Imaging Mass Spectrometry for the Classification of Melanoma Based on BRAF/NRAS Mutational Status
Abstract
1. Introduction
2. Results
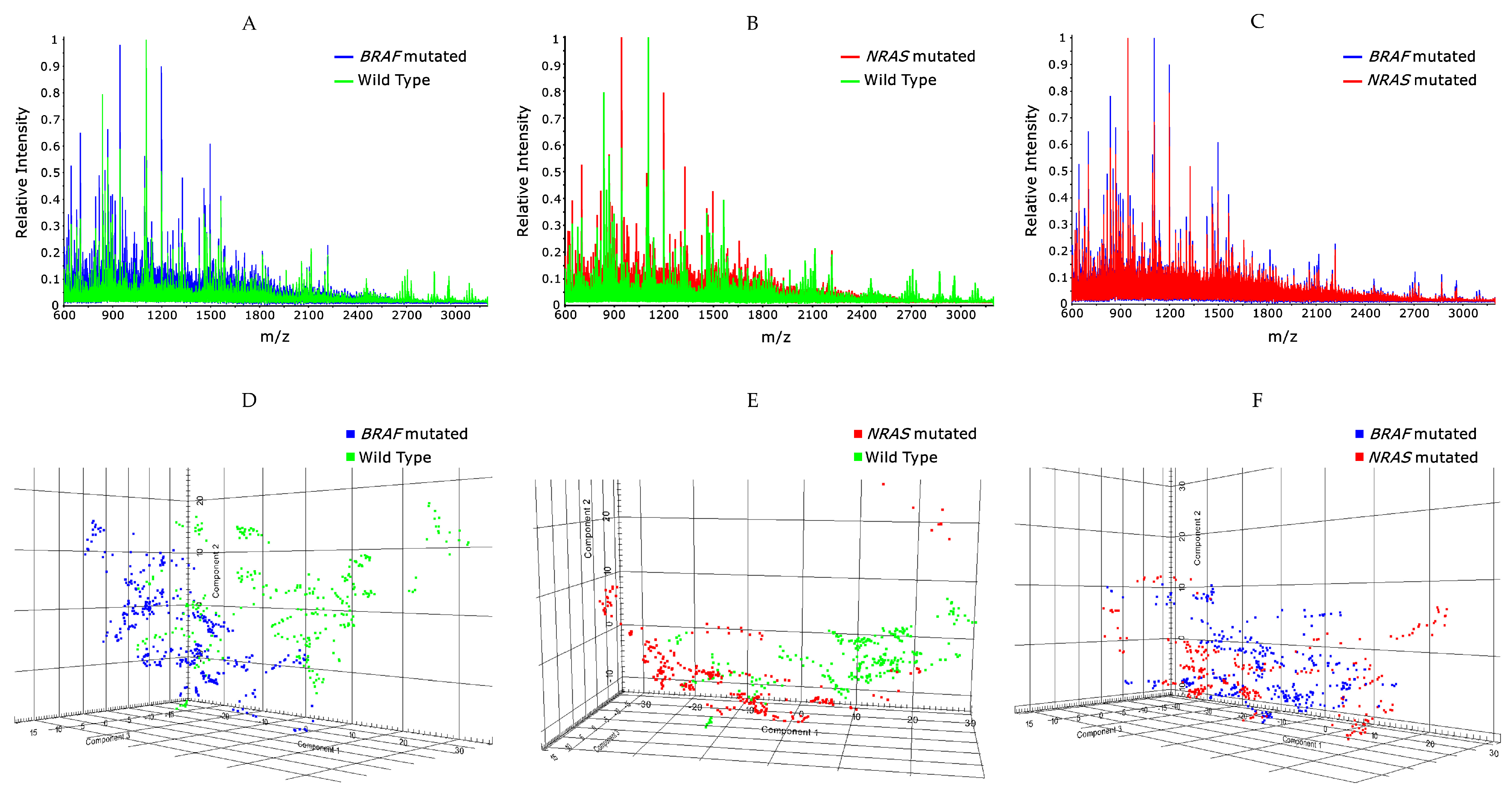
2.1. Classification of BRAF Mutated/Wildtype
2.2. Classification of NRAS Mutated/Wildtype
2.3. Classification of BRAF Mutated and NRAS Mutated Patients
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Reagents and Equipment
4.3. Sample Preparation
4.4. Mass Spectrometry Analysis
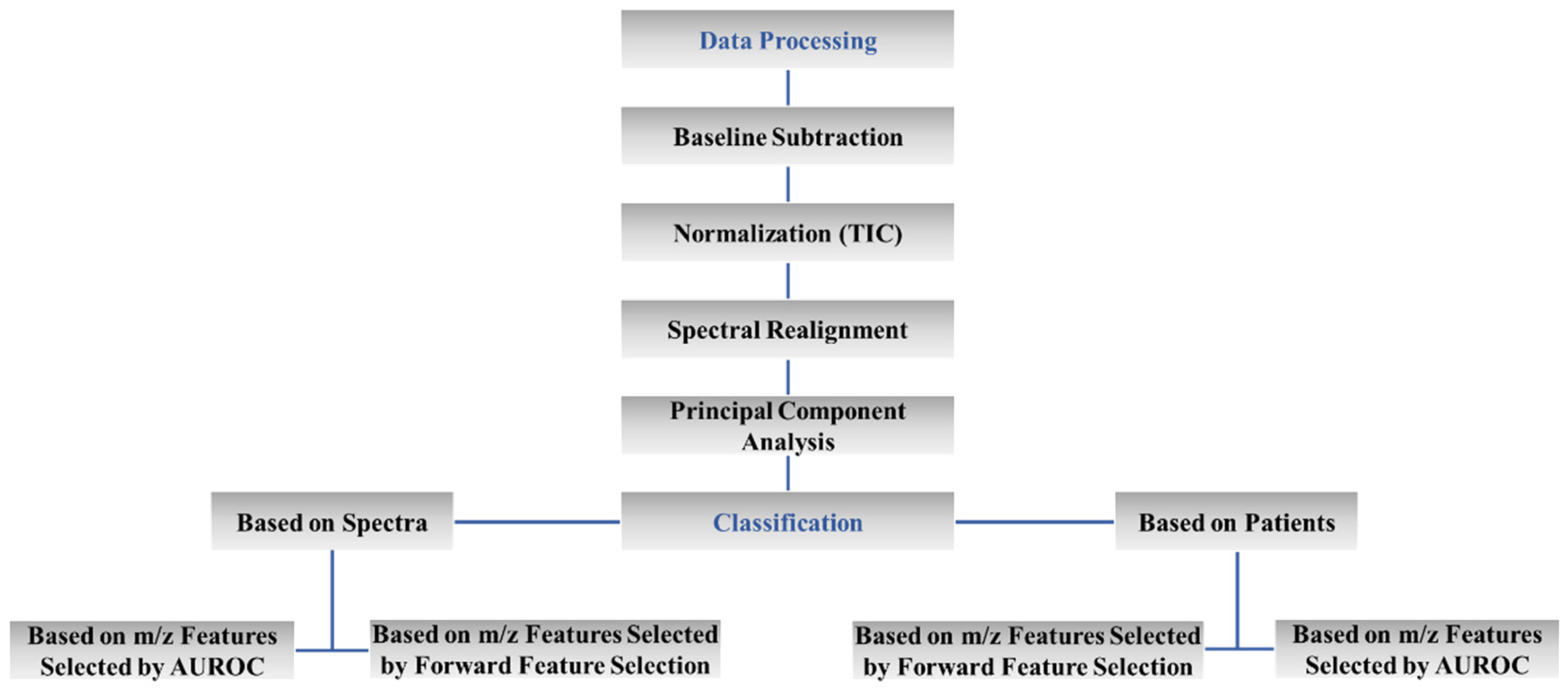
4.5. Classification Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Mesher, D.; Sasieni, P. 13. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br. J. Cancer 2011, 105 (Suppl. 2), S66–S69. [Google Scholar] [CrossRef] [PubMed]
- Cymerman, R.M.; Shao, Y.; Wang, K.; Zhang, Y.; Murzaku, E.C.; Penn, L.A.; Osman, I.; Polsky, D. De Novo vs Nevus-Associated Melanomas: Differences in Associations with Prognostic Indicators and Survival. J. Natl. Cancer Inst. 2016, 108, djw121. [Google Scholar] [CrossRef] [PubMed]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Michielin, O.; van Akkooi, A.C.J.; Ascierto, P.A.; Dummer, R.; Keilholz, U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2019, 30, 1884–1901. [Google Scholar] [CrossRef]
- Teixido, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alos, L. Molecular Markers and Targets in Melanoma. Cells 2021, 10, 2320. [Google Scholar] [CrossRef]
- Cheng, L.; Lopez-Beltran, A.; Massari, F.; MacLennan, G.T.; Montironi, R. Molecular testing for BRAF mutations to inform melanoma treatment decisions: A move toward precision medicine. Mod. Pathol. 2018, 31, 24–38. [Google Scholar] [CrossRef]
- Atlas, C.G. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef]
- Sarkisian, S.; Davar, D. MEK inhibitors for the treatment of NRAS mutant melanoma. Drug Des. Devel. Ther. 2018, 12, 2553–2565. [Google Scholar] [CrossRef]
- Raman, M.; Chen, W.; Cobb, M.H. Differential regulation and properties of MAPKs. Oncogene 2007, 26, 3100–3112. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Falchook, G.S.; Long, G.V.; Kurzrock, R.; Kim, K.B.; Arkenau, T.H.; Brown, M.P.; Hamid, O.; Infante, J.R.; Millward, M.; Pavlick, A.C.; et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: A phase 1 dose-escalation trial. Lancet 2012, 379, 1893–1901. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demidov, L.V.; Hassel, J.C.; Rutkowski, P.; Mohr, P.; et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N. Engl. J. Med. 2012, 367, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Polsky, D.; Cordon-Cardo, C. Oncogenes in melanoma. Oncogene 2003, 22, 3087–3091. [Google Scholar] [CrossRef] [PubMed]
- Pollock, P.M.; Harper, U.L.; Hansen, K.S.; Yudt, L.M.; Stark, M.; Robbins, C.M.; Moses, T.Y.; Hostetter, G.; Wagner, U.; Kakareka, J.; et al. High frequency of BRAF mutations in nevi. Nat. Genet. 2003, 33, 19–20. [Google Scholar] [CrossRef]
- Shain, A.H.; Yeh, I.; Kovalyshyn, I.; Sriharan, A.; Talevich, E.; Gagnon, A.; Dummer, R.; North, J.; Pincus, L.; Ruben, B.; et al. The Genetic Evolution of Melanoma from Precursor Lesions. N. Engl. J. Med. 2015, 373, 1926–1936. [Google Scholar] [CrossRef]
- Reinhardt, J.; Landsberg, J.; Schmid-Burgk, J.L.; Ramis, B.B.; Bald, T.; Glodde, N.; Lopez-Ramos, D.; Young, A.; Ngiow, S.F.; Nettersheim, D.; et al. MAPK Signaling and Inflammation Link Melanoma Phenotype Switching to Induction of CD73 during Immunotherapy. Cancer Res. 2017, 77, 4697–4709. [Google Scholar] [CrossRef]
- Yang, R.; Wang, Z.; Li, J.; Pi, X.; Gao, R.; Ma, J.; Qing, Y.; Zhou, S. The Identification of the Metabolism Subtypes of Skin Cutaneous Melanoma Associated With the Tumor Microenvironment and the Immunotherapy. Front. Cell Dev. Biol. 2021, 9, 707677. [Google Scholar] [CrossRef]
- Cazzato, G.; Lospalluti, L.; Colagrande, A.; Cimmino, A.; Romita, P.; Foti, C.; Demarco, A.; Arezzo, F.; Loizzi, V.; Cormio, G.; et al. Dedifferentiated Melanoma: A Diagnostic Histological Pitfall-Review of the Literature with Case Presentation. Dermatopathology 2021, 8, 51. [Google Scholar] [CrossRef]
- Neumann, E.K.; Djambazova, K.V.; Caprioli, R.M.; Spraggins, J.M. Multimodal Imaging Mass Spectrometry: Next Generation Molecular Mapping in Biology and Medicine. J. Am. Soc. Mass. Spectrom. 2020, 31, 2401–2415. [Google Scholar] [CrossRef]
- Kriegsmann, J.; Casadonte, R.; Kriegsmann, K.; Longuespée, R.; Kriegsmann, M. Mass spectrometry in pathology-Vision for a future workflow. Pathol. Res. Pract. 2018, 214, 1057–1063. [Google Scholar] [CrossRef]
- Hardesty, W.M.; Caprioli, R.M. In situ molecular imaging of proteins in tissues using mass spectrometry. Anal. Bioanal. Chem. 2008, 391, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Hardesty, W.M.; Kelley, M.C.; Mi, D.; Low, R.L.; Caprioli, R.M. Protein signatures for survival and recurrence in metastatic melanoma. J. Proteom. 2011, 74, 1002–1014. [Google Scholar] [CrossRef]
- Casadonte, R.; Kriegsmann, M. Imaging Mass Spectrometry-Based Proteomic Analysis to Differentiate Melanocytic Nevi and Malignant Melanoma. Cancers 2021, 13, 3197. [Google Scholar] [CrossRef] [PubMed]
- Lazova, R.; Seeley, E.H.; Keenan, M.; Gueorguieva, R.; Caprioli, R.M. Imaging mass spectrometry--a new and promising method to differentiate Spitz nevi from Spitzoid malignant melanomas. Am. J. Dermatopathol. 2012, 34, 82–90. [Google Scholar] [CrossRef]
- Lazova, R.; Seeley, E.H.; Kutzner, H.; Scolyer, R.A.; Scott, G.; Cerroni, L.; Fried, I.; Kozovska, M.E.; Rosenberg, A.S.; Prieto, V.G.; et al. Imaging mass spectrometry assists in the classification of diagnostically challenging atypical Spitzoid neoplasms. J. Am. Acad. Dermatol. 2016, 75, 1176–1186. [Google Scholar] [CrossRef]
- Al-Rohil, R.N.; Moore, J.L.; Patterson, N.H.; Nicholson, S.; Verbeeck, N.; Claesen, M.; Muhammad, J.Z.; Caprioli, R.M.; Norris, J.L.; Kantrow, S.; et al. Diagnosis of melanoma by imaging mass spectrometry: Development and validation of a melanoma prediction model. J. Cutan. Pathol. 2021, 48, 1455–1462. [Google Scholar] [CrossRef]
- Alomari, A.K.; Glusac, E.J.; Choi, J.; Hui, P.; Seeley, E.H.; Caprioli, R.M.; Watsky, K.L.; Urban, J.; Lazova, R. Congenital nevi versus metastatic melanoma in a newborn to a mother with malignant melanoma-diagnosis supported by sex chromosome analysis and Imaging Mass Spectrometry. J. Cutan. Pathol. 2015, 42, 757–764. [Google Scholar] [CrossRef]
- Sugihara, Y.; Rivas, D.; Malm, J.; Szasz, M.; Kwon, H.; Baldetorp, B.; Olsson, H.; Ingvar, C.; Rezeli, M.; Fehniger, T.E.; et al. Endogenous expression mapping of malignant melanoma by mass spectrometry imaging. Clin. Transl. Med. 2018, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, Y.; Végvári, A.; Welinder, C.; Jönsson, G.; Ingvar, C.; Lundgren, L.; Olsson, H.; Breslin, T.; Wieslander, E.; Laurell, T.; et al. A new look at drugs targeting malignant melanoma-an application for mass spectrometry imaging. Proteomics 2014, 14, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Jouvet, N.; Jilani, A.; Vongsamphanh, R.; Yang, X.; Yang, S.; Ramotar, D. Human glyceraldehyde-3-phosphate dehydrogenase plays a direct role in reactivating oxidized forms of the DNA repair enzyme APE1. J. Biol. Chem. 2008, 283, 30632–30641. [Google Scholar] [CrossRef] [PubMed]
- Groseclose, M.R.; Massion, P.P.; Chaurand, P.; Caprioli, R.M. High-throughput proteomic analysis of formalin-fixed paraffin-embedded tissue microarrays using MALDI imaging mass spectrometry. Proteomics 2008, 8, 3715–3724. [Google Scholar] [CrossRef]
- Ugurel, S.; Röhmel, J.; Ascierto, P.A.; Flaherty, K.T.; Grob, J.J.; Hauschild, A.; Larkin, J.; Long, G.V.; Lorigan, P.; McArthur, G.A.; et al. Survival of patients with advanced metastatic melanoma: The impact of novel therapies. Eur. J. Cancer 2016, 53, 125–134. [Google Scholar] [CrossRef]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015, 386, 444–451. [Google Scholar] [CrossRef]
- Wagle, N.; Van Allen, E.M.; Treacy, D.J.; Frederick, D.T.; Cooper, Z.A.; Taylor-Weiner, A.; Rosenberg, M.; Goetz, E.M.; Sullivan, R.J.; Farlow, D.N.; et al. MAP kinase pathway alterations in BRAF-mutant melanoma patients with acquired resistance to combined RAF/MEK inhibition. Cancer Discov. 2014, 4, 61–68. [Google Scholar] [CrossRef]
- Lazova, R.; Seeley, E.H. Proteomic Mass Spectrometry Imaging for Skin Cancer Diagnosis. Dermatol. Clin. 2017, 35, 513–519. [Google Scholar] [CrossRef]
- Gruber, F.; Kastelan, M.; Brajac, I.; Saftić, M.; Peharda, V.; Cabrijan, L.; Stanić Zgombić, Z.; Simonić, E. Molecular and genetic mechanisms in melanoma. Coll. Antropol. 2008, 32 Suppl 2, 147–152. [Google Scholar]
- Miller, A.J.; Mihm, M.C., Jr. Melanoma. N. Engl. J. Med. 2006, 355, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Kujirai, T.; Horikoshi, N.; Sato, K.; Maehara, K.; Machida, S.; Osakabe, A.; Kimura, H.; Ohkawa, Y.; Kurumizaka, H. Structure and function of human histone H3.Y nucleosome. Nucleic Acids Res. 2016, 44, 6127–6141. [Google Scholar] [CrossRef]
- Long, G.V.; Menzies, A.M.; Nagrial, A.M.; Haydu, L.E.; Hamilton, A.L.; Mann, G.J.; Hughes, T.M.; Thompson, J.F.; Scolyer, R.A.; Kefford, R.F. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Ellerhorst, J.A.; Greene, V.R.; Ekmekcioglu, S.; Warneke, C.L.; Johnson, M.M.; Cooke, C.P.; Wang, L.E.; Prieto, V.G.; Gershenwald, J.E.; Wei, Q.; et al. Clinical correlates of NRAS and BRAF mutations in primary human melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Jakob, J.A.; Bassett, R.L., Jr.; Ng, C.S.; Curry, J.L.; Joseph, R.W.; Alvarado, G.C.; Rohlfs, M.L.; Richard, J.; Gershenwald, J.E.; Kim, K.B.; et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 2012, 118, 4014–4023. [Google Scholar] [CrossRef] [PubMed]
- Krynetski, E.Y.; Krynetskaia, N.F.; Gallo, A.E.; Murti, K.G.; Evans, W.E. A novel protein complex distinct from mismatch repair binds thioguanylated DNA. Mol. Pharmacol. 2001, 59, 367–374. [Google Scholar] [CrossRef]
- Ramos, D.; Pellín-Carcelén, A.; Agustí, J.; Murgui, A.; Jordá, E.; Pellín, A.; Monteagudo, C. Deregulation of glyceraldehyde-3-phosphate dehydrogenase expression during tumor progression of human cutaneous melanoma. Anticancer. Res. 2015, 35, 439–444. [Google Scholar] [PubMed]
- Gruenbaum, Y.; Aebi, U. Intermediate filaments: A dynamic network that controls cell mechanics. F1000Prime Rep. 2014, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Barak, V.; Goike, H.; Panaretakis, K.W.; Einarsson, R. Clinical utility of cytokeratins as tumor markers. Clin. Biochem. 2004, 37, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Gong, J.; Chen, X.; Xu, M.; Huang, Y.; Wang, L.; Geng, N.; Zhou, Q. Cytokeratin expression in malignant melanoma: Potential application of in-situ hybridization analysis of mRNA. Melanoma Res. 2009, 19, 87–93. [Google Scholar] [CrossRef]
- Xu, K.; Yen, T.; Geczy, C.L. IL-10 Up-Regulates Macrophage Expression of the S100 Protein S100A81. J. Immunol. 2001, 166, 6358–6366. [Google Scholar] [CrossRef]
- Grimbaldeston, M.A.; Geczy, C.L.; Tedla, N.; Finlay-Jones, J.J.; Hart, P.H. S100A8 induction in keratinocytes by ultraviolet A irradiation is dependent on reactive oxygen intermediates. J. Investig. Dermatol. 2003, 121, 1168–1174. [Google Scholar] [CrossRef]
- Sinkala, M.; Nkhoma, P.; Mulder, N.; Martin, D.P. Integrated molecular characterisation of the MAPK pathways in human cancers reveals pharmacologically vulnerable mutations and gene dependencies. Commun. Biol. 2021, 4, 9. [Google Scholar] [CrossRef]
- Zubovits, J.; Buzney, E.; Yu, L.; Duncan, L.M. HMB-45, S-100, NK1/C3, and MART-1 in metastatic melanoma. Hum. Pathol. 2004, 35, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, D.; Chiriboga, L.; Rubin, B.P. Differential expression of S100 protein subtypes in malignant melanoma, and benign and malignant peripheral nerve sheath tumors. J. Cutan. Pathol. 2008, 35, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Kruijff, S.; Hoekstra, H.J. The current status of S-100B as a biomarker in melanoma. Eur. J. Surg. Oncol. 2012, 38, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Sauve, A.C.; Speed, T.P. Normalization, Baseline Correction and Alignment of High-throughput Mass Spectrometry Data. In Proceedings of the Genomic Signal Processing and Statistics Workshop (GENSIPS), Baltimore, MO, USA, 26–28 May 2004; pp. 1–4. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
| Samples Used for Classification | Classification Model | AUROC | Features Selected (n) | Cross-Validation | Accuracy (%) |
|---|---|---|---|---|---|
| Total Spectra, n = 746 BRAF WT, n = 335 BRAF MUT, n = 411 | LDA | ≥0.7 | 947 | LOOCV | 74 |
| k-fold (k = 4) | 66 | ||||
| k-fold (k = 10) | 69 | ||||
| ≥0.8 | 389 | LOOCV | 79 | ||
| k-fold (k = 4) | 76 | ||||
| k-fold (k = 10) | 73 | ||||
| SVM | ≥0.7 | 947 | LOOCV | 80 | |
| k-fold (k = 4) | 80 | ||||
| k-fold (k = 10) | 77 | ||||
| ≥0.8 | 389 | LOOCV | 84 | ||
| k-fold (k = 4) | 80 | ||||
| k-fold (k = 10) | 82 | ||||
| Total Patients, n = 45 BRAF WT, n = 22 BRAF MUT, n = 23 | LDA | ≥0.7 | 1057 | LOOCV | 81 |
| k-fold (k = 4) | 75 | ||||
| k-fold (k = 10) | 81 | ||||
| ≥0.84 | 542 | LOOCV | 84 | ||
| k-fold (k = 4) | 82 | ||||
| k-fold (k = 10) | 82 | ||||
| SVM | ≥0.7 | 1057 | LOOCV | 81 | |
| k-fold (k = 4) | 78 | ||||
| k-fold (k = 10) | 81 | ||||
| ≥0.84 | 542 | LOOCV | 86 | ||
| k-fold (k = 4) | 84 | ||||
| k-fold (k = 10) | 86 |
| Samples Used for Classification | Classification Model | Features Selected ( n) | Cross-Validation | Accuracy (%) |
|---|---|---|---|---|
| Total Spectra, n = 746 BRAF WT, n = 335 BRAF MUT, n = 411 | LDA | 30 | LOOCV | 82 |
| k-fold (k = 4) | 77 | |||
| k-fold (k = 10) | 83 | |||
| 6 | LOOCV | 93 | ||
| k-fold (k = 4) | 92 | |||
| k-fold (k = 10) | 95 | |||
| SVM | 30 | LOOCV | 80 | |
| k-fold (k = 4) | 80 | |||
| k-fold (k = 10) | 81 | |||
| 6 | LOOCV | 95 | ||
| k-fold (k = 4) | 92 | |||
| k-fold (k = 10) | 94 | |||
| Total Patients, n = 45 BRAF WT, n = 22 BRAF MUT, n = 23 | LDA | 30 | LOOCV | 74 |
| k-fold (k = 4) | 61 | |||
| k-fold (k = 10) | 69 | |||
| 3 | LOOCV | 93 | ||
| k-fold (k = 4) | 91 | |||
| k-fold (k = 10) | 93 | |||
| SVM | 30 | LOOCV | 84 | |
| k-fold (k = 4) | 74 | |||
| k-fold (k = 10) | 81 | |||
| 3 | LOOCV | 93 | ||
| k-fold (k = 4) | 94 | |||
| k-fold (k = 10) | 94 |
| Samples Used for Classification | Classification Model | AUROC | Features Selected ( n) | Cross-Validation | Accuracy (%) |
|---|---|---|---|---|---|
| Total Spectra, n = 754 NRAS WT, n = 373 NRAS MUT, n = 381 | LDA | ≥0.7 | 455 | LOOCV | 64 |
| k-fold (k = 4) | 52 | ||||
| k-fold (k = 10) | 61 | ||||
| ≥0.8 | 15 | LOOCV | 76 | ||
| k-fold (k = 4) | 76 | ||||
| k-fold (k = 10) | 77 | ||||
| SVM | ≥0.7 | 455 | LOOCV | 65 | |
| k-fold (k = 4) | 68 | ||||
| k-fold (k = 10) | 63 | ||||
| ≥0.8 | 15 | LOOCV | 76 | ||
| k-fold (k = 4) | 76 | ||||
| k-fold (k = 10) | 76 | ||||
| Total Patients, n = 44 NRAS WT, n = 22 NRAS MUT, n = 22 | LDA | ≥0.8 | 28 | LOOCV | 57 |
| k-fold (k = 4) | 56 | ||||
| k-fold (k = 10) | 58 | ||||
| ≥0.84 | 4 | LOOCV | 84 | ||
| k-fold (k = 4) | 77 | ||||
| k-fold (k = 10) | 77 | ||||
| SVM | ≥0.8 | 28 | LOOCV | 64 | |
| k-fold (k = 4) | 66 | ||||
| k-fold (k = 10) | 65 | ||||
| ≥0.84 | 4 | LOOCV | 82 | ||
| k-fold (k = 4) | 81 | ||||
| k-fold (k = 10) | 82 |
| Samples Used for Classification | Classification Model | Features Selected ( n) | Cross-Validation | Accuracy (%) |
|---|---|---|---|---|
| Total Spectra, n = 754 NRAS WT, n = 373 NRAS MUT, n = 381 | LDA | 50 | LOOCV | 71 |
| k-fold (k = 4) | 75 | |||
| k-fold (k = 10) | 65 | |||
| 27 | LOOCV | 78 | ||
| k-fold (k = 4) | 75 | |||
| k-fold (k = 10) | 79 | |||
| SVM | 50 | LOOCV | 77 | |
| k-fold (k = 4) | 72 | |||
| k-fold (k = 10) | 76 | |||
| 27 | LOOCV | 81 | ||
| k-fold (k = 4) | 79 | |||
| k-fold (k = 10) | 79 | |||
| Total Patients, n = 44 NRAS WT, n = 22 NRAS MUT, n = 22 | LDA | 8 | LOOCV | 64 |
| k-fold (k = 4) | 62 | |||
| k-fold (k = 10) | 63 | |||
| SVM | 8 | LOOCV | 79 | |
| k-fold (k = 4) | 68 | |||
| k-fold (k = 10) | 72 |
| Samples Used for Classification | Classification Model | AUROC | Features Selected ( n) | Cross-Validation | Accuracy (%) |
|---|---|---|---|---|---|
| Total Spectra, n = 792 BRAF MUT, n = 411 NRAS MUT, n = 381 | LDA | ≥0.7 | 10 | LOOCV | 65 |
| k-fold (k = 4) | 62 | ||||
| k-fold (k = 10) | 66 | ||||
| ≥0.73 | 3 | LOOCV | 67 | ||
| k-fold (k = 4) | 68 | ||||
| k-fold (k = 10) | 66 | ||||
| SVM | ≥0.7 | 10 | LOOCV | 69 | |
| k-fold (k = 4) | 67 | ||||
| k-fold (k = 10) | 67 | ||||
| ≥0.73 | 3 | LOOCV | 70 | ||
| k-fold (k = 4) | 65 | ||||
| k-fold (k = 10) | 70 | ||||
| Total Patients, n = 45 BRAF MUT, n = 23 NRAS MUT, n = 22 | LDA | ≥0.7 | 19 | LOOCV | 67 |
| k-fold (k = 4) | 64 | ||||
| k-fold (k = 10) | 61 | ||||
| ≥0.73 | 2 | LOOCV | 71 | ||
| k-fold (k = 4) | 67 | ||||
| k-fold (k = 10) | 69 | ||||
| SVM | ≥0.7 | 19 | LOOCV | 67 | |
| k-fold (k = 4) | 64 | ||||
| k-fold (k = 10) | 61 | ||||
| ≥0.73 | 2 | LOOCV | 67 | ||
| k-fold (k = 4) | 67 | ||||
| k-fold (k = 10) | 67 |
| Samples Used for Classification | Classification Model | Features Selected ( n) | Cross-Validation | Accuracy (%) |
|---|---|---|---|---|
| Total Spectra, n = 792 BRAF MUT, n = 411 NRAS MUT, n = 381 | LDA | 18 | LOOCV | 58 |
| k-fold (k = 4) | 61 | |||
| k-fold (k = 10) | 58 | |||
| 10 | LOOCV | 70 | ||
| k-fold (k = 4) | 67 | |||
| k-fold (k = 10) | 63 | |||
| SVM | 18 | LOOCV | 76 | |
| k-fold (k = 4) | 61 | |||
| k-fold (k = 10) | 72 | |||
| 10 | LOOCV | 71 | ||
| k-fold (k = 4) | 68 | |||
| k-fold (k = 10) | 71 | |||
| Total Patients, n = 45 BRAF MUT, n = 23 NRAS MUT, n = 22 | LDA | 19 | LOOCV | 42 |
| k-fold (k = 4) | 47 | |||
| k-fold (k = 10) | 44 | |||
| 2 | LOOCV | 71 | ||
| k-fold (k = 4) | 71 | |||
| k-fold (k = 10) | 67 | |||
| SVM | 19 | LOOCV | 53 | |
| k-fold (k = 4) | 56 | |||
| k-fold (k = 10) | 52 | |||
| 2 | LOOCV | 69 | ||
| k-fold (k = 4) | 65 | |||
| k-fold (k = 10) | 69 |
| Classification Comparison | m/z | Predictive Protein | FSS Accuracy | AUROC | AVG Intensity BRAF Mutated | AVG Intensity NRAS Mutated | AVG Intensity Wildtype | Log2-Fold Change | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| BRAF MUT/WT | 768.3 | Histone H3.Y | 0.98 | 0.8 | 3.52 | 2.31 | 0.61 | [23] | |
| 1026.5 | Histone H3.Y | 0.89 | 0.92 | 5.63 | 3.18 | 0.82 | [23] | ||
| 1252.6 | Glyceraldehyde-3-phosphate dehydrogenase | 0.97 | 0.81 | 3.91 | 2.88 | 0.44 | [31] | ||
| 1300.6 | Histone H3.Y | 0.9 | 0.85 | 4.16 | 2.65 | 0.65 | [23] | ||
| 1336.6 | Histone H3.3C | 0.95 | 0.75 | 2.64 | 2.09 | 0.33 | [23] | ||
| NRAS MUT/WT | 715.3 | Histone H3.3C | 0.88 | 0.92 | 7.38 | 15.47 | 1.067 | [23] | |
| 733.3 | Histone H3.X | 0.93 | 0.92 | 2.99 | 9.57 | 1.67 | [23] | ||
| 807.3 | Cytochrome c | 0.96 | 0.94 | 5.32 | 13.25 | 1.31 | [23] | ||
| 826.4 | Histone H3.Y | 0.93 | 0.91 | 2.55 | 6.8 | 1.4 | [23] | ||
| 837.4 | Defensin, alpha 6 | 0.94 | 0.83 | 35.48 | 25.22 | −0.49 | [23] | ||
| 910.4 | Histone H3.X | 0.92 | 0.96 | 4.39 | 24.18 | 2.45 | [23] | ||
| 982.4 | Protein S100-A8 (Calgranulin-A) | 0.88 | 0.93 | 6.64 | 24.29 | 1.87 | [23] | ||
| 1082.5 | Keratin, type I cytoskeletal 19 | 0.91 | 0.9 | 4.97 | 14.93 | 1.58 | [32] | ||
| 1091.5 | Histone H3.X | 0.92 | 0.87 | 3.78 | 12.2 | 1.69 | [23] | ||
| 1129.5 | Keratin, type II cytoskeletal 8 or Alpha-enolase | 0.97 | 0.76 | 6.55 | 7.74 | 0.24 | [32] | ||
| 1222.6 | Keratin, type 1 cytoskeletal 19 or Keratin, type 1 cytoskeletal 17 | 0.91 | 0.85 | 5.44 | 9.3 | 0.77 | [32] | ||
| 1259.6 | S100-A11 (Calgizzarin) | 0.93 | 0.86 | 4.12 | 5.44 | 0.39 | [23] | ||
| 1262.6 | Thymosin beta-4 | 0.97 | 0.79 | 5.81 | 7.46 | 0.36 | [23] | ||
| 1512.7 | Thymosin beta-4 | 0.9 | 0.73 | 5.15 | 13.16 | 1.35 | [23] | ||
| 1678.8 | Histone H3.X | 0.86 | 0.7 | 3.94 | 4.35 | 0.14 | [23] | ||
| 1783.8 | Defensin, alpha 3/Heat shock protein beta-1 (HspB1) | 0.93 | 0.74 | 3.63 | 5.86 | 0.69 | [23] | ||
| BRAF MUT/NRAS MUT | 944.4 | Histone H3.3C | 0.76 | 0.94 | 52.37 | 37.35 | 0.48 | [23] | |
| 1825.9 | Glyceraldehyde-3-phosphate dehydrogenase | 0.76 | 0.7 | 4.67 | 6.45 | −0.46 | [31] | ||
| 2169 | Glyceraldehyde-3-phosphate dehydrogenase | 0.74 | 0.88 | 2.91 | 3.7 | −0.34 | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casadonte, R.; Kriegsmann, M.; Kriegsmann, K.; Streit, H.; Meliß, R.R.; Müller, C.S.L.; Kriegsmann, J. Imaging Mass Spectrometry for the Classification of Melanoma Based on BRAF/NRAS Mutational Status. Int. J. Mol. Sci. 2023, 24, 5110. https://doi.org/10.3390/ijms24065110
Casadonte R, Kriegsmann M, Kriegsmann K, Streit H, Meliß RR, Müller CSL, Kriegsmann J. Imaging Mass Spectrometry for the Classification of Melanoma Based on BRAF/NRAS Mutational Status. International Journal of Molecular Sciences. 2023; 24(6):5110. https://doi.org/10.3390/ijms24065110
Chicago/Turabian StyleCasadonte, Rita, Mark Kriegsmann, Katharina Kriegsmann, Helene Streit, Rolf Rüdiger Meliß, Cornelia S. L. Müller, and Joerg Kriegsmann. 2023. "Imaging Mass Spectrometry for the Classification of Melanoma Based on BRAF/NRAS Mutational Status" International Journal of Molecular Sciences 24, no. 6: 5110. https://doi.org/10.3390/ijms24065110
APA StyleCasadonte, R., Kriegsmann, M., Kriegsmann, K., Streit, H., Meliß, R. R., Müller, C. S. L., & Kriegsmann, J. (2023). Imaging Mass Spectrometry for the Classification of Melanoma Based on BRAF/NRAS Mutational Status. International Journal of Molecular Sciences, 24(6), 5110. https://doi.org/10.3390/ijms24065110








