Abstract
Microglia cells are the resident immune cells of the central nervous system. They act as the first-line immune guardians of nervous tissue and central drivers of neuroinflammation. Any homeostatic alteration that can compromise neuron and tissue integrity could activate microglia. Once activated, microglia exhibit highly diverse phenotypes and functions related to either beneficial or harmful consequences. Microglia activation is associated with the release of protective or deleterious cytokines, chemokines, and growth factors that can in turn determine defensive or pathological outcomes. This scenario is complicated by the pathology-related specific phenotypes that microglia can assume, thus leading to the so-called disease-associated microglia phenotypes. Microglia express several receptors that regulate the balance between pro- and anti-inflammatory features, sometimes exerting opposite actions on microglial functions according to specific conditions. In this context, group I metabotropic glutamate receptors (mGluRs) are molecular structures that may contribute to the modulation of the reactive phenotype of microglia cells, and this is worthy of exploration. Here, we summarize the role of group I mGluRs in shaping microglia cells’ phenotype in specific physio-pathological conditions, including some neurodegenerative disorders. A significant section of the review is specifically focused on amyotrophic lateral sclerosis (ALS) since it represents an entirely unexplored topic of research in the field.
1. Role of Microglia in the Central Nervous System
Microglia represent the immune cells resident in the central nervous system (CNS) and are, therefore, the primary immune defense of this central area of the human body. Several studies suggest that microglia mainly originate from the embryonic yolk sac, and this evidence was confirmed in rodent models and humans [1,2,3,4,5]. In mice, microglia precursors enter the CNS around day 9.5 of embryonic life (E9.5) through extravascular pathways, as the first cerebral capillaries appear only on day E10 and are soon closed off from the periphery by E13.5, due to the blood–brain barrier’s (BBB) formation. In humans, microglia penetrate the cerebral cortex by gestational week 4.5 (GW4.5); then, a second wave of microglia infiltration penetrates the embryonic brain via the vasculature at GW12–13 [6,7,8,9,10,11,12]. After entering the brain, microglial cells rapidly proliferate [11,13,14,15] and, by GW22, take on a ramified morphology, becoming fully mature by GW35. Early entry and subsequent brain colonization represent actual events in the initial brain development.
Microglia regulate the number of neural precursors, promote neuron survival, and are involved in the phagocytosis of damaged neurons, synaptic pruning, angiogenesis, synaptogenesis, and the maturation of neural circuits [6,11,16,17,18,19,20,21,22,23]. Microglia have a unique genetic signature with respect to the perivascular, meningeal, and choroidal macrophages in the CNS, probably due to macrophages’ presumed hematopoietic origin and developmental processes [24]. Similarly, pre-natal and post-natal microglia differ from adult microglia [25,26]. Microglia play critical physiological roles during CNS development. Resting microglial cells exhibit numerous ramifications that constantly monitor the surrounding microenvironment, thus maintaining CNS homeostasis by phagocytosing cellular debris [27,28]. Microglia play an essential role in synaptic remodeling through synaptic pruning to optimize neurotransmission processes and are directly involved in the formation and reorganization of neural networks and in providing trophic support to mature neurons [12,20,29].
Microglia can sense the CNS environment by monitoring the signaling pathway molecules that guarantee the physiological crosstalk between microglia and neurons, which is fundamental for the maintenance of cerebral homeostasis [12,30,31,32,33]. A minimal variation in the extracellular milieu composition allows microglia to respond by modulating neuronal activity. The mature homeostatic microglia phenotype progresses in a multiple-step process during CNS development and requires continuous instructions from the adult brain [34,35,36,37,38].
Furthermore, microglia are promptly activated in response to CNS stimuli or pathological conditions and undergo massive morphological and functional changes accompanied by rapid clonal proliferation [39,40,41], a process identified as microglia activation. Activated microglia change their morphology from branched to amoeboid, resembling macrophages circulating in the bloodstream, and migrate toward the lesion site [28,42]. Convergence at the injury site occurs in response to signal molecules released by damaged neurons [42]. Activated microglia-induced neurotoxic effects can occur due to the release of cytotoxic molecules, including pro-inflammatory mediators, such as tumor necrosis factor α (TNF-α) or interferon γ (INFγ), and free radicals, superoxide anion, or nitric oxide (NO), and constituents triggering oxidative stress [43]. Under specific conditions, activated microglia acquire a defined anti-inflammatory phenotype, thus releasing neuroprotective factors [44].
Microglia express membrane receptors for several neurotransmitters [45], allowing them to respond to various external stimuli that determine the cell status. Indeed, some neurotransmitters can influence the activation state of microglia, producing changes in membrane potential as well as in the intracellular calcium concentration, causing the release of cytokines and generating cell motility [45,46]. In homeostatic or resting conditions, microglia exhibit the so-called “surveillant” phenotype, characterized by small cell bodies, limited cellular mobility, and extensive and highly mobile branches to control the surrounding environment [28,42]. This quiescent state is commonly identified as neutral, or “M0” [28,47], and characterized by the low expression of surface markers typical of circulating macrophages, i.e., the common lymphocyte antigen (CD45) and major histocompatibility complex class II (MHCII).
As discussed above, microglia are extremely sensitive to changes in the environment, thus being rapidly activated after exposure to specific signals, such as growth factors, neurotransmitters, or cytokines, that indicate the presence of infection, trauma, neuronal damage, or inflammation [48,49]. Moreover, microglia classify pathogens by recognizing damage-associated molecular patterns (DAMPs), receptor patterns of exogenous microorganisms, or endogenous cells involved in the immune response. As for peripheral macrophages, according to the first proposed nomenclature, the microglia activation state includes at least two distinct phenotypes: M1, described as a pro-inflammatory and neurotoxic phenotype, and M2, also known as the “alternative activation phenotype” [50,51,52], with anti-inflammatory and neuroprotective properties. These two phenotypes differently respond to distinct signals from the microenvironment and, in turn, are involved in producing many effector molecules [53], promoting the transcription of genes that activate cellular defense mechanisms, including the release of inflammatory cytokines and chemokines [50,54].
An example of the adaptative response of microglia cells is the exposure to lipopolysaccharide (LPS) or IFN-γ, which, in vitro, converts microglia into an activated detrimental phenotype, and stimulates the release of pro-inflammatory factors, including interleukin-1α (IL-1α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-12 (IL-12), interleukin-23 (IL-23) cytokines, TNF-α, chemokine (C-C motif) ligand 2 (CCL2), prostaglandin E2 (PGE2), and reactive oxygen species (ROS), through the stimulation of inducible NO synthase (iNOS) [53,55,56]. In contrast, the defensive microglia phenotype is induced by anti-inflammatory cytokines, such as interleukin-4 (IL-4), interleukin-10 (IL-10), or interleukin-13 (IL-13); it suppresses inflammation, promotes the phagocytoses of cell debris, promotes the regeneration of the extracellular matrix, and supports the survival of neurons by releasing protective/trophic factors [52,53,57]. Furthermore, a third microglia activation state, recently defined as the “acquired deactivation phenotype”, represents a different anti-inflammatory phenotype mainly induced by the phagocytosis of apoptotic cells or exposure to anti-inflammatory cytokines, such as IL-10 and transforming growth factor β (TGF-β) [52].
However, this unequivocal classification is inconsistent with the vast repertoire of microglial phenotypes and core functions in different situations, i.e., development, plasticity, ageing, and diseases. As recently reported, considering the coexistence of multiple states, microglia phenotypes occurring in a specific condition should be characterized by more potent analysis tools than those presently applied, such as proteomic, metabolomic, transcriptomic, morphological, and epigenetic ones [58]. Accordingly, a new nomenclature is needed to define the microglia phenotype in each specific physio-pathological environment. This scenario is even more complex since microglia cells can acquire specific activation cellular patterns depending on the pathological environmental conditions in which microglia participate, thus leading to different and peculiar “disease-associated microglia” (DAM) phenotypes [59].
2. Glutamate Receptors Expressed in Microglia
The specific activities of microglia likely result from the cell state, regulated by different environmental stimuli, which in turn activate cellular structures, mainly receptors, that act as sensors of external messengers and trigger several intracellular signals with distinct biological functions [46]. The excellent recent literature reports the presence and the role of many microglia-expressed receptor families. Purinergic, serotoninergic, histaminergic, and cannabinoid receptors are the most relevant in tuning the microglia state by regulating their phenotypic characteristics and functions, including proliferation, branch motility, cytokine release, cell migration, and phagocytosis, in physiological [60,61,62,63,64] and pathological conditions, including neurodegenerative diseases [65,66,67,68,69,70,71]. Although supported by limited evidence in the literature, it is worth mentioning that microglia also express GABAergic, cholinergic, adrenergic, and dopaminergic receptors [45,72].
Glial cells, including microglia, widely express glutamatergic receptors, whose activation exerts numerous crucial effects on the glia themselves and glia–neuron interactions in physiological and pathological conditions [73,74]. Glutamate (GLU) is the primary excitatory amino acid neurotransmitter in the brain. Once released at the presynaptic level, it activates post-synaptic α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), N-methyl D-aspartate (NMDA), and kainate ionotropic receptors to stimulate rapid synaptic transmission [75]. GLU also activates G-protein-coupled metabotropic receptors (mGluRs) with slower signaling transduction kinetics. mGluRs include eight subtypes classified into three groups, termed I, II, and III, based on the sequence homology, signal transduction mechanism, and pharmacological profile [76]. When activated by GLU, mGluRs can generate fine feedback mechanisms at the pre-synaptic level, inhibiting or potentiating the release of GLU itself or other neurotransmitters from heterologous nerve terminals [77,78,79,80]. At the same time, mGluRs regulate critical cellular mechanisms, such as post-synaptic excitatory or inhibitory currents or the activity of glial cells surrounding the synapse, namely astrocytes and microglia [81]. In addition to the numerous actions in glial cells, the activation of mGluRs mediates the interaction between glia and neurons [82,83]. The latter effects are complex and bidirectional, often depending on the implicated mGluR subtypes [83].
Microglia express both ionotropic and metabotropic GLU receptors. These receptors mediate the response to GLU and participate in neuroinflammation and neurodegeneration processes [72]. Microglial NMDA receptors trigger neuroinflammation and neuronal death [84] by also driving pro-inflammatory responses via poly(ADP-ribose) polymerase-1 (PARP-1)/transient receptor potential cation channel subfamily M member 2 (TRMP2) signaling [85]. Thus, the existence of microglial NMDA receptors further offers a link between inflammation and excitotoxicity. Moreover, AMPA and kainate receptor activation triggers microglia reactivity and motor neuron toxicity [86,87,88]. mGluRs in microglia are involved in neuroinflammation [89], acute and chronic neurological disorders, and neurodegenerative diseases [90].
Despite the presence of many mGluR subtypes and their specific functions in finely regulating the cellular processes, in the present review, we focus on the group I mGluRs since they play crucial roles in many physio-pathological situations, as described in the next section. We believe that it is worth exploring their pharmacological or genetic modulation as a potential therapeutic strategy for many traumatic or disease-related conditions.
3. Physio-Pathological Role of Group I Metabotropic GLU Receptors Expressed by Microglia
Metabotropic GLU receptors are organized into three groups, termed I, II, and III, overall including eight subtypes (mGlu1–8 receptors) [76]. Group I include mGlu1 and mGlu5 receptors (henceforth reported as mGluR1 or mGluR5), which couple to a Gq protein, resulting in the activation of phospholipase C (PLC), and inositol triphosphate (IP3) and diacylglycerol (DAG) production. Group I receptors are mainly located in the post-synaptic compartment, and their activation increases cellular excitability. Group II, including mGluR2 and mGluR3, and group III, including mGluR4 and mGluR6–8, couple to Gi/Go proteins and inhibit adenylate cyclase (AC) activity and cyclic adenosine monophosphate (AMP) formation. Due to their widespread expression throughout the nervous system and the modulation of the relevant mechanisms that they participate in, mGluRs represent promising therapeutic targets to shape the microglia phenotype [91,92]. Some mGluR ligands are currently under clinical development regarding the treatment of various disorders, such as X Fragile, schizophrenia, Parkinson’s disease (PD), L-dopa-induced dyskinesias, generalized anxiety disorder, and chronic pain [93,94,95].
Microglia cell lines and primary cultures from the cerebral cortex express mGluR5 mRNA and protein [90,96,97]. Although some evidence indicates that quiescent microglia in the healthy brain do not express mGluR5 [98], after spinal cord or head trauma, activated microglia in the vicinity of the lesion significantly express this receptor [90,99]. Conversely, cultured microglia would not express mGluR1 [90,96], but we will present more information below.
Evidence demonstrating the presence of mGluR1 and mGluR5 in microglia has been obtained using selective receptor agonists and antagonists. The expression of the mGluR5a mRNA and the stimulation of calcium signaling by the mGluR1/5 agonist trans-(1S,3R)-1-amino-1,3-cyclopentane dicarboxylic acid (1S,3R-ACPD) took place in cultured microglia, indicating the expression of the mGluR5a variant in these cells [96]. However, Whittemore et al. [100] obtained conflicting results since they found no intracellular calcium signaling modification in microglia stimulated with 1S,3R-ACPD. The reasons for this discrepancy need to be clarified.
Other group I mGluR agonists trigger the activation of PLC in microglia cultures, which leads, again, to the release of calcium and activation of protein kinase C (PKC) [101]. In turn, PKC activation can cause changes in rectifying potassium channel expression and they shape microglia from the ameboid to the ramified phenotype [102]. Signaling downstream group I mGluRs include mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase 1 (ERK1), and extracellular signal-regulated kinase 2 (ERK2), which are inhibited by selective mGluR5 and mGluR1 antagonists, such as 2-Methyl-6-(phenylethynyl)pyridine (MPEP) and 7-(Hydroxyimino)cyclopropan[b]chromen-1a-carboxylate ethyl ester (CPCCOEt [101,103]). In cultured microglia, the selective activation of mGluR5 by (RS)-2-chloro-5-hidroxyphenylglycine (CHPG), or the combination of the mixed mGluR1/5 agonist [(S)-3,5-Dihydroxyphenylglycine] (DHPG) and the selective mGluR1 antagonist CPCCOEt, attenuated the lipopolysaccharide (LPS) or INFγ-induced activation [97,104], also reducing the accumulation of ROS, the production of TNF-α, and the levels of iNOS with consequent NO release. Moreover, PLC and PKC inhibitors and calcium chelators attenuated the anti-inflammatory events following mGluR5 activation, suggesting that the mGluR5 activation in microglia involves the Gq protein signal transduction pathway [105].
Loane et al. [97] showed that microglia express functional mGluR5, whose activation decreases the release of inflammatory molecules and the impact on neurotoxicity. The inhibition of NADPH oxidase mediated the protective effects of mGluR5 activation in microglia [106], a mechanism of microglia-mediated neurotoxicity common to numerous neurodegenerative diseases [107].
Another molecular pathway linked to the beneficial effects of mGluR5 in microglia is the brain-derived neurotrophic factor/tyrosine-protein kinase B (BDNF/TrKB) cascade [108]. Indeed, the activation of mGluR5 by CHPG protects from oxygen–glucose deprivation (OGD) and reperfusion-induced cytotoxicity, apoptosis, the accumulation of ROS, and the release of inflammatory cytokines in the microglial BV2 cell line [108]. mGluR5 activation also triggers the protein kinase B/glycogen synthase kinase 3β/cAMP-response element binding protein (Akt/GSK-3β/CREB) pathway, resulting in the inhibition of GSK-3β expression, increased phosphorylation of CREB, and reduced expression of inflammation-related genes in microglia cells [109].
Although initially only mRNA coding for mGluR5 was found in microglia cells, and cultured microglia apparently do not express mGluR1, in fact, the mGluR1 subtype appears to be present in this cell population [96], even if less expressed with respect to mGluR5 [105]. In addition to microglia, mGluR1 is also expressed by several cells within the CNS, including neurons, meningeal cells, astrocytes, and T and B cells [96,110], and mGluR1 agonists boost T cell proliferation and promote the activation of the MAPK signaling cascade, increasing inflammation [111]. Additionally, mGluR1 immunoreactivity has been reported in a subset of the microglia/macrophage cell lineage in human multiple sclerosis (MS) lesions [112]. Therefore, mGluR1 antagonists might also have multiple therapeutic applications.
3.1. Role of Group I Metabotropic GLU Receptors in Microglia-Mediated Neuroinflammation
The modulation of microglia’s reactive phenotype and inflammation state has been demonstrated in vitro and predominantly involves the mGluR5 subtype, thus highlighting this receptor as a potential pharmacological target. Byrne et al. demonstrated that mGluR5 stimulation decreased neuroinflammation in vivo after spinal cord injury [105]. Moreover, a single-dose treatment with CHPG significantly improved functional recovery, modulated neuroinflammation, and limited lesion progression after experimental traumatic brain injury, most likely through mGluR5 activation [113]. Fibrinogen-mediated microglial activation was downregulated by the direct activation of mGluR5, providing neuronal protection [114].
Based on the above evidence, new molecules, such as positive allosteric modulators (PAMs), have been developed [115] and the in vivo application has highlighted the importance of reducing inflammation in different pathological conditions. In this context, Loane et al. [116] showed that treatment with mGluR5 PAM VU0360172 significantly reduced neurodegeneration in the mouse hippocampus and improved motor function recovery after controlled cortical impact. These effects were mediated by mGluR5, which reduced cluster of differentiation 68 (CD68) and NADPH oxidase 2 (NOX2) expression and suppressed pro-inflammatory signaling pathways in activated microglia. In addition, VU0360172 treatment shifted the balance between pro-inflammatory and anti-inflammatory microglia activation states toward a protective and pro-reparative phenotype [116]. The pharmacological stimulation of mGluR5 also attenuated the production and release of TNF-α in a Theiler’s murine encephalomyelitis virus (TMEV)-induced model of seizures/epilepsy [117]. The protective role of mGluR5 was recently demonstrated by Carvalho et al. as the specific receptor whose ablation accelerated age-related neuroinflammation and neurodegeneration in a Huntington’s disease mouse model [118]. The α-synuclein-induced microglia inflammation, mimicking PD, was also reduced by the activation of mGluR5 [119], possibly reducing the inflammatory component of this pathology [120]. It is also worth noting that mGluR5 and Toll-like receptor 4 (TLR4), two critical receptors for microglia activation [121], coexist in microglia and that LPS triggers the downregulation of the mGluR5 gene expression by TLR4 activation [122].
3.2. Group I Metabotropic GLU Receptor-Mediated Modulation of Microglia-Released Extracellular Vesicles
Microglia affect neuronal activities by releasing various mediators. Nonetheless, additional intercellular communication mechanisms have been recently described, such as those mediated by microvesicles (MVs), macrovesicles, and exosomes [123,124,125]. The extracellular vesicles released by microglia significantly differ in size, content, and intracellular organelle origin. They are formed either constitutively or, as in the case of microglia, under stimulating conditions, including the activation of receptors expressed at the cell membrane [126,127,128,129]. Vesicles from microglia diffuse into the extracellular space, shuttling toward recipient cells, cytokines, miRNAs, lipids, and other factors [126,130,131,132]. Although the precise kinetics of this interaction are only partially known, the release of vesicles by microglia has been clearly described [124,133,134,135]. Released extracellular vesicles mediate neurotoxicity mainly by transferring pro-inflammatory cytokines [130,136] or even modulating protein aggregation, as in the case of β-amyloid [137]. Furthermore, extracellular vesicles were increased in the human and rodent cerebrospinal fluid during cerebral inflammation, supporting their role in the spread of the inflammatory process [135,138] and in neurodegenerative diseases [139,140,141,142].
Indeed, we need to learn more about the role of group I mGluR activation or blockade in the modulation of the release of extracellular vesicles and their cargo from microglia cells. Activating the P2X7 receptor triggers the release of MVs from microglia, and the same occurs after the stimulation of mGluR5 by CHPG [143]. Of note, LPS-activated microglia blunted the mGluR5 effect, possibly indicating the downregulation of the receptor after LPS stimulation. MVs produced by microglia exposed to CHPG significantly increased the rotenone-induced MN neurotoxicity, suggesting a pivotal role of mGluR5 in regulating the cargo rather than the number of released MVs. Interestingly, miR146a was upregulated in microglia cells by pro-inflammatory signals [144,145] and highly expressed in the MV cargo after microglial mGluR5 activation [143].
4. Role of Group I mGluRs Expressed by Microglia Cells in Specific Traumatic and Pathological Conditions
Based on the evidence in the literature, the group I mGluRs represent key regulators in different pathological conditions, directly or indirectly influencing the microglial activation state. As schematically illustrated in Figure 1, the following section aims to elucidate the effect triggered by the positive or negative modulation of mGluR1 and mGluR5, also expressed by microglia cells, focusing on their potential exploitation as druggable targets for therapeutic interventions.
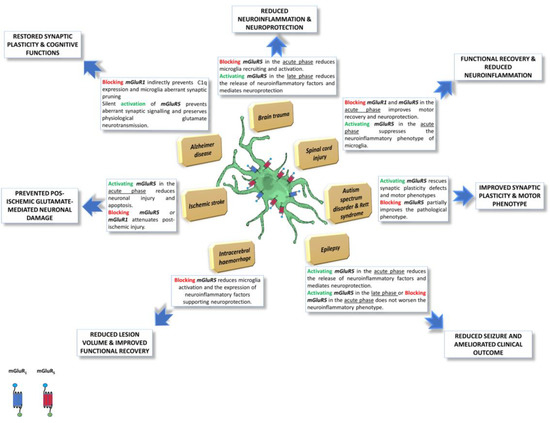
Figure 1.
Schematic representation of microglia-expressed group I mGluRs effects, in different pathological conditions. Group I mGluRs are key regulators in different pathological conditions, directly or indirectly influencing the microglia cell activation state. Pharmacological or genetic modulation of Group I mGluRs, also expressed by microglia cells, can affect the disease course, with often dual or biphasic effects, depending on the time window of intervention and duration of the treatment, overall being a promising tool to develop new pharmacological drugs. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unreported license “https://creativecommons.org/licenses/by/3.0/ (accessed on 19 January 2023)”.
4.1. Brain Trauma
Traumatic brain injury (TBI) is a common and often life-threatening clinical condition [146,147], that begins within seconds or minutes after the traumatic insult and can last for days, weeks, and potentially months or years [148]. After an injury, microglia cells undergo marked morphological and behavioral changes, switching from branched to amoeboid cells, followed by proliferation and migration to the lesion site [42].
Evidence in the literature highlights a dual role of mGluRs in TBI. In the acute phase, Yang et al. [149] demonstrated that the genetic ablation of mGluR5 slowed down the progression of cerebral inflammation by reducing the activation of circulating immune cells and their massive infiltration through the BBB in the traumatic area, thereby decreasing the onset of the neuroinflammatory state and improving the clinical scenario. Mechanistically, the mGluR5 absence blocks the signaling pathway mediated by PKC and causes the inhibition of chemokine expression [150]. In the delayed phase, stimulation of microglial mGluR5 beneficially modulates the neuroinflammation-linked microglia-mediated neurodegenerative mechanisms. Moreover, Byrnes et al. [113] demonstrated that microglia-expressed mGluR5 is more reactive to CHPG after TBI, thus inhibiting the persistent post-injury neuroinflammation and reducing it. Indeed, treatment with CHPG significantly improved long-term sensorimotor and cognitive recovery while reducing the number of activated microglia cells, the persistent post-injury neuroinflammation, and the tissue loss after trauma. The beneficial effects can be attributed to the activation of mGluR5 since the administration of a selective mGluR5 antagonist blocked this effect. However, further investigation is needed to better explain how the selective modulation of mGluR5 expressed by microglia cells can directly impact brain injury.
4.2. Spinal Cord Injury
Spinal cord injury (SCI) is a severe pathological condition leading to cell damage and death [151]. During SCI, CNS-resident microglia become rapidly reactive and release cytokines, leukotrienes, prostaglandins, NO, and superoxide radicals that produce neurotoxic and neurodegenerative effects [152].
Although the microglia-expressed group I mGluRs play a crucial role in shaping inflammation in SCI, we need to learn more about these potential targets. The expression of mGluRs changes after SCI [153], which most likely also occurs in microglia. In particular, mGluR1 expression increases with time following injury, while mGluR5 and mGluR2/3 decrease acutely or chronically, respectively. The opposite effect of mGluR1 and mGluR2/3/5 could contribute to balancing the clinical outcome after SCI. In 2002, Mills et al. [154] showed that a single treatment with the mGluR1 selective antagonist LY367385 improved locomotor scores and attenuated the development of mechanical allodynia. On the other hand, treatment with the selective mGluR5 antagonist MPEP attenuated the development of thermal hyperalgesia, although it did not affect locomotion and mechanical allodynia. The positive effects of the two antagonists suggest distinct acute pathophysiological roles for the two group I mGluR subtypes after SCI. However, further investigation is needed into the direct involvement of mGluR1 and mGluR5 expressed by microglia and the effect triggered by their pharmacological blockade.
Subsequently, the results published by Byrnes et al. [155] provided further evidence, showing that mGluR5 receptor agonists could significantly improve histopathological and functional outcomes after SCI. As an in vitro proof-of-concept, the authors showed that the activation of spinal cord microglia by stimulating mGluR5 with CHPG suppressed the expression of inflammatory markers, such as iNOS, NO, galectin-3, and TNF-α, and reduced microglia neurotoxicity. The above evidence suggests that the pharmacological activation of mGluR5 after SCI may contribute to the protective effects via an anti-inflammatory mechanism enacted by microglia cells. However, we cannot exclude the simultaneous in vivo effects of CHPG on circulating macrophages and other CNS cells, such as astrocytes, that clearly express high mGluR5 levels, especially under pathological conditions.
It is worth recalling that the influence of microglia and macrophages in SCI is a controversial topic. The type of treatment, acute or chronic, with pharmacological tools acting on group I mGluRs, can lead to neuroprotective or neurotoxic effects. Indeed, the differential activation of microglia and macrophages at different stages after SCI can cause the loss or preservation of the tissue affected by the trauma [156], thus indicating that the neuroprotective effect of microglia modulation after SCI is closely related to the pathological timing. The anti-inflammatory outcomes indicate that mGluR5 activation can have multiple neuroprotective effects, mainly during the early phase after SCI. Notably, as mGluR5 is also expressed by neurons, oligodendrocytes, and astrocytes, selective agonists can positively act in vivo as multimodal drugs [90].
4.3. Autism Spectrum Disorder
Autism spectrum disorders (ASDs) are severe conditions affecting childhood neurological development with a multifactorial and polygenic etiology [157]. Zantomio and co-Authors [158] showed the presence of glutamatergic signaling alterations, with particular attention to the mGluR5 receptor and the downstream pathways.
mGluR knockout mice and syndromic and non-syndromic forms of ASD were studied, with a specific incidence in X fragile and Rett’s syndrome [159,160].
In 2009, Blaylock et al. introduced the term “immunoexcitotoxicity” to describe neuronal damage resulting from microglia activation [161]. The increase in the microglia cell number in the fronto-insular, visual, and dorsolateral prefrontal cortex [162,163] in the post-mortem brains of ASD patients, and the presence of activated microglia in the cerebellum [164], manifests a chronic inflammation status sustained by pathologically activated microglia cells.
The link between neuroinflammation and ASD is also supported by the increase in pro-inflammatory cytokines in the blood and CSF [165,166,167]. mGluR5 represents a central actor in the synapse alteration and neuroinflammation observed in ASD. Moreover, the downstream mGluR5 signaling pathways are closely related to many rare gene variants associated with the pathogenesis of ASD, such as the coding elements SHANK1, SHANK2, and SHANK3 [168,169,170].
The subtle correlation between ASD, microglia, and mGluRs is supported by the hypothesis that mGluR5 and the Fragile X Messenger Ribonucleoprotein 1 (FMRP) may exert opposite actions on neuronal plasticity. However, the direct role of mGluR5 in maintaining microglia homeostasis during development has yet to be fully elucidated. Chana et al. demonstrated that the decreased expression of mGluR5 represents a key pathophysiological hallmark in ASD since it regulates the microglia cell number and synaptic pruning during development, with the preservation of appropriate connectivity for normal brain functioning [171]. The Grm5 gene expression was significantly decreased in ASD and associated with increased pro-inflammatory markers. These studies provide evidence of a connection between mGluR5 signaling, microglia cells, and a syndromic form of ASD in the X Fragile mouse model. Nevertheless, further investigation is needed to fully elucidate the role of microglia and mGluRs in this context.
4.4. Rett’s Syndrome
Another neuropathological condition characterized by a severe cognitive deficit often associated with an ASD context is Rett’s syndrome. An alteration in the regulatory role of the methyl-CpG binding protein 2 (MeCP2) gene, indirectly linked to mGluR5, is part of the disease etiology [172]. In Rett’s syndrome, mGluR5 signaling is reduced, accompanied by immune dysregulation [160,173]. MeCP2 phosphorylation is required to modulate synaptic scaling via mGluR5 activation [160]. Indeed, microglial cells lacking MeCP2 showed increased neurotoxicity supported by the release of neurotoxic factors without altering their morphology and the proliferation rate [174]. Interestingly, when MeCP2 was restored only in microglia via bone marrow transplantation, both microglial dysfunction and Rett’s syndrome behavioral symptoms were significantly ameliorated [175]. This finding indicates that the microglia-limited MeCP2 deficit is sufficient to produce the symptoms of Rett’s syndrome and increases the importance of microglia in non-syndromic ASD forms, thus providing evidence that manipulating the immune response can have significant therapeutic potential.
Further results from Gogliotti et al. demonstrated that the mGluR5 PAM VU0462807 could rescue synaptic plasticity and motor defects in a mouse model of Rett’s syndrome [176]. Conversely, the chronic treatment of MeCP2 KO mice with the mGluR5 negative allosteric modulator (NAM) 2-chloro-4-((2,5-dimethyl-1-(4-(trifluoromethoxy) phenyl)-1H-imidazol-4-yl)ethinyl)pyridine (CTEP) partially improved the pathological phenotypes of mice and reduced the upregulated MeCP2-linked gene level, establishing a potential mechanistic link between MeCP2-dependent transcription repression and the mGluR5 pharmacological modulation [177]. Therefore, the microglia-expressed mGluR5 could represent a challenging target for the modulation of Rett’s syndrome and other cognitive deficits.
4.5. Epilepsy
Several studies exploiting Theiler’s murine encephalomyelitis virus (TMEV) and other epilepsy rodent models demonstrated the importance of microglia neuroinflammation in the development of seizures [178,179,180,181,182,183]. TMEV infection causes the infiltration of circulating immune cells in the brain [184] and the activation of microglia [178,179].
Interestingly, the stimulation of mGluR5 by the PAM VU0360172 reduced the production of microglial L-6 and TNF-α and generated neuroprotective effects [97,185]. Short-term treatment with VU0360172, from days 0 to 3 after infection, reduced seizures, while long-term treatment, up to 8 days after infection, did not further reduce seizures in TMEV-infected mice. These experiments showed that the stimulation of mGluR5 suppresses the production of TNF-α at seizure onset; thus, one possible mechanism supporting the improvement in TMEV-infected mouse clinical outcomes after short-term treatment is the reduced amount of TNF-α released by microglia and macrophages. Interestingly, treating TMEV-infected mice with the mGluR5 NAM 3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP) did not worsen seizures, probably because blocking mGluR5 did not increase the TNF-α levels, or the further production of TNF-α by microglia or macrophages did not exacerbate the seizures.
These studies show that inflammation is essential in the development of seizures and epilepsy, which do not depend solely on viral infection, and again supports the role of microglia and the importance of modulating mGluR5 signaling within an early, critical therapeutic window.
4.6. Intracerebral Hemorrhage
Hemorrhage in the brain parenchyma, here termed intracerebral hemorrhage (ICH), often has devastating consequences [186], with long-term neuronal damage and neurological deficits [187,188].
After ICH, the activation and aggregation of microglia are the most common expressions of the immune response. They are among the earliest and most significant events in the maintenance of post-ICH damage [189,190]. Resting microglia increase the expression of mGluR5 significantly only after brain injury [99,191]. The upregulation of mGluR5 induced by ICH promotes the activation of microglia, facilitating the release of the inflammatory cytokines TNF-α and IL-6 close to the lesion site. Therefore, reducing the reactive microglia by in vivo pharmacologically inhibiting mGluR5 can preserve neuronal death and promote functional recovery after stroke. In line with this, several observations support the therapeutic potential of mGluR5 antagonists, which, via reduced cytoplasmic calcium mobilization [121], can modulate microglia’s activation state and, consequently, reduce neurodegeneration and neuronal apoptosis [192,193,194,195].
Very recently, Rahman et al. confirmed this occurrence, showing that reactive microglia highly expressed mGluR5 after ICH and that the in vivo inhibition of mGluR5 by MTEP resulted in attenuated microglial activation and reduced cytokine release [196]. Notably, this effect translated into a marked reduction in cell apoptosis and neurodegeneration, a significant decrease in the lesion volume, and improved functional recovery [196].
Although previous studies reported that mGluR5 stimulation reduces microglia activation and the associated inflammatory response [105,116,185], the above results are consistent with contrasting evidence that mGluR5 blockade reduced microglial activation [121,197,198,199]. The different experimental models adopted and the diverse time windows of the pharmacological treatments might partly explain these discrepancies [197].
Overall, there is a broad consensus that after ICH, mGluR5 attenuation decreases the activated microglia, inflammatory response, neuronal death, and neurological deficit. Therefore, mGluR5 blockade may represent a potential strategy to treat ICH, and microglia indeed represent a potential target for mGluR ligands to modulate the microglia-activated phenotype in ICH. However, further pre-clinical evidence is needed to confirm this hypothesis and clarify the contrasting results in the literature.
4.7. Ischemic Stroke
Ischemia caused by stroke is one of the leading causes of mortality and long-term disability worldwide [200]. The modulation of the GLU-mediated apoptotic pathway is one of the most promising strategies for the development of new drugs. Indeed, excessive GLU release after ischemic injury triggers an excitotoxic cascade, which leads to receptor-mediated neuronal cell death [201,202]. Ischemic injury consists of three phases [203]. Whereas neuronal necrosis occurs early after stroke, delayed neuronal death emerges several hours, days, or weeks after the primary damage due to apoptosis [204]. During the subacute phase of ischemic stroke (24–72 h after onset), vasogenic oedema begins [205]. Weeks after ischemia onset, the chronic phase leads to additional tissue damage and may result in delayed neurodegeneration triggered by oxidative stress and immune activation.
Microglia modify the expression of GLU receptors only after priming conditions [206]. During brain hypoxia and ischemia, microglia release elevated amounts of GLU and other factors that, in turn, influence GLU homeostasis [207], thus directly contributing to the excitotoxic injury.
Experimental evidence indicates that mGluR5 play different roles in modelling cerebral ischemia conditions. Interestingly, the mGluR5 mRNA level significantly increased in the middle cerebral artery occlusion (MCAO) model of ischemia [208]. Since microglia are activated after ischemic stroke and mGluRs may represent a potential target for modulating the microglia’s detrimental phenotype, the pharmacological intervention of group I mGluRs may provide an alternative approach to reducing GLU-mediated microglia activation and neuronal cell death after ischemic stroke. A research report by Bao et al. highlighted that MPEP and CHPG proved a therapeutic potential for treating stroke when acutely administered after focal cerebral ischemia by attenuating apoptotic cell death, although they, respectively, inhibit and activate the receptor [198]. Confirming the role of mGluR5 in ischemic stroke, the pre-ischemic reduction in mGluR5 mRNA levels by exercise training was demonstrated to produce ischemic tolerance in a rat model of MCAO through the PKC-α-GLT-1-GLU interconnected pathways [208,209]. Recently, Cavallo et al. showed that the mGluR5 PAM VU0092273 significantly reduced in vitro the hippocampal neuron injury in the OGD model of ischemia via the PI3K/Akt pathway and the molecular switch of AMPA receptors that indirectly reduce the Ca2+ influx [210]. The same group previously provided a different viewpoint when studying the neuroprotective mechanism enacted by mGluR1 antagonists, predicting that the pharmacological blockade indirectly attenuates post-ischemic injury by enhancing the GABA-mediated neurotransmission, which differs from the pathways reported when activating or blocking mGluR5 in microglial cells [211].
Considering the above studies and the fact that microglia express both mGluR1 and mGluR5, the beneficial effects could result from a complex interplay of factors that indeed involve microglia, since they actively contribute to the pathological scenario of ischemic stroke. In turn, the pharmacological modulation of mGluR1 and mGluR5 provides an exciting perspective for new therapeutic interventions. However, dissecting the contributions of the two microglial receptors and determining which would be the most efficacious for clinical application remains challenging.
4.8. Alzheimer’s Disease
Alzheimer’s disease involves extensive neuroinflammation, neuron and synapse loss, and memory impairment [212]. Microglia play a central role in inducing and maintaining synaptic plasticity and connections, via synaptic pruning [213]. On the other hand, they can also affect synaptic efficiency in neurological disorders, as demonstrated in transgenic AD mouse models [72,214]. The molecular mechanism of this aspect is unknown, although β-amyloid induced microglia activation and neuroinflammation, enhancing the expression of neuroligin 1 transcription repressors, thus reducing GLU synapses in the hippocampus [215]. Altered microglia–neuron interaction impairs synaptic pruning and the correct brain network development, which are associated with social interaction deficits and altered behavioral phenotypes in rodents [216].
FMRP is an abundant mRNA-binding protein in the brain, that modulates the transport and local translation of synaptic mRNA [217,218], and the increased expression of FMRP has been recently reported in a transgenic mouse model of AD [219]. The upregulation of mGluR1 induces FMRP dephosphorylation and facilitates the local translation of synaptic complement component 1q (C1q) mRNA, the initiator of the classical complement activation pathway promoting microglia-operated synaptic pruning [220,221], consequently increasing the phagocytosis of hippocampal glutamatergic synapses and contributing to cognitive dysfunction in AD rodent models [222]. Accordingly, inhibiting C1q or blocking the microglial complement receptor CR3 reduced the microglia-operated synapse phagocytosis and synaptic loss in the early stage of the disease [223]. Indeed, indirect suppression of p-FMRP de-phosphorylation by inhibiting the mGluR1 transduction signals decreases the expression of synaptic C1q and microglial phagocytosis, favoring the recovery of GLU transmission and cognitive abilities, in rats treated with amyloid fragments [224].
On the other hand, Spurrier et al. recently demonstrated that treatment with the mGluR5 silent allosteric modulator BMS-98492 prevents β-amyloid oligomer-induced aberrant synaptic signaling while preserving the physiological GLU response and restoring synaptic density in AD mouse models [225].
To conclude, the key role of microglia cells in AD, leading to the non-physiological activation of synaptic pruning processes and contributing to a progressive cognitive deficit, is now clear, but the basis for this immune-mediated synaptic attack remains to be elucidated. In this scenario, mGluR1 is pathologically upregulated in microglia and can participate in increased complement-mediated synaptic phagocytosis. However, as depicted in many other traumatic or pathological conditions, the direct role of microglia-expressed group I mGluRs and their involvement in C1q-mediated synaptic phagocytosis in AD has yet to be clarified.
5. Microglia and Amyotrophic Lateral Sclerosis
5.1. The Multifactorial and Multicellular Facets of Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by the progressive loss of upper and lower motor neurons (MNs). In the early phase, symptoms are muscle weakness, followed by a gradual loss of muscle control and contraction, atrophy, and paralysis, which lead to death by respiratory failure [226]. Around 90% of cases of ALS are sporadic or unrelated to a specific etiological or hereditary genetic cause. However, 10% of patients are familial, attributable to specific transmissible genetic mutations [227,228]. The identification of more than 40 genes mutated in ALS, affecting numerous cellular functions, has allowed the generation of different animal models useful for pre-clinical research [229]. Mutations in the superoxide dismutase 1 (SOD1) enzyme were the first identified [230] and SOD1-mutated rodents are still the most used models since they recapitulate the ALS pathology better than others. The most recently discovered and frequent ALS-related mutation relates to a repeated hexanucleotide expansion (GGGGCC) in the C9orf72 gene [231,232]. Many other gene mutations have been identified over the years linked to ALS, the most relevant being fused in sarcoma (FUS) protein [233] and binding TAR-DNA-43 (TDP-43) protein [234] mutations.
At present, the approved therapeutic drugs are riluzole, edaravone, and tofersen. Riluzole was proposed to reduce glutamate neurotransmission but only prolongs the lifespan by 2–3 months [235,236]. Edaravone, which reduces oxidative stress, slightly improves motor function scores in patients but only when administered in the early stages of the disease [237,238]. The most recent, tofersen, is an anti-sense oligonucleotide inhibiting SOD1 synthesis. A recent phase 3 clinical trial demonstrated that by initiating the administration of the anti-sense oligonucleotide in early symptomatic patients carrying SOD1 mutations, the drug effectively slowed the decline in clinical and respiratory function and increased muscle strength, improving the quality of life in SOD1-mutated familial patients [239].
Although the initial ALS approach was mainly focused on neurons, growing and unequivocal evidence indicates that non-neuronal cells also play a key role in the pathogenesis of ALS, thus contributing to the definition of ALS as a non-cell-autonomous disease [240,241,242,243,244,245]. Glial cells, such as astrocytes and microglia, can participate in the local inflammatory response with peripheral lymphocytes and macrophages. They acquire a reactive phenotype, migrate to the lesion site, proliferate, and secrete pro-inflammatory and neurotoxic mediators [246,247,248,249,250,251,252,253,254]. Glial activation modifies the expression of a wide range of soluble molecules, such as cytokines and chemokines, DAMPs, reactive nitrogen species (RNS), and ROS, giving rise to profound changes in fundamental aspects of the interactions between glia and neurons [255].
Microglia become reactive before the symptomatic phase of the disease [52,256], concomitantly with the first loss of neuromuscular junctions [257] and MNs [258]. The SOD1G93A mouse model of ALS demonstrated that the disease onset is associated with microglial activation and TNF-α, IL-6, and IL-1β production, suggesting that the SOD1 mutant protein may trigger a pathogenic response in microglia [259]. On the other hand, microglia can also play a protective role in ALS by secreting anti-inflammatory factors, such as IL-4 and IL-10, and growth factors, thus achieving a crucial balance between the pathogenic and protective phenotypes [260,261]. Likewise, astrocytes and oligodendrocytes represent essential cells in maintaining CNS homeostasis and axonal and neuronal integrity; nevertheless, they undergo functional and molecular alterations in ALS, thus sustaining the degeneration and death of MNs [262,263,264].
5.2. The Dual Role of Microglia in Amyotrophic Lateral Sclerosis
Several in vivo studies have shown that circulating microglia cells increase during disease progression and change their activation state [240]; thus, using the former nomenclature, they have been extensively classified as M1- (pro-inflammatory) or M2 (anti-inflammatory)-like phenotypes in ALS [260,265,266]. The microglia phenotype, activated in response to different microenvironmental signals, always includes a balanced ratio between the pro-inflammatory and the anti-inflammatory phenotypes [267,268,269,270], involving the production of different effector molecules [53].
Interestingly, mutant SOD1G93A microglia in ALS differ from wtSOD1 lipopolysaccharide (LPS)-activated microglia and M1/M2 macrophages, defining an ALS-specific phenotype [268] and confirming that the M1/M2 paradigm is an oversimplification of the genuine status, in line with the actual consensus [58,271]. The different microglial cell phenotypes in ALS are mainly based on the characterization of their morphology. The cells in the first phase of the disease, defined as “supervisor” microglia, are characterized by short and poorly branched processes and show an anti-inflammatory profile and overexpression of interleukin IL-10 [272]. In later phases of the disease, microglia show large cell bodies with short and dense processes [273]. Moreover, in the spinal cord of the SOD1G93A mouse, the expression of anti-inflammatory phenotype markers was strongly reduced during the pre-symptomatic phases, while it became more evident in the late symptomatic stage of the disease only [260,274].
Reactive microglia appear hyper-activated in ALS [275]. The toxic activity may be attributed to intracellular mutated proteins, such as TDP-43 and SOD1 [248,267,276]. In particular, the expression of mutated SOD1 (mSOD1) in microglia contributes to the phenotype shift during disease progression. Accordingly, mSOD1-expressing microglia exhibit neuroprotective features during the early phase, improving neuronal survival, while, in the late stage of the disease, mSOD1 microglia exhibit neurotoxic activity [267]. NF-kB, the primary regulator of neuroinflammation, whose expression can be triggered by mutant proteins, plays a central role in the activation of microglia [277]. Upregulation of NF-kB in WT microglia induces gliosis and indirect MN death, both in vitro and in vivo. In contrast, the downregulation of NF-kB in microglia protects MNs from death in vitro and prolongs survival in ALS-affected mice by shifting microglia to anti-inflammatory activity. These data suggest that microglia induce MN death through the activation of the classic NF-kB pathway, which might therefore represent an excellent target in the therapeutic strategies for ALS [277,278]. Several findings indicate that, during ALS progression, microglia cells do not undergo a stage-dependent transition [268]; instead, they show the coexistence of the different phenotypes, thus producing a peculiar functional profile as a complex outcome of multiple regulation factors. It will be very intriguing to further explore the modulatory effect exerted by group I mGluRs on the NF-kB pathway, thus potentially unveiling a pharmacological alternative to the genetic manipulation aimed at modifying microglia cells’ phenotypes.
5.3. Glutamatergic Neurotransmission and Microglial Group I Metabotropic Glutamate Receptors in Amyotrophic Lateral Sclerosis
The excitotoxic role of glutamate was one of the first etiopathological mechanisms studied in ALS [279,280,281,282,283]. In line with this, riluzole was, for a long time, the only drug approved for ALS, which modestly improves the disease course through the unspecific inhibition of glutamatergic neurotransmission [284].
Microglia express ionotropic and metabotropic receptors mediating the response to glutamate and participating in neuroinflammation and neurodegeneration [72,83]. In ALS, MNs are damaged in the motor cortex, brain stem, and spinal cord. This damage is also supported by the selective dysfunction of astrocyte glutamate reuptake, further contributing to excitotoxicity and MN death [285]. In 2004, Zhao et al. investigated the effects of primary mouse microglia cells activated by LPS or ALS patients’ IgG immune complexes on MN survival. Microglia indirectly damaged MNs by increasing their susceptibility to glutamate through a reduction in astrocyte reuptake [86]. On the other hand, the release of TNF-α induced by activated microglia produced the significant release of autocrine glutamate, mainly through gap junctions and supported by the upregulation of glutaminase, which generates a direct excitotoxic effect toward MN [286]. The link between microglia and glutamate was further confirmed by the ability of riluzole to modulate the activation of primary rat microglia cell cultures by decreasing the release of LPS-induced pro-inflammatory markers and increasing the production of neuroprotective factors, such as IL-4; thus, this suggests that riluzole could be neuroprotective in ALS via microglia-mediated mechanisms [287].
The release of glutamate by activated microglia occurs mainly by exploiting the cystine/glutamate antiporter (xCT/Slc7a11), the expression of which is increased during disease progression and associated with enhanced inflammation, in microglia cells from the post-mortem spinal cord tissue of ALS patients [288]. Instead, xCT/Slc7a11 is not expressed in MNs [288]. Mesci et al. demonstrated that the genetic deletion of xCT/Slc7a11 in mice decreased the microglia-associated pro-inflammatory factors, such as NO, TNF-α, and IL-6, while increasing the expression of the neuroprotective marker chitinase-like protein 3 (Chil3, known also as Ym1). Surprisingly, the deletion of xCT/Slc7a11 in mutated SOD1G37R ALS mice anticipated the onset but significantly slowed down the progression of the disease, consistent with the dual role of microglia and glutamate’s downstream effects during disease progression [288].
Differing from other pathological or traumatic conditions, the involvement of group I mGluRs in the modulation of the microglia phenotype in ALS is poorly documented. Nevertheless, mGluR1, mGluR5, and microglia cells are indeed a promising target for ALS treatment and other neurodegenerative diseases in which neuroinflammation plays a pivotal role [73,90,92,270]. In ALS patients, mGluR1 and mGluR5 mRNAs are abundantly expressed in the dorsal horn rather than in the ventral horn of the spinal cord. Of note, spared MNs express abundant mGluR5, while vulnerable MNs do not [289]. Aronica et al. showed that mGluR1 and mGluR5 were highly represented in neuronal cells throughout the human spinal cord, with mGluR1 having high expression in ventral horn neurons. In contrast, intense mGluR5 immunoreactivity was observed in the dorsal horns [290]. This different CNS area- and cell-specific expression of group I mGluRs highlights an intriguing clue possibly linked to the selective vulnerability of MNs in ALS [291,292,293,294]. Regarding glial cells, only sparse astrocytes showed weak to moderate staining for mGluR1 and mGluR5 in the spinal cords of healthy patients [290,295]. In ALS patients, the mGluR1 and mGluR5 immunolabeling intensity increased in cells with an astroglia morphology in the grey and white matter. At the same time, their expression in neurons was comparable to that observed in healthy subjects [290,295].
Although the role of mGluR5 in regulating astrocyte function and their neurotoxic phenotype during ALS progression was largely investigated [296,297,298,299,300,301], there is only one paper describing mGluRs affecting microglia functionality [122]. Berger et al. examined in vitro the modulation of mGluRs expressed by microglial cells in two distinct models of inflammatory conditions, including microglia cell cultures obtained from rats expressing the SOD1G93A ALS-linked mutation. As expected, SOD1G93A microglia were characterized by increased neuroinflammation and enhanced reactivity. The results showed that the mGluR5 mRNA was upregulated in microglia cell cultures prepared from the brains of neonatal SOD1G93A rats. Interestingly, the exposure to LPS, mimicking an inflammatory environment, increased mGluR3 and decreased mGluR5 gene expression in both SOD1G93A and wtSOD1 microglia [122]. This evidence indicates that an inflammatory environment may trigger the opposite regulation of mGluR subtype gene expression. These events seem particularly robust in SOD1G93A microglia cultures. Thus, it will be crucial to consider the possible cell-specific receptor expression and localization when considering these therapeutic targets in ALS.
More recently, Wang et al. developed radiotracers that selectively target mGluR5 [302], allowing their characterization, distribution, and functional binding properties by in vivo micro-PET in different experimental animal models, including SOD1G93A mice [303,304]. Exploiting this technique, Brownell et al. described the expression pattern and the effects obtained by the modulation of mGluR5 during progressive degeneration in ALS mice carrying the SOD1G93A mutation [305]. In detail, concomitantly using specific ligands for mGluR5 ([18F]FPEB) and activated microglia cells ([11C]PBR 28) during the inflammatory response, they evidenced that inflammation and mGluR5 expression, colocalizing with the IBA1-positive microglia, were enhanced in the hippocampus, striatum, frontal cortex, and spinal cord of SOD1G93A mice. These data highlighted the role of GLU in promoting the inflammatory response in ALS, through the activation of mGluR5, expressed by microglia cells. Of note, the enhanced expression of mGluR5 in the brains of SOD1G93A mice couples with the inflammation observed in ALS patients by using the same [11C]PBR28 ligand [306].
A significant contribution to our understanding of the potential role of group I mGluRs in modulating the disease progression and microglia reactivity in ALS comes from our research group. Using the SOD1G93A mouse model, we first demonstrated that the expression and function of mGluR1 and mGluR5 were enhanced at glutamatergic synapses in the spinal cord at the early pre-symptomatic and late symptomatic stages of the disease [78,79]. These alterations could further exacerbate the excessive glutamatergic neurotransmission previously demonstrated in the spinal cords of SOD1G93A mice [281,283,307]. In subsequent studies, genetically halving mGluR1 and mGluR5, or ablating mGluR5, significantly ameliorated disease progression and survival probability in SODG93A mice [308,309,310], and produced a reduction in astrogliosis and microgliosis, always accompanied by positive outcomes for the ALS phenotype. In vivo, the beneficial effects can hardly be ascribed to a specific cell subtype; however, we postulated that the observed modulation of reactive astrocytes and microglia could represent a potential contribution to the improved MN survival [308,309,310]. Unfortunately, the total genetic ablation of mGluR1 receptor in the SOD1G93A mouse model produced a very serious ataxic and detrimental phenotype (unpublished results), thus excluding possible therapeutic approaches using mGluR1 pharmacological antagonists. Then, we tested the effect of the chronic oral administration of the mGluR5 NAM CTEP [311]. Differing from the mGluR5 genetic ablation, the pharmacological treatment was started after symptom onset and maintained until the late symptomatic stage of the disease [311]. CTEP dose-dependently ameliorated the survival and clinical course in SOD1G93A mice. Of relevance, paralleling the genetic studies, chronic treatment with CTEP also reduced astrogliosis and microglia proliferation in the spinal cords of SOD1G93A mice, possibly contributing to the amelioration of the extracellular noxious milieu toward MNs, thus in turn reducing the disease severity.
Considering the dual role of microglia during ALS progression and the fact that blocking mGluR5 before or after disease onset, by genetic or pharmacological strategies, respectively, always ameliorated disease progression and reduced glial reactivity, uncertainty about the mixed effects of dampening mGluR5 in ALS arises. The negative modulation of group I mGluRs should differently affect astrocyte or microglia cells early in the pathology, when microglia probably possess an anti-inflammatory phenotype and astrocytes should start to be reactive, with respect to the late symptomatic stages, when both astrocytes and microglia are detrimental for MNs.
Studying microglia cells isolated acutely at specific stages of the disease from the spinal cords of SOD1G93A mice partially lacking mGluR5 allowed us to gather interesting, still unpublished results. We analyzed the cell phenotype at the pre-symptomatic and late disease stages to characterize the cell shift during the disease progression in SOD1G93A mice, highlighting possible age- and sex-related differences. We also verified the impact of the genetic ablation of mGluR5 on the microglia phenotype. As expected, the expression of numerous microglia activation markers changed during disease progression, depicting a unique ALS microglia signature with no particular evidence of sex differences. Of note, mGluR5 dampening seems to strongly modify the microglia’s bioenergetic metabolism by promoting aerobic metabolism and decreasing the antioxidant response.
The studies mentioned above, including our microglia-specific effects of group I mGluR modulation, are worth considering in the potential therapeutic application of mGluR5-targeted drugs to be exploited for ALS and other neurodegenerative diseases characterized by glial activation and neuroinflammatory features. Due to the subtle modifications that the microglia phenotype may undergo during specific ALS stages, and the uncertain role played by mGluRs, the only way to shed light on this complex scenario would be to expand the studies by exploiting more powerful and “omic” approaches, including transcriptomics, proteomics, metabolomics, and epigenomics, besides those adopted till now, as schematically proposed in Figure 2.
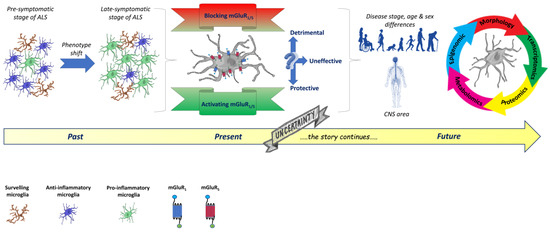
Figure 2.
Past, present, and future perspectives to be further investigated in dissecting the role of mGluRs in the modulation of microglia phenotype changes during ALS progression. Past. Microglia phenotypes in ALS are mainly characterized by their different morphologies, depicting an unbalanced M1/M2 polarization ratio. During the early non-symptomatic stage of ALS, in response to different CNS microenvironmental signals, microglia cells acquire an anti-inflammatory phenotype, characterized by short and poorly branched processes and defined as M2, involved in the production of different protective effectors. The complex interplay between pathological signals from various cell types triggers a precocious microglia shift that acquires a detrimental pro-inflammatory phenotype, characterized by large cell bodies with short and dense processes, defined as M1, involving the production of several toxic factors. Present. During ALS progression, microglia cells do not undergo a stage-dependent discrete transition, but, instead, they show the coexistence of many different phenotypes with peculiar functional profiles, thus depicting a unique ALS microglia signature. Group I mGluR NAM and PAM modulate the microglia phenotype, ameliorating the disease outcome in experimental models of ALS. However, their most effective application mode, and the optimal intervention time window, still need to be determined. Future. Due to the subtle modifications that the microglia phenotype undergoes during specific ALS stages and the uncertain role played by mGluRs, “multiomic” investigation strategies, such as transcriptomic, proteomic, metabolomic, and epigenomic, are needed to shed light on this very complex scenario. These new, powerful approaches will hopefully unveil further details of tissue specificity, age-related diversity, and sex-dependent responses to a specific mGluR-based therapeutic intervention aimed at modulating the microglia phenotype. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unreported license https://creativecommons.org/licenses/by/3.0/ (accessed on 19 January 2023).
5.4. Microglia Characteristics Dictate the Therapeutic Strategies in ALS
The responses of individual cell types to diseases in the CNS should be considered when using mGluR agonists or antagonists for therapeutic interventions, and the different approaches should be tailored depending on the specific pathological condition, on the optimal timing for the maximal efficacy, and on the cell subtype that needs to be targeted.
This aspect is particularly true in ALS, where the microglial cell activation and neuroinflammatory state play a prominent role. Progress has been recently made because of the growing interest in glial cells, particularly in microglia, and the development of innovative approaches targeting these specific cells. However, microglia cell signaling remains enigmatic, and many aspects of their pathophysiology still need to be unveiled. Different therapeutic strategies have been applied to reduce the pro-inflammatory activity of microglia and counteract the progression of the disease. Unfortunately, these anti-inflammatory therapies have had only modest success. The non-univocal role played by microglia during the disease might explain this partial failure.
To emphasize the crucial role of the phenotype balance of microglia cells in ALS and the importance of efficacious modulation to obtain therapeutic effects, we report the paradigm of minocycline administration, an antibiotic belonging to the tetracycline class. When minocycline was administered early before the detrimental shift toward a neuroinflammatory phenotype, it prevented microglia activation in ALS [312,313,314]. Instead, when administered later during the progression of the pathology, when microglia had already acquired a neurotoxic phenotype, the drug failed to counteract the pathological symptoms and even increased microgliosis [315]. Consistently, inhibiting the microglial function becomes a sound strategy only when the different stages of microglia polarization are considered, targeting the harmful factors or promoting the anti-inflammatory phenotype. Although very challenging, the normalization of the microglia polarization balance, inhibiting the pro-inflammatory phenotype, and simultaneously boosting the activation of protective microglia cells, appears to be one of the most promising therapeutic perspectives in the treatment of ALS [316,317,318,319].
Neurotransmitters, growth factors, cytokines, and their pathways lead to microglia reactivity regulation, but, moving deeper into the brain region, metabolic pathways and environmental factors are still a crucial challenge. Moreover, the design of tools able to antagonize the molecular triggers underlying microglia activation in the optimal time window is critical to ALS. Thus, the main future challenge will be to reveal the multifaceted aspects of microglia during disease progression and to indicate the correct time window in which to intervene, considering the local environment, which also affects the specific responses of microglia or other surrounding cells, positively amplifying or worsening the targeted approach. For instance, the local permissive milieu created by microglia is fundamental to guaranteeing the success of different therapeutic strategies aimed at reducing demyelination [320]. The above considerations justify the need for multimodal actions to normalize altered processes directly involving microglia, and the surrounding cells. Moreover, the individuation of specific time windows related to disease progression and possible sex-related differences in ALS are still fundamental aspects to be considered for future research.
Group I mGluRs are potentially druggable targets for ALS treatment because they effectively counteract the downstream consequences of excessive glutamate and represent fine receptor modulators, thus avoiding the well-known side effects generated by drugs acting at the ionotropic glutamate receptors. Moreover, exploiting a combination of mGlu5 and mGlu1 receptor NAMs, which would reduce excitatory transmission and glia reactivity, and mGluR3 PAMs, able to induce the production of neurotrophic factors, could represent an even more intriguing opportunity to reduce MN degeneration and glia reactivity in ALS.
6. Conclusions
The pharmacological or genetic modulation of group I mGluRs expressed by microglia cells represents an attractive multipotential therapeutic strategy for acute traumatic and chronic neurodegenerative disorders. Although the literature has frequently investigated the roles of mGluRs and related therapeutic approaches focusing on neurons, other cell types, including astrocytes and microglia, express these receptors. Many neuroprotective strategies aimed at modulating the aberrant reactive state of these cells have highlighted microglia as a new target to improve clinical outcomes in different pathological conditions, particularly neurodegenerative diseases characterized by prominent neuroinflammatory hallmarks. Group I mGluR modulation reduces inflammation, excitotoxicity, necroptosis, and apoptotic cell death. In this review, we present, a comprehensive overview describing the multifaced effects obtained by modulating group I mGluRs expressed in microglia cells in several acute or chronic CNS pathological conditions, such as trauma, spinal cord injury, cognitive disorders, epilepsy, intracerebral hemorrhage, ischemic stroke, Alzheimer’s disease, and ALS for the first time.
The pipelines of many pharmaceutical companies consider different strategies based on the group I mGluR modulation in treating neurological diseases. Unfortunately, none of these experimental compounds passed the final step for approval. ALS represents a neurodegenerative disease with urgent unmet therapeutic needs; thus, the possibility of exploring new approaches to cure it, i.e., the pharmacological modulation of group I mGluRs to counteract the aberrant glial reactivity, is a very appealing strategy.
As depicted in the present review, the further collection of pre-clinical and clinical evidence will be essential to optimize group I mGluRs as multipotential targets to modulate the complex balance of microglia phenotypes in CNS disorders.
Author Contributions
Conceptualization, M.B., M.M. and T.B.; writing—original draft preparation, M.B. and T.B.; writing—review and editing, G.B., M.M. and T.B.; supervision, M.M. and G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are very grateful to the Foundation Bellandi Bernardoni, Genoa, Italy, for the support. The authors also acknowledge the undergraduate student Francesca Caratti of the Department of Pharmacy, University of Genoa, for her very helpful contribution in selecting and organizing the literature dataset for the preparation of the review draft.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alliot, F.; Godin, I.; Pessac, B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Dev. Brain Res. 1999, 117, 145–152. [Google Scholar] [CrossRef]
- Rezaie, P.; Patel, K.; Male, D.K. Microglia in the human fetal spinal cord--patterns of distribution, morphology and phenotype. Dev. Brain Res. 1999, 115, 71–81. [Google Scholar] [CrossRef]
- Chan, W.Y.; Kohsaka, S.; Rezaie, P. The origin and cell lineage of microglia: New concepts. Brain Res. Rev. 2007, 53, 344–354. [Google Scholar] [CrossRef]
- Schulz, C.; Gomez Perdiguero, E.; Chorro, L.; Szabo-Rogers, H.; Cagnard, N.; Kierdorf, K.; Prinz, M.; Wu, B.; Jacobsen, S.E.W.; Pollard, J.W.; et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012, 336, 86–90. [Google Scholar] [CrossRef]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Hölscher, C.; et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef]
- Monier, A.; Adle-Biassette, H.; Delezoide, A.-L.; Evrard, P.; Gressens, P.; Verney, C. Entry and distribution of microglial cells in human embryonic and fetal cerebral cortex. J. Neuropathol. Exp. Neurol. 2007, 66, 372–382. [Google Scholar] [CrossRef]
- Verney, C.; Monier, A.; Fallet-Bianco, C.; Gressens, P. Early microglial colonization of the human forebrain and possible involvement in periventricular white-matter injury of preterm infants. J. Anat. 2010, 217, 436–448. [Google Scholar] [CrossRef]
- Kurz, H.; Christ, B. Embryonic CNS macrophages and microglia do not stem from circulating, but from extravascular precursors. Glia 1998, 22, 98–102. [Google Scholar] [CrossRef]
- Navascués, J.; Calvente, R.; Marín-Teva, J.L.; Cuadros, M.A. Entry, dispersion and differentiation of microglia in the developing central nervous system. An. Acad. Bras. Cienc. 2000, 72, 91–102. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- Swinnen, N.; Smolders, S.; Avila, A.; Notelaers, K.; Paesen, R.; Ameloot, M.; Brône, B.; Legendre, P.; Rigo, J.-M. Complex invasion pattern of the cerebral cortex bymicroglial cells during development of the mouse embryo. Glia 2013, 61, 150–163. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia development and function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef]
- Kim, I.; Mlsna, L.M.; Yoon, S.; Le, B.; Yu, S.; Xu, D.; Koh, S. A postnatal peak in microglial development in the mouse hippocampus is correlated with heightened sensitivity to seizure triggers. Brain Behav. 2015, 5, e00403. [Google Scholar] [CrossRef]
- Nikodemova, M.; Kimyon, R.S.; De, I.; Small, A.L.; Collier, L.S.; Watters, J.J. Microglial numbers attain adult levels after undergoing a rapid decrease in cell number in the third postnatal week. J. Neuroimmunol. 2015, 278, 280–288. [Google Scholar] [CrossRef]
- Eyo, U.B.; Miner, S.A.; Weiner, J.A.; Dailey, M.E. Developmental changes in microglial mobilization are independent of apoptosis in the neonatal mouse hippocampus. Brain. Behav. Immun. 2016, 55, 49–59. [Google Scholar] [CrossRef]
- Wakselman, S.; Béchade, C.; Roumier, A.; Bernard, D.; Triller, A.; Bessis, A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J. Neurosci. 2008, 28, 8138–8143. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Cunningham, C.L.; Martínez-Cerdeño, V.; Noctor, S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef]
- Parkhurst, C.N.; Yang, G.; Ninan, I.; Savas, J.N.; Yates, J.R., 3rd; Lafaille, J.J.; Hempstead, B.L.; Littman, D.R.; Gan, W.-B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013, 155, 1596–1609. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Fujita, Y.; Tanaka, T.; Nakamura, Y.; Kikuta, J.; Ishii, M.; Yamashita, T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 2013, 16, 543–551. [Google Scholar] [CrossRef]
- Pont-Lezica, L.; Beumer, W.; Colasse, S.; Drexhage, H.; Versnel, M.; Bessis, A. Microglia shape corpus callosum axon tract fasciculation: Functional impact of prenatal inflammation. Eur. J. Neurosci. 2014, 39, 1551–1557. [Google Scholar] [CrossRef]
- Squarzoni, P.; Oller, G.; Hoeffel, G.; Pont-Lezica, L.; Rostaing, P.; Low, D.; Bessis, A.; Ginhoux, F.; Garel, S. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014, 8, 1271–1279. [Google Scholar] [CrossRef]
- Hattori, Y. The behavior and functions of embryonic microglia. Anat. Sci. Int. 2022, 97, 1–14. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Matcovitch-Natan, O.; Winter, D.R.; Giladi, A.; Vargas Aguilar, S.; Spinrad, A.; Sarrazin, S.; Ben-Yehuda, H.; David, E.; Zelada González, F.; Perrin, P.; et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016, 353, aad8670. [Google Scholar] [CrossRef]
- Yoo, H.-J.; Kwon, M.-S. Aged Microglia in Neurodegenerative Diseases: Microglia Lifespan and Culture Methods. Front. Aging Neurosci. 2021, 13, 766267. [Google Scholar] [CrossRef]
- Neumann, H.; Kotter, M.R.; Franklin, R.J.M. Debris clearance by microglia: An essential link between degeneration and regeneration. Brain 2009, 132, 288–295. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Bessis, A.; Béchade, C.; Bernard, D.; Roumier, A. Microglial control of neuronal death and synaptic properties. Glia 2007, 55, 233–238. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Perry, V.H. Microglial physiology: Unique stimuli, specialized responses. Annu. Rev. Immunol. 2009, 27, 119–145. [Google Scholar] [CrossRef]
- Li, Y.; Du, X.-F.; Liu, C.-S.; Wen, Z.-L.; Du, J.-L. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev. Cell 2012, 23, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Eyo, U.B.; Dailey, M.E. Microglia: Key elements in neural development, plasticity, and pathology. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2013, 8, 494–509. [Google Scholar] [CrossRef] [PubMed]
- Eyo, U.B.; Wu, L.-J. Bidirectional microglia-neuron communication in the healthy brain. Neural Plast. 2013, 2013, 456857. [Google Scholar] [CrossRef] [PubMed]
- Baxter, P.S.; Dando, O.; Emelianova, K.; He, X.; McKay, S.; Hardingham, G.E.; Qiu, J. Microglial identity and inflammatory responses are controlled by the combined effects of neurons and astrocytes. Cell Rep. 2021, 34, 108882. [Google Scholar] [CrossRef] [PubMed]
- Bennett, F.C.; Bennett, M.L.; Yaqoob, F.; Mulinyawe, S.B.; Grant, G.A.; Hayden Gephart, M.; Plowey, E.D.; Barres, B.A. A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron 2018, 98, 1170–1183.e8. [Google Scholar] [CrossRef]
- Elmore, M.R.P.; Najafi, A.R.; Koike, M.A.; Dagher, N.N.; Spangenberg, E.E.; Rice, R.A.; Kitazawa, M.; Matusow, B.; Nguyen, H.; West, B.L.; et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 2014, 82, 380–397. [Google Scholar] [CrossRef]
- Gosselin, D.; Link, V.M.; Romanoski, C.E.; Fonseca, G.J.; Eichenfield, D.Z.; Spann, N.J.; Stender, J.D.; Chun, H.B.; Garner, H.; Geissmann, F.; et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 2014, 159, 1327–1340. [Google Scholar] [CrossRef]
- Gosselin, D.; Skola, D.; Coufal, N.G.; Holtman, I.R.; Schlachetzki, J.C.M.; Sajti, E.; Jaeger, B.N.; O’Connor, C.; Fitzpatrick, C.; Pasillas, M.P.; et al. An environment-dependent transcriptional network specifies human microglia identity. Science 2017, 356, aal3222. [Google Scholar] [CrossRef]
- Füger, P.; Hefendehl, J.K.; Veeraraghavalu, K.; Wendeln, A.-C.; Schlosser, C.; Obermüller, U.; Wegenast-Braun, B.M.; Neher, J.J.; Martus, P.; Kohsaka, S.; et al. Microglia turnover with aging and in an Alzheimer’s model via long-term in vivo single-cell imaging. Nat. Neurosci. 2017, 20, 1371–1376. [Google Scholar] [CrossRef]
- Jordão, M.J.C.; Sankowski, R.; Brendecke, S.M.; Sagar; Locatelli, G.; Tai, Y.-H.; Tay, T.L.; Schramm, E.; Armbruster, S.; Hagemeyer, N.; et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 2019, 363, aat7554. [Google Scholar] [CrossRef]
- Tay, T.L.; Mai, D.; Dautzenberg, J.; Fernández-Klett, F.; Lin, G.; Sagar; Datta, M.; Drougard, A.; Stempfl, T.; Ardura-Fabregat, A.; et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci. 2017, 20, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef]
- Block, M.L.; Hong, J.-S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamashita, T. Neuroprotective function of microglia in the developing brain. Neuronal Signal. 2021, 5, NS20200024. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Pocock, J.M.; Kettenmann, H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007, 30, 527–535. [Google Scholar] [CrossRef]
- Timmerman, R.; Burm, S.M.; Bajramovic, J.J. An Overview of in vitro Methods to Study Microglia. Front. Cell. Neurosci. 2018, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.A. The multifaceted profile of activated microglia. Mol. Neurobiol. 2009, 40, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Mosser, C.-A.; Baptista, S.; Arnoux, I.; Audinat, E. Microglia in CNS development: Shaping the brain for the future. Prog. Neurobiol. 2017, 149–150, 1–20. [Google Scholar] [CrossRef]
- Colton, C.A. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2009, 4, 399–418. [Google Scholar] [CrossRef]
- Colton, C.A.; Wilcock, D.M. Assessing activation states in microglia. CNS Neurol. Disord. Drug Targets 2010, 9, 174–191. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Du, L.; Zhang, Y.; Chen, Y.; Zhu, J.; Yang, Y.; Zhang, H.-L. Role of Microglia in Neurological Disorders and Their Potentials as a Therapeutic Target. Mol. Neurobiol. 2017, 54, 7567–7584. [Google Scholar] [CrossRef]
- Kigerl, K.A.; de Rivero Vaccari, J.P.; Dietrich, W.D.; Popovich, P.G.; Keane, R.W. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 2014, 258, 5–16. [Google Scholar] [CrossRef]
- Bagasra, O.; Michaels, F.H.; Zheng, Y.M.; Bobroski, L.E.; Spitsin, S.V.; Fu, Z.F.; Tawadros, R.; Koprowski, H. Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc. Natl. Acad. Sci. USA 1995, 92, 12041–12045. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization—New prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Castellano, B.; Bosch-Queralt, M.; Almolda, B.; Villacampa, N.; González, B. Purine Signaling and Microglial Wrapping. Adv. Exp. Med. Biol. 2016, 949, 147–165. [Google Scholar] [CrossRef]
- Calovi, S.; Mut-Arbona, P.; Sperlágh, B. Microglia and the Purinergic Signaling System. Neuroscience 2019, 405, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Lillo, A.; Rivas-Santisteban, R.; Reyes-Resina, I.; Navarro, G. Microglial Adenosine Receptors: From Preconditioning to Modulating the M1/M2 Balance in Activated Cells. Cells 2021, 10, 1124. [Google Scholar] [CrossRef]
- Krabbe, G.; Matyash, V.; Pannasch, U.; Mamer, L.; Boddeke, H.W.G.M.; Kettenmann, H. Activation of serotonin receptors promotes microglial injury-induced motility but attenuates phagocytic activity. Brain. Behav. Immun. 2012, 26, 419–428. [Google Scholar] [CrossRef]
- Iida, T.; Yanai, K.; Yoshikawa, T. Histamine and Microglia. Curr. Top. Behav. Neurosci. 2022, 59, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Volonté, C.; Apolloni, S.; Parisi, C.; Amadio, S. Purinergic contribution to amyotrophic lateral sclerosis. Neuropharmacology 2016, 104, 180–193. [Google Scholar] [CrossRef]
- Woods, L.T.; Ajit, D.; Camden, J.M.; Erb, L.; Weisman, G.A. Purinergic receptors as potential therapeutic targets in Alzheimer’s disease. Neuropharmacology 2016, 104, 169–179. [Google Scholar] [CrossRef]
- El Oussini, H.; Bayer, H.; Scekic-Zahirovic, J.; Vercruysse, P.; Sinniger, J.; Dirrig-Grosch, S.; Dieterlé, S.; Echaniz-Laguna, A.; Larmet, Y.; Müller, K.; et al. Serotonin 2B receptor slows disease progression and prevents degeneration of spinal cord mononuclear phagocytes in amyotrophic lateral sclerosis. Acta Neuropathol. 2016, 131, 465–480. [Google Scholar] [CrossRef]
- Barata-Antunes, S.; Cristóvão, A.C.; Pires, J.; Rocha, S.M.; Bernardino, L. Dual role of histamine on microglia-induced neurodegeneration. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 764–769. [Google Scholar] [CrossRef]
- Mecha, M.; Carrillo-Salinas, F.J.; Feliú, A.; Mestre, L.; Guaza, C. Microglia activation states and cannabinoid system: Therapeutic implications. Pharmacol. Ther. 2016, 166, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Komorowska-Müller, J.A.; Schmöle, A.-C. CB2 Receptor in Microglia: The Guardian of Self-Control. Int. J. Mol. Sci. 2020, 22, 19. [Google Scholar] [CrossRef]
- Duffy, S.S.; Hayes, J.P.; Fiore, N.T.; Moalem-Taylor, G. The cannabinoid system and microglia in health and disease. Neuropharmacology 2021, 190, 108555. [Google Scholar] [CrossRef]
- Liu, H.; Leak, R.K.; Hu, X. Neurotransmitter receptors on microglia. Stroke Vasc. Neurol. 2016, 1, 52–58. [Google Scholar] [CrossRef]
- Volonté, C.; Amadio, S.; Fabbrizio, P.; Apolloni, S. Functional microglia neurotransmitters in amyotrophic lateral sclerosis. Semin. Cell Dev. Biol. 2019, 94, 121–128. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Zhang, B.; Zhu, J.; Zhou, Z.; Cui, L. Regulation of microglia by glutamate and its signal pathway in neurodegenerative diseases. Drug Discov. Today 2020, 25, 1074–1085. [Google Scholar] [CrossRef]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999, 51, 7–61. [Google Scholar]
- Willard, S.S.; Koochekpour, S. Glutamate, Glutamate Receptors, and Downstream Signaling Pathways. Int. J. Biol. Sci. 2013, 9, 948. [Google Scholar] [CrossRef]
- Bragina, L.; Bonifacino, T.; Bassi, S.; Milanese, M.; Bonanno, G.; Conti, F. Differential expression of metabotropic glutamate and GABA receptors at neocortical glutamatergic and GABAergic axon terminals. Front. Cell. Neurosci. 2015, 9, 345. [Google Scholar] [CrossRef]
- Giribaldi, F.; Milanese, M.; Bonifacino, T.; Anna Rossi, P.I.; Di Prisco, S.; Pittaluga, A.; Tacchetti, C.; Puliti, A.; Usai, C.; Bonanno, G. Group I metabotropic glutamate autoreceptors induce abnormal glutamate exocytosis in a mouse model of amyotrophic lateral sclerosis. Neuropharmacology 2013, 66, 253–263. [Google Scholar] [CrossRef]
- Bonifacino, T.; Rebosio, C.; Provenzano, F.; Torazza, C.; Balbi, M.; Milanese, M.; Raiteri, L.; Usai, C.; Fedele, E.; Bonanno, G. Enhanced function and overexpression of metabotropic glutamate receptors 1 and 5 in the spinal Cord of the SOD1G93A mouse model of amyotrophic lateral sclerosis during disease progression. Int. J. Mol. Sci. 2019, 20, 4552. [Google Scholar] [CrossRef]
- Olivero, G.; Vergassola, M.; Cisani, F.; Roggeri, A.; Pittaluga, A. Presynaptic Release-regulating Metabotropic Glutamate Receptors: An Update. Curr. Neuropharmacol. 2020, 18, 655–672. [Google Scholar] [CrossRef]
- Conn, P.J.; Pin, J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 205–237. [Google Scholar] [CrossRef]
- D’Antoni, S.; Berretta, A.; Bonaccorso, C.M.; Bruno, V.; Aronica, E.; Nicoletti, F.; Catania, M.V. Metabotropic glutamate receptors in glial cells. Neurochem. Res. 2008, 33, 2436–2443. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Copani, A.; Nicoletti, F.; Sortino, M.A.; Caraci, F. Metabotropic Glutamate Receptors in Glial Cells: A New Potential Target for Neuroprotection? Front. Mol. Neurosci. 2018, 11, 414. [Google Scholar] [CrossRef]
- Kaindl, A.M.; Degos, V.; Peineau, S.; Gouadon, E.; Chhor, V.; Loron, G.; Le Charpentier, T.; Josserand, J.; Ali, C.; Vivien, D.; et al. Activation of microglial N-methyl-D-aspartate receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann. Neurol. 2012, 72, 536–549. [Google Scholar] [CrossRef]
- Raghunatha, P.; Vosoughi, A.; Kauppinen, T.M.; Jackson, M.F. Microglial NMDA receptors drive pro-inflammatory responses via PARP-1/TRMP2 signaling. Glia 2020, 68, 1421–1434. [Google Scholar] [CrossRef]
- Zhao, W.; Xie, W.; Le, W.; Beers, D.R.; He, Y.; Henkel, J.S.; Simpson, E.P.; Yen, A.A.; Xiao, Q.; Appel, S.H. Activated microglia initiate motor neuron injury by a nitric oxide and glutamate-mediated mechanism. J. Neuropathol. Exp. Neurol. 2004, 63, 964–977. [Google Scholar] [CrossRef]
- Ceprian, M.; Fulton, D. Glial Cell AMPA Receptors in Nervous System Health, Injury and Disease. Int. J. Mol. Sci. 2019, 20, 2450. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Zhu, J. Kainic Acid-induced neurotoxicity: Targeting glial responses and glia-derived cytokines. Curr. Neuropharmacol. 2011, 9, 388–398. [Google Scholar] [CrossRef]
- Fazio, F.; Ulivieri, M.; Volpi, C.; Gargaro, M.; Fallarino, F. Targeting metabotropic glutamate receptors for the treatment of neuroinflammation. Curr. Opin. Pharmacol. 2018, 38, 16–23. [Google Scholar] [CrossRef]
- Byrnes, K.R.; Loane, D.J.; Faden, A.I. Metabotropic glutamate receptors as targets for multipotential treatment of neurological disorders. Neurother. J. Am. Soc. Exp. Neurother. 2009, 6, 94–107. [Google Scholar] [CrossRef]
- Conn, P.J. Physiological roles and therapeutic potential of metabotropic glutamate receptors. Ann. N. Y. Acad. Sci. 2003, 1003, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, G.; Bruno, V. Metabotropic glutamate receptor involvement in the pathophysiology of amyotrophic lateral sclerosis: New potential drug targets for therapeutic applications. Curr. Opin. Pharmacol. 2018, 38, 65–71. [Google Scholar] [CrossRef]
- Nicoletti, F.; Bockaert, J.; Collingridge, G.L.; Conn, P.J.; Ferraguti, F.; Schoepp, D.D.; Wroblewski, J.T.; Pin, J.P. Metabotropic glutamate receptors: From the workbench to the bedside. Neuropharmacology 2011, 60, 1017–1041. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.M.; Lindemann, L.; Jønch, A.E.; Apostol, G.; Bear, M.F.; Carpenter, R.L.; Crawley, J.N.; Curie, A.; Des Portes, V.; Hossain, F.; et al. Drug development for neurodevelopmental disorders: Lessons learned from fragile X syndrome. Nat. Rev. Drug Discov. 2018, 17, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-D.; Wang, N.; Han, J.; Shen, Y. Group 1 Metabotropic Glutamate Receptors in Neurological and Psychiatric Diseases: Mechanisms and Prospective. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2022, 28, 453–468. [Google Scholar] [CrossRef]
- Biber, K.; Laurie, D.J.; Berthele, A.; Sommer, B.; Tölle, T.R.; Gebicke-Härter, P.J.; Van Calker, D.; Boddeke, H.W.G.M. Expression and signaling of group I metabotropic glutamate receptors in astrocytes and microglia. J. Neurochem. 1999, 72, 1671–1680. [Google Scholar] [CrossRef]
- Loane, D.J.; Stoica, B.A.; Pajoohesh-Ganji, A.; Byrnes, K.R.; Faden, A.I. Activation of metabotropic glutamate receptor 5 modulates microglial reactivity and neurotoxicity by inhibiting NADPH oxidase. J. Biol. Chem. 2009, 284, 15629–15639. [Google Scholar] [CrossRef] [PubMed]
- Mudo, G.; Trovato-Salinaro, A.; Caniglia, G.; Cheng, Q.; Condorelli, D.F. Cellular localization of mGluR3 and mGluR5 mRNAs in normal and injured rat brain. Brain Res. 2007, 1149, 1–13. [Google Scholar] [CrossRef]
- Drouin-Ouellet, J.; Brownell, A.-L.L.; Saint-Pierre, M.; Fasano, C.; Emond, V.; Trudeau, L.-E.E.; Lévesque, D.; Cicchetti, F. Neuroinflammation is associated with changes in glial mGluR5 expression and the development of neonatal excitotoxic lesions. Glia 2011, 59, 188–199. [Google Scholar] [CrossRef]
- Whittemore, E.R.; Korotzer, A.R.; Etebari, A.; Cotman, C.W. Carbachol increases intracellular free calcium in cultured rat microglia. Brain Res. 1993, 621, 59–64. [Google Scholar] [CrossRef]
- Karim, F.; Wang, C.C.; Gereau, R.W. 4th Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J. Neurosci. 2001, 21, 3771–3779. [Google Scholar] [CrossRef]
- Eder, C. Ion channels in microglia (brain macrophages). Am. J. Physiol. 1998, 275, C327–C342. [Google Scholar] [CrossRef]
- Warwick, H.K.; Nahorski, S.R.; Challiss, R.A.J. Group I metabotropic glutamate receptors, mGlu1a and mGlu5a, couple to cyclic AMP response element binding protein (CREB) through a common Ca2+- and protein kinase C-dependent pathway. J. Neurochem. 2005, 93, 232–245. [Google Scholar] [CrossRef]
- Farso, M.C.; O’Shea, R.D.; Beart, P.M. Evidence group I mGluR drugs modulate the activation profile of lipopolysaccharide-exposed microglia in culture. Neurochem. Res. 2009, 34, 1721–1728. [Google Scholar] [CrossRef]
- Byrnes, K.R.; Stoica, B.; Loane, D.J.; Riccio, A.; Davis, M.I.; Faden, A.I. Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity. Glia 2009, 57, 550–560. [Google Scholar] [CrossRef]
- Loane, D.J.; Stoica, B.A.; Byrnes, K.R.; Jeong, W.; Faden, A.I. Activation of mGluR5 and inhibition of NADPH oxidase improves functional recovery after traumatic brain injury. J. Neurotrauma 2013, 30, 403–412. [Google Scholar] [CrossRef]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurother. J. Am. Soc. Exp. Neurother. 2010, 7, 354–365. [Google Scholar] [CrossRef]
- Ye, X.; Yu, L.; Zuo, D.; Zhang, L.; Zu, J.; Hu, J.; Tang, J.; Bao, L.; Cui, C.; Zhang, R.; et al. Activated mGluR5 protects BV2 cells against OGD/R induced cytotoxicity by modulating BDNF-TrkB pathway. Neurosci. Lett. 2017, 654, 70–79. [Google Scholar] [CrossRef]
- Bhat, S.A.; Henry, R.J.; Blanchard, A.C.; Stoica, B.A.; Loane, D.J.; Faden, A.I. Enhanced Akt/GSK-3β/CREB signaling mediates the anti-inflammatory actions of mGluR5 positive allosteric modulators in microglia and following traumatic brain injury in male mice. J. Neurochem. 2021, 156, 225–248. [Google Scholar] [CrossRef]
- Hermans, E.; Challiss, R.A. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: Prototypic family C G-protein-coupled receptors. Biochem. J. 2001, 359, 465–484. [Google Scholar] [CrossRef]
- Pacheco, R.; Ciruela, F.; Casadó, V.; Mallol, J.; Gallart, T.; Lluis, C.; Franco, R. Group I metabotropic glutamate receptors mediate a dual role of glutamate in T cell activation. J. Biol. Chem. 2004, 279, 33352–33358. [Google Scholar] [CrossRef]
- Geurts, J.J.G.; Wolswijk, G.; Bö, L.; van der Valk, P.; Polman, C.H.; Troost, D.; Aronica, E. Altered expression patterns of group I and II metabotropic glutamate receptors in multiple sclerosis. Brain 2003, 126, 1755–1766. [Google Scholar] [CrossRef]
- Byrnes, K.R.; Loane, D.J.; Stoica, B.A.; Zhang, J.; Faden, A.I. Delayed mGluR5 activation limits neuroinflammation and neurodegeneration after traumatic brain injury. J. Neuroinflamm. 2012, 9, 43. [Google Scholar] [CrossRef]
- Piers, T.M.; Heales, S.J.; Pocock, J.M. Positive allosteric modulation of metabotropic glutamate receptor 5 down-regulates fibrinogen-activated microglia providing neuronal protection. Neurosci. Lett. 2011, 505, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Stoica, B.A.; Hanscom, M.; Kabadi, S.V.; Faden, A.I. Positive allosteric modulators (PAMs) of metabotropic glutamate receptor 5 (mGluR5) attenuate microglial activation. CNS Neurol. Disord. Drug Targets 2014, 13, 558–566. [Google Scholar] [CrossRef]
- Loane, D.J.; Stoica, B.A.; Tchantchou, F.; Kumar, A.; Barrett, J.P.; Akintola, T.; Xue, F.; Conn, P.J.; Faden, A.I. Novel mGluR5 positive allosteric modulator improves functional recovery, attenuates neurodegeneration, and alters microglial polarization after experimental traumatic brain injury. Neurother. J. Am. Soc. Exp. Neurother. 2014, 11, 857–869. [Google Scholar] [CrossRef]
- Hanak, T.J.; Libbey, J.E.; Doty, D.J.; Sim, J.T.; DePaula-Silva, A.B.; Fujinami, R.S. Positive modulation of mGluR5 attenuates seizures and reduces TNF-α(+) macrophages and microglia in the brain in a murine model of virus-induced temporal lobe epilepsy. Exp. Neurol. 2019, 311, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.G.; Alves-Silva, J.; de Souza, J.M.; Real, A.L.C.V.; Doria, J.G.; Vieira, E.L.M.; Gomes, G.F.; de Oliveira, A.C.; Miranda, A.S.; Ribeiro, F.M. Metabotropic glutamate receptor 5 ablation accelerates age-related neurodegeneration and neuroinflammation. Neurochem. Int. 2019, 126, 218–228. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Fan, J.-K.; Gu, L.; Yang, H.-M.; Zhan, S.-Q.; Zhang, H. Metabotropic glutamate receptor 5 inhibits α-synuclein-induced microglia inflammation to protect from neurotoxicity in Parkinson’s disease. J. Neuroinflamm. 2021, 18, 23. [Google Scholar] [CrossRef]
- Oh, S.J.; Ahn, H.; Jung, K.-H.; Han, S.J.; Nam, K.R.; Kang, K.J.; Park, J.-A.; Lee, K.C.; Lee, Y.J.; Choi, J.Y. Evaluation of the Neuroprotective Effect of Microglial Depletion by CSF-1R Inhibition in a Parkinson’s Animal Model. Mol. Imaging Biol. 2020, 22, 1031–1042. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, R.; Yan, H.; Yin, H.; Wu, X.; Tan, Y.; Li, L. Metabotropic glutamate receptor 5 modulates calcium oscillation and innate immune response induced by lipopolysaccharide in microglial cell. Neuroscience 2014, 281, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.V.; Dumont, A.O.; Focant, M.C.; Vergouts, M.; Sternotte, A.; Calas, A.-G.G.; Goursaud, S.; Hermans, E. Opposite regulation of metabotropic glutamate receptor 3 and metabotropic glutamate receptor 5 by inflammatory stimuli in cultured microglia and astrocytes. Neuroscience 2012, 205, 29–38. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R.; Sheridan, G.K. Extracellular vesicles and intercellular communication in the central nervous system. FEBS Lett. 2021, 595, 1391–1410. [Google Scholar] [CrossRef]
- Pistono, C.; Bister, N.; Stanová, I.; Malm, T. Glia-Derived Extracellular Vesicles: Role in Central Nervous System Communication in Health and Disease. Front. Cell Dev. Biol. 2020, 8, 623771. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Bergamini, G.; Rajendran, L. Cell-to-cell Communication by Extracellular Vesicles: Focus on Microglia. Neuroscience 2019, 405, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, L.; Giacomelli, C.; Marchetti, L.; Martini, C. Microglia extracellular vesicles: Focus on molecular composition and biological function. Biochem. Soc. Trans. 2021, 49, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Bastoni, M.; Nigro, A.; Podini, P.; Finardi, A.; Casella, G.; Ramesh, M.; Farina, C.; Verderio, C.; Furlan, R. Cytokines Stimulate the Release of Microvesicles from Myeloid Cells Independently from the P2X7 Receptor/Acid Sphingomyelinase Pathway. Front. Immunol. 2018, 9, 204. [Google Scholar] [CrossRef]
- Peña-Altamira, L.E.; Polazzi, E.; Giuliani, P.; Beraudi, A.; Massenzio, F.; Mengoni, I.; Poli, A.; Zuccarini, M.; Ciccarelli, R.; Di Iorio, P.; et al. Release of soluble and vesicular purine nucleoside phosphorylase from rat astrocytes and microglia induced by pro-inflammatory stimulation with extracellular ATP via P2X(7) receptors. Neurochem. Int. 2018, 115, 37–49. [Google Scholar] [CrossRef]
- Glebov, K.; Löchner, M.; Jabs, R.; Lau, T.; Merkel, O.; Schloss, P.; Steinhäuser, C.; Walter, J. Serotonin stimulates secretion of exosomes from microglia cells. Glia 2015, 63, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Pravettoni, E.; Colombo, A.; Schenk, U.; Möller, T.; Matteoli, M.; Verderio, C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J. Immunol. 2005, 174, 7268–7277. [Google Scholar] [CrossRef]
- Tang, L.; Wu, Y.; Fang, C.; Qu, P.; Gao, Z. NDRG2 promoted secreted miR-375 in microvesicles shed from M1 microglia, which induced neuron damage. Biochem. Biophys. Res. Commun. 2016, 469, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Coleman, L.G.J.; Zou, J.; Crews, F.T. Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J. Neuroinflamm. 2017, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Turola, E.; Furlan, R.; Bianco, F.; Matteoli, M.; Verderio, C. Microglial microvesicle secretion and intercellular signaling. Front. Physiol. 2012, 3, 149. [Google Scholar] [CrossRef] [PubMed]
- Prada, I.; Furlan, R.; Matteoli, M.; Verderio, C. Classical and unconventional pathways of vesicular release in microglia. Glia 2013, 61, 1003–1017. [Google Scholar] [CrossRef]
- Gabrielli, M.; Raffaele, S.; Fumagalli, M.; Verderio, C. The multiple faces of extracellular vesicles released by microglia: Where are we 10 years after? Front. Cell. Neurosci. 2022, 16, 984690. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, S.; Lombardi, M.; Verderio, C.; Fumagalli, M. TNF Production and Release from Microglia via Extracellular Vesicles: Impact on Brain Functions. Cells 2020, 9, 2145. [Google Scholar] [CrossRef]
- Joshi, P.; Turola, E.; Ruiz, A.; Bergami, A.; Libera, D.D.; Benussi, L.; Giussani, P.; Magnani, G.; Comi, G.; Legname, G.; et al. Microglia convert aggregated amyloid-β into neurotoxic forms through the shedding of microvesicles. Cell Death Differ. 2014, 21, 582–593. [Google Scholar] [CrossRef]
- Verderio, C.; Muzio, L.; Turola, E.; Bergami, A.; Novellino, L.; Ruffini, F.; Riganti, L.; Corradini, I.; Francolini, M.; Garzetti, L.; et al. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann. Neurol. 2012, 72, 610–624. [Google Scholar] [CrossRef]
- Aires, I.D.; Ribeiro-Rodrigues, T.; Boia, R.; Ferreira-Rodrigues, M.; Girão, H.; Ambrósio, A.F.; Santiago, A.R. Microglial Extracellular Vesicles as Vehicles for Neurodegeneration Spreading. Biomolecules 2021, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tan, X.; Li, S.; Al-Nusaif, M.; Le, W. Role of Glia-Derived Extracellular Vesicles in Neurodegenerative Diseases. Front. Aging Neurosci. 2021, 13, 765395. [Google Scholar] [CrossRef]
- Croese, T.; Furlan, R. Extracellular vesicles in neurodegenerative diseases. Mol. Aspects Med. 2018, 60, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Prada, I.; Joshi, P.; Falcicchia, C.; D’Arrigo, G.; Rutigliano, G.; Battocchio, E.; Zenatelli, R.; Tozzi, F.; Radeghieri, A.; et al. Microglial large extracellular vesicles propagate early synaptic dysfunction in Alzheimer’s disease. Brain 2022, 145, 2849–2868. [Google Scholar] [CrossRef]
- Beneventano, M.; Spampinato, S.F.; Merlo, S.; Chisari, M.; Platania, P.; Ragusa, M.; Purrello, M.; Nicoletti, F.; Sortino, M.A. Shedding of Microvesicles from Microglia Contributes to the Effects Induced by Metabotropic Glutamate Receptor 5 Activation on Neuronal Death. Front. Pharmacol. 2017, 8, 812. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Cui, J.G.; Dua, P.; Pogue, A.I.; Bhattacharjee, S.; Lukiw, W.J. Differential expression of miRNA-146a-regulated inflammatory genes in human primary neural, astroglial and microglial cells. Neurosci. Lett. 2011, 499, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J.; Zhao, Y.; Cui, J.G. An NF-kappaB-sensitive micro RNA-146a-mediated inflammatory circuit in Alzheimer disease and in stressed human brain cells. J. Biol. Chem. 2008, 283, 31315–31322. [Google Scholar] [CrossRef] [PubMed]
- Corps, K.N.; Roth, T.L.; McGavern, D.B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015, 72, 355–362. [Google Scholar] [CrossRef]
- Ghajar, J. Traumatic brain injury. Lancet 2000, 356, 923–929. [Google Scholar] [CrossRef]
- Bramlett, H.M.; Dietrich, W.D. Progressive damage after brain and spinal cord injury: Pathomechanisms and treatment strategies. Prog. Brain Res. 2007, 161, 125–141. [Google Scholar] [CrossRef]
- Yang, T.; Liu, Y.-W.; Zhao, L.; Wang, H.; Yang, N.; Dai, S.-S.; He, F. Metabotropic glutamate receptor 5 deficiency inhibits neutrophil infiltration after traumatic brain injury in mice. Sci. Rep. 2017, 7, 9998. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, L.; Qu, Y.; Huo, K.; Jiang, X.; Fei, Z. The selective mGluR5 agonist CHPG protects against traumatic brain injury in vitro and in vivo via ERK and Akt pathway. Int. J. Mol. Med. 2012, 29, 630–636. [Google Scholar] [CrossRef]
- Ban, J.; Sámano, C.; Mladinic, M.; Munitic, I. Glia in amyotrophic lateral sclerosis and spinal cord injury: Common therapeutic targets. Croat. Med. J. 2019, 60, 109–120. [Google Scholar] [CrossRef]
- Abdanipour, A.; Tiraihi, T.; Taheri, T.; Kazemi, H. Microglial activation in rat experimental spinal cord injury model. Iran. Biomed. J. 2013, 17, 214–220. [Google Scholar] [CrossRef]
- Mills, C.D.; Fullwood, S.D.; Hulsebosch, C.E. Changes in metabotropic glutamate receptor expression following spinal cord injury. Exp. Neurol. 2001, 170, 244–257. [Google Scholar] [CrossRef]
- Mills, C.D.; Johnson, K.M.; Hulsebosch, C.E. Group I metabotropic glutamate receptors in spinal cord injury: Roles in neuroprotection and the development of chronic central pain. J. Neurotrauma 2002, 19, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, K.R.; Stoica, B.; Riccio, A.; Pajoohesh-Ganji, A.; Loane, D.J.; Faden, A.I. Activation of metabotropic glutamate receptor 5 improves recovery after spinal cord injury in rodents. Ann. Neurol. 2009, 66, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Rolls, A.; Shechter, R.; London, A.; Segev, Y.; Jacob-Hirsch, J.; Amariglio, N.; Rechavi, G.; Schwartz, M. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: A role in microglia/macrophage activation. PLoS Med. 2008, 5, e171. [Google Scholar] [CrossRef] [PubMed]
- Hallmayer, J.; Cleveland, S.; Torres, A.; Phillips, J.; Cohen, B.; Torigoe, T.; Miller, J.; Fedele, A.; Collins, J.; Smith, K.; et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry 2011, 68, 1095–1102. [Google Scholar] [CrossRef]
- Zantomio, D.; Chana, G.; Laskaris, L.; Testa, R.; Everall, I.; Pantelis, C.; Skafidas, E. Convergent evidence for mGluR5 in synaptic and neuroinflammatory pathways implicated in ASD. Neurosci. Biobehav. Rev. 2015, 52, 172–177. [Google Scholar] [CrossRef]
- Gipson, T.T.; Johnston, M.V. Plasticity and mTOR: Towards restoration of impaired synaptic plasticity in mTOR-related neurogenetic disorders. Neural Plast. 2012, 2012, 486402. [Google Scholar] [CrossRef]
- Zhong, X.; Li, H.; Chang, Q. MeCP2 phosphorylation is required for modulating synaptic scaling through mGluR5. J. Neurosci. 2012, 32, 12841–12847. [Google Scholar] [CrossRef]
- Blaylock, R.L.; Strunecka, A. Immune-glutamatergic dysfunction as a central mechanism of the autism spectrum disorders. Curr. Med. Chem. 2009, 16, 157–170. [Google Scholar] [CrossRef]
- Tetreault, N.A.; Hakeem, A.Y.; Jiang, S.; Williams, B.A.; Allman, E.; Wold, B.J.; Allman, J.M. Microglia in the cerebral cortex in autism. J. Autism Dev. Disord. 2012, 42, 2569–2584. [Google Scholar] [CrossRef]
- Morgan, J.T.; Chana, G.; Pardo, C.A.; Achim, C.; Semendeferi, K.; Buckwalter, J.; Courchesne, E.; Everall, I.P. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol. Psychiatry 2010, 68, 368–376. [Google Scholar] [CrossRef]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef]
- Toscano, C.V.A.; Barros, L.; Lima, A.B.; Nunes, T.; Carvalho, H.M.; Gaspar, J.M. Neuroinflammation in autism spectrum disorders: Exercise as a “pharmacological” tool. Neurosci. Biobehav. Rev. 2021, 129, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Hughes, H.K.; Moreno, R.J.; Ashwood, P. Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD). Brain. Behav. Immun. 2023, 108, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The influence of neuroinflammation in Autism Spectrum Disorder. Brain. Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Xie, H.M.; Perin, J.C.; Takahashi, N.; Murphy, K.; Wenocur, A.S.; D’arcy, M.; O’Hara, R.J.; Goldmuntz, E.; Grice, D.E.; et al. Rare structural variation of synapse and neurotransmission genes in autism. Mol. Psychiatry 2012, 17, 402–411. [Google Scholar] [CrossRef]
- Verpelli, C.; Dvoretskova, E.; Vicidomini, C.; Rossi, F.; Chiappalone, M.; Schoen, M.; Di Stefano, B.; Mantegazza, R.; Broccoli, V.; Böckers, T.M.; et al. Importance of Shank3 protein in regulating metabotropic glutamate receptor 5 (mGluR5) expression and signaling at synapses. J. Biol. Chem. 2011, 286, 34839–34850. [Google Scholar] [CrossRef]
- Qin, Y.; Du, Y.; Chen, L.; Liu, Y.; Xu, W.; Liu, Y.; Li, Y.; Leng, J.; Wang, Y.; Zhang, X.-Y.; et al. A recurrent SHANK1 mutation implicated in autism spectrum disorder causes autistic-like core behaviors in mice via downregulation of mGluR1-IP3R1-calcium signaling. Mol. Psychiatry 2022, 27, 2985–2998. [Google Scholar] [CrossRef]
- Chana, G.; Laskaris, L.; Pantelis, C.; Gillett, P.; Testa, R.; Zantomio, D.; Burrows, E.L.; Hannan, A.J.; Everall, I.P.; Skafidas, E. Decreased expression of mGluR5 within the dorsolateral prefrontal cortex in autism and increased microglial number in mGluR5 knockout mice: Pathophysiological and neurobehavioral implications. Brain. Behav. Immun. 2015, 49, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, S.; Boggio, E.M.; Grosso, S.; Lonetti, G.; Forlani, G.; Stefanelli, G.; Calcagno, E.; Morello, N.; Landsberger, N.; Biffo, S.; et al. Reduced AKT/mTOR signaling and protein synthesis dysregulation in a Rett syndrome animal model. Hum. Mol. Genet. 2011, 20, 1182–1196. [Google Scholar] [CrossRef]
- Pecorelli, A.; Cervellati, C.; Cordone, V.; Hayek, J.; Valacchi, G. Compromised immune/inflammatory responses in Rett syndrome. Free Radic. Biol. Med. 2020, 152, 100–106. [Google Scholar] [CrossRef]
- Maezawa, I.; Jin, L.-W. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J. Neurosci. 2010, 30, 5346–5356. [Google Scholar] [CrossRef]
- Derecki, N.C.; Cronk, J.C.; Kipnis, J. The role of microglia in brain maintenance: Implications for Rett syndrome. Trends Immunol. 2013, 34, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Gogliotti, R.G.; Senter, R.K.; Rook, J.M.; Ghoshal, A.; Zamorano, R.; Malosh, C.; Stauffer, S.R.; Bridges, T.M.; Bartolome, J.M.; Daniels, J.S.; et al. mGlu5 positive allosteric modulation normalizes synaptic plasticity defects and motor phenotypes in a mouse model of Rett syndrome. Hum. Mol. Genet. 2016, 25, 1990–2004. [Google Scholar] [CrossRef]
- Tao, J.; Wu, H.; Coronado, A.A.; de Laittre, E.; Osterweil, E.K.; Zhang, Y.; Bear, M.F. Negative Allosteric Modulation of mGluR5 Partially Corrects Pathophysiology in a Mouse Model of Rett Syndrome. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 11946–11958. [Google Scholar] [CrossRef] [PubMed]
- Cusick, M.F.; Libbey, J.E.; Patel, D.C.; Doty, D.J.; Fujinami, R.S. Infiltrating macrophages are key to the development of seizures following virus infection. J. Virol. 2013, 87, 1849–1860. [Google Scholar] [CrossRef]
- Kirkman, N.J.; Libbey, J.E.; Wilcox, K.S.; White, H.S.; Fujinami, R.S. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia 2010, 51, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.C.; Wallis, G.; Dahle, E.J.; McElroy, P.B.; Thomson, K.E.; Tesi, R.J.; Szymkowski, D.E.; West, P.J.; Smeal, R.M.; Patel, M.; et al. Hippocampal TNFα Signaling Contributes to Seizure Generation in an Infection-Induced Mouse Model of Limbic Epilepsy. eNeuro 2017, 4, ENEURO.0105-17.2017. [Google Scholar] [CrossRef]
- Pauletti, A.; Terrone, G.; Shekh-Ahmad, T.; Salamone, A.; Ravizza, T.; Rizzi, M.; Pastore, A.; Pascente, R.; Liang, L.-P.; Villa, B.R.; et al. Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain 2017, 140, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- Bertani, I.; Iori, V.; Trusel, M.; Maroso, M.; Foray, C.; Mantovani, S.; Tonini, R.; Vezzani, A.; Chiesa, R. Inhibition of IL-1β Signaling Normalizes NMDA-Dependent Neurotransmission and Reduces Seizure Susceptibility in a Mouse Model of Creutzfeldt-Jakob Disease. J. Neurosci. 2017, 37, 10278–10289. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Lang, B.; Aronica, E. Immunity and Inflammation in Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 6, a022699. [Google Scholar] [CrossRef]
- Howe, C.L.; Lafrance-Corey, R.G.; Sundsbak, R.S.; Lafrance, S.J. Inflammatory monocytes damage the hippocampus during acute picornavirus infection of the brain. J. Neuroinflamm. 2012, 9, 50. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Sun, B.-L.; Liu, J.-K.; Yang, M.-F.; Li, D.-W.; Fang, J.; Zhang, S.; Yuan, Q.-L.; Huang, S.-L. Activation of mGluR5 Attenuates Microglial Activation and Neuronal Apoptosis in Early Brain Injury After Experimental Subarachnoid Hemorrhage in Rats. Neurochem. Res. 2015, 40, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Manno, E.M. Update on intracerebral hemorrhage. Continuum 2012, 18, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Broderick, J.P.; Brott, T.G.; Duldner, J.E.; Tomsick, T.; Huster, G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 1993, 24, 987–993. [Google Scholar] [CrossRef]
- Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003, 4, 399–415. [Google Scholar] [CrossRef]
- Lan, X.; Han, X.; Li, Q.; Yang, Q.-W.; Wang, J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat. Rev. Neurol. 2017, 13, 420–433. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Lu, H.; Yang, Q.; Wu, H.; Wang, J. Microglial Polarization and Inflammatory Mediators After Intracerebral Hemorrhage. Mol. Neurobiol. 2017, 54, 1874–1886. [Google Scholar] [CrossRef]
- Barres, B.A. The Mystery and Magic of Glia: A Perspective on Their Roles in Health and Disease. Neuron 2008, 60, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-H.; Ho, S.-C.; Yeh, K.-Y.; Pawlak, C.R.; Chang, H.-M.; Ho, Y.-J.; Lai, T.-J.; Wu, F.-Y. Blockade of metabotropic glutamate receptors inhibits cognition and neurodegeneration in an MPTP-induced Parkinson’s disease rat model. Pharmacol. Biochem. Behav. 2012, 102, 64–71. [Google Scholar] [CrossRef]
- Makarewicz, D.; Duszczyk, M.; Gadamski, R.; Danysz, W.; Łazarewicz, J.W. Neuroprotective potential of group I metabotropic glutamate receptor antagonists in two ischemic models. Neurochem. Int. 2006, 48, 485–490. [Google Scholar] [CrossRef]
- Abushik, P.A.; Niittykoski, M.; Giniatullina, R.; Shakirzyanova, A.; Bart, G.; Fayuk, D.; Sibarov, D.A.; Antonov, S.M.; Giniatullin, R. The role of NMDA and mGluR5 receptors in calcium mobilization and neurotoxicity of homocysteine in trigeminal and cortical neurons and glial cells. J. Neurochem. 2014, 129, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Overk, C.R.; Cartier, A.; Shaked, G.; Rockenstein, E.; Ubhi, K.; Spencer, B.; Price, D.L.; Patrick, C.; Desplats, P.; Masliah, E. Hippocampal neuronal cells that accumulate α-synuclein fragments are more vulnerable to Aβ oligomer toxicity via mGluR5--implications for dementia with Lewy bodies. Mol. Neurodegener. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Yang, J.; Luan, Y.; Qiu, Z.; Zhang, J.; Lu, H.; Chen, X.; Liu, Y. Attenuation of Acute Intracerebral Hemorrhage-Induced Microglial Activation and Neuronal Death Mediated by the Blockade of Metabotropic Glutamate Receptor 5 In Vivo. Neurochem. Res. 2020, 45, 1230–1243. [Google Scholar] [CrossRef]
- Li, H.; Zhang, N.; Sun, G.; Ding, S. Inhibition of the group I mGluRs reduces acute brain damage and improves long-term histological outcomes after photothrombosis-induced ischaemia. ASN Neuro 2013, 5, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.L.; Williams, A.J.; Faden, A.I.; Tortella, F.C. Selective mGluR5 receptor antagonist or agonist provides neuroprotection in a rat model of focal cerebral ischemia. Brain Res. 2001, 922, 173–179. [Google Scholar] [CrossRef]
- Szydlowska, K.; Kaminska, B.; Baude, A.; Parsons, C.G.; Danysz, W. Neuroprotective activity of selective mGlu1 and mGlu5 antagonists in vitro and in vivo. Eur. J. Pharmacol. 2007, 554, 18–29. [Google Scholar] [CrossRef]
- Law, H.C.H.; Szeto, S.S.W.; Quan, Q.; Zhao, Y.; Zhang, Z.; Krakovska, O.; Lui, L.T.; Zheng, C.; Lee, S.M.-Y.; Siu, K.W.M.; et al. Characterization of the Molecular Mechanisms Underlying the Chronic Phase of Stroke in a Cynomolgus Monkey Model of Induced Cerebral Ischemia. J. Proteome Res. 2017, 16, 1150–1166. [Google Scholar] [CrossRef]
- Buchan, A.M.; Pulsinelli, W.A. Septo-hippocampal deafferentation protects CA1 neurons against ischemic injury. Brain Res. 1990, 512, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Guyot, L.L.; Diaz, F.G.; O’Regan, M.H.; Song, D.; Phillis, J.W. The effect of streptozotocin-induced diabetes on the release of excitotoxic and other amino acids from the ischemic rat cerebral cortex. Neurosurgery 2001, 48, 381–385. [Google Scholar] [CrossRef]
- Andreeva, K.; Zhang, M.; Fan, W.; Li, X.; Chen, Y.; Rebolledo-Mendez, J.D.; Cooper, N.G. Time-dependent Gene Profiling Indicates the Presence of Different Phases for Ischemia/Reperfusion Injury in Retina. Ophthalmol. Eye Dis. 2014, 6, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Hu, R.; Csernansky, C.A.; Liu, X.Z.; Hsu, C.Y.; Choi, D.W. Additive neuroprotective effects of dextrorphan and cycloheximide in rats subjected to transient focal cerebral ischemia. Brain Res. 1996, 718, 233–236. [Google Scholar] [CrossRef]
- Wetterling, F.; Chatzikonstantinou, E.; Tritschler, L.; Meairs, S.; Fatar, M.; Schad, L.R.; Ansar, S. Investigating potentially salvageable penumbra tissue in an in vivo model of transient ischemic stroke using sodium, diffusion, and perfusion magnetic resonance imaging. BMC Neurosci. 2016, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Matute, C. Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 1997, 17, 290–300. [Google Scholar] [CrossRef]
- Domercq, M.; Sánchez-Gómez, M.V.; Sherwin, C.; Etxebarria, E.; Fern, R.; Matute, C. System xc- and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J. Immunol. 2007, 178, 6549–6556. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Yang, S.-D.; Li, W.-B.; Ren, S.-Q.; Zhang, J.; Zhang, F. Pre-ischemic treadmill training alleviates brain damage via GLT-1-mediated signal pathway after ischemic stroke in rats. Neuroscience 2014, 274, 393–402. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, Y.; Jia, J.; Hu, Y.-S. Pre-ischemic treadmill training induces tolerance to brain ischemia: Involvement of glutamate and ERK1/2. Molecules 2010, 15, 5246–5257. [Google Scholar] [CrossRef]
- Cavallo, D.; Landucci, E.; Gerace, E.; Lana, D.; Ugolini, F.; Henley, J.M.; Giovannini, M.G.; Pellegrini-Giampietro, D.E. Neuroprotective effects of mGluR5 activation through the PI3K/Akt pathway and the molecular switch of AMPA receptors. Neuropharmacology 2020, 162, 107810. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini-Giampietro, D.E. The distinct role of mGlu1 receptors in post-ischemic neuronal death. Trends Pharmacol. Sci. 2003, 24, 461–470. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Kettenmann, H.; Kirchhoff, F.; Verkhratsky, A. Microglia: New roles for the synaptic stripper. Neuron 2013, 77, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Bie, B.; Wu, J.; Yang, H.; Xu, J.J.; Brown, D.L.; Naguib, M. Epigenetic suppression of neuroligin 1 underlies amyloid-induced memory deficiency. Nat. Neurosci. 2014, 17, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Paolicelli, R.C.; Sforazzini, F.; Weinhard, L.; Bolasco, G.; Pagani, F.; Vyssotski, A.L.; Bifone, A.; Gozzi, A.; Ragozzino, D.; et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014, 17, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.T.J.; Wilkinson, K.D.; Reines, D.; Warren, S.T. FMR1 protein: Conserved RNP family domains and selective RNA binding. Science 1993, 262, 563–566. [Google Scholar] [CrossRef]
- Bassell, G.J.; Warren, S.T. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron 2008, 60, 201–214. [Google Scholar] [CrossRef]
- Hamilton, A.; Zamponi, G.W.; Ferguson, S.S.G. Glutamate receptors function as scaffolds for the regulation of β-amyloid and cellular prion protein signaling complexes. Mol. Brain 2015, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Lui, H.; Zhang, J.; Makinson, S.R.; Cahill, M.K.; Kelley, K.W.; Huang, H.-Y.; Shang, Y.; Oldham, M.C.; Martens, L.H.; Gao, F.; et al. Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia via Complement Activation. Cell 2016, 165, 921–935. [Google Scholar] [CrossRef]
- Stephan, A.H.; Barres, B.A.; Stevens, B. The complement system: An unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci. 2012, 35, 369–389. [Google Scholar] [CrossRef]
- Bie, B.; Wu, J.; Foss, J.F.; Naguib, M. Activation of mGluR1 Mediates C1q-Dependent Microglial Phagocytosis of Glutamatergic Synapses in Alzheimer’s Rodent Models. Mol. Neurobiol. 2019, 56, 5568–5585. [Google Scholar] [CrossRef]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.V.; Huang, W.; Buffington, S.A.; Hsu, C.-C.; Bonnen, P.E.; Placzek, A.N.; Sidrauski, C.; Krnjević, K.; Kaufman, R.J.; Walter, P.; et al. Translational control of mGluR-dependent long-term depression and object-place learning by eIF2α. Nat. Neurosci. 2014, 17, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Spurrier, J.; Nicholson, L.; Fang, X.T.; Stoner, A.J.; Toyonaga, T.; Holden, D.; Siegert, T.R.; Laird, W.; Allnutt, M.A.; Chiasseu, M.; et al. Reversal of synapse loss in Alzheimer mouse models by targeting mGluR5 to prevent synaptic tagging by C1Q. Sci. Transl. Med. 2022, 14, eabi8593. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef]
- Renton, A.E.; Chiò, A.; Traynor, B.J. State of play in amyotrophic lateral sclerosis genetics. Nat. Publ. Gr. 2013, 17, 17–23. [Google Scholar] [CrossRef]
- Rowland, L.P.; Shneider, N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001, 344, 1688–1700. [Google Scholar] [CrossRef]
- Bonifacino, T.; Zerbo, R.A.; Balbi, M.; Torazza, C.; Frumento, G.; Fedele, E.; Bonanno, G.; Milanese, M. Nearly 30 Years of Animal Models to Study Amyotrophic Lateral Sclerosis: A Historical Overview and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 12236. [Google Scholar] [CrossRef]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.X.; et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef]
- DeJesus-Hernandez, M.; Mackenzie, I.R.; Boeve, B.F.; Boxer, A.L.; Baker, M.; Rutherford, N.J.; Nicholson, A.M.; Finch, N.C.A.; Flynn, H.; Adamson, J.; et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef]
- Renton, A.E.; Majounie, E.; Waite, A.; Simón-Sánchez, J.; Rollinson, S.; Gibbs, J.R.; Schymick, J.C.; Laaksovirta, H.; van Swieten, J.C.; Myllykangas, L.; et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011, 72, 257–268. [Google Scholar] [CrossRef]
- Kwiatkowski, T.J.J.; Bosco, D.A.; Leclerc, A.L.; Tamrazian, E.; Vanderburg, C.R.; Russ, C.; Davis, A.; Gilchrist, J.; Kasarskis, E.J.; Munsat, T.; et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 2009, 323, 1205–1208. [Google Scholar] [CrossRef]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Bucchia, M.; Ramirez, A.; Parente, V.; Simone, C.; Nizzardo, M.; Magri, F.; Dametti, S.; Corti, S. Therapeutic development in amyotrophic lateral sclerosis. Clin. Ther. 2015, 37, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Mitchell, J.D.; Moore, D.H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst. Rev. 2012, 2012, CD001447. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Yamashita, S.; Ueyama, H.; Ishizaki, M.; Maeda, Y.; Ando, Y. Long-term effects of edaravone on survival of patients with amyotrophic lateral sclerosis. eNeurologicalSci 2018, 11, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Aoki, M.; Tsuji, S.; Itoyama, Y.; Sobue, G.; Togo, M.; Hamada, C.; Tanaka, M.; Akimoto, M.; Nakamura, K.; et al. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017, 16, 505–512. [Google Scholar] [CrossRef]
- Benatar, M.; Wuu, J.; Andersen, P.M.; Bucelli, R.C.; Andrews, J.A.; Otto, M.; Farahany, N.A.; Harrington, E.A.; Chen, W.; Mitchell, A.A.; et al. Correction to: Design of a Randomized, Placebo-Controlled, Phase 3 Trial of Tofersen Initiated in Clinically Presymptomatic SOD1 Variant Carriers: The ATLAS Study. Neurother. J. Am. Soc. Exp. Neurother. 2022, 19, 1686. [Google Scholar] [CrossRef]
- Van Harten, A.C.M.M.; Phatnani, H.; Przedborski, S. Non-cell-autonomous pathogenic mechanisms in amyotrophic lateral sclerosis. Trends Neurosci. 2021, 44, 658–668. [Google Scholar] [CrossRef]
- Li, L.; Acioglu, C.; Heary, R.F.; Elkabes, S. Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain. Behav. Immun. 2021, 91, 740–755. [Google Scholar] [CrossRef]
- Kim, C.; Yousefian-Jazi, A.; Choi, S.-H.; Chang, I.; Lee, J.; Ryu, H. Non-Cell Autonomous and Epigenetic Mechanisms of Huntington’s Disease. Int. J. Mol. Sci. 2021, 22, 12499. [Google Scholar] [CrossRef]
- Seol, Y.; Ki, S.; Ryu, H.L.; Chung, S.; Lee, J.; Ryu, H. How Microglia Manages Non-cell Autonomous Vicious Cycling of Aβ Toxicity in the Pathogenesis of AD. Front. Mol. Neurosci. 2020, 13, 593724. [Google Scholar] [CrossRef]
- Lim, S.; Kim, H.-J.; Kim, D.-K.; Lee, S.-J. Non-cell-autonomous actions of α-synuclein: Implications in glial synucleinopathies. Prog. Neurobiol. 2018, 169, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, H.; Polymenidou, M.; Cleveland, D.W. Non–cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 2009, 187, 761–772. [Google Scholar] [CrossRef]
- Vaz, S.H.; Pinto, S.; Sebastião, A.M.; Brites, D. Astrocytes in Amyotrophic Lateral Sclerosis; Araki, T., Ed.; Exon Publications: Brisbane, Australia, 2021; ISBN 978-0-6450017-7-8. [Google Scholar]
- McGeer, P.L.; McGeer, E.G. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve 2002, 26, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Beers, D.R.; Henkel, J.S.; Xiao, Q.; Zhao, W.; Wang, J.; Yen, A.A.; Siklos, L.; McKercher, S.R.; Appel, S.H. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2006, 103, 16021–16026. [Google Scholar] [CrossRef]
- Beers, D.R.; Henkel, J.S.; Zhao, W.; Wang, J.; Appel, S.H. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc. Natl. Acad. Sci. USA 2008, 105, 15558–15563. [Google Scholar] [CrossRef]
- Chiu, I.M.; Phatnani, H.; Kuligowski, M.; Tapia, J.C.; Carrasco, M.A.; Zhang, M.; Maniatis, T.; Carroll, M.C. Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice. Proc. Natl. Acad. Sci. USA 2009, 106, 20960–20965. [Google Scholar] [CrossRef] [PubMed]
- Lincecum, J.M.; Vieira, F.G.; Wang, M.Z.; Thompson, K.; De Zutter, G.S.; Kidd, J.; Moreno, A.; Sanchez, R.; Carrion, I.J.; Levine, B.A.; et al. From transcriptome analysis to therapeutic anti-CD40L treatment in the SOD1 model of amyotrophic lateral sclerosis. Nat. Genet. 2010, 42, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Philips, T.; Rothstein, J.D. Glial cells in amyotrophic lateral sclerosis. Exp. Neurol. 2014, 262, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Beers, D.R.; Hooten, K.G.; Sieglaff, D.H.; Zhang, A.; Kalyana-Sundaram, S.; Traini, C.M.; Halsey, W.S.; Hughes, A.M.; Sathe, G.M.; et al. Characterization of Gene Expression Phenotype in Amyotrophic Lateral Sclerosis Monocytes Supplemental content. JAMA Neurol. 2017, 74, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Beers, D.R.; Appel, S.H. Immune dysregulation in amyotrophic lateral sclerosis: Mechanisms and emerging therapies. Lancet Neurol. 2019, 18, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Becher, B.; Spath, S.; Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2017, 17, 49–59. [Google Scholar] [CrossRef]
- Brites, D.; Vaz, A.R. Microglia centered pathogenesis in ALS: Insights in cell interconnectivity. Front. Cell. Neurosci. 2014, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Gerber, Y.N.; Sabourin, J.C.; Hugnot, J.P.; Perrin, F.E. Unlike Physical Exercise, Modified Environment Increases the Lifespan of SOD1G93A Mice However Both Conditions Induce Cellular Changes. PLoS ONE 2012, 7, e45503. [Google Scholar] [CrossRef]
- Alexianu, M.E.; Kozovska, M.; Appel, S.H. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology 2001, 57, 1282–1289. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef]
- Beers, D.R.; Zhao, W.; Liao, B.; Kano, O.; Wang, J.; Huang, A.; Appel, S.H.; Henkel, J.S. Neuroinflammation modulates distinct regional and temporal clinical responses in ALS mice. Brain. Behav. Immun. 2011, 25, 1025–1035. [Google Scholar] [CrossRef]
- Lewis, C.-A.; Manning, J.; Rossi, F.; Krieger, C. The Neuroinflammatory Response in ALS: The Roles of Microglia and T Cells. Neurol. Res. Int. 2012, 2012, 803701. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.R.A.; Ansorge, O.; Strong, M.; Bilbao, J.; Zinman, L.; Ang, L.-C.; Baker, M.; Stewart, H.; Eisen, A.; Rademakers, R.; et al. Pathological heterogeneity in amyotrophic lateral sclerosis with FUS mutations: Two distinct patterns correlating with disease severity and mutation. Acta Neuropathol. 2011, 122, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Karki, P.; Smith, K.; Johnson, J.; Lee, E. Astrocyte-derived growth factors and estrogen neuroprotection: Role of transforming growth factor-$α$ in estrogen-induced upregulation of glutamate transporters in astrocytes. Mol. Cell. Endocrinol. 2014, 389, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, E.; Bonifacino, T.; Raffaele, S.; Milanese, M.; Morgante, E.; Bonanno, G.; Abbracchio, M.P.; Fumagalli, M. Abnormal upregulation of gpr17 receptor contributes to oligodendrocyte dysfunction in SOD1G93A mice. Int. J. Mol. Sci. 2020, 21, 2395. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- Michelucci, A.; Heurtaux, T.; Grandbarbe, L.; Morga, E.; Heuschling, P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J. Neuroimmunol. 2009, 210, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Zhao, W.; Beers, D.R.; Henkel, J.S.; Appel, S.H. Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp. Neurol. 2012, 237, 147–152. [Google Scholar] [CrossRef]
- Chiu, I.M.; Morimoto, E.T.A.A.; Goodarzi, H.; Liao, J.T.; O’Keeffe, S.; Phatnani, H.P.; Muratet, M.; Carroll, M.C.; Levy, S.; Tavazoie, S.; et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep. 2013, 4, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Beers, D.R.; Appel, S.H. Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2013, 8, 888–899. [Google Scholar] [CrossRef]
- Geloso, M.C.; Corvino, V.; Marchese, E.; Serrano, A.; Michetti, F.; D’Ambrosi, N. The dual role of microglia in ALS: Mechanisms and therapeutic approaches. Front. Aging Neurosci. 2017, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci. 2016, 19, 987–991. [Google Scholar] [CrossRef]
- Gravel, M.; Béland, L.-C.C.; Ve Soucy, G.; Abdelhamid, E.; Rahimian, R.; Gravel, C.; Kriz, J.; Soucy, G.; Abdelhamid, E.; Rahimian, R.; et al. Il-10 controls early microglial phenotypes and disease onset in ALS caused by misfolded superoxide dismutase 1. J. Neurosci. 2016, 36, 1031–1048. [Google Scholar] [CrossRef]
- Ohgomori, T.; Yamada, J.; Takeuchi, H.; Kadomatsu, K.; Jinno, S. Comparative morphometric analysis of microglia in the spinal cord of SOD1(G93A) transgenic mouse model of amyotrophic lateral sclerosis. Eur. J. Neurosci. 2016, 43, 1340–1351. [Google Scholar] [CrossRef]
- Cunha, C.; Santos, C.; Gomes, C.; Fernandes, A.; Correia, A.M.; Sebastião, A.M.; Vaz, A.R.; Brites, D. Downregulated Glia Interplay and Increased miRNA-155 as Promising Markers to Track ALS at an Early Stage. Mol. Neurobiol. 2018, 55, 4207–4224. [Google Scholar] [CrossRef]
- D’Ambrosi, N.; Finocchi, P.; Apolloni, S.; Cozzolino, M.; Ferri, A.; Padovano, V.; Pietrini, G.; Carrì, M.T.; Volonté, C. The proinflammatory action of microglial P2 receptors is enhanced in SOD1 models for amyotrophic lateral sclerosis. J. Immunol. 2009, 183, 4648–4656. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhao, W.; Beers, D.R.; Yen, A.A.; Xie, W.; Henkel, J.S.; Appel, S.H. Mutant SOD1(G93A) microglia are more neurotoxic relative to wild-type microglia. J. Neurochem. 2007, 102, 2008–2019. [Google Scholar] [CrossRef] [PubMed]
- Frakes, A.E.; Ferraiuolo, L.; Haidet-Phillips, A.M.; Schmelzer, L.; Braun, L.; Miranda, C.J.; Ladner, K.J.; Bevan, A.K.; Foust, K.D.; Godbout, J.P.; et al. Microglia induce motor neuron death via the classical NF-$κ$B pathway in amyotrophic lateral sclerosis. Neuron 2014, 81, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Beers, D.R.; Bell, S.; Wang, J.; Wen, S.; Baloh, R.H.; Appel, S.H. TDP-43 activates microglia through NF-κB and NLRP3 inflammasome. Exp. Neurol. 2015, 273, 24–35. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Woodhouse, A.; Kirkcaldie, M.T.K.; Vickers, J.C. Excitotoxicity in ALS: Overstimulation, or overreaction? Exp. Neurol. 2016, 275 Pt 1, 162–171. [Google Scholar] [CrossRef]
- Pál, B.; Rose, C.R.; Wittner, L.; Vaz, S.H.; Armada-Moreira, A.; Gomes, J.I.; Campos Pina, C.; Savchak, O.K.; Gonçalves-Ribeiro, J.; Rei, N.; et al. Going the Extra (Synaptic) Mile: Excitotoxicity as the Road Toward Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 90. [Google Scholar] [CrossRef]
- Bonifacino, T.; Musazzi, L.; Milanese, M.; Seguini, M.; Marte, A.; Gallia, E.; Cattaneo, L.; Onofri, F.; Popoli, M.; Bonanno, G. Altered mechanisms underlying the abnormal glutamate release in amyotrophic lateral sclerosis at a pre-symptomatic stage of the disease. Neurobiol. Dis. 2016, 95, 122–133. [Google Scholar] [CrossRef]
- Milanese, M.; Bonifacino, T.; Fedele, E.; Rebosio, C.; Cattaneo, L.; Benfenati, F.; Usai, C.; Bonanno, G. Exocytosis regulates trafficking of GABA and glycine heterotransporters in spinal cord glutamatergic synapses: A mechanism for the excessive heterotransporter-induced release of glutamate in experimental amyotrophic lateral sclerosis. Neurobiol. Dis. 2015, 74, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Milanese, M.; Zappettini, S.; Onofri, F.; Musazzi, L.; Tardito, D.; Bonifacino, T.; Messa, M.; Racagni, G.; Usai, C.; Benfenati, F.; et al. Abnormal exocytotic release of glutamate in a mouse model of amyotrophic lateral sclerosis. J. Neurochem. 2011, 116, 1028–1042. [Google Scholar] [CrossRef]
- Oskarsson, B.; Gendron, T.F.; Staff, N.P. Amyotrophic Lateral Sclerosis: An Update for 2018. Mayo Clin. Proc. 2018, 93, 1617–1628. [Google Scholar] [CrossRef]
- Pehar, M.; Harlan, B.A.; Killoy, K.M.; Vargas, M.R. Role and Therapeutic Potential of Astrocytes in Amyotrophic Lateral Sclerosis. Curr. Pharm. Des. 2017, 23, 5010–5021. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Jin, S.; Wang, J.; Zhang, G.; Kawanokuchi, J.; Kuno, R.; Sonobe, Y.; Mizuno, T.; Suzumura, A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J. Biol. Chem. 2006, 281, 21362–21368. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-S.; Ferreira, R.; Lively, S.; Schlichter, L.C. Microglial SK3 and SK4 currents and activation state are modulated by the neuroprotective drug, riluzole. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2013, 8, 227–237. [Google Scholar] [CrossRef]
- Mesci, P.; Zaïdi, S.; Lobsiger, C.S.; Millecamps, S.; Escartin, C.; Seilhean, D.; Sato, H.; Mallat, M.; Boillée, S. System xC- is a mediator of microglial function and its deletion slows symptoms in amyotrophic lateral sclerosis mice. Brain 2015, 138, 53–68. [Google Scholar] [CrossRef]
- Tomiyama, M.; Kimura, T.; Maeda, T.; Tanaka, H.; Furusawa, K.; Kurahashi, K.; Matsunaga, M. Expression of metabotropic glutamate receptor mRNAs in the human spinal cord: Implications for selective vulnerability of spinal motor neurons in amyotrophic lateral sclerosis. J. Neurol. Sci. 2001, 189, 65–69. [Google Scholar] [CrossRef]
- Aronica, E.; Catania, M.V.; Geurts, J.; Yankaya, B.; Troost, D. Immunohistochemical localization of group I and II metabotropic glutamate receptors in control and amyotrophic lateral sclerosis human spinal cord: Upregulation in reactive astrocytes. Neuroscience 2001, 105, 509–520. [Google Scholar] [CrossRef]
- Valerio, A.; Ferrario, M.; Paterlini, M.; Liberini, P.; Moretto, G.; Cairns, N.J.; Pizzi, M.; Spano, P.F. Spinal cord mGlu1a receptors: Possible target for amyotrophic lateral sclerosis therapy. Pharmacol. Biochem. Behav. 2002, 73, 447–454. [Google Scholar] [CrossRef]
- Anneser, J.M.H.; Ince, P.G.; Shaw, P.J.; Borasio, G.D. Differential expression of mGluR5 in human lumbosacral motoneurons. Neuroreport 2004, 15, 271–273. [Google Scholar] [CrossRef]
- Anneser, J.M.H.; Chahli, C.; Borasio, G.D. Protective effect of metabotropic glutamate receptor inhibition on amyotrophic lateral sclerosis-cerebrospinal fluid toxicity in vitro. Neuroscience 2006, 141, 1879–1886. [Google Scholar] [CrossRef]
- Ma, L.; Ostrovsky, H.; Miles, G.; Lipski, J.; Funk, G.D.; Nicholson, L.F.B. Differential expression of group I metabotropic glutamate receptors in human motoneurons at low and high risk of degeneration in amyotrophic lateral sclerosis. Neuroscience 2006, 143, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Anneser, J.M.H.; Chahli, C.; Ince, P.G.; Borasio, G.D.; Shaw, P.J. Glial proliferation and metabotropic glutamate receptor expression in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2004, 63, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Planas-Fontánez, T.M.; Dreyfus, C.F.; Saitta, K.S. Reactive Astrocytes as Therapeutic Targets for Brain Degenerative Diseases: Roles Played by Metabotropic Glutamate Receptors. Neurochem. Res. 2020, 45, 541–550. [Google Scholar] [CrossRef]
- Vergouts, M.; Doyen, P.J.; Peeters, M.; Opsomer, R.; Hermans, E. Constitutive downregulation protein kinase C epsilon in hSOD1(G93A) astrocytes influences mGluR5 signaling and the regulation of glutamate uptake. Glia 2018, 66, 749–761. [Google Scholar] [CrossRef]
- Rossi, D.; Brambilla, L.; Valori, C.F.; Roncoroni, C.; Crugnola, A.; Yokota, T.; Bredesen, D.E.; Volterra, A. Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ. 2008, 15, 1691–1700. [Google Scholar] [CrossRef]
- Vermeiren, C.; De Hemptinne, I.; Vanhoutte, N.; Tilleux, S.; Maloteaux, J.-M.M.; Hermans, E.; Hemptinne, I.; Vanhoutte, N.; Tilleux, S.; Maloteaux, J.-M.M.; et al. Loss of metabotropic glutamate receptor-mediated regulation of glutamate transport in chemically activated astrocytes in a rat model of amyotrophic lateral sclerosis. J. Neurochem. 2006, 96, 719–731. [Google Scholar] [CrossRef]
- D’Antoni, S.; Berretta, A.; Seminara, G.; Longone, P.; Giuffrida-Stella, A.M.; Battaglia, G.; Sortino, M.A.; Nicoletti, F.; Catania, M. V A prolonged pharmacological blockade of type-5 metabotropic glutamate receptors protects cultured spinal cord motor neurons against excitotoxic death. Neurobiol. Dis. 2011, 42, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Martorana, F.; Brambilla, L.; Valori, C.F.; Bergamaschi, C.; Roncoroni, C.; Aronica, E.; Volterra, A.; Bezzi, P.; Rossi, D. The BH4 domain of Bcl-X(L) rescues astrocyte degeneration in amyotrophic lateral sclerosis by modulating intracellular calcium signals. Hum. Mol. Genet. 2012, 21, 826–840. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Tueckmantel, W.; Zhu, A.; Pellegrino, D.; Brownell, A.-L. Synthesis and preliminary biological evaluation of 3-[(18)F]fluoro-5-(2-pyridinylethynyl)benzonitrile as a PET radiotracer for imaging metabotropic glutamate receptor subtype 5. Synapse 2007, 61, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, D.; Cicchetti, F.; Wang, X.; Zhu, A.; Yu, M.; Saint-Pierre, M.; Brownell, A.-L. Modulation of dopaminergic and glutamatergic brain function: PET studies on parkinsonian rats. J. Nucl. Med. 2007, 48, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Pernaute, R.; Wang, J.-Q.; Kuruppu, D.; Cao, L.; Tueckmantel, W.; Kozikowski, A.; Isacson, O.; Brownell, A.-L. Enhanced binding of metabotropic glutamate receptor type 5 (mGluR5) PET tracers in the brain of parkinsonian primates. Neuroimage 2008, 42, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Brownell, A.L.; Kuruppu, D.; Kil, K.E.; Jokivarsi, K.; Poutiainen, P.; Zhu, A.; Maxwell, M. PET imaging studies show enhanced expression of mGluR5 and inflammatory response during progressive degeneration in ALS mouse model expressing SOD1-G93A gene. J. Neuroinflamm. 2015, 12, 217. [Google Scholar] [CrossRef]
- Zürcher, N.R.; Loggia, M.L.; Lawson, R.; Chonde, D.B.; Izquierdo-Garcia, D.; Yasek, J.E.; Akeju, O.; Catana, C.; Rosen, B.R.; Cudkowicz, M.E.; et al. Increased in vivo glial activation in patients with amyotrophic lateral sclerosis: Assessed with [(11)C]-PBR28. NeuroImage. Clin. 2015, 7, 409–414. [Google Scholar] [CrossRef]
- Milanese, M.; Zappettini, S.; Jacchetti, E.; Bonifacino, T.; Cervetto, C.; Usai, C.; Bonanno, G. In vitro activation of GAT1 transporters expressed in spinal cord gliosomes stimulates glutamate release that is abnormally elevated in the SOD1/G93A(+) mouse model of amyotrophic lateral sclerosis. J. Neurochem. 2010, 113, 489–501. [Google Scholar] [CrossRef]
- Milanese, M.; Giribaldi, F.; Melone, M.; Bonifacino, T.; Musante, I.; Carminati, E.; Rossi, P.I.A.; Vergani, L.; Voci, A.; Conti, F.; et al. Knocking down metabotropic glutamate receptor 1 improves survival and disease progression in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2014, 64, 48–59. [Google Scholar] [CrossRef]
- Bonifacino, T.; Cattaneo, L.; Gallia, E.; Puliti, A.; Melone, M.; Provenzano, F.; Bossi, S.; Musante, I.; Usai, C.; Conti, F.; et al. In-vivo effects of knocking-down metabotropic glutamate receptor 5 in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Neuropharmacology 2017, 123, 433–445. [Google Scholar] [CrossRef]
- Bonifacino, T.; Provenzano, F.; Gallia, E.; Ravera, S.; Torazza, C.; Bossi, S.; Ferrando, S.; Puliti, A.; Van Den Bosch, L.; Bonanno, G.; et al. In-vivo genetic ablation of metabotropic glutamate receptor type 5 slows down disease progression in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2019, 129, 79–92. [Google Scholar] [CrossRef]
- Milanese, M.; Bonifacino, T.; Torazza, C.; Provenzano, F.; Kumar, M.; Ravera, S.; Arianna; Zerbo, R.; Frumento, G.; Balbi, M.; et al. Blocking glutamate mGlu 5 receptors with the negative allosteric modulator CTEP improves disease course in SOD1 G93A mouse model of amyotrophic lateral sclerosis. Br. J. Pharmacol. 2021, 178, 3747–3764. [Google Scholar] [CrossRef]
- Kriz, J.; Nguyen, M.D.; Julien, J.-P. Minocycline slows disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2002, 10, 268–278. [Google Scholar] [CrossRef]
- Van Den Bosch, L.; Tilkin, P.; Lemmens, G.; Robberecht, W. Minocycline delays disease onset and mortality in a transgenic model of ALS. Neuroreport 2002, 13, 1067–1070. [Google Scholar] [CrossRef]
- Zhu, S.; Stavrovskaya, I.G.; Drozda, M.; Kim, B.Y.S.; Ona, V.; Li, M.; Sarang, S.; Liu, A.S.; Hartley, D.M.; Wu, D.C.; et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 2002, 417, 74–78. [Google Scholar] [CrossRef]
- Keller, A.F.; Gravel, M.; Kriz, J. Treatment with minocycline after disease onset alters astrocyte reactivity and increases microgliosis in SOD1 mutant mice. Exp. Neurol. 2011, 228, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Song, G.J.; Suk, K. Pharmacological Modulation of Functional Phenotypes of Microglia in Neurodegenerative Diseases. Front. Aging Neurosci. 2017, 9, 139. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, B.; Yang, L.-E.; Zhang, C. Platycodigenin as Potential Drug Candidate for Alzheimer’s Disease via Modulating Microglial Polarization and Neurite Regeneration. Molecules 2019, 24, 3207. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, Y.-Z.; Wang, G.-Q.; Li, D.-D.; Shi, J.-S.; Zhang, F. Targeting MAPK Pathways by Naringenin Modulates Microglia M1/M2 Polarization in Lipopolysaccharide-Stimulated Cultures. Front. Cell. Neurosci. 2018, 12, 531. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xiao, L.; Zhong, Z.; Wang, L.; Li, Z.; Pan, X.; Liu, Z. Astaxanthin acts via LRP-1 to inhibit inflammation and reverse lipopolysaccharide-induced M1/M2 polarization of microglial cells. Oncotarget 2017, 8, 69370–69385. [Google Scholar] [CrossRef] [PubMed]
- Gelosa, P.; Bonfanti, E.; Castiglioni, L.; Delgado-Garcia, J.M.; Gruart, A.; Fontana, L.; Gotti, M.; Tremoli, E.; Lecca, D.; Fumagalli, M.; et al. Improvement of fiber connectivity and functional recovery after stroke by montelukast, an available and safe anti-asthmatic drug. Pharmacol. Res. 2019, 142, 223–236. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).