Emerging Strategies in Proteolysis-Targeting Chimeras (PROTACs): Highlights from 2022
Abstract
1. Introduction
- Selectivity and controllability;
- Cell permeability;
- Linker flexibility;
- Druggability;
- PROTAC-based approaches.
2. Discussion
2.1. Modalities with Improved Selectivity and Controllability
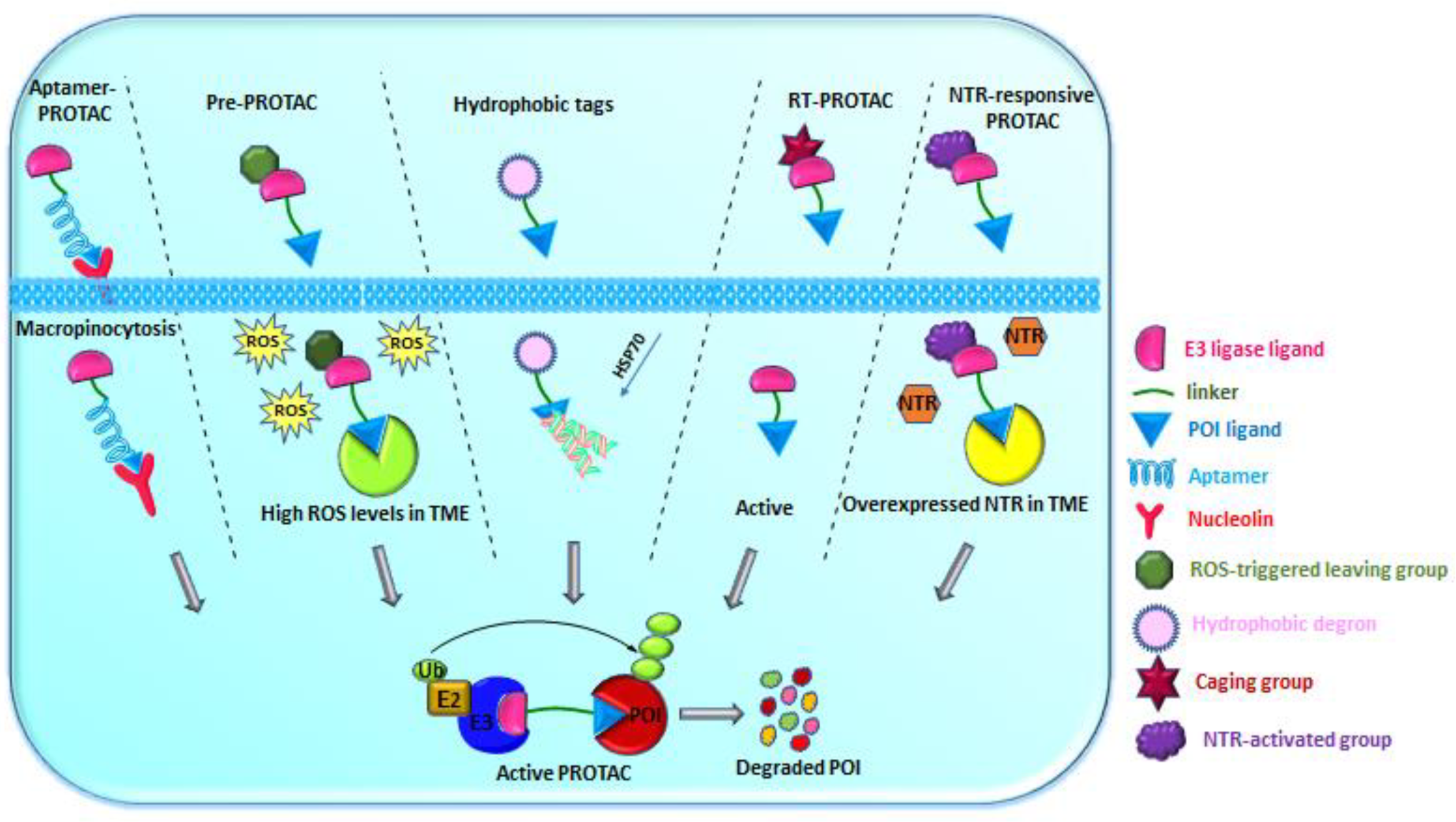
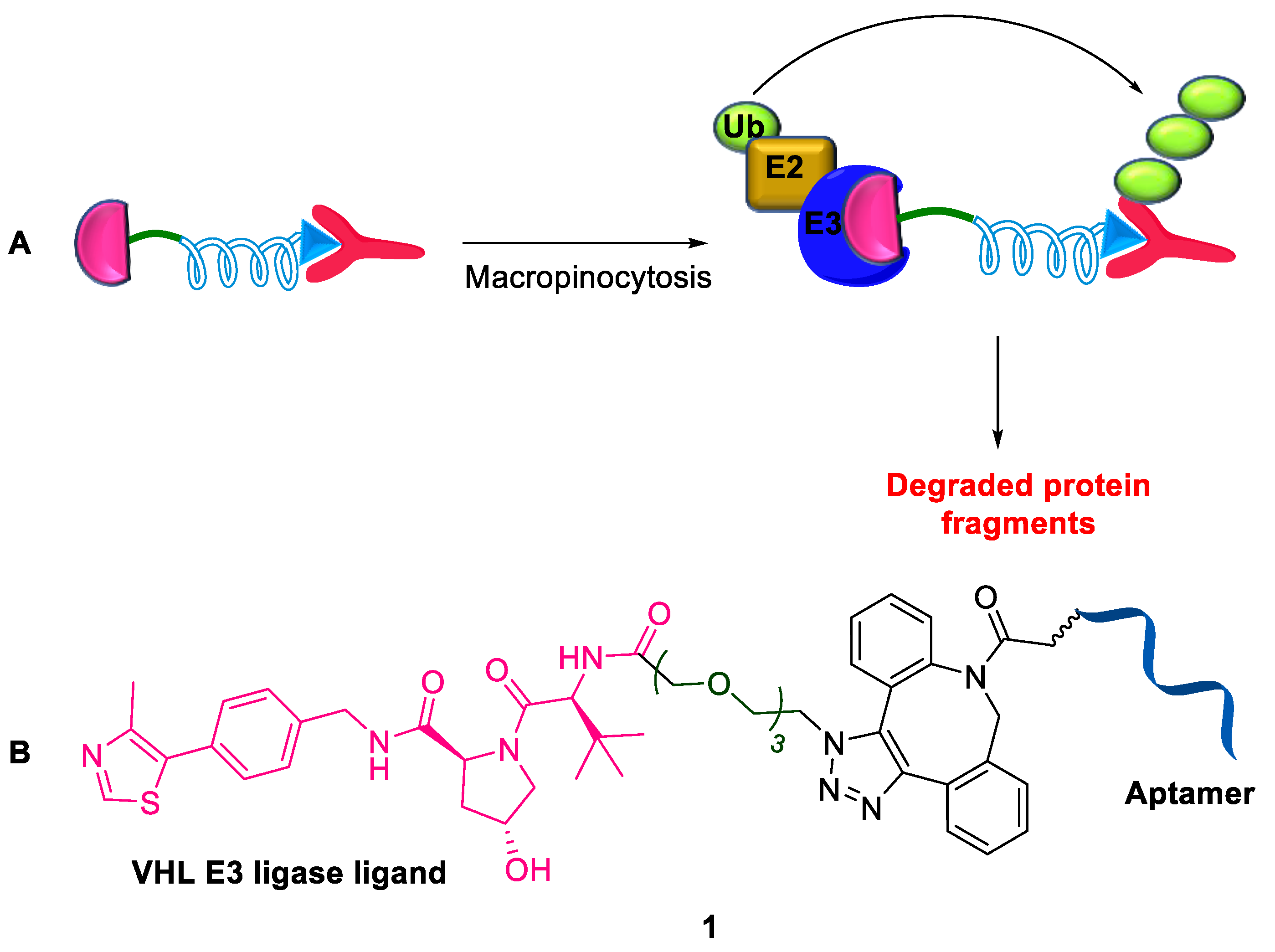
2.1.1. Aptamer-Based PROTAC
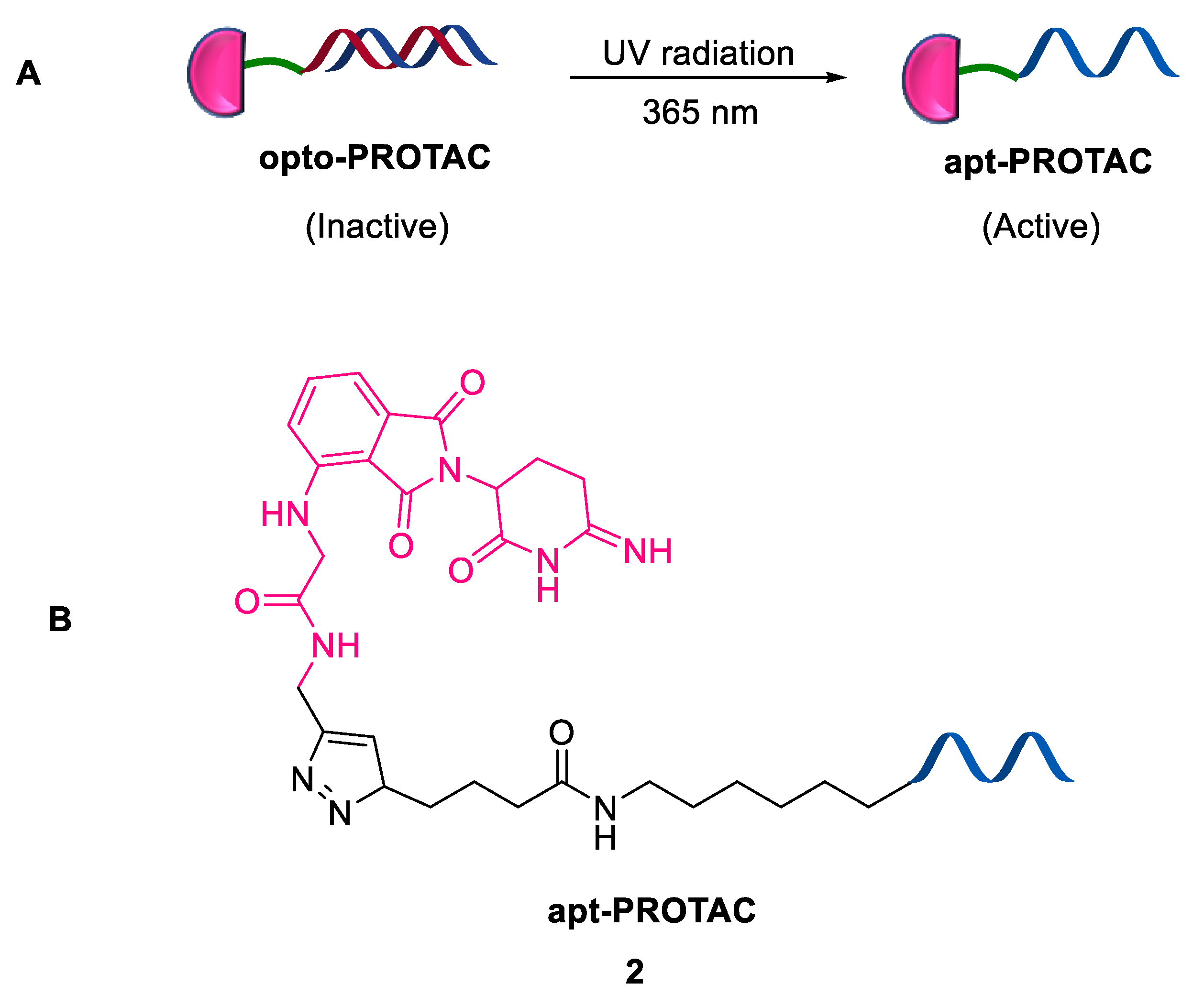
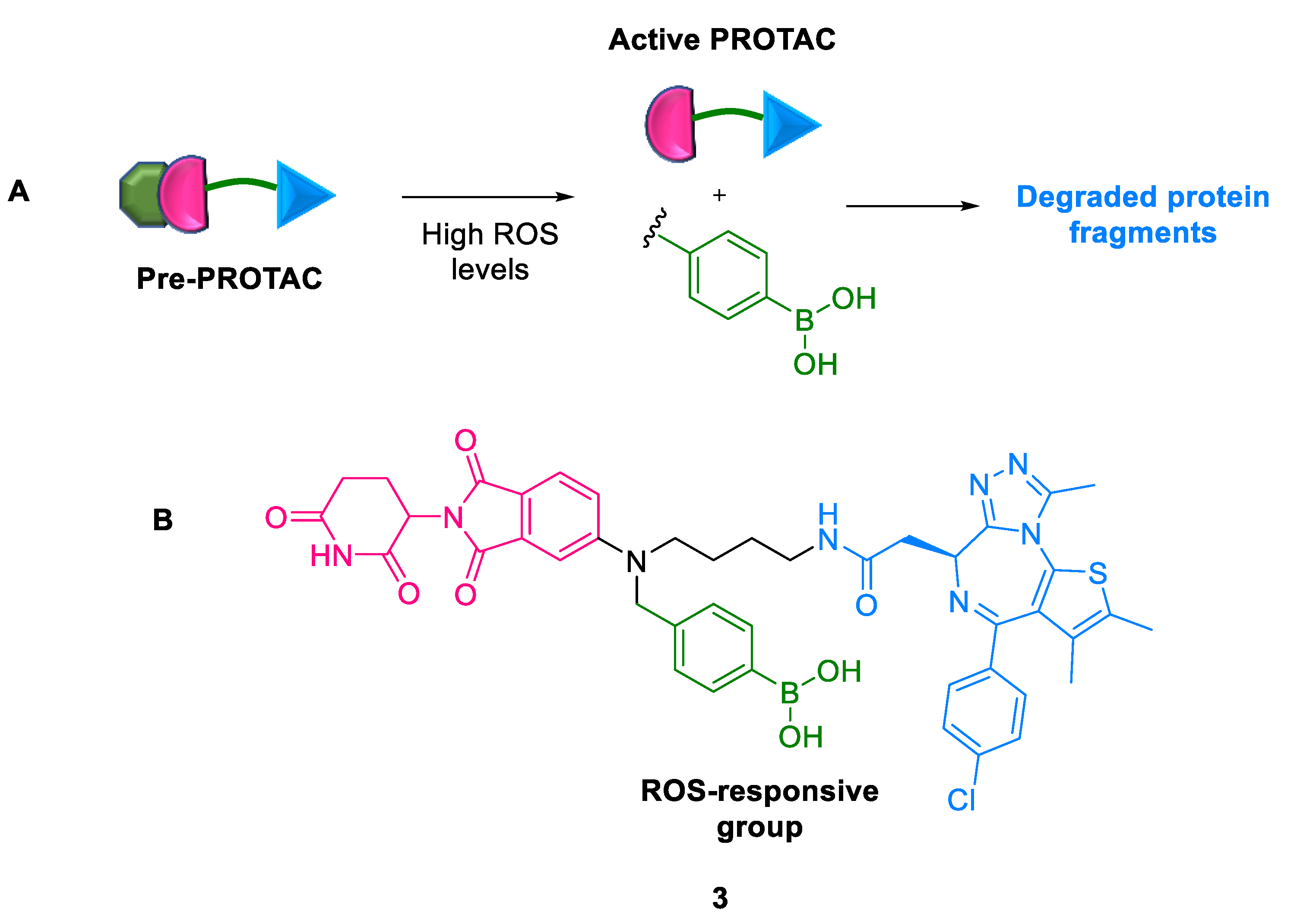
2.1.2. Pre-PROTACs
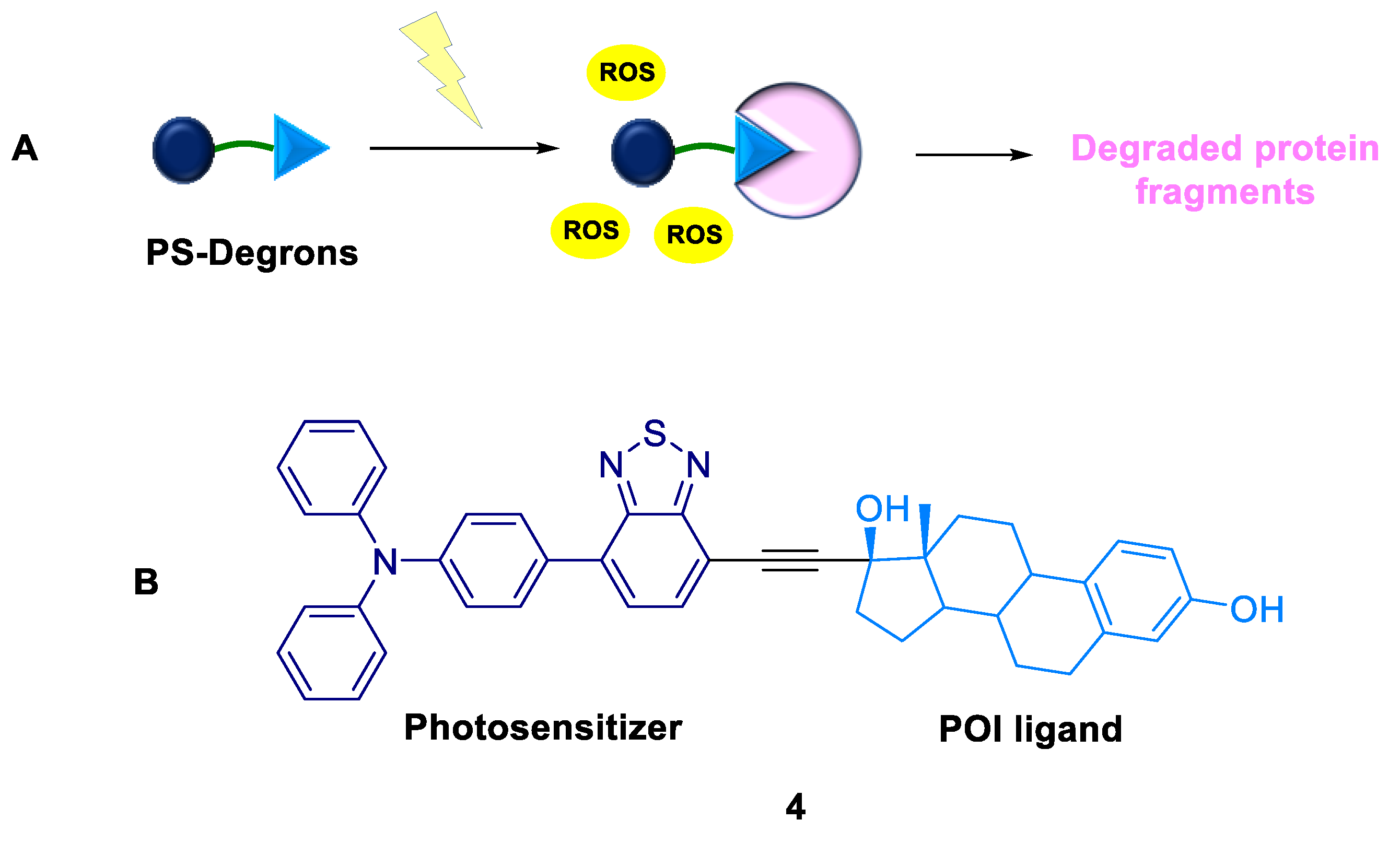
2.1.3. PS-Degrons
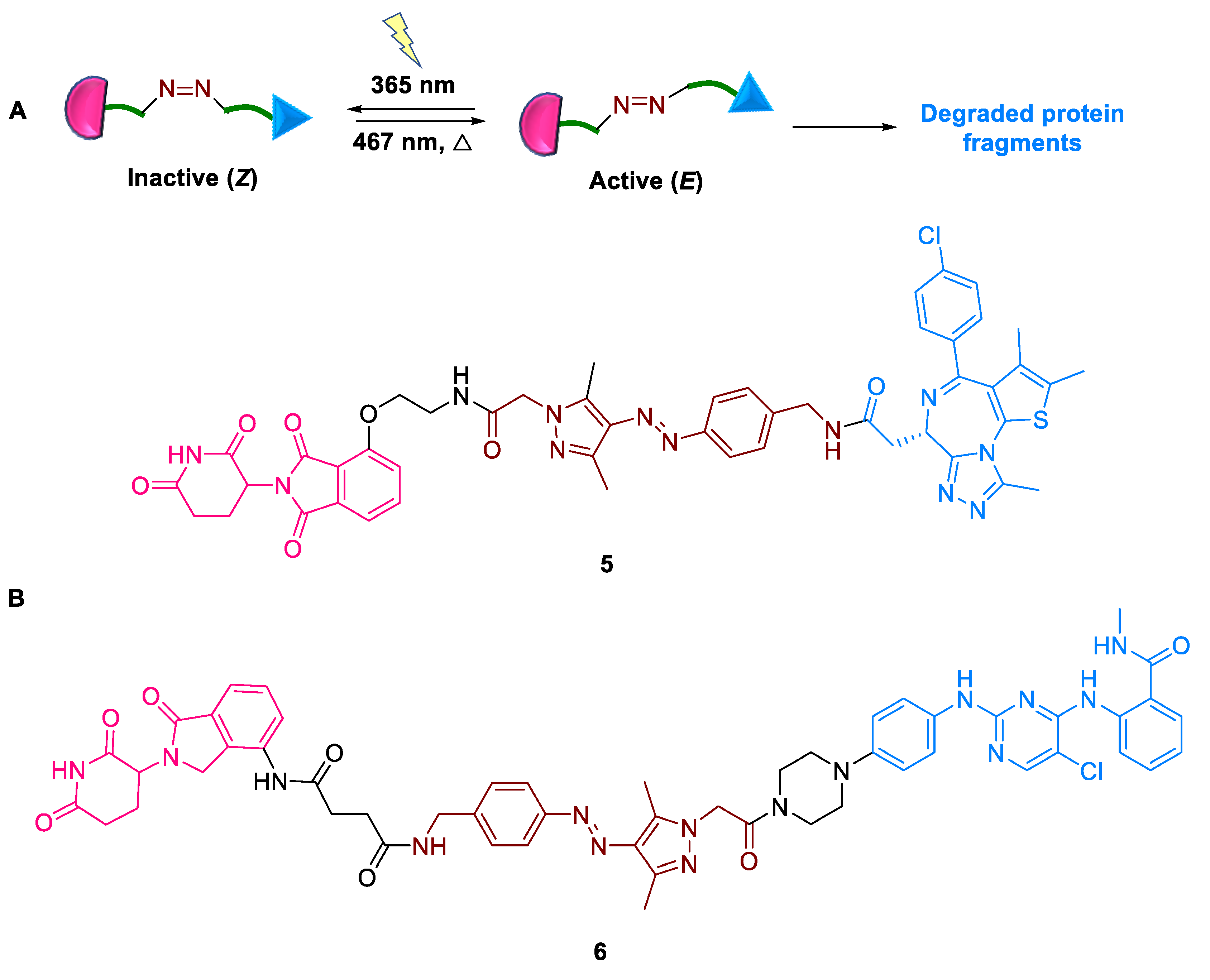
2.1.4. AP-PROTACs
2.1.5. RT-PROTACs
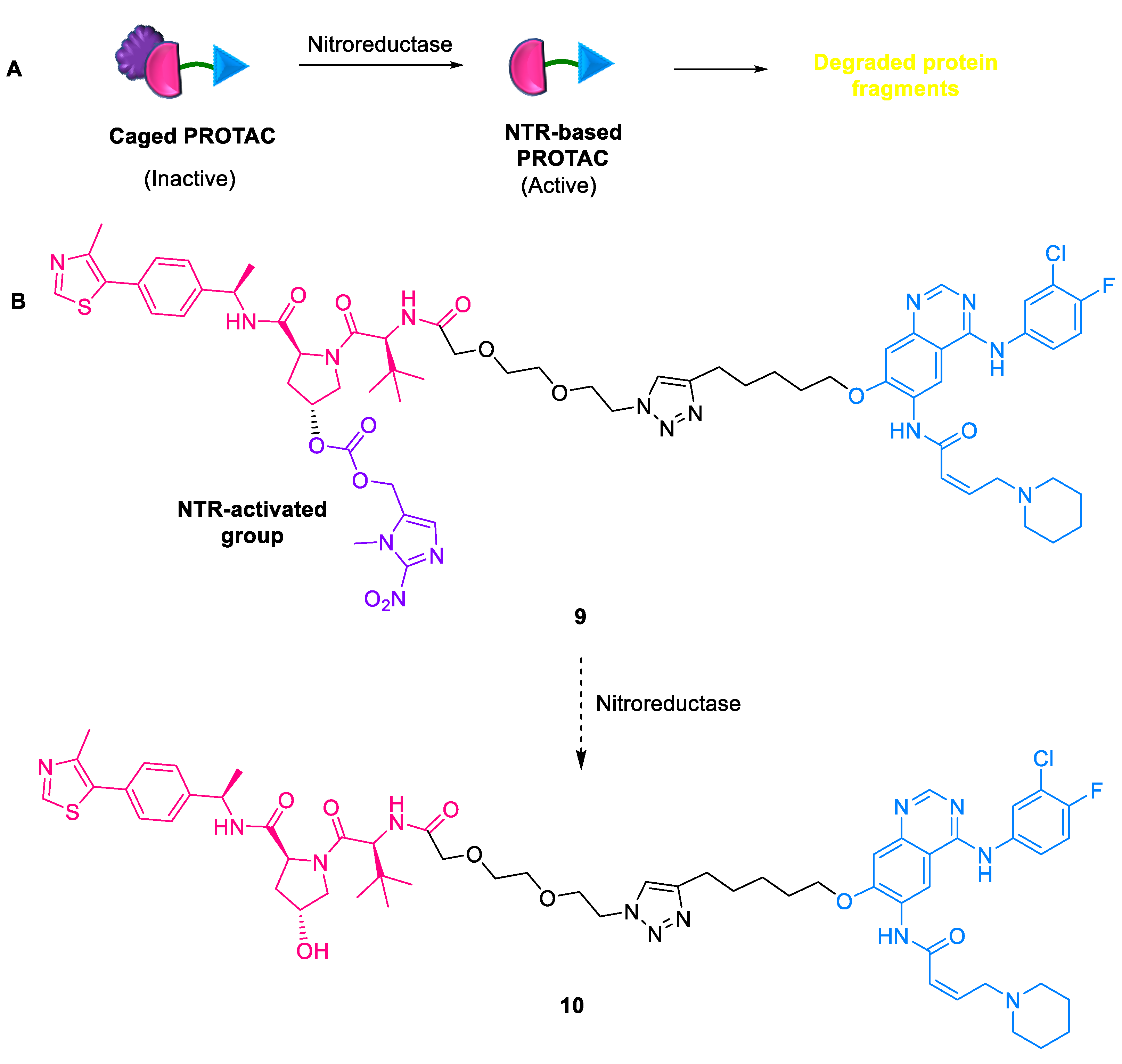
2.1.6. NTR-Based PROTACs
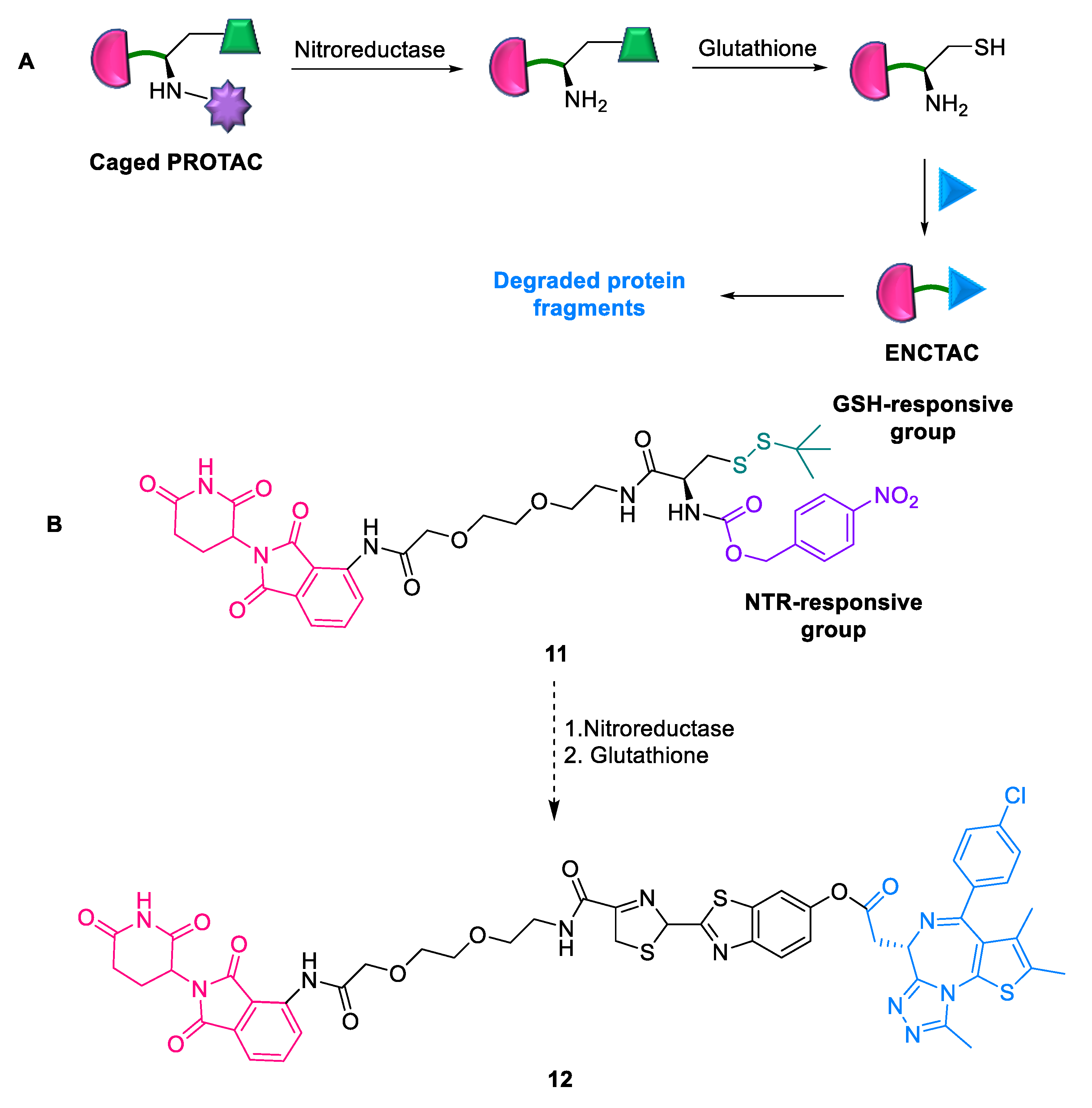
2.1.7. ENCTACs
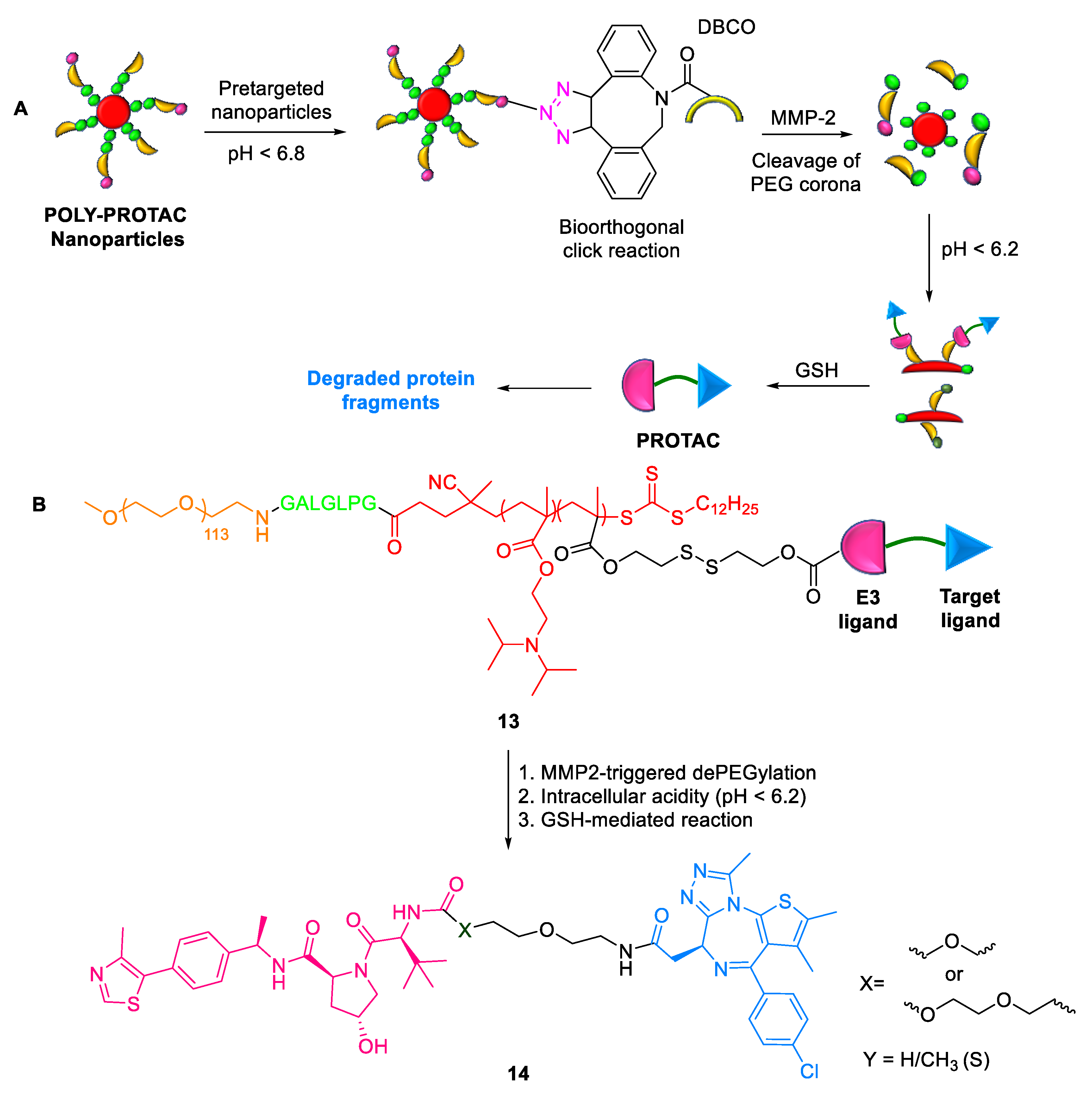
2.1.8. POLY-PROTACs
2.1.9. Smart Nano-PROTACs
2.2. Modalities with Improved Linker Flexibility
Split-PROTACs
2.3. Modalities with Improved Cell Permeability
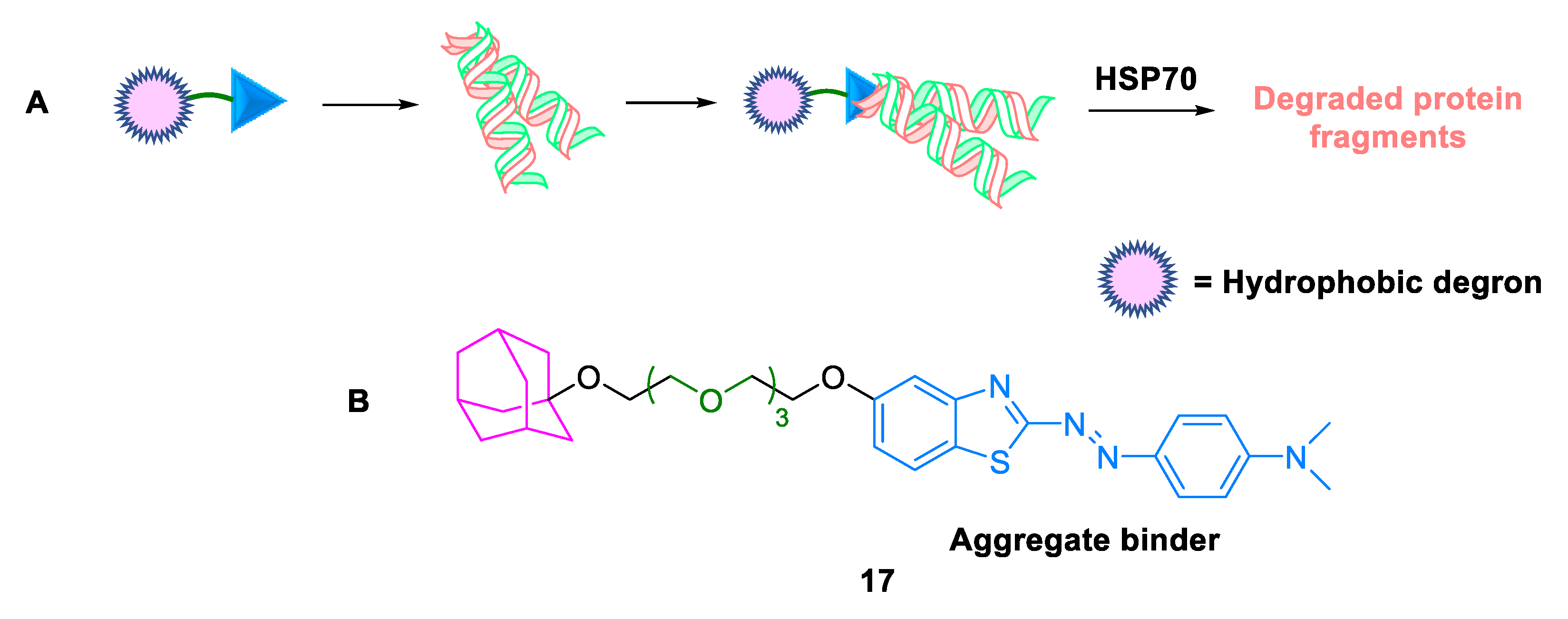
2.3.1. Hydrophobic Tags
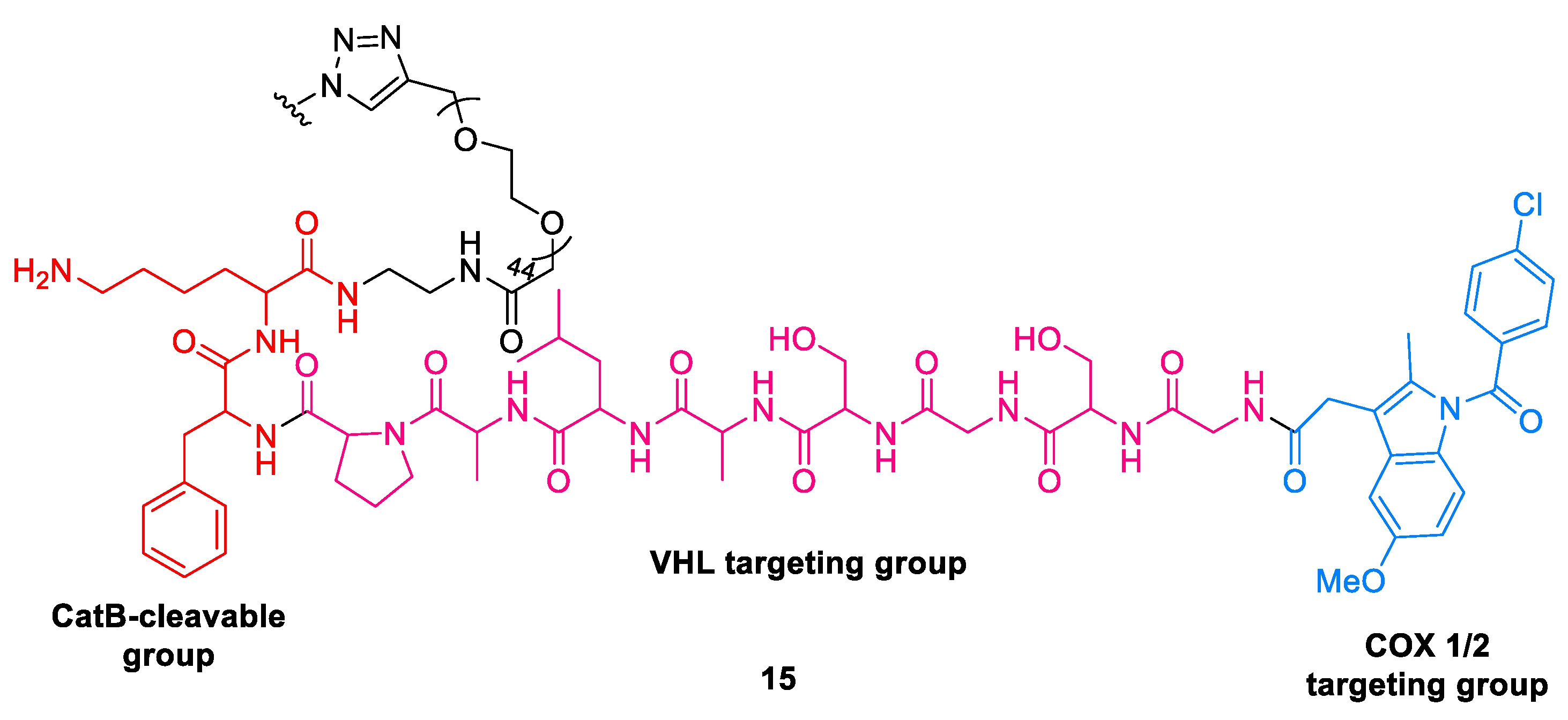
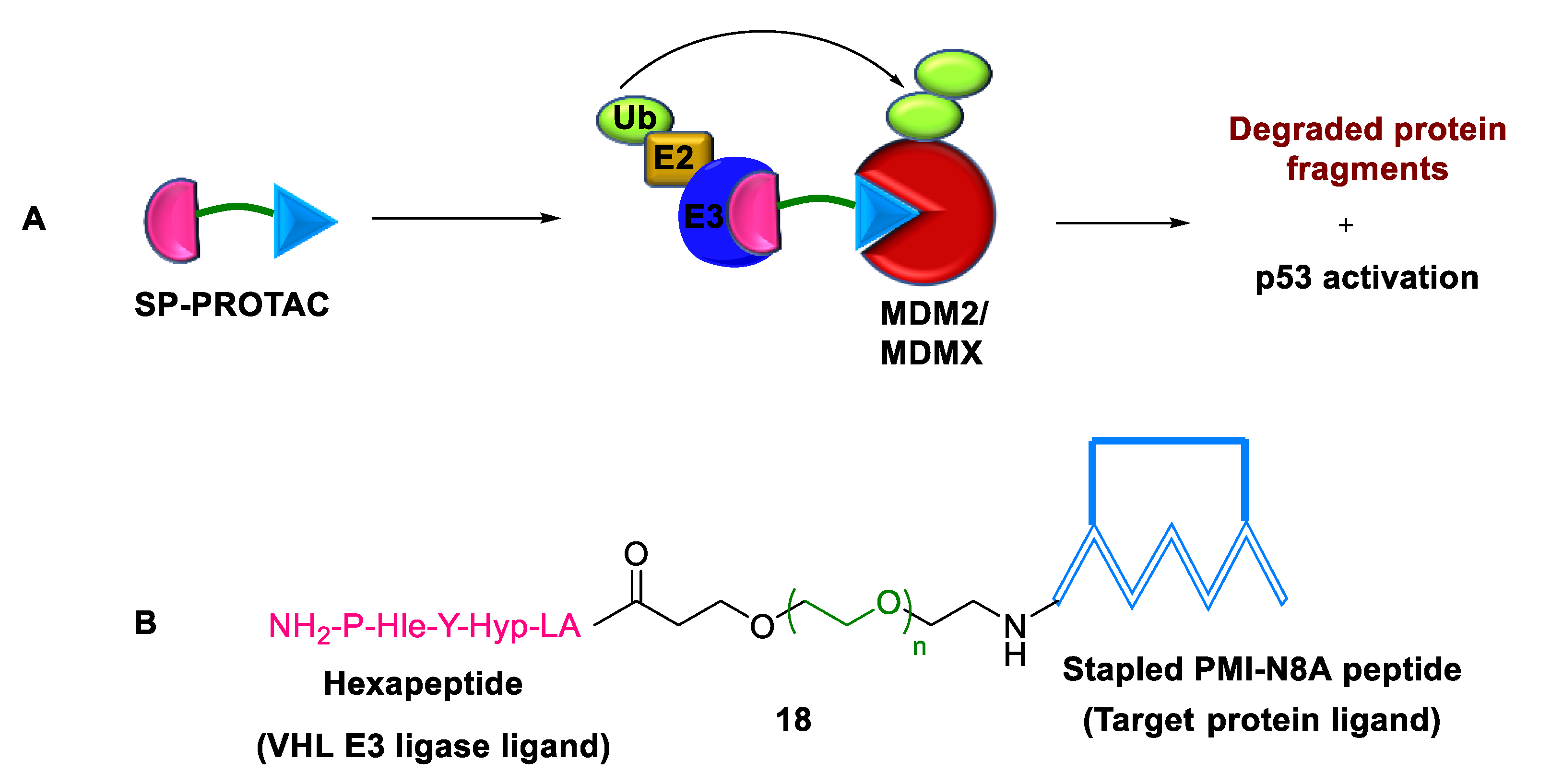
2.3.2. Stapled PROTACs
2.4. Modalities with Improved Druggability
2.4.1. Bridged PROTACs
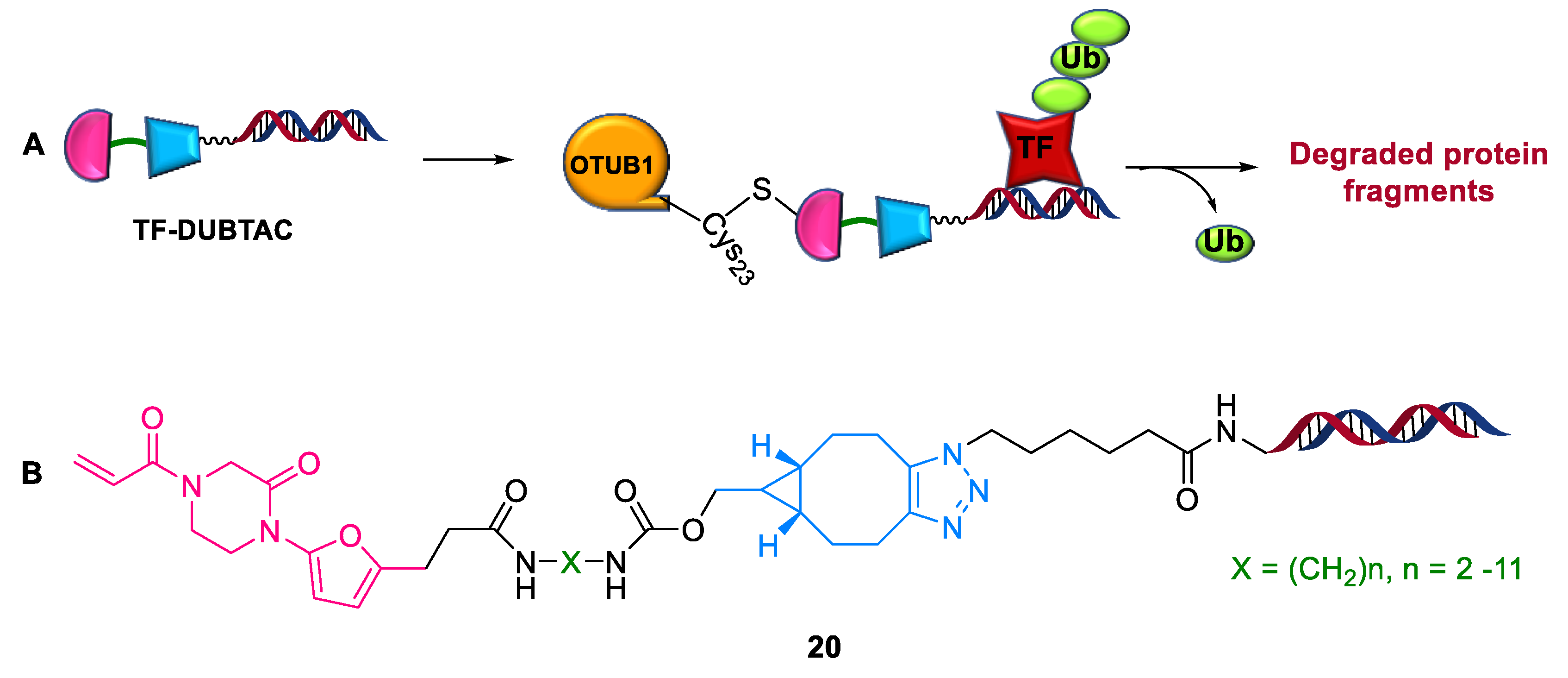
2.4.2. TF-DUBTACs
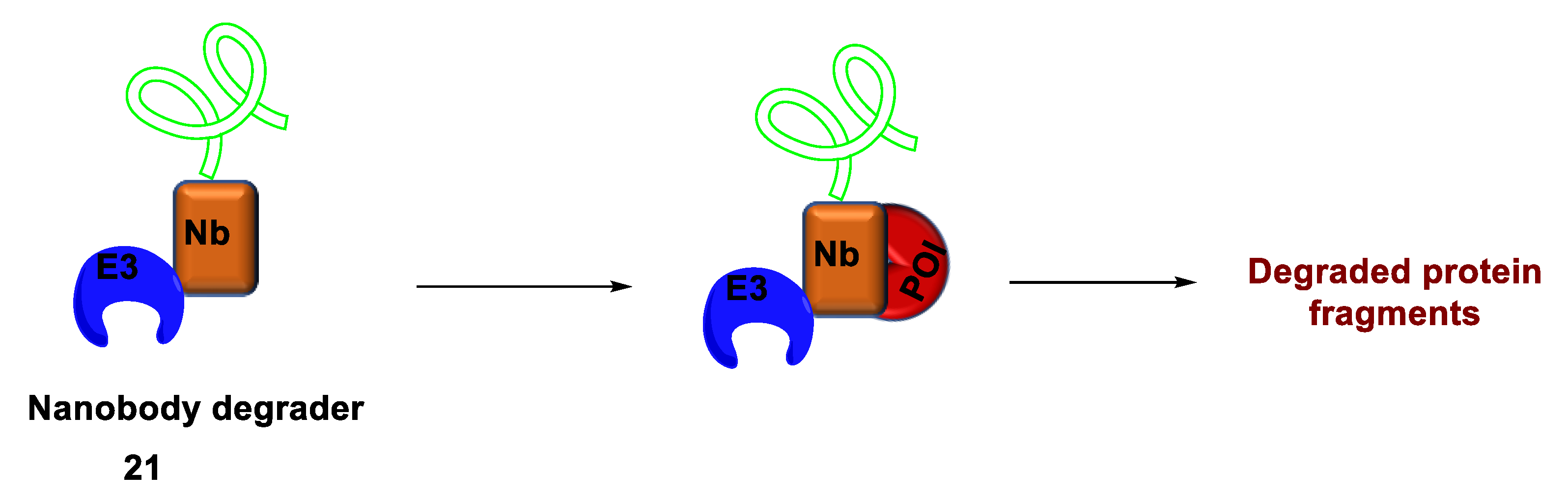
2.4.3. Nanobody-Based Degrader
2.5. PROTAC-Based Approaches
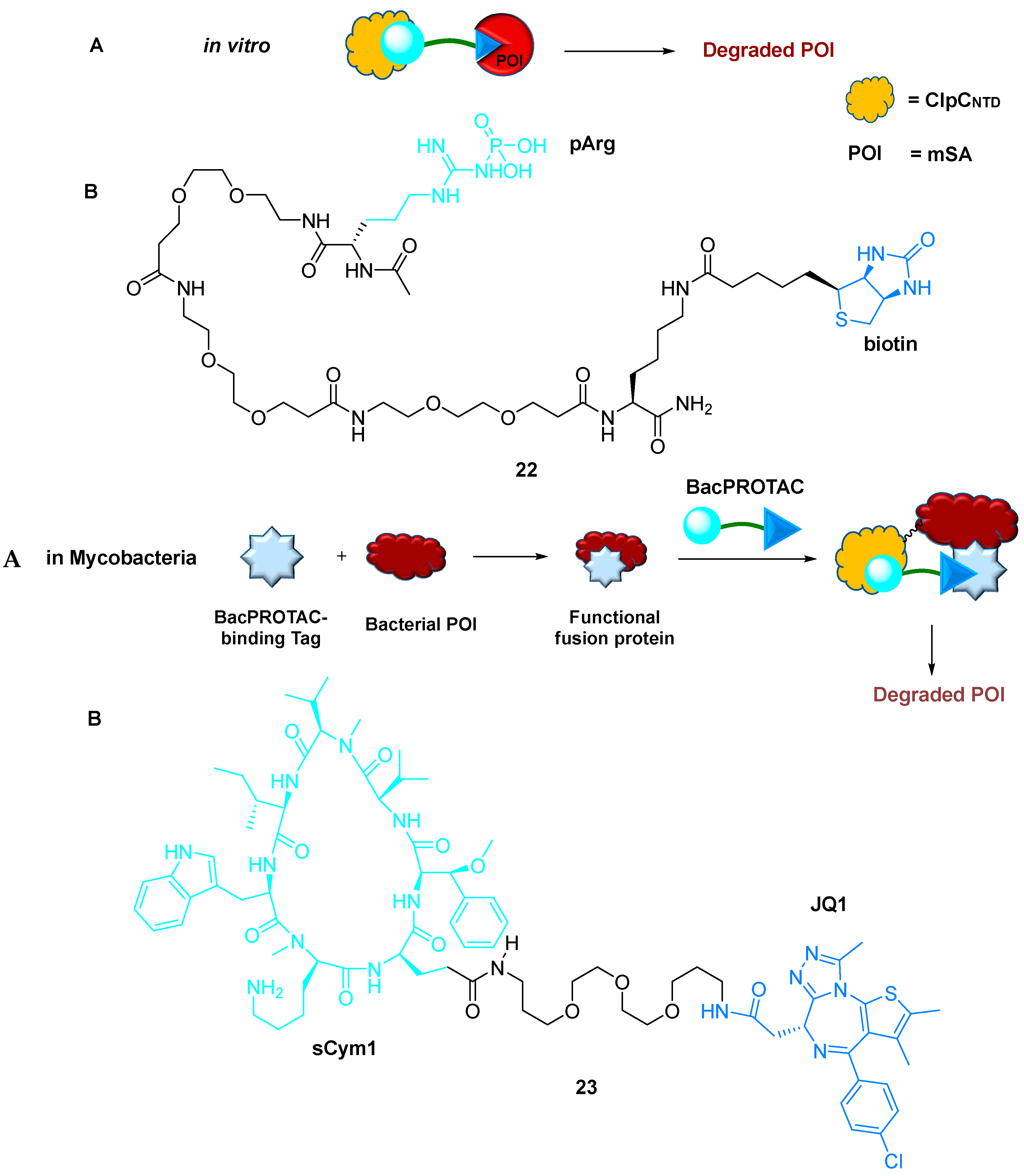
2.5.1. BacPROTACs
2.5.2. DUB-Based Degrader
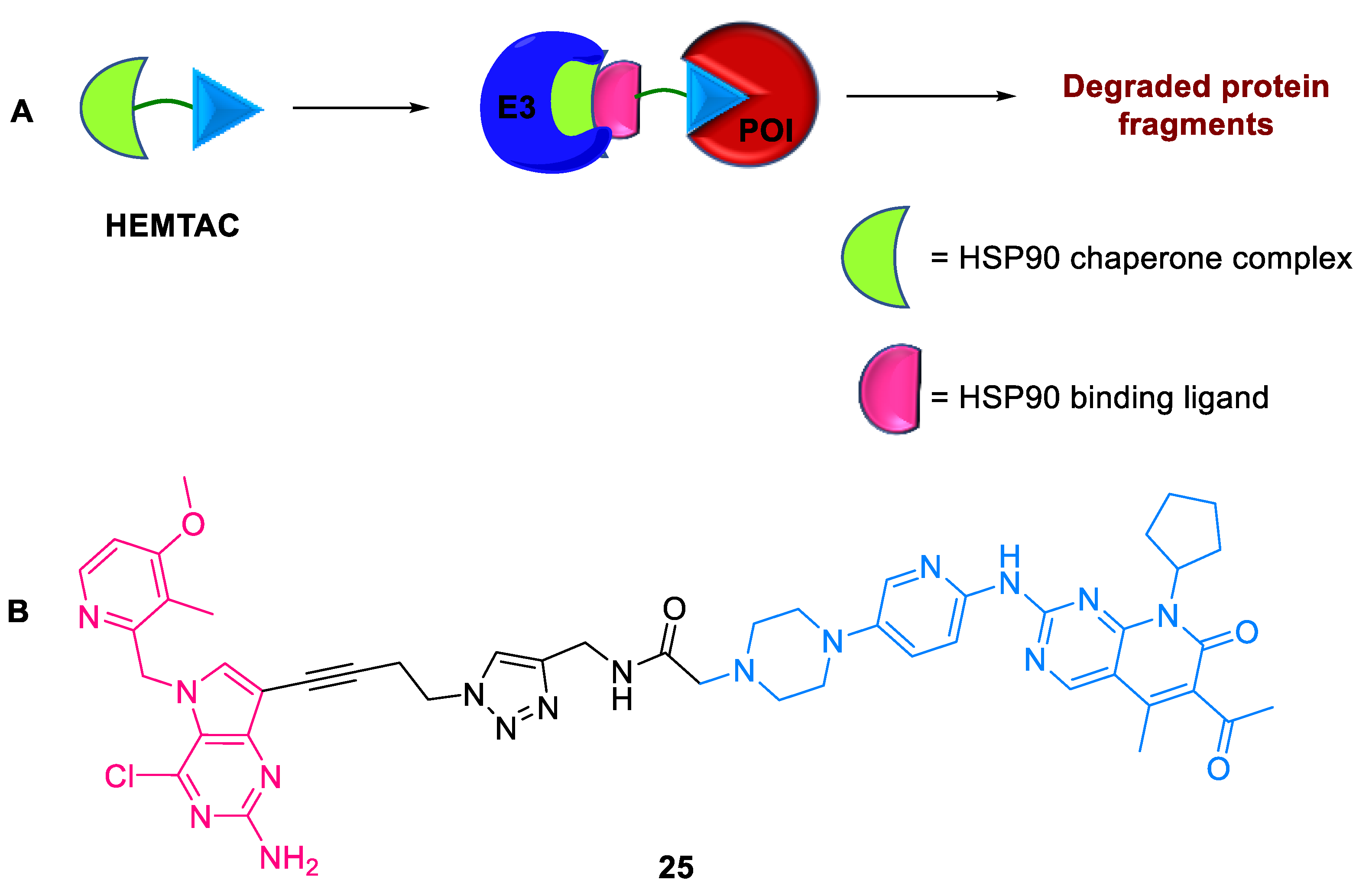
2.5.3. HEMTACs
3. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blanco, M.J.; Gardinier, K.M. New Chemical Modalities and Strategic Thinking in Early Drug Discovery. ACS Med. Chem. Lett. 2020, 11, 228–231. [Google Scholar] [CrossRef]
- Cohen, P.; Cross, D.; Janne, P.A. Kinase drug discovery 20 years after imatinib: Progress and future directions. Nat. Rev. Drug Discov. 2021, 20, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Burslem, G.M.; Crews, C.M. Small-Molecule Modulation of Protein Homeostasis. Chem. Rev. 2017, 117, 11269–11301. [Google Scholar] [CrossRef]
- Dang, C.V.; Reddy, E.P.; Shokat, K.M.; Soucek, L. Drugging the ‘undruggable’ cancer targets. Nature Reviews Cancer 2017, 17, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Overington, J.P.; Al-Lazikani, B.; Hopkins, A.L. How Many Drug Targets are There? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef]
- Crews, C.M. Targeting the Undruggable Proteome: The Small Molecules of my Dreams. Chem. Biol. 2010, 17, 551–555. [Google Scholar] [CrossRef]
- Gurevich, E.; Gurevich, V. Beyond Traditional Pharmacology: New Tools and Approaches. Br. J. Pharmacol. 2015, 172, 3229–3241. [Google Scholar] [CrossRef] [PubMed]
- Glickman, M.H.; Ciechanover, A. The Ubiquitin-Proteasome Proteolytic Pathway: Destruction for the Sake of Construction. Physiol. Rev. 2002, 82, 373–428. [Google Scholar] [CrossRef]
- Samarasinghe, K.T.G.; Crews, C.M. Targeted protein degradation: A promise for undruggable proteins. Cell Chem. Biol. 2021, 28, 934–951. [Google Scholar] [CrossRef]
- Sun, X.; Rao, Y. PROTACs as Potential Therapeutic Agents for Cancer Drug Resistance. Biochemistry 2020, 59, 240–249. [Google Scholar] [CrossRef]
- Bekes, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. The PROTAC GoldRush. Nat. Biotechnol. 2022, 40, 12–16. [Google Scholar] [CrossRef]
- Zhong, Y.; Chi, F.; Wu, H.; Liu, Y.; Xie, Z.; Huang, W.; Shi, W.; Qian, H. Emerging Targeted Protein Degradation Tools For Innovative Drug Discovery: From Classical Protacs To The Novel And Beyond. Eur. J. Med. Chem. 2022, 231, 114142–114165. [Google Scholar] [CrossRef] [PubMed]
- Kevin, M.; Muireann, C.; Andrew, X.Z.; Fiona, P.; Castaldi, M.P.; Goran, D.; Helen, B.; Clay, S.; Pete, N. Proteolysis-targeting chimeras in drug development: A safety perspective. Br. J. Pharm. 2020, 177, 1709–1718. [Google Scholar]
- Cotton, A.D.; Nguyen, D.P.; Gramespacher, J.A.; Seiple IBWells, J.A. Development of antibody-based PROTACs for the degradation of the cell-surface immune checkpoint protein PD-L1. J. Am. Chem. Soc. 2021, 143, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.P.; Pragya, A.; Robert, A.B.; Jinhua, C.; Geoffrey, D.R.; Gauri, D.; Isabel, F.; Karen, E.G.; Amrita, V.K.; Susan, K.; et al. Antibody conjugation of a chimeric BET degrader enables in vivo activity. ChemMedChem 2020, 15, 17–25. [Google Scholar]
- Jing, L.; He, C.; Yi, L.; Yudao, S.; Fanye, M.; Kaniskan, H.U.; Jian, J.; Wenyi, W. Cancer selective target degradation by folate-caged PROTACs. J. Am. Chem. Soc. 2021, 143, 7380–7387. [Google Scholar]
- Nalawansha, D.A.; Crews, C.M. PROTACs: An Emerging Therapeutic Modality in Precision Medicine. Cell Chem. Biol. 2020, 27, 998–1014. [Google Scholar] [CrossRef]
- Nimjee, S.M.; White, R.R.; Becker, R.C.; Sullenger, B.A. Aptamers as therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 61–79. [Google Scholar] [CrossRef]
- Koutsioumpa, M.; Papadimitriou, E. Cell Surface Nucleolin as a target for Anti-cancer Therapies. Recent Pat. Anti-Cancer Drug Discov. 2014, 9, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Ling, L.; Xia, W.; Huimin, L.; Yibin, Z.; Tiantian, X.; Hui, Z.; Xiaodong, L.; Tianhuan, P.; Xing, S.; et al. Development of a novel PROTAC using the nucleic acid aptamer as a targeting ligand for tumor selective degradation of nucleolin. Mol. Ther. Nucleic Acids 2022, 30, 66–79. [Google Scholar]
- Reyes-Reyes, E.M.; Teng, Y.; Bates, P.J. A new paradigm for aptamer therapeutic AS1411 action: Uptake by macropinocytosis and its stimulation by a nucleolin-dependent mechanism. Cancer Res. 2010, 70, 8617–8629. [Google Scholar] [CrossRef]
- He, S.; Gao, F.; Ma, J.; Ma, H.; Dong, G.; Sheng, C. Aptamer-PROTAC conjugates (APCs) for tumor-specific targeting in breast cancer. Angew Chem. Int. Ed. Engl. 2021, 60, 23299–23305. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.; Tuerk, C.; Gold, L. SELEXION. Systematic evolution of ligands by exponential enrichment with integrated optimization by non-linear analysis. J. Mol. Biol. 1991, 222, 739–761. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.I.; Arangundy-Franklin, S.; Holliger, P. Towards applications of synthetic genetic polymers in diagnosis and therapy. Curr. Opin. Chem. Biol. 2014, 22, 79–84. [Google Scholar] [CrossRef]
- Li, L.; Jiang, Y.; Cui, C.; Yang, Y.; Zhang, P.; Stewart, K.; Pan, X.; Li, X.; Yang, L.; Qiu, L.; et al. Modulating aptamer specificity with pH-responsive DNA bonds. J. Am. Chem. Soc. 2018, 140, 13335–13339. [Google Scholar] [CrossRef] [PubMed]
- Biniuri, Y.; Luo, G.F.; Fadeev, M.; Wulf, V.; Willner, I. Redox-switchable binding properties of the ATP-aptamer. J. Am. Chem. Soc. 2019, 141, 15567–15576. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, G.; Liu, Q.; Liu, W.; Weng, Y.; Zhao, Y.; Qu, F.; Li, L.; Huang, Y. A photo-triggerable aptamer nanoswitch for spatiotemporal controllable siRNA delivery. Nanoscale 2020, 12, 10939–10943. [Google Scholar] [CrossRef]
- Miao, C.; Ping, Z.; Yun, K.; Jingrui, L.; Yan, L.; Yao, Z.; Jie, R.; Jun, Z.; Yan, C.; Songbo, X. Inducible Degradation of Oncogenic Nucleolin Using an Aptamer-Based PROTAC. J. Med. Chem. 2023, 66, 1339–1348. [Google Scholar]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumor development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Hydrogen peroxide reactivity and specificity in thiol-based cell signalling. Biochem. Soc. Trans. 2020, 48, 745–754. [Google Scholar] [CrossRef]
- Haixia, L.; Chaowei, R.; Renhong, S.; Huihui, W.; Yuexiong, Z.; Xiaobao, Y.; Biao, J.; Hongli, C. Reactive oxygen species-responsive Pre-PROTAC for tumor-specific protein degradation. Chem. Commun. 2022, 58, 10072–10075. [Google Scholar]
- Silong, Z.; Yuanyuan, L.; Tao, L.; Yu, Z.; Haimei, L.; Zhengzai, C.; Na, P.; Yi, L.; Juan, X.; Huan, H. Activable Targeted Protein Degradation Platform Based on Light-triggered Singlet Oxygen. J. Med. Chem. 2022, 65, 3632–3643. [Google Scholar]
- Reynders, M.T.D. Optical control of targeted protein degradation. Cell Chem. Biol. 2021, 28, 969–986. [Google Scholar] [CrossRef] [PubMed]
- Qisi, Z.; Cyrille, S.K.; Milon, M.; Jake, L.G.; Jennifer, R.B.; Sergei, K.; Mikhail, I.; Christopher, P.T.; Leran, Z.; Daniel, C.; et al. Light-mediated multi-target protein degradation using arylazopyrazolephotoswitchable PROTACs (AP-PROTACs). Chem. Commun. 2022, 58, 10933–10936. [Google Scholar]
- Chunrong, Y.; Yuchen, Y.; Yujie, L.; Qiankun, N.; Jinghong, L. Radiotherapy-Triggered Proteolysis Targeting Chimera Prodrug Activation in Tumors. J. Am. Chem. Soc. 2023, 145, 385–391. [Google Scholar]
- Singleton, D.C.; Macann, A.; Wilson, W.R. Therapeutic targeting of the hypoxic tumor microenvironment. Nat. Rev. Clin. Oncol. 2021, 18, 751–772. [Google Scholar] [CrossRef]
- Shi, S.; Yu, D.; Yi, Z.; Jing, N.; Zeyu, C.; Xiaonan, W.; Feihuang, Q.; Yi, D.; Gengchen, Y.; Yunze, W.; et al. Rational Design for Nitroreductase (NTR)-Responsive Proteolysis Targeting Chimeras (PROTACs) Selectively Targeting Tumor Tissues. J. Med. Chem. 2022, 65, 5057–5071. [Google Scholar] [CrossRef]
- Thang, C.D.; Jun, W.L.; Caixia, S.; Songhan, L.; Khoa, T.K.; Seok, T.L.; Yu, Y.O.; Yuet, P.K.; Jia, J.M.; Yuguang, M.; et al. Hypoxia deactivates epigenetic feedbacks via enzyme derived clicking proteolysis-targeting chimeras. Sci. Adv. 2022, 8, eabq2216. [Google Scholar]
- Jing, G.; Bo, H.; Qiwen, Z.; Lei, Y.; Xingyu, J.; Zhifeng, Z.; Xutong, L.; Tianfeng, X.; Mingyue, Z.; Yi-Hung, C.; et al. Engineered bioorthogonal POLY-PROTAC nanoparticles for tumor-specific protein degradation and precise cancer therapy. Nat. Comm. 2022, 13, 4318. [Google Scholar]
- Chi, Z.; Shasha, H.; Ziling, Z.; Penghui, C.; Kanyi, P. Smart Nano-PROTACs Reprogram Tumor Microenvironment for ActivatablePhoto-metabolic Cancer Immunotherapy. Angew. Chemie. 2021, 61, e202114957. [Google Scholar]
- Weijun, G.; Thomas, K. Applications and Limitations of Oxime-Linked “Split PROTACs”. ChemBioChem. 2022, 23, e202200275. [Google Scholar]
- Lebraud, H.; Wright, D.J.; Johnson, C.N.; Heightman, T.D. Protein Degradation by In-Cell Self-Assembly of Proteolysis Targeting Chimeras. ACS Cent. Sci. 2016, 2, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Keigo, H.; Hiroko, Y.; Shusuke, T.; Yugo, M.; Tatsuya, N.; Kenji, O.; Mayumi, I.; Kayoko, K.; Yui, I.; Shinsaku, N.; et al. Conversion of a PROTAC Mutant Huntingtin Degrader into Small Molecule Hydrophobic Tags Focusing on Drug-like Properties. ACS Med. Chem. Lett. 2022, 13, 396–402. [Google Scholar]
- Gustafson, J.L.; Neklesa, T.K.; Cox, C.S.; Roth, A.G.; Buckley, D.L.; Tae, H.S.; Sundberg, T.B.; Stagg, D.B.; Hines, J.; McDonnell, D.P.; et al. Small-Molecule-Mediated Degradation of the Androgen Receptor through Hydrophobic Tagging. Angew. Chem. Int. Ed. 2015, 54, 9659–9662. [Google Scholar] [CrossRef]
- Si, C.; Xiang, L.; Yinghua, L.; Xing, Y.; Chenchen, G.; Songyan, G.; Jinyang, L.; Bohan, M.; Zhe, W.; Wuyuan, L.; et al. Design of stapled peptide-based PROTACs for MDM2/MDMX atypical degradation and tumor Suppression. Theranostics 2022, 12, 6665–6681. [Google Scholar]
- Luh, L.M.; Scheib, U.; Juenemann, K.; Wortmann, L.; Brands, M.; Cromm, P.M. Prey for the Proteasome: Targeted Protein Degradation-A Medicinal Chemist’s Perspective. Angew. Chem. Int. Ed. Engl. 2020, 59, 15448–15466. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Kaniskan, H.Ü.; Xie, L.; Chen, X.; Jin, J.; Wenyi, W. TFPROTACs Enable Targeted Degradation of Transcription Factors. J. Am. Chem. Soc. 2021, 143, 8902–8910. [Google Scholar] [CrossRef]
- Ghidini, A.; Cléry, A.; Halloy, F.; Allain, F.H.T.; Hall, J. RNAPROTACs: Degraders of RNA-Binding Proteins. Angew. Chem. Int. Ed. 2021, 60, 3163–3169. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yue, Z.; Hyerin, Y.; Xiaobao, Y.; Kwang-Su, P.; Ling, X.; Poulikos, I.P.; Xiaoran, H.; Yue, X.; Xian, C.; et al. Bridged Proteolysis Targeting Chimera (PROTAC) Enables Degradation of Undruggable Targets. J. Am. Chem. Soc. 2022, 144, 22622–22632. [Google Scholar]
- Jing, L.; Xufen, Y.; He, C.; Kaniskan, H.U.; Ling, X.; Xian, C.; Jian, J.; Wenyi, W. TF-DUBTACs Stabilize Tumor Suppressor Transcription Factors. J. Am. Chem. Soc. 2022, 144, 12934–12941. [Google Scholar]
- McMahon, C.; Baier, A.S.; Pascolutti, R.; Wegrecki, M.; Zheng, S.; Ong, J.X.; Erlandson, S.C.; Hilger, D.; Rasmussen, S.G.F.; Ring, A.M.; et al. Yeast surface display platform for rapid discovery of conformationally selective nanobodies. Nat. Struct. Mol. Biol. 2018, 25, 289–296. [Google Scholar] [CrossRef]
- Orkin, S.H. Molecular Medicine: Found in Translation. Med 2021, 2, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Platt, O.S.; Brambilla, D.J.; Rosse, W.F.; Milner, P.F.; Castro, O.; Steinberg, M.H.; Klug, P.P. Mortality in Sickle Cell Disease—Life Expectancy and Risk Factors for Early Death. New Engl. J. Med. 1994, 330, 1639–1644. [Google Scholar] [CrossRef]
- Liu, N.; Hargreaves, V.V.; Zhu, Q.; Kurland, J.V.; Hong, J.; Kim, W.; Sher, F.; Macias-Trevino, C.; Rogers, J.M.; Kurita, R.; et al. Direct Promoter Repression by BCL11A Controls the Fetal to Adult Hemoglobin Switch. Cell 2018, 173, 430–442. [Google Scholar] [CrossRef]
- Shen, F.; Zheng, G.; Setegne, M.; Tenglin, K.; Izadi, M.; Xie, H.; Zhai, L.; Orkin, S.H.; Dassama, L.M.K. A Cell-Permeant Nanobody-Based Degrader That Induces Fetal Hemoglobin. ACS Cent. Sci. 2022, 8, 1695–1703. [Google Scholar] [CrossRef]
- Trentini, D.B.; Suskiewicz, M.J.; Heuck, A.; Kurzbauer, R.; Deszcz, L.; Mechtler, K.; Clausen, T. Arginine phosphorylation marks proteins for degradation by a Clp protease. Nature 2016, 539, 48–53. [Google Scholar] [CrossRef]
- Francesca, E.M.; Stefan, K.; Julia, L.; Sabryna, J.; David, M.H.; Stepan, O.; Anastasia, O.; Juliane, K.; Robert, K.; Lukas, J.; et al. BacPROTACs mediate targeted protein degradation in bacteria. Cell 2022, 185, 2338–2353. [Google Scholar]
- Arunima, M.; Izidor, S.; Martina, G.; Patricia, L.; Matic, P.; Sophie, W.; Rabea, V.; Michael, G.; Jan, K.; Christian, S. Targeting the deubiquitinase USP7 for degradation with PROTACs. Chem. Commun. 2022, 58, 8858–8861. [Google Scholar]
- Verba, K.A.; Wang, R.Y.-R.; Arakawa, A.; Liu, Y.; Shirouzu, M.; Yokoyama, S.; Agard, D.A. STRUCTURAL BIOLOGY Atomic structure of Hsp90-Cdc37-Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science 2016, 352, 1542–1547. [Google Scholar] [CrossRef]
- Verba, K.A.; Agard, D.A. How Hsp90 and Cdc37 Lubricate Kinase Molecular Switches. Trends Biochem. Sci. 2017, 42, 799–811. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, Y.-H.; Huang, F.-Y.; Mei, W.-L.; Liu, Q.; Wang, C.-C.; Lin, Y.-Y.; Huang, C.; Li, Y.-N.; Dai, H.-F.; et al. 3′-epi-12 beta-hydroxyfroside, a new cardenolide, induces cytoprotective autophagy via blocking the Hsp90/Akt/mTOR axis in lung cancer cells. Theranostics 2018, 8, 2044–2060. [Google Scholar] [CrossRef]
- Matyskiela, M.E.; Lu, G.; Ito, T.; Pagarigan, B.; Lu, C.-C.; Miller, K.; Fang, W.; Wang, N.-Y.; Nguyen, D.; Houston, J.; et al. A novel cereblon modulator recruits GSPT1 to the CRL4(CRBN) ubiquitin ligase. Nature 2016, 535, 252. [Google Scholar] [CrossRef]
- Churcher, I. Protac-Induced Protein Degradation in Drug Discovery: Breaking the Rules or Just Making New Ones? J. Med. Chem. 2018, 61, 444–452. [Google Scholar] [CrossRef]
- Zhang, L.; Riley-Gillis, B.; Vijay, P.; Shen, Y. Acquired Resistance to BET-PROTACs (Proteolysis-Targeting Chimeras) Caused by Genomic Alterations in Core Components of E3 Ligase Complexes. Mol. Cancer Ther. 2019, 18, 1302–1311. [Google Scholar] [CrossRef]
- Li, Z.; Ma, S.; Zhang, S.; Ma, Z.; Du, L.; Li, M. Targeted Protein Degradation Induced by HEMTACs Based on HSP90. J. Med. Chem. 2023, 66, 733–751. [Google Scholar] [CrossRef]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef]
- Classon, M.; Harlow, E. The retinoblastoma tumor suppressor in development and cancer. Nat. Rev. Cancer 2002, 2, 910–991. [Google Scholar] [CrossRef]
- Malumbres, M.; Sotillo, R.; Santamaría, D.; Galán, J.; Cerezo, A.; Ortega, S.; Dubus, P.; Barbacid, M. Mammalian cells cycle without the D-type cyclin-elependent kinases Cdk4 and Cdk6. Cell 2004, 118, 493–504. [Google Scholar] [CrossRef]
- Fassl, A.; Geng, Y.; Sicinski, P. CDK4 and CDK6 kinases: From basic science to cancer therapy. Science 2022, 375, eabc1495. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamatam, R.; Shin, D. Emerging Strategies in Proteolysis-Targeting Chimeras (PROTACs): Highlights from 2022. Int. J. Mol. Sci. 2023, 24, 5190. https://doi.org/10.3390/ijms24065190
Tamatam R, Shin D. Emerging Strategies in Proteolysis-Targeting Chimeras (PROTACs): Highlights from 2022. International Journal of Molecular Sciences. 2023; 24(6):5190. https://doi.org/10.3390/ijms24065190
Chicago/Turabian StyleTamatam, Rekha, and Dongyun Shin. 2023. "Emerging Strategies in Proteolysis-Targeting Chimeras (PROTACs): Highlights from 2022" International Journal of Molecular Sciences 24, no. 6: 5190. https://doi.org/10.3390/ijms24065190
APA StyleTamatam, R., & Shin, D. (2023). Emerging Strategies in Proteolysis-Targeting Chimeras (PROTACs): Highlights from 2022. International Journal of Molecular Sciences, 24(6), 5190. https://doi.org/10.3390/ijms24065190




