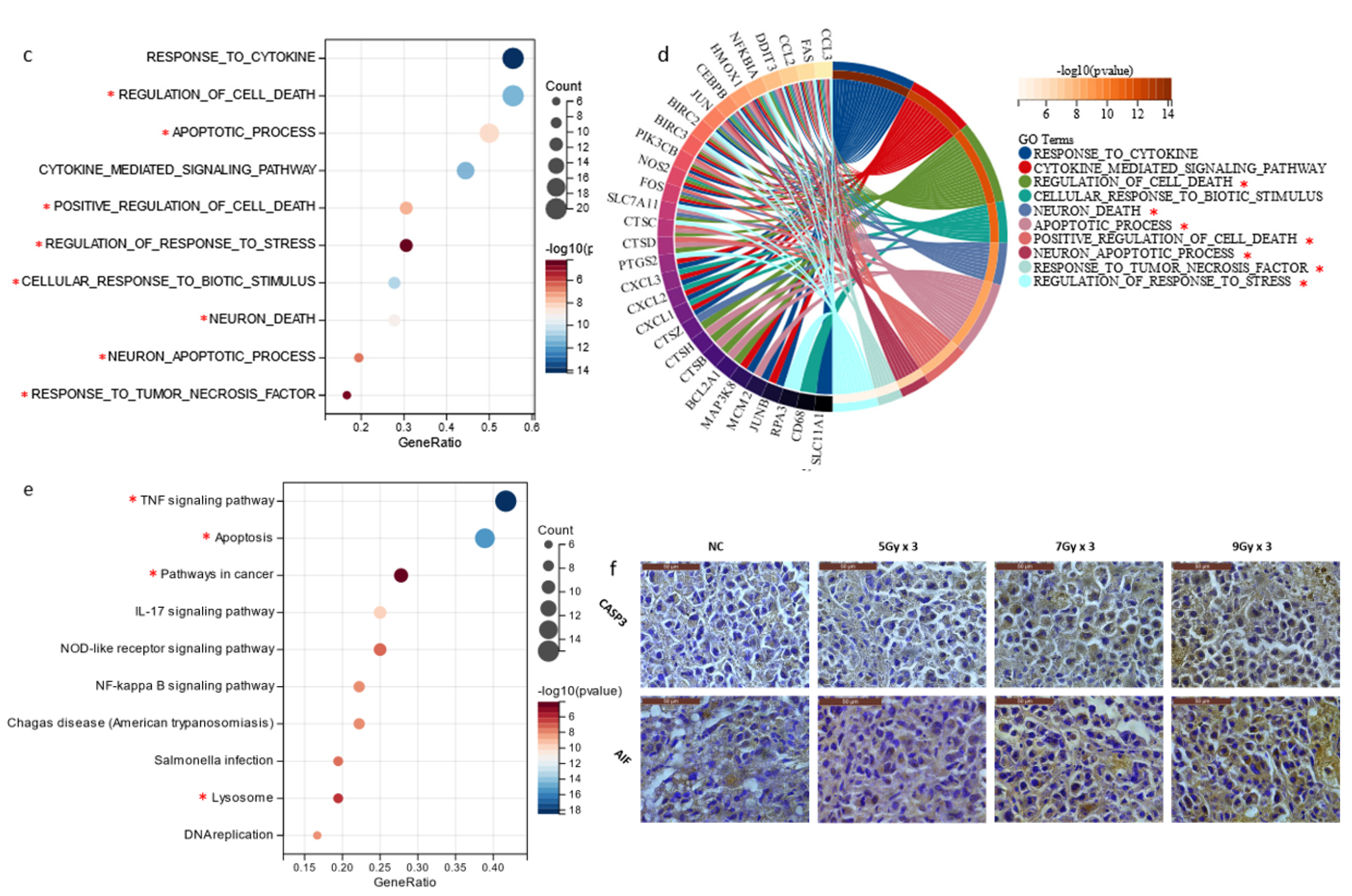
2.1. Radiation-Inhibited Tumor Growth and Induced Apoptosis In Vivo
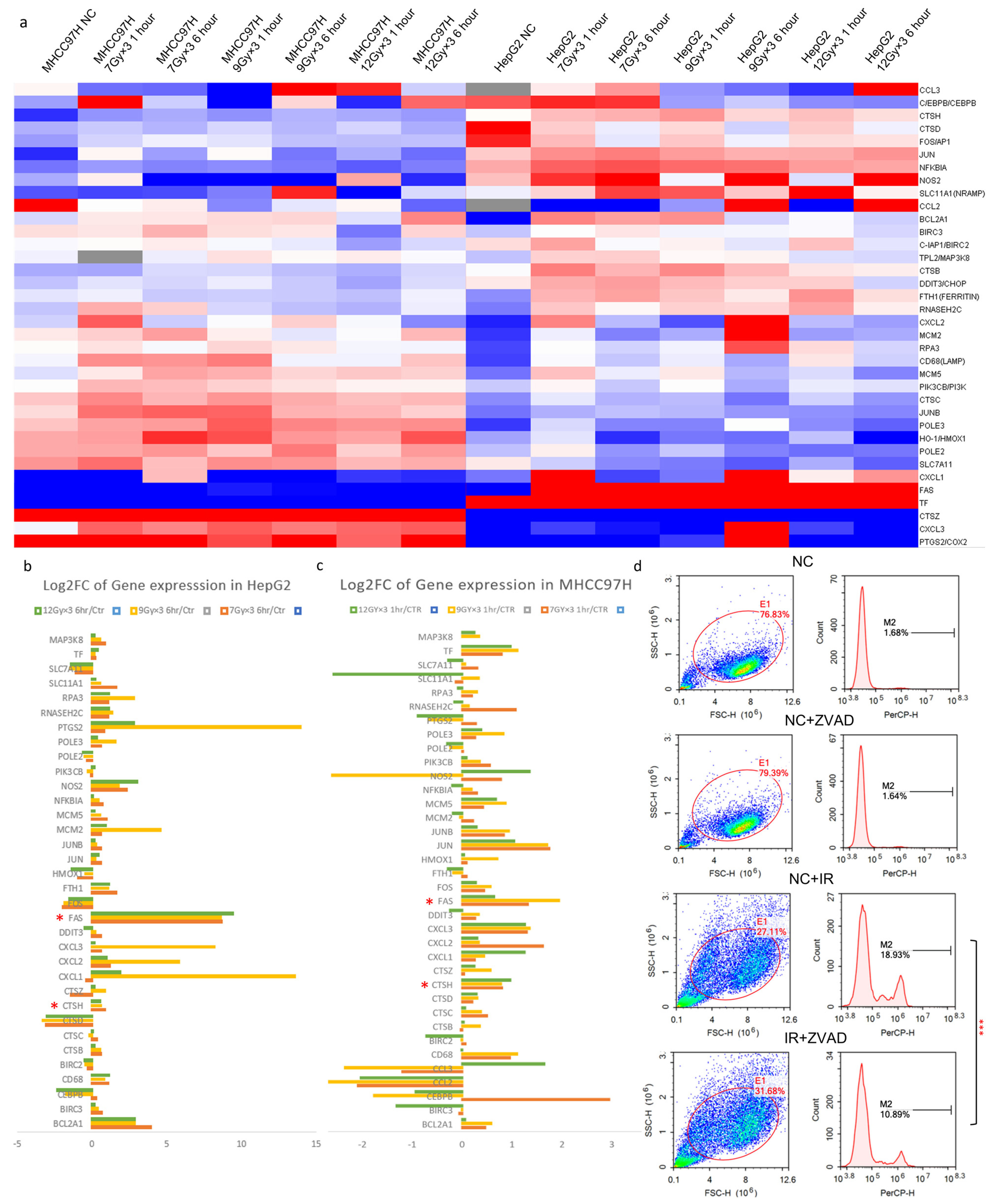
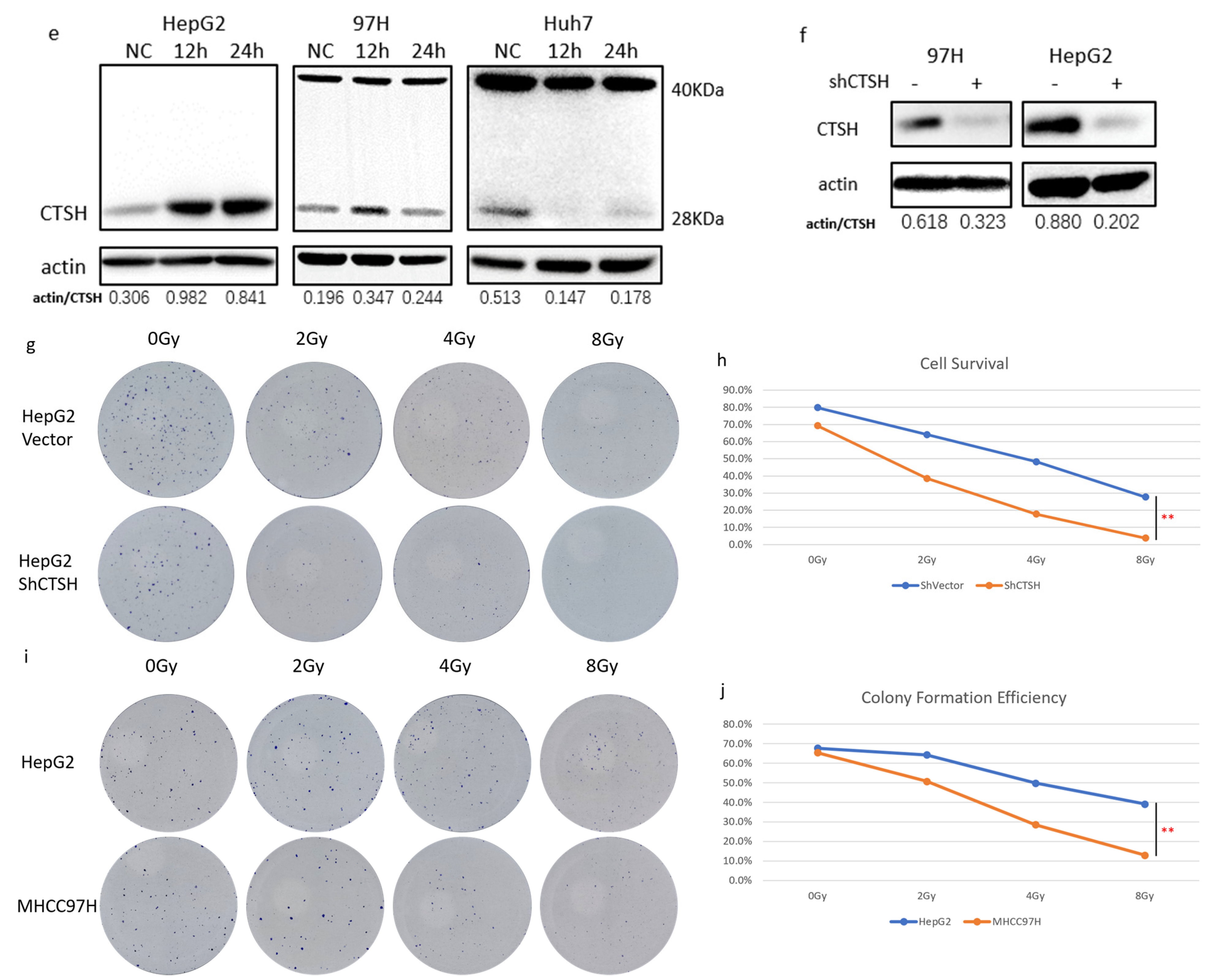
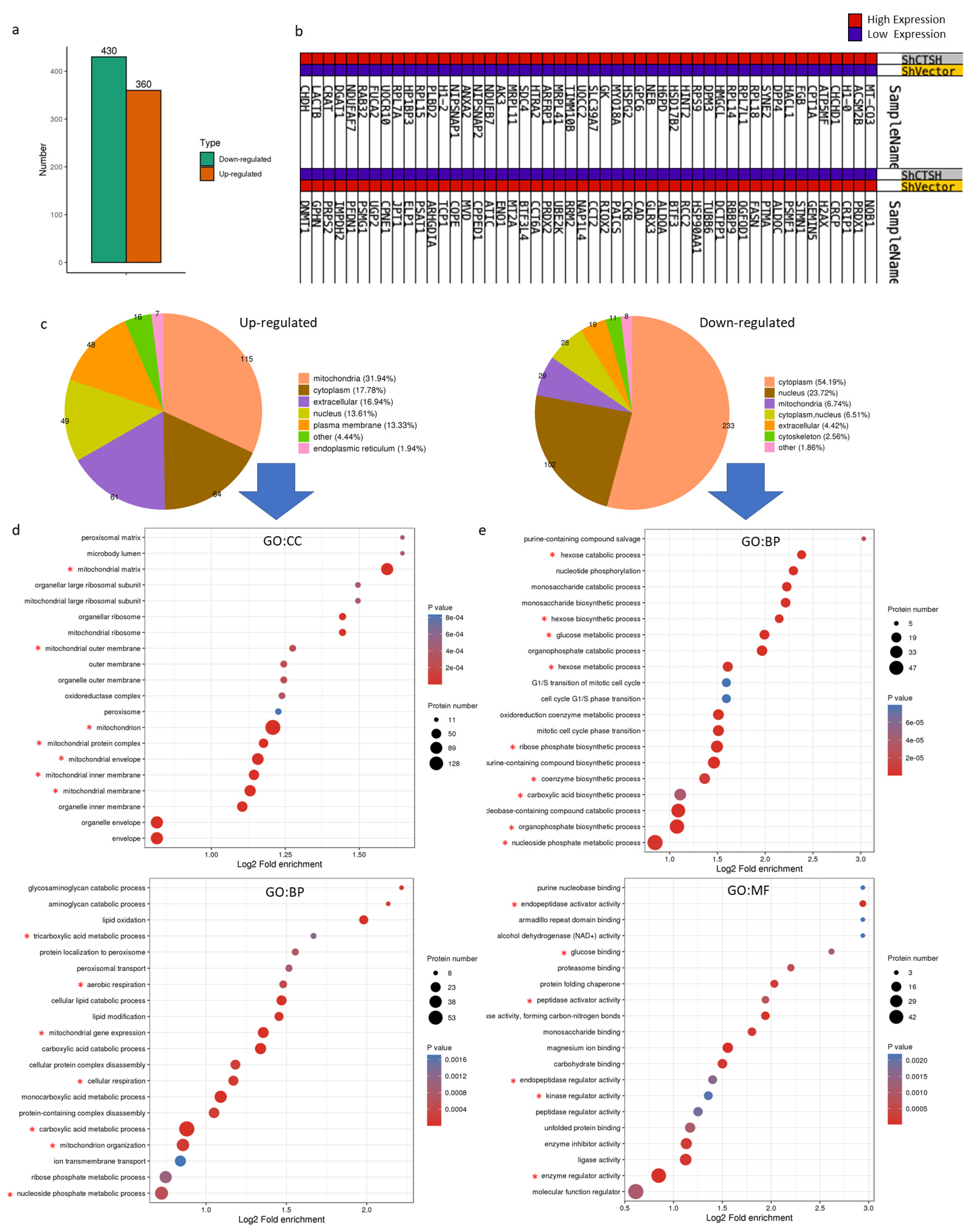
2.2. CTSH Participated in Radioresistance Regulation of HCC Cells
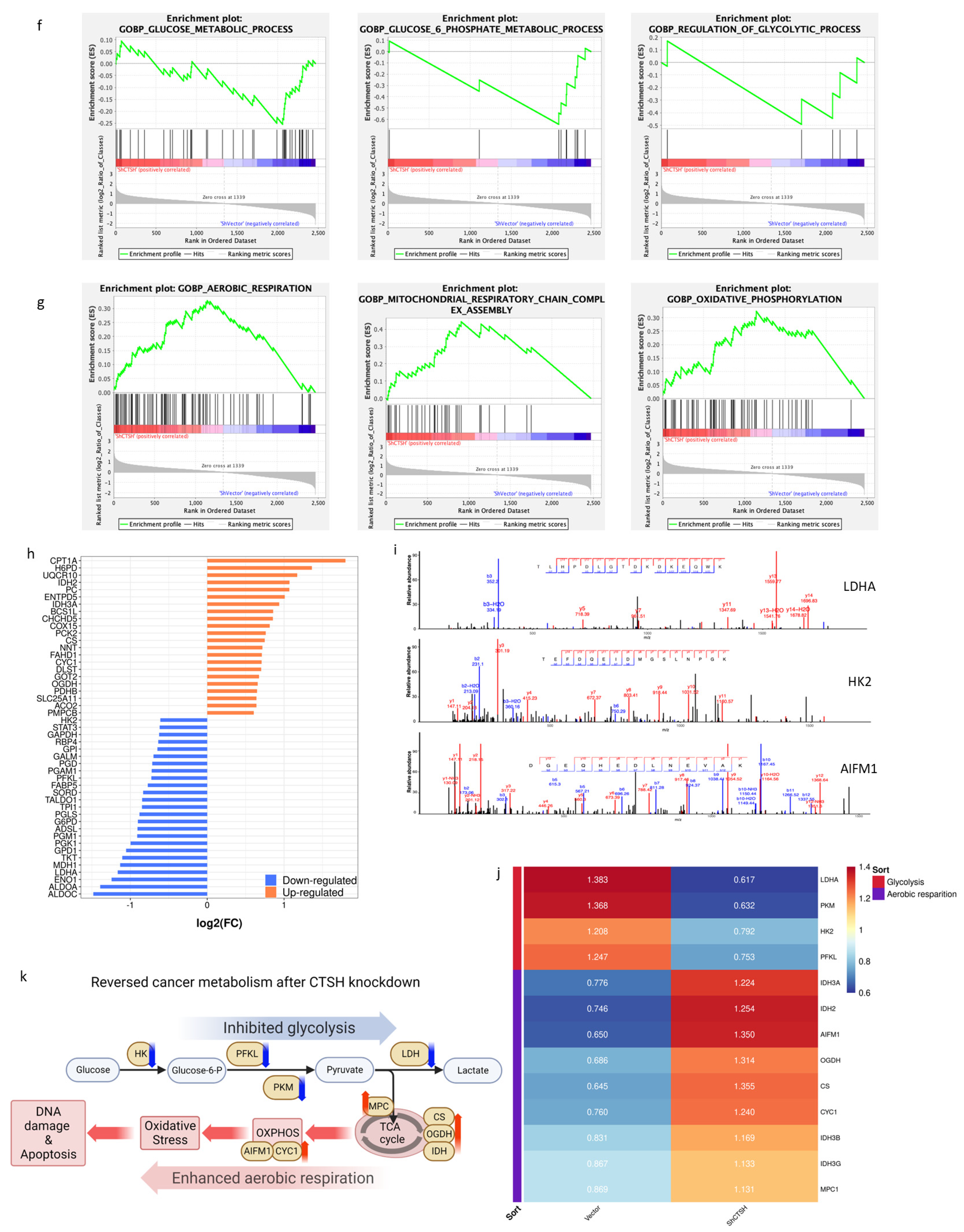
2.3. Restrained Glycolysis and Promoted Aerobic Respiration Inhibited Radioresistance of HepG2 Cells
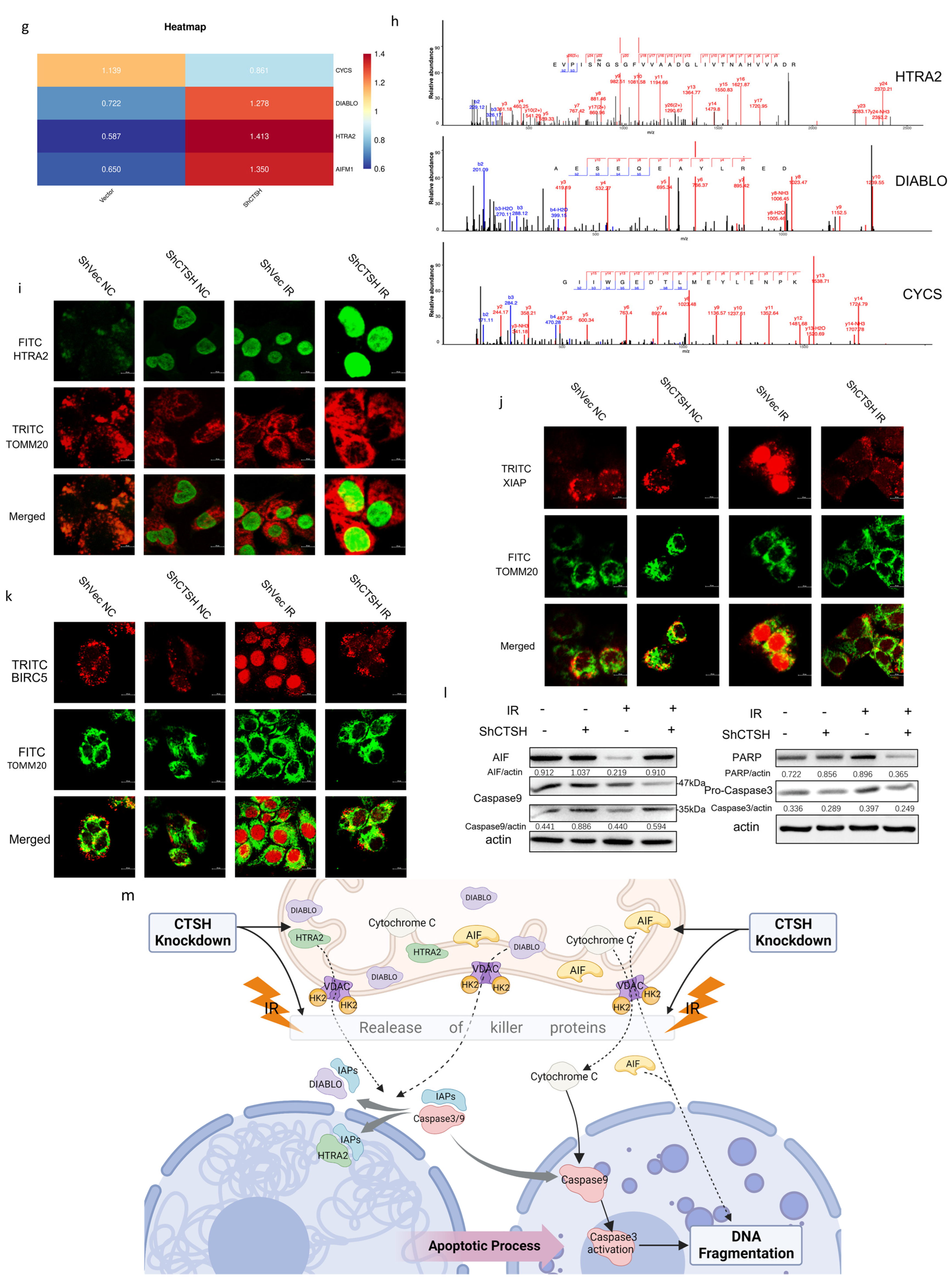
2.4. Knockdown of CTSH and Enhanced Aerobic Respiration Promoted Radiation-Induced Apoptosis via IAP Inhibition and AIF Signal
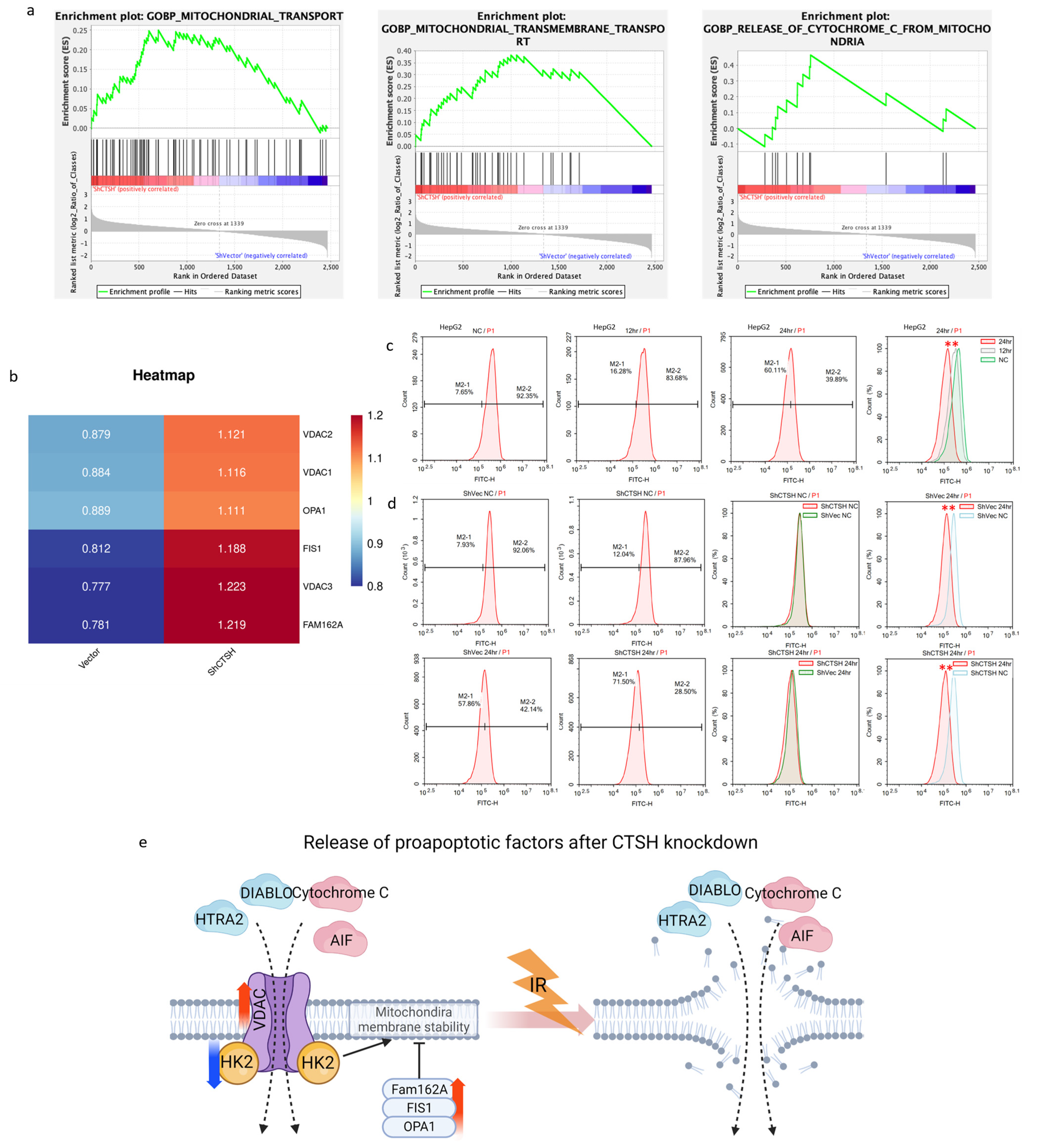
2.5. CTSH Knockdown Changed Mitochondrial Membrane Permeability and Stability in Proapoptotic Signaling
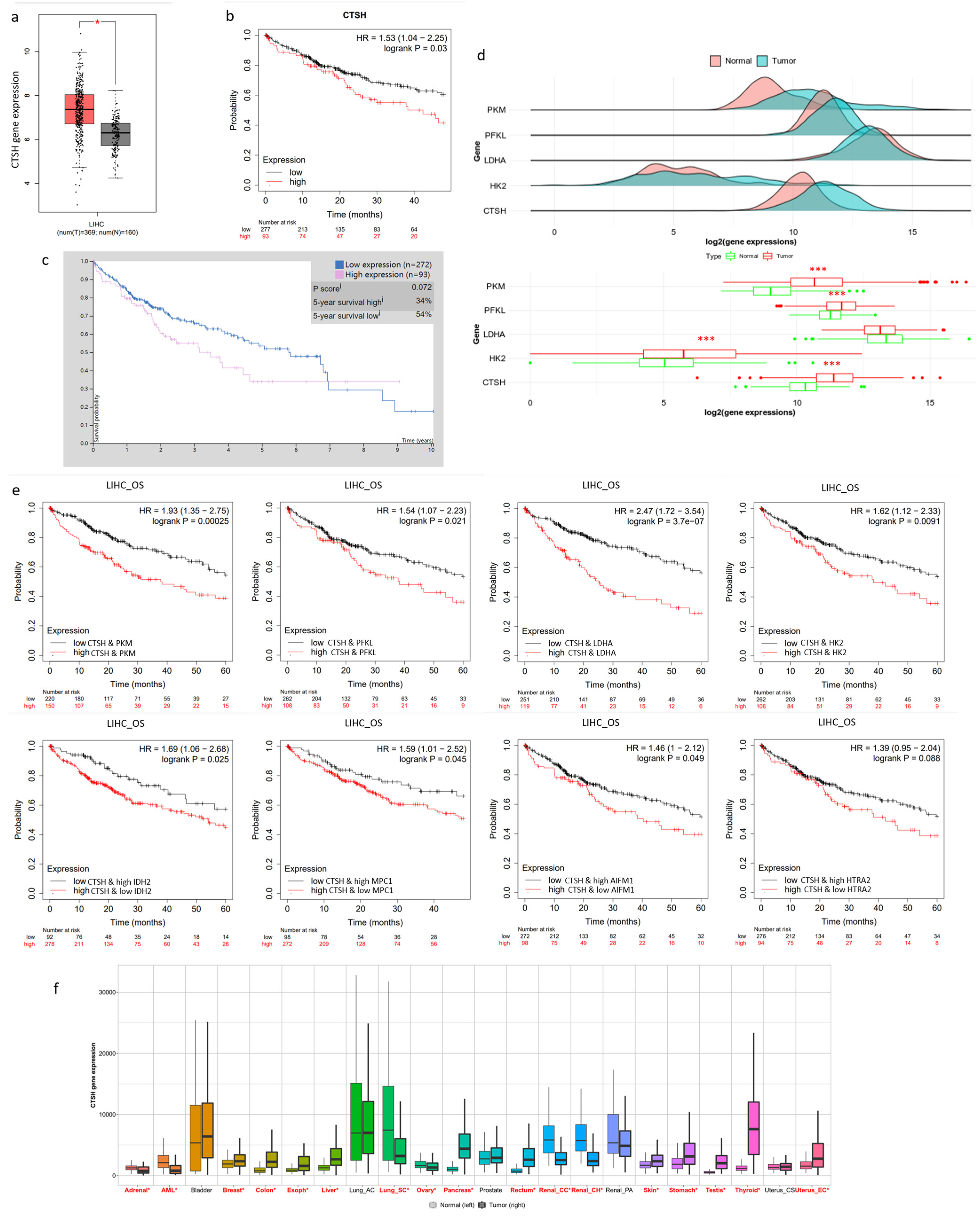
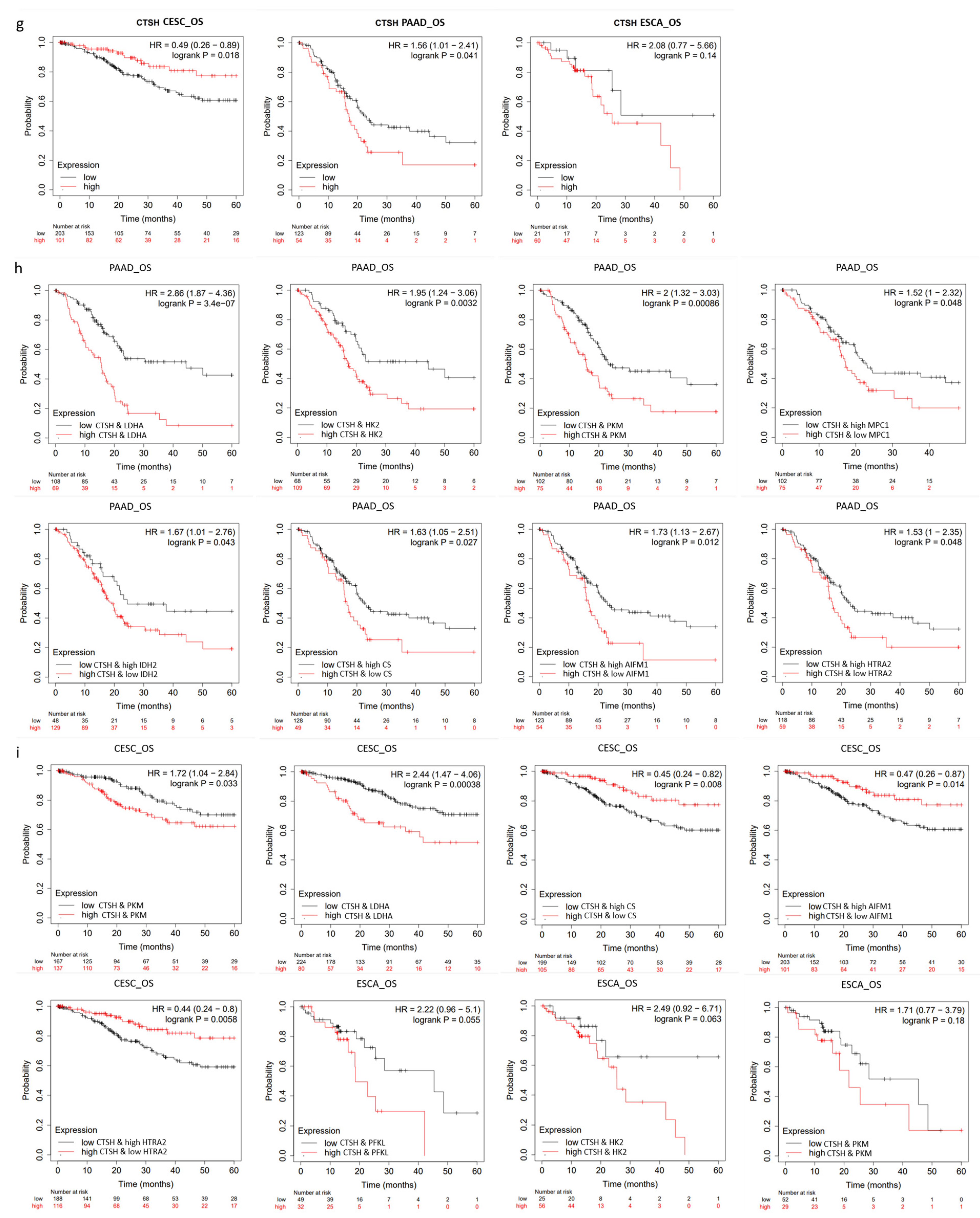
2.6. CTSH and Targets Were Correlated with Tumorigenesis and Poor Prognosis
3. Discussion
3.1. CTSH-Modulated Metabolic Switch from Aerobic Respiration to Glycolysis Is Important for Radioresistance of HCC
3.2. CTSH Knockdown Promotes Apoptosis Following Reversed Metabolic Switch
3.3. CTSH Together with Its Targets Show Potential Value in HCC Treatment
Limitations of the Study
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Western Blot
4.3. Cell Lines and Cell Culture
4.4. Irradiation
4.5. Analysis of Mitochondrial Membrane Potential (MMP)
4.6. Analysis of Apoptosis by Cytometry
4.7. Lentiviral Production
4.8. Transfection
4.9. Cell-Death Analysis by Trypan Blue Staining
4.10. Immunofluorescence Co-Localization Assay
4.11. Orthotopic Tumor Model Establishment
4.12. Bioinformatic Analyses
4.13. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajyaguru, D.J.; Borgert, A.J.; Smith, A.L.; Thomes, R.M.; Conway, P.D.; Halfdanarson, T.R.; Truty, M.J.; Kurup, A.N.; Go, R.S. Radiofrequency Ablation Versus Stereotactic Body Radiotherapy for Localized Hepatocellular Carcinoma in Nonsurgically Managed Patients: Analysis of the National Cancer Database. J. Clin. Oncol. 2018, 36, 600–608. [Google Scholar] [CrossRef]
- Wahl, D.R.; Stenmark, M.H.; Tao, Y.; Pollom, E.L.; Caoili, E.M.; Lawrence, T.S.; Schipper, M.J.; Feng, M. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J. Clin. Oncol. 2016, 34, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Jiang, Y.; Zhang, X.; Feng, S.; Zhou, B.; Ye, X.; Xing, H.; Xu, Y.; Shi, J.; Guo, W.; et al. Neoadjuvant Three-Dimensional Conformal Radiotherapy for Resectable Hepatocellular Carcinoma with Portal Vein Tumor Thrombus: A Randomized, Open-Label, Multicenter Controlled Study. J. Clin. Oncol. 2019, 37, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Venters, C.C.; Di, C.; Pinto, A.M.; Wan, L.; Younis, I.; Cai, Z.; Arai, C.; So, B.R.; Duan, J.; et al. U1 snRNP regulates cancer cell migration and invasion in vitro. Nat. Commun. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Xiao, L.; Tang, M.; Bai, F.; Li, J.; Li, L.; Shi, F.; Li, N.; Li, Y.; Du, Q.; et al. Targeting CPT1A-mediated fatty acid oxidation sensitizes nasopharyngeal carcinoma to radiation therapy. Theranostics 2018, 8, 2329–2347. [Google Scholar] [CrossRef]
- Rashmi, R.; Huang, X.; Floberg, J.M.; Elhammali, A.E.; McCormick, M.L.; Patti, G.J.; Spitz, D.R.; Schwarz, J.K. Radioresistant Cervical Cancers Are Sensitive to Inhibition of Glycolysis and Redox Metabolism. Cancer Res. 2018, 78, 1392–1403. [Google Scholar] [CrossRef]
- Gunda, V.; Souchek, J.; Abrego, J.; Shukla, S.K.; Goode, G.D.; Vernucci, E.; Dasgupta, A.; Chaika, N.V.; King, R.J.; Li, S.; et al. MUC1-Mediated Metabolic Alterations Regulate Response to Radiotherapy in Pancreatic Cancer. Clin. Cancer Res. 2017, 23, 5881–5891. [Google Scholar] [CrossRef]
- Zhou, W.; Yao, Y.; Scott, A.J.; Wilder-Romans, K.; Dresser, J.J.; Werner, C.K.; Sun, H.; Pratt, D.; Sajjakulnukit, P.; Zhao, S.G.; et al. Purine metabolism regulates DNA repair and therapy resistance in glioblastoma. Nat. Commun. 2020, 11, 3811. [Google Scholar] [CrossRef]
- Souchek, J.J.; Baine, M.J.; Lin, C.; Rachagani, S.; Gupta, S.; Kaur, S.; Lester, K.; Zheng, D.; Chen, S.; Smith, L.; et al. Unbiased analysis of pancreatic cancer radiation resistance reveals cholesterol biosynthesis as a novel target for radiosensitisation. Br. J. Cancer 2014, 111, 1139–1149. [Google Scholar] [CrossRef]
- Liu, Y.; Murray-Stewart, T.; Casero, R.A., Jr.; Kagiampakis, I.; Jin, L.; Zhang, J.; Wang, H.; Che, Q.; Tong, H.; Ke, J.; et al. Targeting hexokinase 2 inhibition promotes radiosensitization in HPV16 E7-induced cervical cancer and suppresses tumor growth. Int. J. Oncol. 2017, 50, 2011–2023. [Google Scholar] [CrossRef]
- Park, J.H.; Pyun, W.Y.; Park, H.W. Cancer Metabolism: Phenotype, Signaling and Therapeutic Targets. Cells 2020, 9, 2308. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; ME, L.L. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, D.; Xiao, M.; Zhu, Y.; Zhou, J.; Cao, K. Long noncoding RNA LINC00518 induces radioresistance by regulating glycolysis through an miR-33a-3p/HIF-1α negative feedback loop in melanoma. Cell Death Dis. 2021, 12, 245. [Google Scholar] [CrossRef]
- Shimura, T.; Noma, N.; Sano, Y.; Ochiai, Y.; Oikawa, T.; Fukumoto, M.; Kunugita, N. AKT-mediated enhanced aerobic glycolysis causes acquired radioresistance by human tumor cells. Radiother. Oncol. 2014, 112, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Nagasawa, T.; Kuwahara, Y.; Torii, S.; Igarashi, K.; Roudkenar, M.H.; Roushandeh, A.M.; Kurimasa, A.; Sato, T. MiR-7-5p Is Involved in Ferroptosis Signaling and Radioresistance Thru the Generation of ROS in Radioresistant HeLa and SAS Cell Lines. Int. J. Mol. Sci. 2021, 22, 8300. [Google Scholar] [CrossRef]
- Kim, B.M.; Hong, Y.; Lee, S.; Liu, P.; Lim, J.H.; Lee, Y.H.; Lee, T.H.; Chang, K.T.; Hong, Y. Therapeutic Implications for Overcoming Radiation Resistance in Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 26880–26913. [Google Scholar] [CrossRef]
- Ferlini, C.; D’Amelio, R.; Scambia, G. Apoptosis induced by ionizing radiation. The biological basis of radiosensitivity. Subcell Biochem. 2002, 36, 171–186. [Google Scholar]
- Conus, S.; Simon, H.U. Cathepsins: Key modulators of cell death and inflammatory responses. Biochem. Pharmacol. 2008, 76, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Adair, B.; Reinheckel, T. Specialized roles for cysteine cathepsins in health and disease. J. Clin. Investig. 2010, 120, 3421–3431. [Google Scholar] [CrossRef] [PubMed]
- Gocheva, V.; Chen, X.; Peters, C.; Reinheckel, T.; Joyce, J.A. Deletion of cathepsin H perturbs angiogenic switching, vascularization and growth of tumors in a mouse model of pancreatic islet cell cancer. Biol. Chem. 2010, 391, 937–945. [Google Scholar] [CrossRef]
- Lu, W.D.; Funkelstein, L.; Toneff, T.; Reinheckel, T.; Peters, C.; Hook, V. Cathepsin H functions as an aminopeptidase in secretory vesicles for production of enkephalin and galanin peptide neurotransmitters. J. Neurochem. 2012, 122, 512–522. [Google Scholar]
- Cirman, T.; Oresić, K.; Mazovec, G.D.; Turk, V.; Reed, J.C.; Myers, R.M.; Salvesen, G.S.; Turk, B. Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J. Biol. Chem. 2004, 279, 3578–3587. [Google Scholar] [CrossRef] [PubMed]
- Droga-Mazovec, G.; Bojic, L.; Petelin, A.; Ivanova, S.; Romih, R.; Repnik, U.; Salvesen, G.S.; Stoka, V.; Turk, V.; Turk, B. Cysteine cathepsins trigger caspase-dependent cell death through cleavage of bid and antiapoptotic Bcl-2 homologues. J. Biol. Chem. 2008, 283, 19140–19150. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, M.E.; Bird, P.I.; Peters, C.; Reinheckel, T.; Trapani, J.A.; Sutton, V.R. Cathepsin H is an additional convertase of pro-granzyme B. J. Biol. Chem. 2010, 285, 20514–20519. [Google Scholar] [CrossRef]
- Zou, Y.; Yamagishi, M.; Horikoshi, I.; Ueno, M.; Gu, X.; Perez-Soler, R. Enhanced therapeutic effect against liver W256 carcinosarcoma with temperature-sensitive liposomal adriamycin administered into the hepatic artery. Cancer Res. 1993, 53, 3046–3051. [Google Scholar] [PubMed]
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, H.; Zhang, J.; Volk, A.; Zhang, S.; Wei, W.; Zhang, S.; Breslin, P.; Zhang, J. JTNF-α/Fas-RIP-1-induced cell death signaling separates murine hematopoietic stem cells/progenitors into 2 distinct populations. Blood 2011, 118, 6057–6067. [Google Scholar] [CrossRef]
- Li, H.; Fan, J.; Zhao, Y.; Yang, J.; Xu, H.; Manthari, R.K.; Cheng, X.; Wang, J.; Wang, J. Calcium alleviates fluoride-induced kidney damage via FAS/FASL, TNFR/TNF, DR5/TRAIL pathways in rats. Ecotoxicol. Environ. Saf. 2021, 226, 112851. [Google Scholar] [CrossRef]
- Chiara, F.; Castellaro, D.; Marin, O.; Petronilli, V.; Brusilow, W.S.; Juhaszova, M.; Sollott, S.J.; Forte, M.; Bernardi, P.; Rasola, A. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS ONE 2008, 3, e1852. [Google Scholar] [CrossRef]
- Kim, J.; Yu, L.; Chen, W.; Xu, Y.; Wu, M.; Todorova, D.; Tang, Q.; Feng, B.; Jiang, L.; He, J.; et al. Wild-Type p53 Promotes Cancer Metabolic Switch by Inducing PUMA-Dependent Suppression of Oxidative Phosphorylation. Cancer Cell 2019, 35, 191–203.e8. [Google Scholar] [CrossRef]
- Bartke, T.; Pohl, C.; Pyrowolakis, G.; Jentsch, S. Dual role of BRUCE as an antiapoptotic IAP and a chimeric E2/E3 ubiquitin ligase. Mol. Cell 2004, 14, 801–811. [Google Scholar] [CrossRef]
- Balakrishnan, M.P.; Cilenti, L.; Mashak, Z.; Popat, P.; Alnemri, E.S.; Zervos, A.S. THAP5 is a human cardiac-specific inhibitor of cell cycle that is cleaved by the proapoptotic Omi/HtrA2 protease during cell death. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H643–H653. [Google Scholar] [CrossRef]
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000, 102, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Sevrioukova, I.F. Structure/Function Relations in AIFM1 Variants Associated with Neurodegenerative Disorders. J. Mol. Biol. 2016, 428, 3650–3665. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, D.; Sevrioukova, I.; Invernizzi, F.; Lamperti, C.; Mora, M.; D’Adamo, P.; Novara, F.; Zuffardi, O.; Uziel, G.; Zeviani, M. Severe X-linked mitochondrial encephalomyopathy associated with a mutation in apoptosis-inducing factor. Am. J. Hum. Genet. 2010, 86, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Kalpage, H.A.; Bazylianska, V.; Recanati, M.A.; Fite, A.; Liu, J.; Wan, J.; Mantena, N.; Malek, M.H.; Podgorski, I.; Heath, E.I.; et al. Tissue-specific regulation of cytochrome c by post-translational modifications: Respiration, the mitochondrial membrane potential, ROS, and apoptosis. Faseb J. 2019, 33, 1540–1553. [Google Scholar] [CrossRef]
- Ow, Y.P.; Green, D.R.; Hao, Z.; Mak, T.W. Cytochrome c: Functions beyond respiration. Nat. Rev. Mol. Cell Biol. 2008, 9, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Hiller, S.; Garces, R.G.; Malia, T.J.; Orekhov, V.Y.; Colombini, M.; Wagner, G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science 2008, 321, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, J.Y.; Suk, K.; Park, J.H. Identification of the hypoxia-inducible factor 1 alpha-responsive HGTD-P gene as a mediator in the mitochondrial apoptotic pathway. Mol. Cell Biol. 2004, 24, 3918–3927. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Fox, R.J.; Burwell, L.S.; Yoon, Y. Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hFis1. J. Cell Sci. 2005, 118 Pt 18, 4141–4151. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Wai, T.; Baker, M.J.; Kladt, N.; Schauss, A.C.; Rugarli, E.; Langer, T. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J. Cell Biol. 2014, 204, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J.; Holmuhamedov, E. Voltage-dependent anion channel (VDAC) as mitochondrial governator—Thinking outside the box. Biochim. Biophys. Acta 2006, 1762, 181–190. [Google Scholar] [CrossRef]
- Yu, L.; Sun, Y.; Li, J.; Wang, Y.; Zhu, Y.; Shi, Y.; Fan, X.; Zhou, J.; Bao, Y.; Xiao, J.; et al. Silencing the Girdin gene enhances radio-sensitivity of hepatocellular carcinoma via suppression of glycolytic metabolism. J. Exp. Clin. Cancer Res. 2017, 36, 110. [Google Scholar] [CrossRef]
- Shao, Y.; Song, X.; Jiang, W.; Chen, Y.; Ning, Z.; Gu, W.; Jiang, J. MicroRNA-621 Acts as a Tumor Radiosensitizer by Directly Targeting SETDB1 in Hepatocellular Carcinoma. Mol. Ther. 2019, 27, 355–364. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Ding, Y.; Li, Q.; Wang, R.; Liu, T.; Sun, Q.; Yang, H.; Peng, S.; Wang, W.; et al. MiR-20a Induces Cell Radioresistance by Activating the PTEN/PI3K/Akt Signaling Pathway in Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 1132–1140. [Google Scholar] [CrossRef]
- Bamodu, O.A.; Chang, H.-L.; Ong, J.-R.; Lee, W.-H.; Yeh, C.-T.; Tsai, J.-T. Elevated PDK1 expression drives PI3K/AKT/MTOR signaling promotes radiation-resistant and dedifferentiated phenotype of hepatocellular carcinoma. Cells 2020, 9, 746. [Google Scholar] [CrossRef]
- Deng, G.; Mou, T.; He, J.; Chen, D.; Lv, D.; Liu, H.; Yu, J.; Wang, S.; Li, G. Circular RNA circRHOBTB3 acts as a sponge for miR-654-3p inhibiting gastric cancer growth. J. Exp. Clin. Cancer Res. 2020, 39, 1. [Google Scholar] [CrossRef]
- Shen, L.; Sun, X.; Fu, Z.; Yang, G.; Li, J.; Yao, L. The fundamental role of the p53 pathway in tumor metabolism and its implication in tumor therapy. Clin. Cancer Res. 2012, 18, 1561–1567. [Google Scholar] [CrossRef]
- Wang, M.; Chen, X.; Tan, P.; Wang, Y.; Pan, X.; Lin, T.; Jiang, Y.; Wang, B.; Xu, H.; Wang, Y.; et al. Acquired semi-squamatization during chemotherapy suggests differentiation as a therapeutic strategy for bladder cancer. Cancer Cell 2022, 40, 1044–1059.e8. [Google Scholar] [CrossRef]
- Di, Y.Q.; Han, X.L.; Kang, X.L.; Wang, D.; Chen, C.H.; Wang, J.X.; Zhao, X.F. Autophagy triggers CTSD (cathepsin D) maturation and localization inside cells to promote apoptosis. Autophagy 2021, 17, 1170–1192. [Google Scholar] [CrossRef] [PubMed]
- Chwieralski, C.E.; Welte, T.; Bühling, F. Cathepsin-regulated apoptosis. Apoptosis 2006, 11, 143–149. [Google Scholar] [CrossRef]
- Faubion, W.A.; Gores, G.J. Death receptors in liver biology and pathobiology. Hepatology 1999, 29, 1–4. [Google Scholar] [CrossRef]
- Fløyel, T.; Brorsson, C.; Nielsen, L.B.; Miani, M.; Bang-Berthelsen, C.H.; Friedrichsen, M.; Overgaard, A.J.; Berchtold, L.A.; Wiberg, A.; Poulsen, P.; et al. CTSH regulates β-cell function and disease progression in newly diagnosed type 1 diabetes patients. Proc. Natl. Acad. Sci. USA 2014, 111, 10305–10310. [Google Scholar] [CrossRef] [PubMed]
- Fløyel, T.; Mirza, A.H.; Kaur, S.; Frørup, C.; Yarani, R.; Størling, J.; Pociot, F. The Rac2 GTPase contributes to cathepsin H-mediated protection against cytokine-induced apoptosis in insulin-secreting cells. Mol. Cell Endocrinol. 2020, 518, 110993. [Google Scholar] [CrossRef] [PubMed]
- Pospisilik, J.A.; Knauf, C.; Joza, N.; Benit, P.; Orthofer, M.; Cani, P.D.; Ebersberger, I.; Nakashima, T.; Sarao, R.; Neely, G.; et al. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 2007, 131, 476–491. [Google Scholar] [CrossRef]
- Kesavardhana, S.; Malireddi, R.K.S.; Kanneganti, T.D. Caspases in Cell Death, Inflammation, and Pyroptosis. Annu. Rev. Immunol. 2020, 38, 567–595. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Han, D.; Liu, J.; Chen, C.; Dong, L.; Liu, Y.; Chang, R.; Huang, X.; Liu, Y.; Wang, J.; Dougherty, U.; et al. Anti-tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature 2019, 566, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Kang, S.C. Molecular dynamic simulation (MDS) and in vitro cathepsin-B inhibitory activity of decrusin angelate, ibuprofen, and thymol. Nat. Prod. Res. 2022, 36, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Luo, S.; Libby, P.; Shi, G.P. Cathepsin L-selective inhibitors: A potentially promising treatment for COVID-19 patients. Pharmacol. Ther. 2020, 213, 107587. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Gao, Y. Early changes in the urine proteome in a rat liver tumour model. Peer J. 2020, 8, e8462. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A.; da Silva, M.E.; da Silva, R.A.; Justo, G.Z.; Gomes-Marcondes, M.C.; Aoyama, H. Inhibition of tumor growth by quercetin with increase of survival and prevention of cachexia in Walker 256 tumor-bearing rats. Biochem. Biophys. Res. Commun. 2011, 406, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, L.; Zhang, D.; Jin, Q.; Gao, M.; Wu, T.; Feng, Y.; Ni, Y.; Yin, Z.; Zhang, J. Synthesis and Evaluation of Diindole-Based MRI Contrast Agent for In Vivo Visualization of Necrosis. Mol. Imaging Biol. 2020, 22, 593–601. [Google Scholar] [CrossRef]
- Camargo, C.A.; Gomes-Marcondes, M.C.; Wutzki, N.C.; Aoyama, H. Naringin inhibits tumor growth and reduces interleukin-6 and tumor necrosis factor α levels in rats with Walker 256 carcinosarcoma. Anticancer Res. 2012, 32, 129–133. [Google Scholar]
- Sun, D.S.; Chen, J.H.; Ling, R.; Yao, Q.; Wang, L.; Ma, Z.; Li, Y. Treatment of hepatoma with liposome-encapsulated adriamycin administered into hepatic artery of rats. World J. Gastroenterol. 2006, 12, 4741–4744. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Qu, S.; Liang, Z.; Liu, Y.; Chen, H.; Ma, S.; Liu, X. Cathepsin H Knockdown Reverses Radioresistance of Hepatocellular Carcinoma via Metabolic Switch Followed by Apoptosis. Int. J. Mol. Sci. 2023, 24, 5257. https://doi.org/10.3390/ijms24065257
Chen Q, Qu S, Liang Z, Liu Y, Chen H, Ma S, Liu X. Cathepsin H Knockdown Reverses Radioresistance of Hepatocellular Carcinoma via Metabolic Switch Followed by Apoptosis. International Journal of Molecular Sciences. 2023; 24(6):5257. https://doi.org/10.3390/ijms24065257
Chicago/Turabian StyleChen, Qiao, Shugen Qu, Zhenzhen Liang, Yi Liu, Huajian Chen, Shumei Ma, and Xiaodong Liu. 2023. "Cathepsin H Knockdown Reverses Radioresistance of Hepatocellular Carcinoma via Metabolic Switch Followed by Apoptosis" International Journal of Molecular Sciences 24, no. 6: 5257. https://doi.org/10.3390/ijms24065257
APA StyleChen, Q., Qu, S., Liang, Z., Liu, Y., Chen, H., Ma, S., & Liu, X. (2023). Cathepsin H Knockdown Reverses Radioresistance of Hepatocellular Carcinoma via Metabolic Switch Followed by Apoptosis. International Journal of Molecular Sciences, 24(6), 5257. https://doi.org/10.3390/ijms24065257




