Surface-Modified Inhaled Microparticle-Encapsulated Celastrol for Enhanced Efficacy in Malignant Pleural Mesothelioma
Abstract
1. Introduction
2. Results
2.1. Formulation of Celastrol Microparticles (Cela MPs)
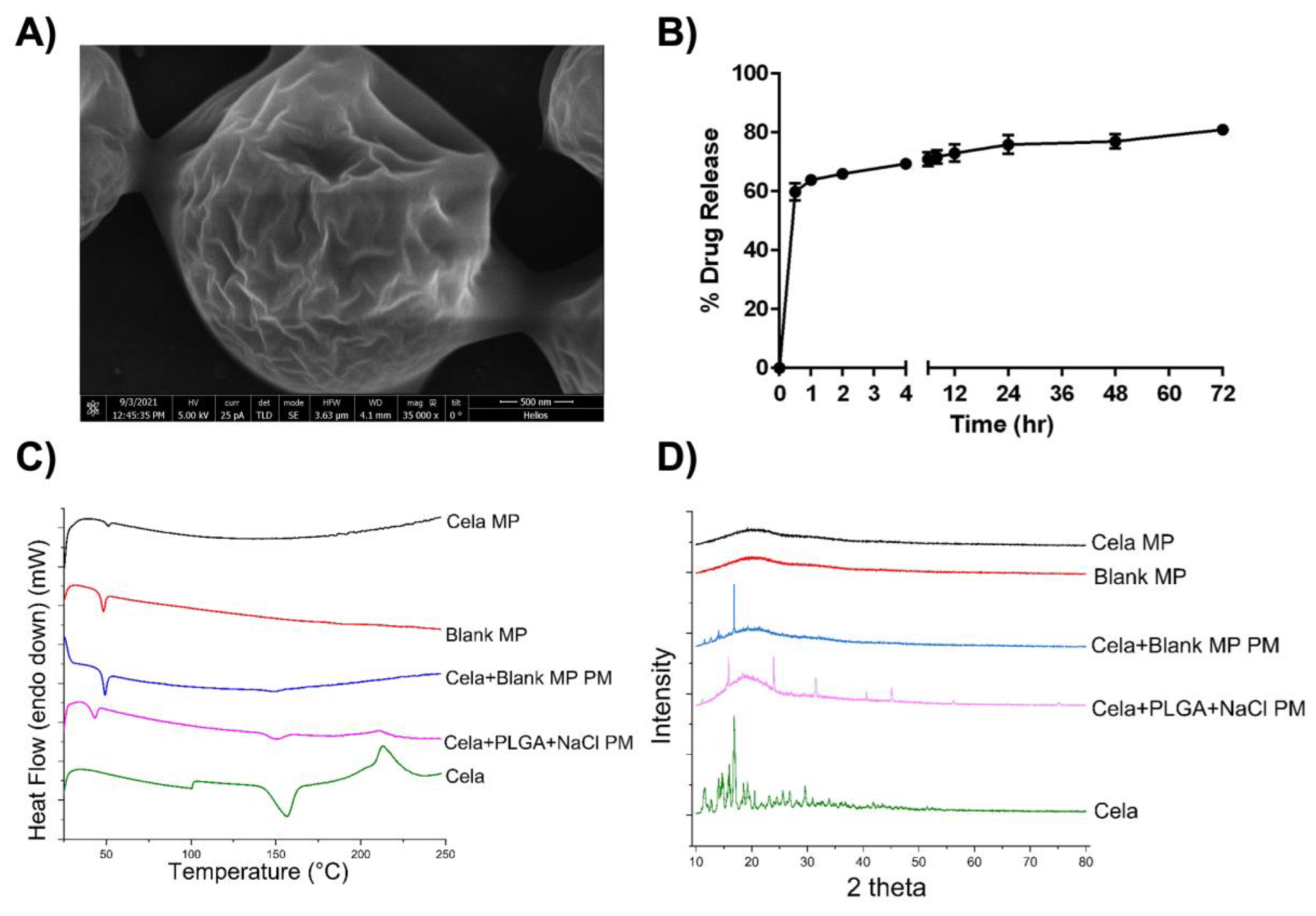
2.2. Morphological Analysis
2.3. In Vitro Release Study
2.4. Differential Scanning Calorimetry (DSC) Studies
2.5. X-ray Diffraction Studies (XRD)
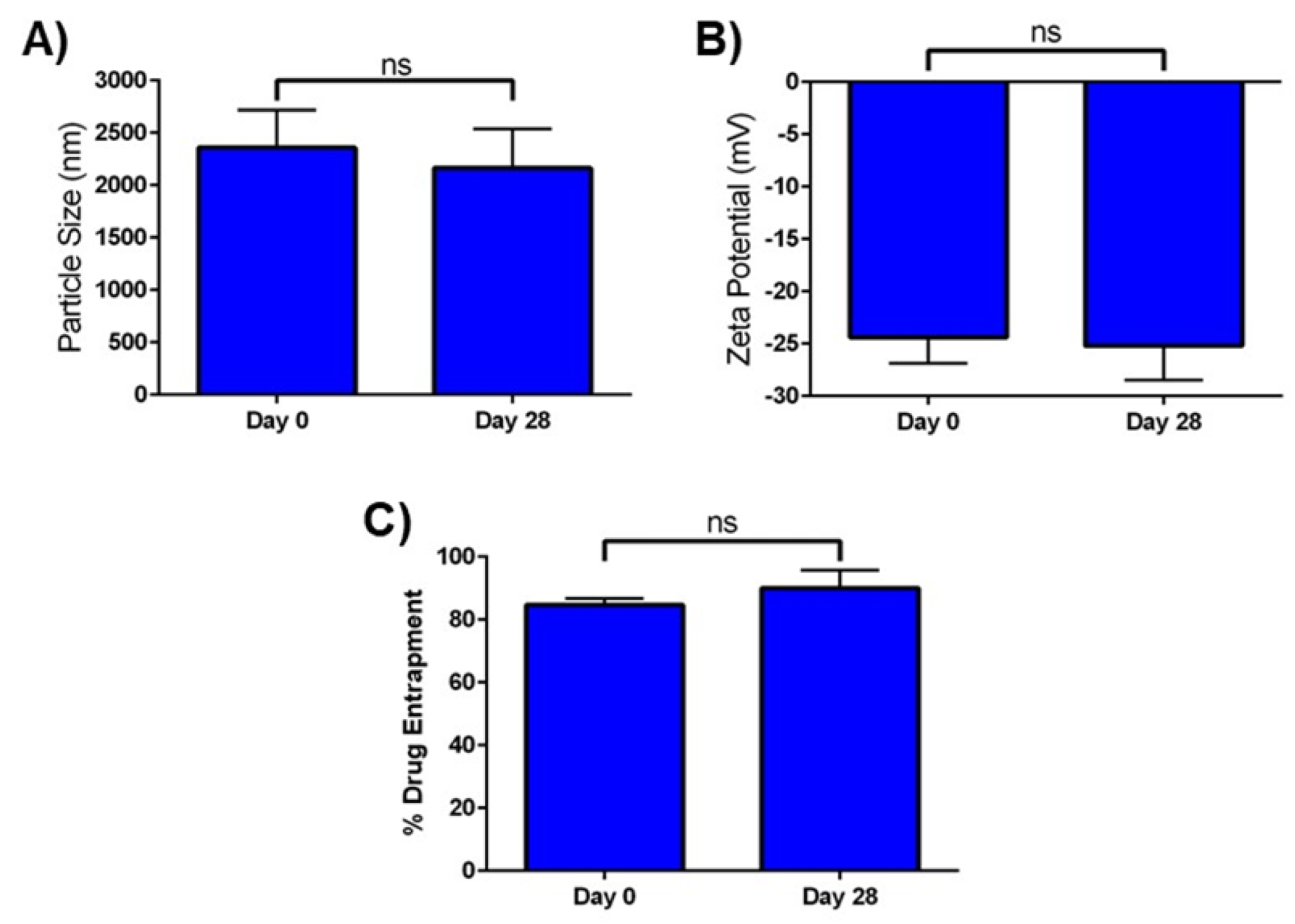
2.6. In Vitro Stability
2.7. Aerosolization of Cela MPs
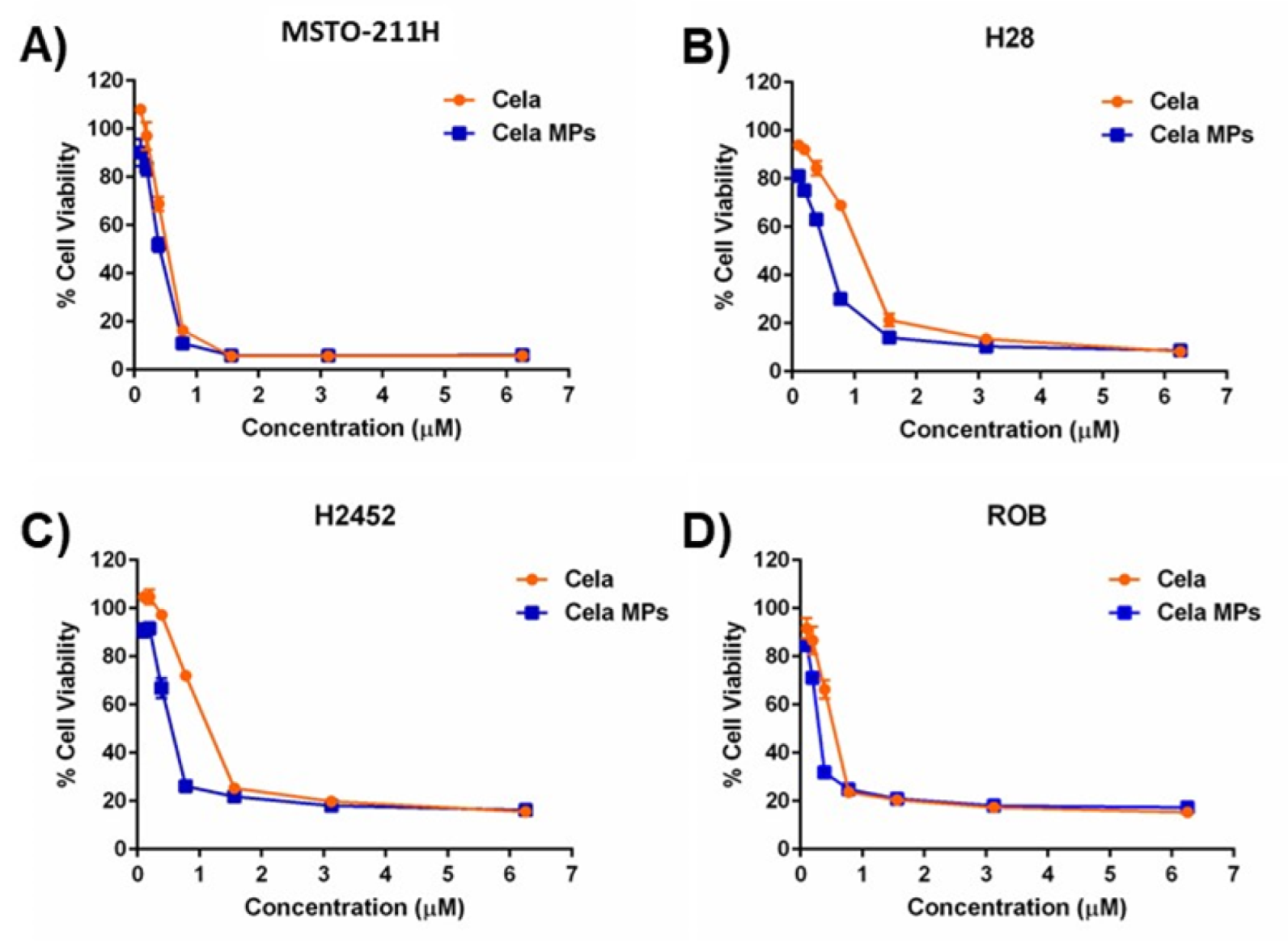
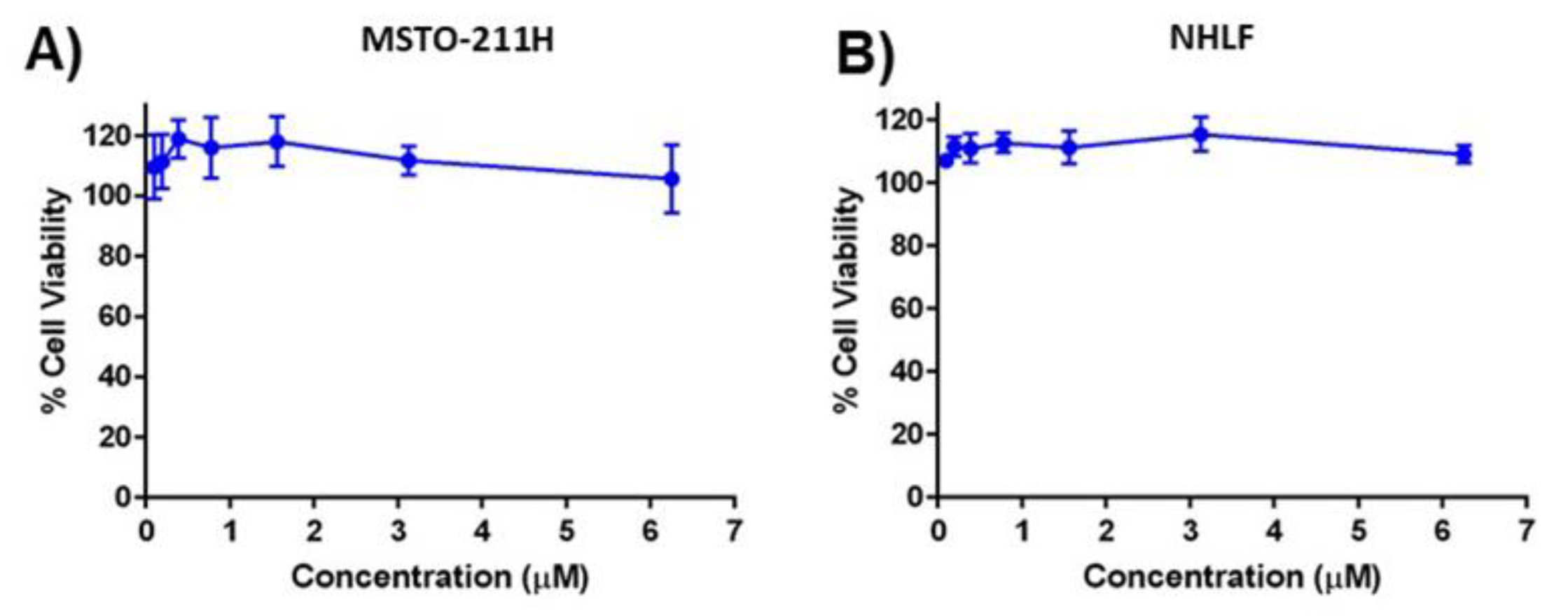
2.8. Cytotoxicity Studies
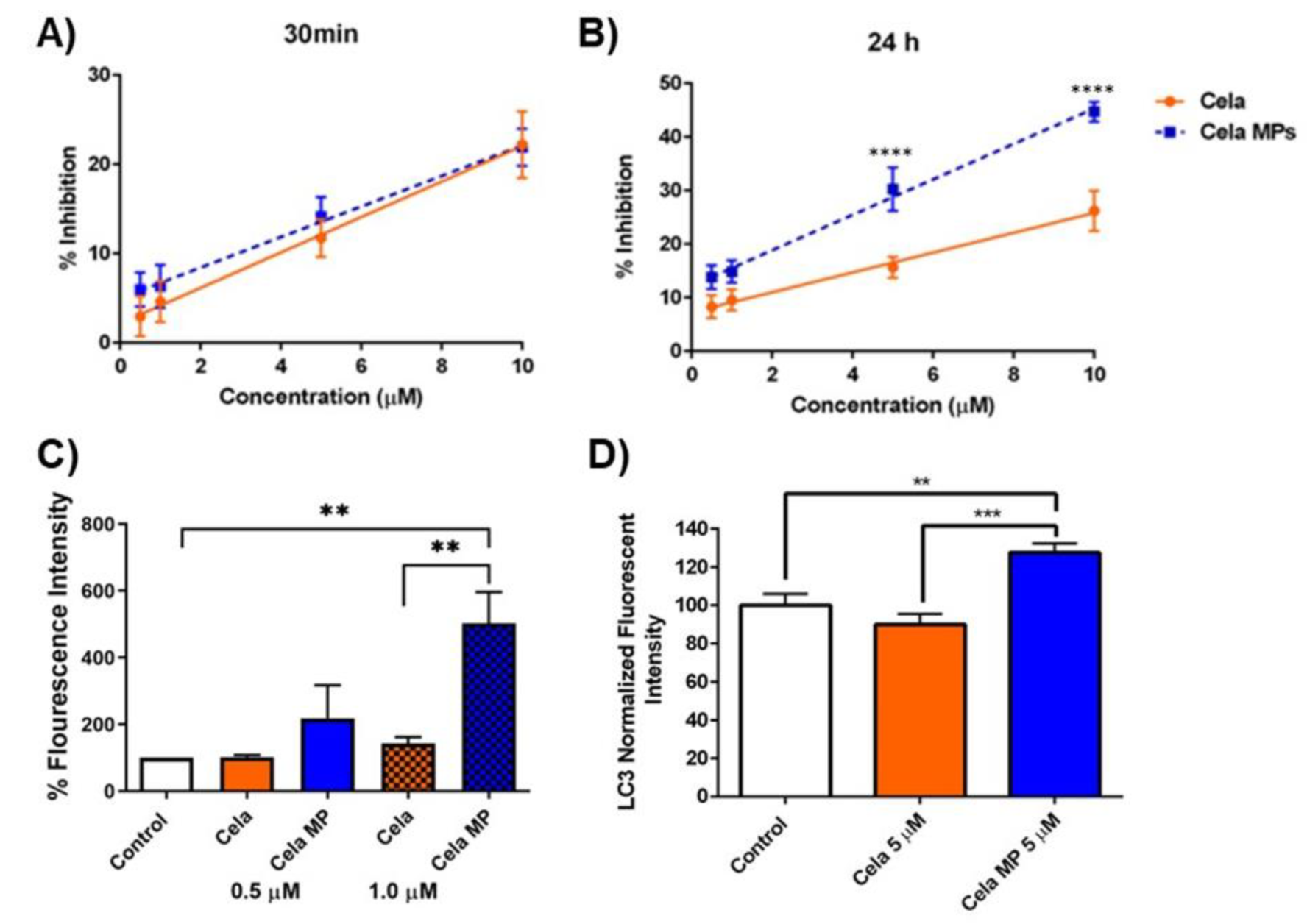
2.9. Mechanistic Studies
2.9.1. DPPH Assay
2.9.2. Caspase-3 Assay
2.9.3. Effect of Cela and Cela MP on Cellular Autophagy
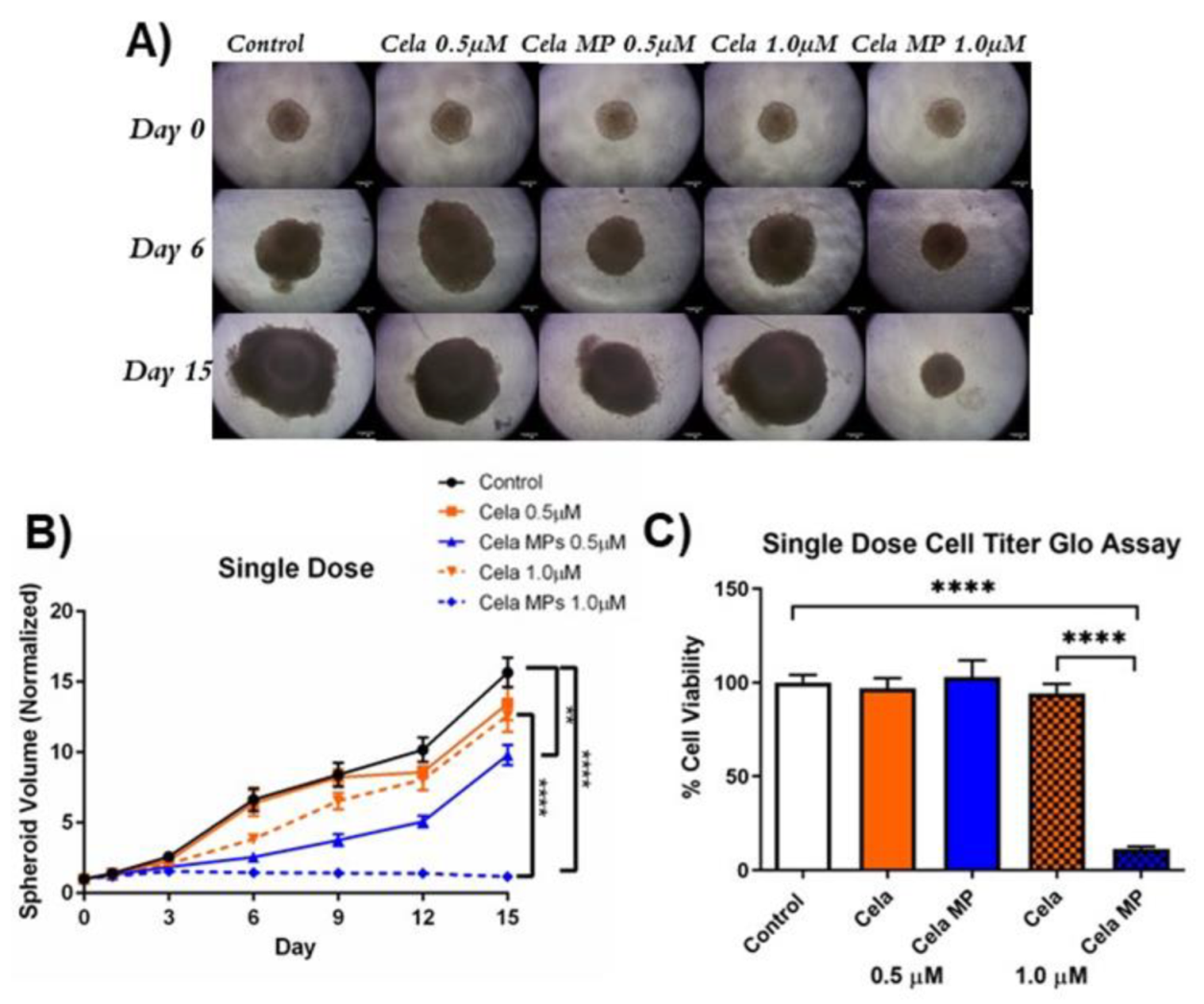
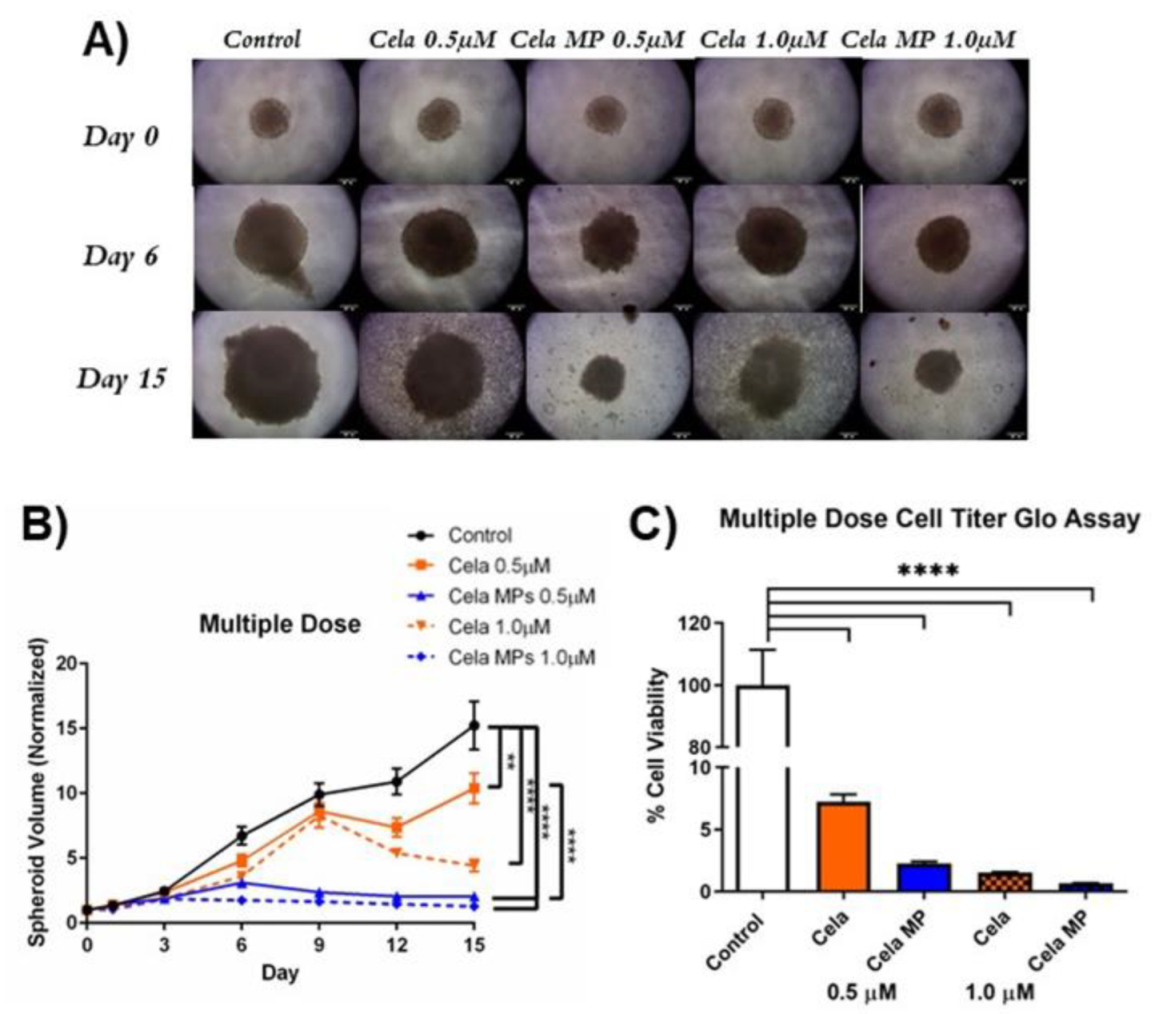
2.10. Determination of Cela MP Efficacy in 3D Spheroid Model
2.10.1. Tumor Volume Reduction Studies
2.10.2. Cell Viability Assay
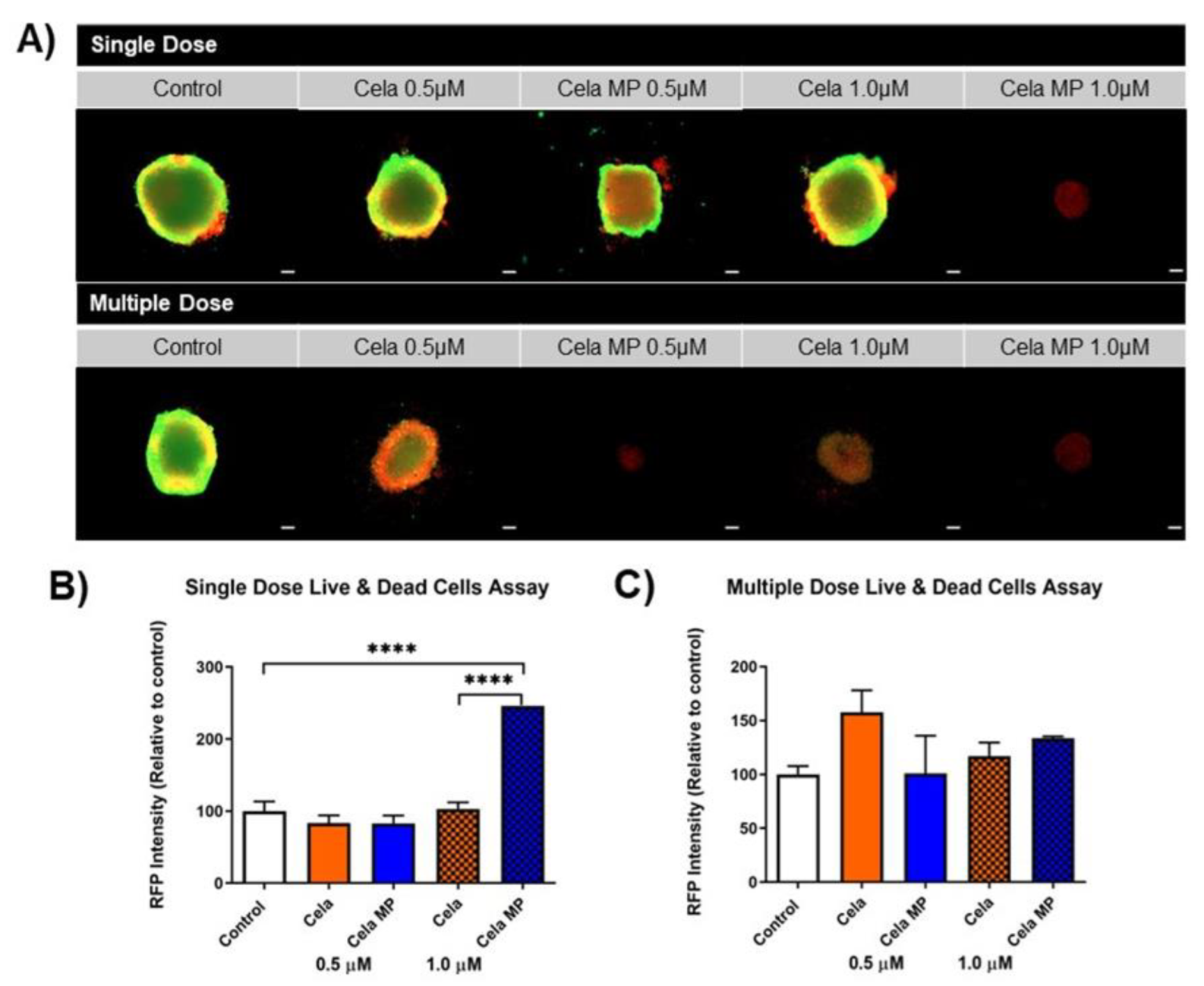
2.10.3. Live/Dead Assay
3. Discussion
4. Materials and Methods
4.1. Materials & Cell Lines
4.2. UPLC Method for Celastrol (Cela) Content
4.3. Formulation of Celastrol Microparticles (Cela MPs)
4.4. Characterization of Cela MPs
4.4.1. Physiochemical Characterization: Particle Size (PS), Polydispersity Index (PDI), and zeta Potential
4.4.2. Drug Content
4.4.3. Morphological Analysis
4.4.4. In Vitro Release Study
4.4.5. Differential Scanning Calorimetry (DSC) Studies
4.4.6. X-ray Diffraction Studies (XRD)
4.5. In Vitro Stability Study
4.6. Aerosolization, Aerodynamic Properties, and Inhalability of Cela MPs
4.7. In Vitro Cell Culture Studies for the Determination of Anti-Cancer Efficacy
Cytotoxicity Studies
4.8. Mechanistic Studies
4.8.1. DPPH Antioxidant Assay
4.8.2. Caspase-3 Assay
4.8.3. Effect of Cela and Cela MP on Cellular Autophagy
4.9. Determination of Cela MP Efficacy in 3D Tumor Spheroid Model
4.9.1. Cell Titer-Glo Cell Viability Study
4.9.2. Live/Dead Cell Assay
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| Cela | Celastrol |
| Cela MP | Celastrol microparticle |
| DCM | dichloromethane |
| DMSO | dimethyl sulfoxide |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DSC | differential scanning calorimetry |
| ED | emitted dose |
| %EE | encapsulation efficiency |
| FBS | fetal bovine serum |
| FPF | Fine Particle Fraction |
| GSD | Geometric standard deviation |
| IC50 | 50% inhibition concentration |
| MM | Malignant mesothelioma |
| MMAD | Mass median aerodynamic diameter |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| MPM | Malignant mesothelioma |
| NaCl | Sodium chloride |
| NaHCO3 | sodium bicarbonate |
| NGI | Next Generation Impactor |
| NHLF | Normal human lung fibroblast |
| OPA | orthophosphoric acid |
| PBS | phosphate-buffered saline |
| PDI | polydispersity index |
| PEI | polyethyleneimine |
| PLGA | poly(lactic-co-glycolic acid) |
| PS | particle size |
| PVA | poly(vinyl alcohol) |
| SEM | Scanning electron microscopy |
| SLF | Simulated Lung Fluid |
| SLS | sodium lauryl sulfate |
| XRD | X-ray diffraction |
References
- Berzenji, L.; Van Schil, P. Multimodality treatment of malignant pleural mesothelioma. F1000Research 2018, 7, 1681. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Ruan, J.; Zheng, Y.; Xiang, D.; Li, N.; Hu, J.; Shen, J.; Deng, Y.; Yao, J.; Zhao, P.; et al. Assessment of Global Trends in the Diagnosis of Mesothelioma From 1990 to 2007. JAMA Netw. Open 2021, 4, e2120360. [Google Scholar] [CrossRef] [PubMed]
- Malignant Mesothelioma Cancer|Stages, Prognosis, Treatment [Internet]. Available online: https://www.mesothelioma.com/mesothelioma/ (accessed on 2 May 2022).
- Kulkarni, N.; Vaidya, B.; Parvathaneni, V.; Bhanja, D.; Gupta, V. Repurposing Quinacrine for Treatment of Malignant Mesothelioma: In-Vitro Therapeutic and Mechanistic Evaluation. Int. J. Mol. Sci. 2020, 21, 6306. [Google Scholar] [CrossRef] [PubMed]
- Key Statistics About Malignant Mesothelioma [Internet]. Available online: https://www.cancer.org/cancer/malignant-mesothelioma/about/key-statistics.html (accessed on 26 July 2022).
- Survival Rates for Mesothelioma [Internet]. Available online: https://www.cancer.org/cancer/malignant-mesothelioma/detection-diagnosis-staging/survival-statistics.html (accessed on 2 May 2022).
- Lim, H.Y.; Ong, P.S.; Wang, L.; Goel, A.; Ding, L.; Wong, A.L.-A.; Ho, P.C.-L.; Sethi, G.; Xiang, X.; Goh, B.C. Celastrol in cancer therapy: Recent developments, challenges and prospects. Cancer Lett. 2021, 521, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Gu, J.; Chen, C.; Duanmu, J.; Miao, J.; Yao, W.; Tao, J.; Tu, M.; Xiong, B.; et al. Celastrol exerts anti-inflammatory effect in liver fibrosis via activation of AMPK-SIRT3 signalling. J. Cell. Mol. Med. 2019, 24, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Pace, S.; Zhang, K.; Jordan, P.M.; Bilancia, R.; Wang, W.; Börner, F.; Hofstetter, R.K.; Potenza, M.; Kretzer, C.; Gerstmeier, J.; et al. Anti-inflammatory celastrol promotes a switch from leukotriene biosynthesis to formation of specialized pro-resolving lipid mediators. Pharmacol. Res. 2021, 167, 105556. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, M.; Wang, B.; Su, Z.; Guo, B.; Qin, L.; Zhang, W.; Zheng, R. The Nrf2-NLRP3-caspase-1 axis mediates the neuroprotective effects of Celastrol in Parkinson’s disease. Redox Biol. 2021, 47, 102134. [Google Scholar] [CrossRef]
- Lin, M.-W.; Lin, C.C.; Chen, Y.-H.; Yang, H.-B.; Hung, S.-Y. Celastrol Inhibits Dopaminergic Neuronal Death of Parkinson’s Disease through Activating Mitophagy. Antioxidants 2019, 9, 37. [Google Scholar] [CrossRef]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Kanabar, D.D.; Patel, K.; Ayehunie, S.; Gupta, V. Enhanced solubility, stability, permeation and anti-cancer efficacy of Celastrol-β-cyclodextrin inclusion complex. J. Mol. Liq. 2020, 318, 113936. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Luo, W.; Chen, S.; Lin, F.; Zhang, X.; Fan, S.; Shen, X.; Wang, Y.; Liang, G. Celastrol induces ROS-mediated apoptosis via directly targeting peroxiredoxin-2 in gastric cancer cells. Theranostics 2020, 10, 10290–10308. [Google Scholar] [CrossRef]
- Shen, B.; Chen, H.-B.; Zhou, H.-G.; Wu, M.-H. Celastrol induces caspase-dependent apoptosis of hepatocellular carcinoma cells by suppression of mammalian target of rapamycin. J. Tradit. Chin. Med. 2021, 41, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, R.; Cheng, L.; Xu, H. Celastrol inhibit the proliferation, invasion and migration of human cervical HeLa cancer cells through down-regulation of MMP-2 and MMP-9. J. Cell Mol. Med. 2021, 25, 5335–5338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Z.; Qi, H.; Chen, L.; Wang, T.; Mao, X.; Shi, H.; Chen, H.; Zhong, M.; Shi, X.; et al. Celastrol upregulated ATG7 triggers autophagy via targeting Nur77 in colorectal cancer. Phytomedicine 2022, 104. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Qian, B.; Pang, J.; Tan, Z.-B.; Zhao, K.; Lei, T. Celastrol Induces Apoptosis and Autophagy via the AKT/mTOR Signaling Pathway in the Pituitary ACTH-secreting Adenoma Cells. Curr. Med Sci. 2022, 42, 387–396. [Google Scholar] [CrossRef]
- Wang, X.; Wan, W.; Lu, J.; Zhang, Y.; Quan, G.; Pan, X.; Liu, P. Inhalable cryptotanshinone spray-dried swellable microparticles for pulmonary fibrosis therapy by regulating TGF-β1/Smad3, STAT3 and SIRT3 pathways. Eur. J. Pharm. Biopharm. 2022, 172, 177–1792. [Google Scholar] [CrossRef]
- Alipour, S.; Montaseri, H.; Tafaghodi, M. Inhalable, large porous PLGA microparticles loaded with paclitaxel: Preparation, in vitro and in vivo characterization. J. Microencapsul. 2015, 32, 661–668. [Google Scholar] [CrossRef]
- Sheth, P.; Stein, S.W.; Myrdal, P.B. Factors Influencing Aerodynamic Particle Size Distribution of Suspension Pressurized Metered Dose Inhalers. AAPS PharmSciTech 2014, 16, 192–201. [Google Scholar] [CrossRef]
- Ngan, C.L.; Asmawi, A.A. Lipid-based pulmonary delivery system: A review and future considerations of formulation strategies and limitations. Drug Deliv. Transl. Res. 2018, 8, 1527–1544. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Han, H.; Chen, J.; Wang, Y.; Li, Q.; Wang, Y. Disulfiram-loaded porous PLGA microparticle for inhibiting the proliferation and migration of non-small-cell lung cancer. Int. J. Nanomed. 2017, 12, 827–837. [Google Scholar] [CrossRef]
- Hu, Y.; Li, M.; Zhang, M.; Jin, Y. Inhalation treatment of idiopathic pulmonary fibrosis with curcumin large porous microparticles. Int. J. Pharm. 2018, 551, 212–222. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, L.; Su, J.; Sun, Y.; Zhang, L.; Li, J.; Beck-Broichsitter, M.; Muenster, U.; Chen, L.; Mao, S. Engineering large porous microparticles with tailored porosity and sustained drug release behavior for inhalation. Eur. J. Pharm. Biopharm. 2020, 155, 139–146. [Google Scholar] [CrossRef]
- Ungaro, F.; Bianca, R.D.D.V.; Giovino, C.; Miro, A.; Sorrentino, R.; Quaglia, F.; La Rotonda, M.I. Insulin-loaded PLGA/cyclodextrin large porous particles with improved aerosolization properties: In vivo deposition and hypoglycaemic activity after delivery to rat lungs. J. Control. Release 2009, 135, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.J.; Lee, J.; Seo, J.Y.; Rhim, T.; Kim, S.-H.; Yoon, H.J.; Lee, K.Y. Preparation of budesonide-loaded porous PLGA microparticles and their therapeutic efficacy in a murine asthma model. J. Control. Release 2011, 150, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Byeon, H.J.; Kim, T.H.; Lee, E.S.; Oh, K.T.; Shin, B.S.; Lee, K.C.; Youn, Y.S. Doxorubicin-loaded highly porous large PLGA microparticles as a sustained- release inhalation system for the treatment of metastatic lung cancer. Biomaterials 2012, 33, 5574–5583. [Google Scholar] [CrossRef]
- Shi, X.; Li, C.; Gao, S.; Zhang, L.; Han, H.; Zhang, J.; Shi, W.; Li, Q. Combination of doxorubicin-based chemotherapy and polyethylenimine/p53 gene therapy for the treatment of lung cancer using porous PLGA microparticles. Colloids Surf. B Biointerfaces 2014, 122, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, Y.; Zhang, H.; Zhou, W.; Ma, G. A novel strategy for the preparation of porous microspheres and its application in peptide drug loading. J. Colloid Interface Sci. 2016, 478, 46–53. [Google Scholar] [CrossRef]
- Gallo, L.; Ramírez-Rigo, M.V.; Bucalá, V. Development of porous spray-dried inhalable particles using an organic solvent-free technique. Powder Technol. 2018, 342, 642–652. [Google Scholar] [CrossRef]
- Li, P.; Zhou, X.; Qu, D.; Guo, M.; Fan, C.; Zhou, T.; Ling, Y. Preliminary study on fabrication, characterization and synergistic anti-lung cancer effects of self-assembled micelles of covalently conjugated celastrol–polyethylene glycol–ginsenoside Rh2. Drug Deliv. 2017, 24, 834–845. [Google Scholar] [CrossRef]
- Ayyoob, M.; Kim, Y.J. Effect of Chemical Composition Variant and Oxygen Plasma Treatments on the Wettability of PLGA Thin Films, Synthesized by Direct Copolycondensation. Polymers 2018, 10, 1132. [Google Scholar] [CrossRef]
- Deng, Y.-N.; Shi, J.; Liu, J.; Qu, Q.-M. Celastrol protects human neuroblastoma SH-SY5Y cells from rotenone-induced injury through induction of autophagy. Neurochem. Int. 2013, 63, 1–9. [Google Scholar] [CrossRef]
- Lee, H.-W.; Jang, K.S.B.; Choi, H.J.; Jo, A.; Cheong, J.-H.; Chun, K.-H. Celastrol inhibits gastric cancer growth by induction of apoptosis and autophagy. BMB Rep. 2014, 47, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Zhang, J.; Sun, L.-L.; Li, B.-H.; Gao, H.-L.; Xie, T.; Zhang, N.; Ye, Z.-M. Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells: An in vitro and in vivo study. Cell Death Dis. 2015, 6, e1604. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.S.; Parvathaneni, V.; Shukla, S.K.; Barasa, L.; Perron, J.C.; Yoganathan, S.; Muth, A.; Gupta, V. Tyrosine kinase inhibitor conjugated quantum dots for non-small cell lung cancer (NSCLC) treatment. Eur. J. Pharm. Sci. 2019, 133, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Kulkarni, N.S.; Chan, A.; Parvathaneni, V.; Farrales, P.; Muth, A.; Gupta, V. Metformin-Encapsulated Liposome Delivery System: An Effective Treatment Approach against Breast Cancer. Pharmaceutics 2019, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, J.; Xu, Z.; Chen, L.; Luo, R.; Zhang, C.; Gao, F.; Zhang, J.; Fu, C. Celastrol: A Review of Useful Strategies Overcoming its Limitation in Anticancer Application. Front. Pharmacol. 2020, 11, 558741. [Google Scholar] [CrossRef]
- Cascão, R.; Fonseca, J.E.; Moita, L.F. Celastrol: A Spectrum of Treatment Opportunities in Chronic Diseases. Front. Med. 2017, 4, 69. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Mukherjee, T.; Bishayee, A. Molecular targets of celastrol in cancer: Recent trends and advancements. Crit. Rev. Oncol. 2018, 128, 70–81. [Google Scholar] [CrossRef]
- Cascão, R.; Carvalho, T.; Goncalves, J.; Moita, L.; Fonseca, J. AB0096 Efficacy and safety of oral administration of pure celastrol in aia rats. Ann. Rheum. Dis. 2017, 76, 1080. [Google Scholar] [CrossRef]
- Hou, W.; Liu, B.; Xu, H. Celastrol: Progresses in structure-modifications, structure-activity relationships, pharmacology and toxicology. Eur. J. Med. Chem. 2020, 189, 112081. [Google Scholar] [CrossRef]
- Thakur, P.; Sonawane, S.S.; Sonawane, S.H.; Bhanvase, B.A. Nanofluids-based delivery system, encapsulation of nanoparticles for stability to make stable nanofluids. In Encapsulation of Active Molecules and Their Delivery System [Internet]; Elsevier: Amsterdam, The Netherlands, 2020; p. 141. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128193631000090 (accessed on 20 July 2022).
- Heijerman, H.; Westerman, E.; Conway, S.; Touw, D. Inhaled medication and inhalation devices for lung disease in patients with cystic fibrosis: A European consensus. J. Cyst. Fibros. 2009, 8, 295–315. [Google Scholar] [CrossRef]
- Champion, J.A.; Walker, A.; Mitragotri, S. Role of Particle Size in Phagocytosis of Polymeric Microspheres. Pharm. Res. 2008, 25, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bajaj, N.; Xu, P.; Ohn, K.; Tsifansky, M.D.; Yeo, Y. Development of highly porous large PLGA microparticles for pulmonary drug delivery. Biomaterials 2009, 30, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Aina, A.; Boukari, Y.; Doughty, S.; Morris, A.; Billa, N. Effect of volume of porogens on the porosity of PLGA scaffolds in pH-controlled environment. Pharm. Dev. Technol. 2018, 23, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cao, Y.-P.; Feng, X.-Q.; Gao, H. Mechanics of morphological instabilities and surface wrinkling in soft materials: A review. Soft Matter 2012, 8, 5728–5745. [Google Scholar] [CrossRef]
- Lechanteur, A.; Evrard, B. Influence of Composition and Spray-Drying Process Parameters on Carrier-Free DPI Properties and Behaviors in the Lung: A review. Pharmaceutics 2020, 12, 55. [Google Scholar] [CrossRef]
- Li, M.; Joung, D.; Hughes, B.; Waldman, S.D.; Kozinski, J.A.; Hwang, D.K. Wrinkling Non-Spherical Particles and Its Application in Cell Attachment Promotion. Sci. Rep. 2016, 6, 30463. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Tedesco, E.; Benetti, F.; Mabondzo, A.; Montagner, I.M.; Marigo, I.; Gonzalez-Touceda, D.; Tovar, S.; Diéguez, C.; Santander-Ortega, M.J.; et al. Rational design of polyarginine nanocapsules intended to help peptides overcoming intestinal barriers. J. Control. Release 2017, 263, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Kulkarni, N.S.; Farrales, P.; Kanabar, D.D.; Parvathaneni, V.; Kunda, N.K.; Muth, A.; Gupta, V. Sorafenib Loaded Inhalable Polymeric Nanocarriers against Non-Small Cell Lung Cancer. Pharm. Res. 2020, 37, 1–19. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Sanna, V.; Chamcheu, J.C.; Pala, N.; Mukhtar, H.; Sechi, M.; Siddiqui, I.A. Nanoencapsulation of natural triterpenoid celastrol for prostate cancer treatment. Int. J. Nanomed. 2015, ume 10, 6835–6846. [Google Scholar] [CrossRef]
- Zhan, S.; Paik, A.; Onyeabor, F.; Ding, B.; Prabhu, S.; Wang, J. Oral Bioavailability Evaluation of Celastrol-Encapsulated Silk Fibroin Nanoparticles Using an Optimized LC-MS/MS Method. Molecules 2020, 25, 3422. [Google Scholar] [CrossRef] [PubMed]
- Surber, M.W.; Beck, S.; Pham, S.; Marsden, A.T.; Gandi, S.K.; Baily, J.; McElroy, M.C. Inhaled nintedanib is well-tolerated and delivers key pharmacokinetic parameters required to treat bleomycin-induced pulmonary fibrosis. Pulm. Pharmacol. Ther. 2020, 63, 101938. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Farrales, P.T.; Kunda, N.K.; Muth, A.; Gupta, V. Systematic Development and Optimization of Inhalable Pirfenidone Liposomes for Non-Small Cell Lung Cancer Treatment. Pharmaceutics 2020, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kanabar, D.D.; Muth, A.; Gupta, V. Cyclodextrin Complexation for Enhanced Stability and Non-invasive Pulmonary Delivery of Resveratrol—Applications in Non-small Cell Lung Cancer Treatment. AAPS PharmSciTech 2020, 21, 1–14. [Google Scholar] [CrossRef]
- Elbatanony, R.S.; Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Chauhan, G.; Kunda, N.K.; Gupta, V. Afatinib-loaded inhalable PLGA nanoparticles for localized therapy of non-small cell lung cancer (NSCLC)—development and in-vitro efficacy. Drug Deliv. Transl. Res. 2020, 11, 927–943. [Google Scholar] [CrossRef]
- Pulivendala, G.; Bale, S.; Godugu, C. Inhalation of sustained release microparticles for the targeted treatment of respiratory diseases. Drug Deliv. Transl. Res. 2019, 10, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Chew, N.Y.K.; Chan, H.-K. Use of Solid Corrugated Particles to Enhance Powder Aerosol Performance. Pharm. Res. 2001, 18, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.K. What is the role of particle morphology in pharmaceutical powder aerosols? Expert Opin. Drug. Deliv. 2008, 5, 909–914. [Google Scholar] [CrossRef]
- Chew, N.Y.; Chan, H.-K. The Role of Particle Properties in Pharmaceutical Powder Inhalation Formulations. J. Aerosol Med. 2002, 15, 325–330. [Google Scholar] [CrossRef]
- Brasen, J.C.; Olsen, L.F.; Hallett, M.B. Cell surface topology creates high Ca2+ signalling microdomains. Cell Calcium 2010, 47, 339–349. [Google Scholar] [CrossRef]
- Edlund, A.F.; Swanson, R.; Preuss, D. Pollen and Stigma Structure and Function: The Role of Diversity in Pollination. Plant Cell 2004, 16, S84–S97. [Google Scholar] [CrossRef] [PubMed]
- Jannuzzi, A.T.; Kara, M.; Alpertunga, B. Celastrol ameliorates acetaminophen-induced oxidative stress and cytotoxicity in HepG2 cells. Hum. Exp. Toxicol. 2017, 37, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, R.; Satoh, K.; Nakata, T.; Shindo, T.; Kikuchi, N.; Satoh, T.; Shimokawa, H. Identification of Celastrol as a Novel Therapeutic Agent for Pulmonary Arterial Hypertension and Right Ventricular Failure Through Suppression of Bsg (Basigin)/CyPA (Cyclophilin A). Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhong, Q.; Chen, X.; Wu, X.; Sha, R.; Song, G.; Zhang, C.; Chen, X. Neuroprotective Effects of Celastrol on Transient Global Cerebral Ischemia Rats via Regulating HMGB1/NF-κB Signaling Pathway. Front. Neurosci. 2020, 14, 847. [Google Scholar] [CrossRef] [PubMed]
- Divya, T.; Dineshbabu, V.; Soumyakrishnan, S.; Sureshkumar, A.; Sudhandiran, G. Celastrol enhances Nrf2 mediated antioxidant enzymes and exhibits anti-fibrotic effect through regulation of collagen production against bleomycin-induced pulmonary fibrosis. Chem. Interactions 2016, 246, 52–62. [Google Scholar] [CrossRef]
- Wang, C.; Shi, C.; Yang, X.; Yang, M.; Sun, H.; Wang, C. Celastrol suppresses obesity process via increasing antioxidant capacity and improving lipid metabolism. Eur. J. Pharmacol. 2014, 744, 52–58. [Google Scholar] [CrossRef]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kulkarni, N.S.; Muth, A.; Kunda, N.K.; Gupta, V. Inhalable resveratrol-cyclodextrin complex loaded biodegradable nanoparticles for enhanced efficacy against non-small cell lung cancer. Int. J. Biol. Macromol. 2020, 164, 638–650. [Google Scholar] [CrossRef]
- Los, M.J.; Mozoluk, M.; Ferrari, D.; Stepczynska, A.; Stroh, C.; Renz, A.; Herceg, Z.; Wang, Z.-Q.; Schulze-Osthoff, K. Activation and Caspase-mediated Inhibition of PARP: A Molecular Switch between Fibroblast Necrosis and Apoptosis in Death Receptor Signaling. Mol. Biol. Cell 2002, 13, 978–988. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Cui, W.; Wang, W.; Zhang, H.; Liu, L.; Zhang, Z.; Li, Z.; Ying, G.; Zhang, N.; et al. Visualization of caspase-3-like activity in cells using a genetically encoded fluorescent biosensor activated by protein cleavage. Nat. Commun. 2013, 4, 2157. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef]
- Mizushima, N. The pleiotropic role of autophagy: From protein metabolism to bactericide. Cell Death Differ. 2005, 12, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.-E.; Kim, Y.-S.; Jung, J.-W.; Kwon, S.-J.; Park, D.-S.; Cha, B.-K.; Oh, S.-H.; Yoon, K.-H.; Jeong, E.-T.; Kim, H.-R. Inhibition of autophagy potentiates pemetrexed and simvastatin-induced apoptotic cell death in malignant mesothelioma and non-small cell lung cancer cells. Oncotarget 2015, 6, 29482–29496. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, M.T.; Ryan, K.M. The multiple roles of autophagy in cancer. Carcinog. 2011, 32, 955–963. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, P.; Wang, X.; Wang, L.; Zhu, Y.; Song, Y.; Gao, W. Celastrol mediates autophagy and apoptosis via the ROS/JNK and Akt/mTOR signaling pathways in glioma cells. J. Exp. Clin. Cancer Res. CR 2019, 38, 184. [Google Scholar] [CrossRef]
- Ferreira, L.; Gaspar, V.; Mano, J. Design of spherically structured 3D in vitro tumor models -Advances and prospects. Acta Biomater. 2018, 75, 11–34. [Google Scholar] [CrossRef]
- Li, Q.; Verschraegen, C.F.; Mendoza, J.; Hassan, R. Cytotoxic activity of the recombinant anti-mesothelin immunotoxin, SS1(dsFv)PE38, towards tumor cell lines established from ascites of patients with peritoneal mesotheliomas. Anticancer. Res. 2004, 24, 1327–1336. [Google Scholar]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Gupta, V. Metformin-loaded chitosomes for treatment of malignant pleural mesothelioma–A rare thoracic cancer. Int. J. Biol. Macromol. 2020, 160, 128–141. [Google Scholar] [CrossRef]
- Vaidya, B.; Kulkarni, N.S.; Shukla, S.K.; Parvathaneni, V.; Chauhan, G.; Damon, J.K.; Sarode, A.; Garcia, J.V.; Kunda, N.; Mitragotri, S.; et al. Development of inhalable quinacrine loaded bovine serum albumin modified cationic nanoparticles: Repurposing quinacrine for lung cancer therapeutics. Int. J. Pharm. 2020, 577, 118995. [Google Scholar] [CrossRef]
- Kulkarni, N.S.; Chauhan, G.; Goyal, M.; Sarvepalli, S.; Gupta, V. Development of gelatin methacrylate (GelMa) hydrogels for versatile intracavitary applications. Biomater. Sci. 2022, 10, 4492–4507. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.C.; Cacabelos, R.; Lombardi, V.R.; Álvarez, X.A.; Vigo, C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2001, 25, 1341–1357. [Google Scholar] [CrossRef]
- You, D.; Jeong, Y.; Yoon, S.Y.; A Kim, S.; Kim, S.W.; Nam, S.J.; Kim, S. Celastrol attenuates the inflammatory response by inhibiting IL-1β expression in triple-negative breast cancer cells. Oncol. Rep. 2021, 45, 89. [Google Scholar] [CrossRef] [PubMed]
- Bufu, T.; Di, X.; Yilin, Z.; Gege, L.; Xi, C.; Ling, W. Celastrol inhibits colorectal cancer cell proliferation and migration through suppression of MMP3 and MMP7 by the PI3K/AKT signaling pathway. Anti-Cancer Drugs 2018, 29, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhao, N.; Xu, L.; Ye, P.; Nan, X.; Zhou, H.; Shi, Z. Celastrol inhibits the growth of ovarian cancer cells in vitro and In Vivo. Gynecol. Oncol. 2019, 154, 101. [Google Scholar] [CrossRef]
- Brüningk, S.C.; Rivens, I.; Box, C.; Oelfke, U.; ter Haar, G. 3D tumour spheroids for the prediction of the effects of radiation and hyperthermia treatments. Sci. Rep. 2020, 10, 1653. [Google Scholar] [CrossRef]
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186. [Google Scholar] [CrossRef]
- Roper, S.J.; Coyle, B. Establishing an In Vitro 3D Spheroid Model to Study Medulloblastoma Drug Response and Tumor Dissemination. Curr. Protoc. 2022, 2, e357. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Chilamakuri, R.; Kulkarni, N.S.; Wang, X.; Agarwal, S.; Gupta, V. Repurposing clofazimine for malignant pleural mesothelioma treatment — In-vitro assessment of efficacy and mechanism of action. Life Sci. 2022, 306, 120843. [Google Scholar] [CrossRef]
| No. | A1 | O1 | A2 | % Entrapment Efficiency | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|---|---|
| F6 | Water + 1% NaCl | 2 mg drug in DCM and DMSO | 1% PVA | 72.8 ± 6.1 | 2076 ± 390 | 0.3 ± 0.2 | −36.6 ± 3.8 |
| Cell Line | IC50 (µM) | |
|---|---|---|
| Cela | Cela MP | |
| MSTO-211H | 3.6 ± 0.4 | 2.7 ± 0.3 * |
| H28 | 12.9 ± 3.3 | 3.8 ± 0.6 ** |
| H2452 | 18.7 ± 0.1 | 3.8 ± 0.1 **** |
| ROB | 3.5 ± 0.2 | 2.3 ± 0.3 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chauhan, G.; Tacderas, A.R.L.; Muth, A.; Gupta, V. Surface-Modified Inhaled Microparticle-Encapsulated Celastrol for Enhanced Efficacy in Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2023, 24, 5204. https://doi.org/10.3390/ijms24065204
Wang X, Chauhan G, Tacderas ARL, Muth A, Gupta V. Surface-Modified Inhaled Microparticle-Encapsulated Celastrol for Enhanced Efficacy in Malignant Pleural Mesothelioma. International Journal of Molecular Sciences. 2023; 24(6):5204. https://doi.org/10.3390/ijms24065204
Chicago/Turabian StyleWang, Xuechun, Gautam Chauhan, Alison R. L. Tacderas, Aaron Muth, and Vivek Gupta. 2023. "Surface-Modified Inhaled Microparticle-Encapsulated Celastrol for Enhanced Efficacy in Malignant Pleural Mesothelioma" International Journal of Molecular Sciences 24, no. 6: 5204. https://doi.org/10.3390/ijms24065204
APA StyleWang, X., Chauhan, G., Tacderas, A. R. L., Muth, A., & Gupta, V. (2023). Surface-Modified Inhaled Microparticle-Encapsulated Celastrol for Enhanced Efficacy in Malignant Pleural Mesothelioma. International Journal of Molecular Sciences, 24(6), 5204. https://doi.org/10.3390/ijms24065204







