Muscle Cell Insulin Resistance Is Attenuated by Rosmarinic Acid: Elucidating the Mechanisms Involved
Abstract
1. Introduction
2. Results
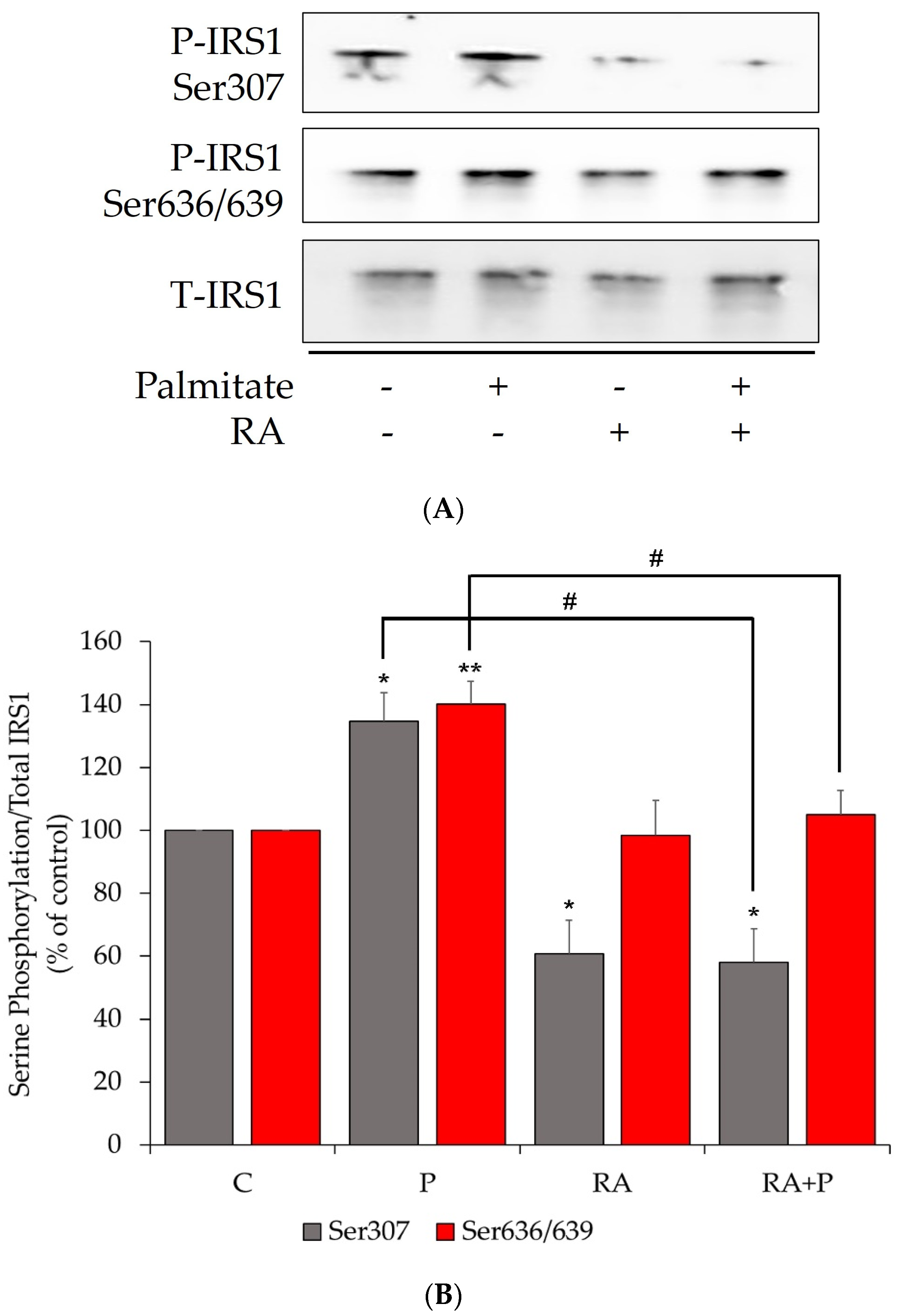
2.1. The Palmitate-Induced Serine Phosphorylation of Insulin Receptor Substrate-1 (IRS-1) Is Prevented by Rosmarinic Acid Treatment
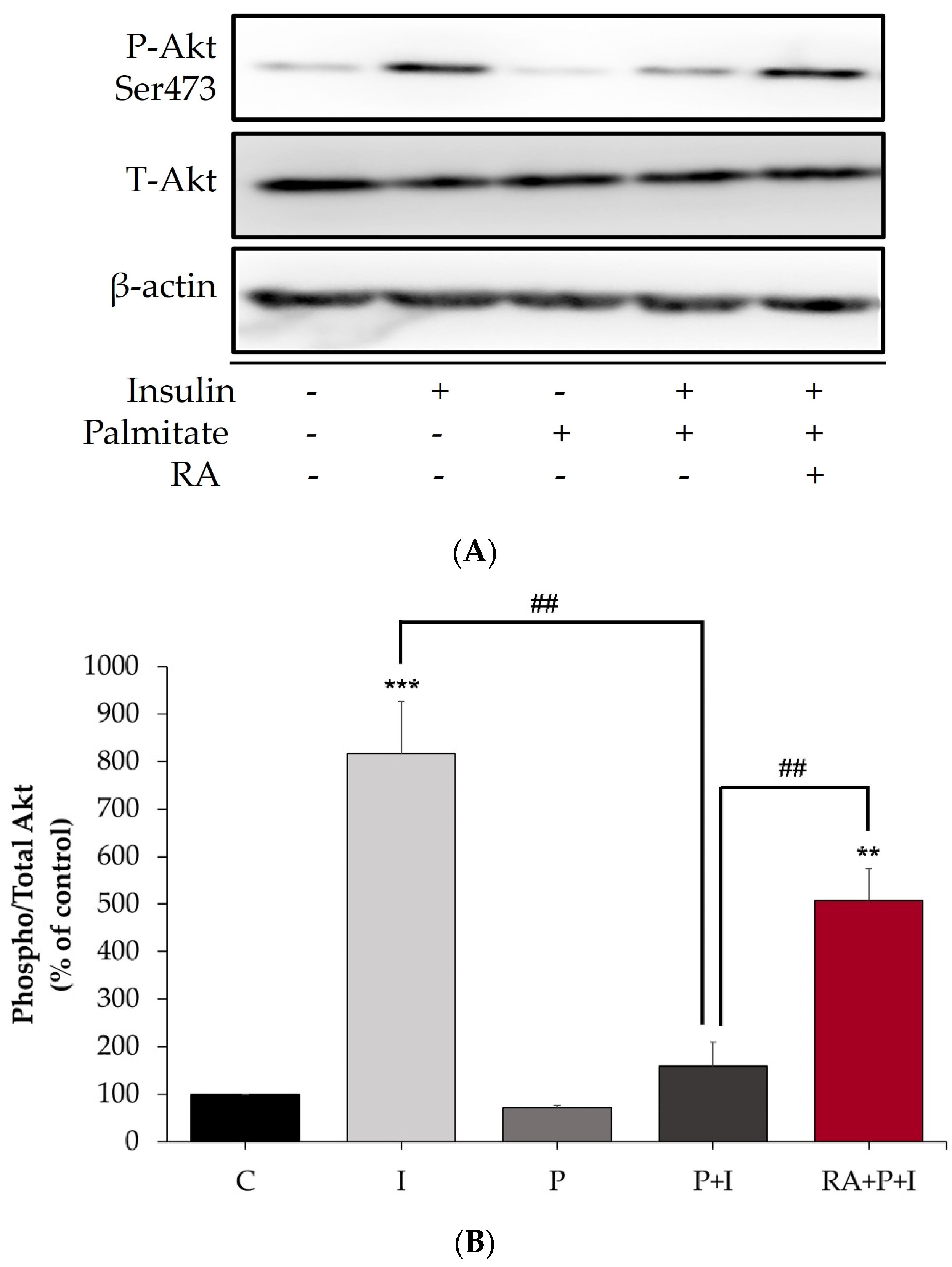
2.2. The Insulin-Stimulated Akt Phosphorylation in Palmitate-Treated Myotubes Is Restored with Rosmarinic Acid
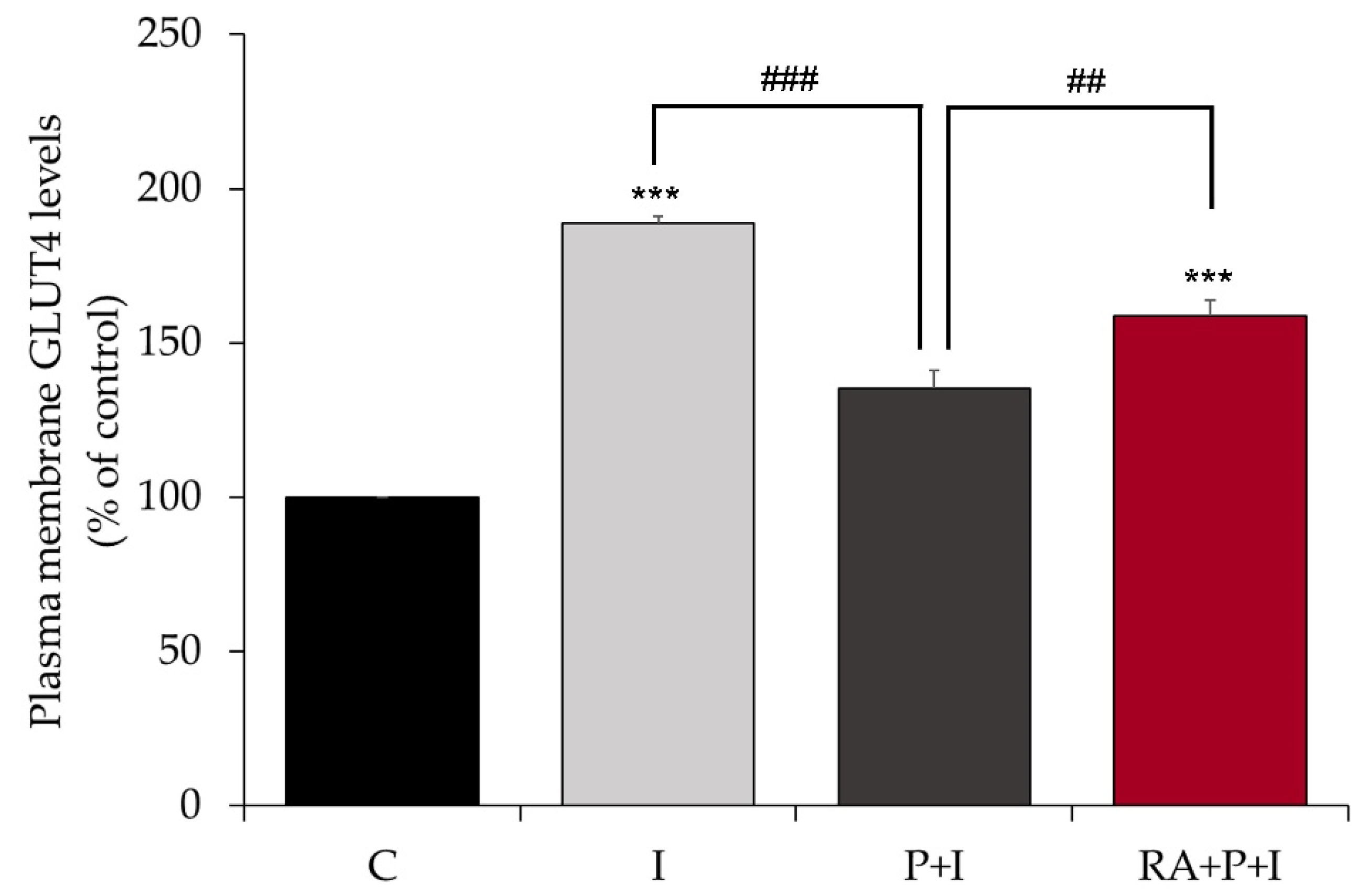
2.3. Rosmarinic Acid Restores Insulin-Stimulated GLUT4 Translocation to Plasma Membrane in Palmitate-Treated Myotubes
2.4. Rosmarinic Acid Restores the Insulin-Stimulated Glucose Uptake in Palmitate-Treated Myotubes
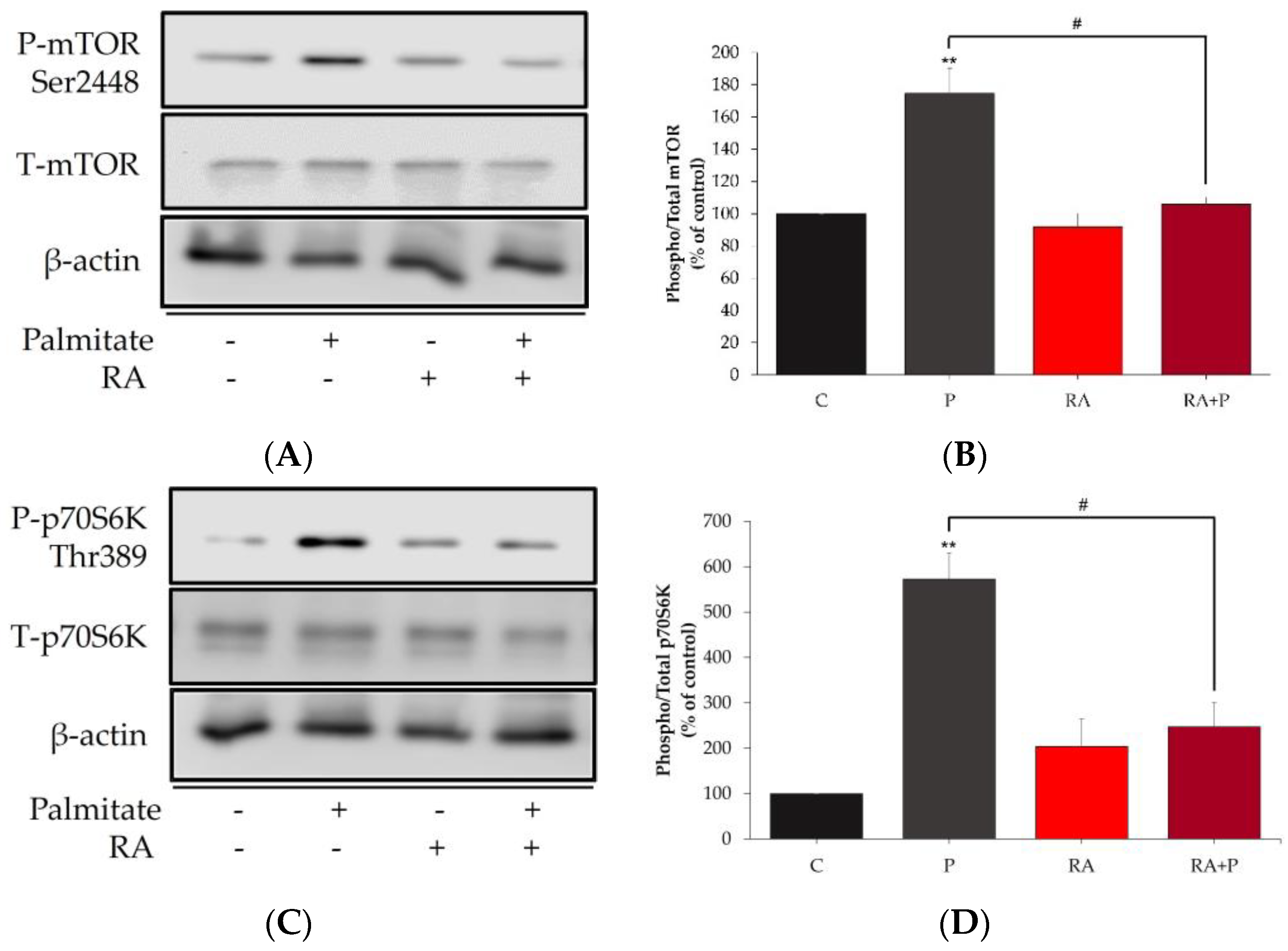
2.5. The Palmitate-Induced Phosphorylation/Activation of mTOR and p70S6K Is Prevented in the Presence of Rosmarinic Acid
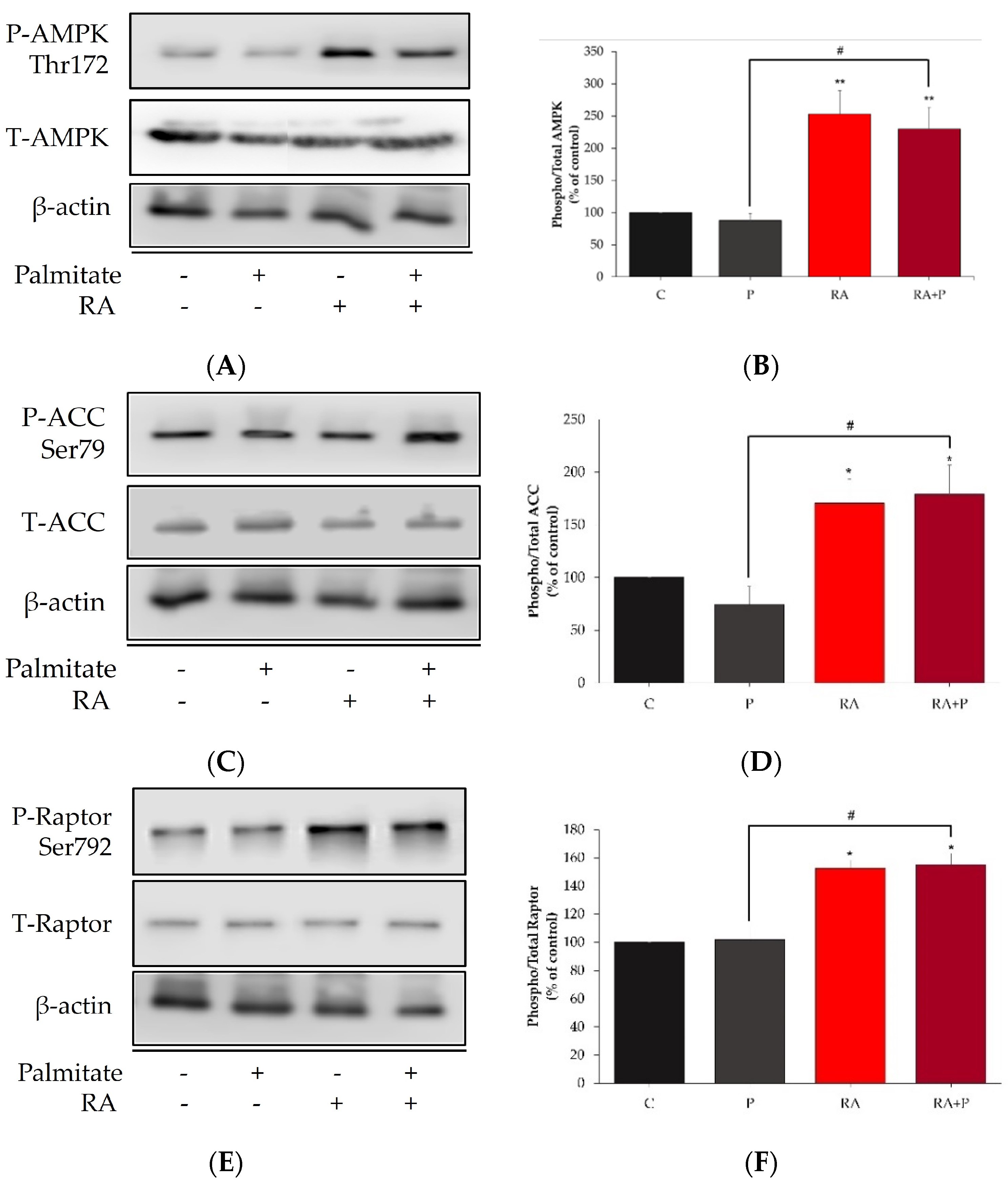
2.6. Rosmarinic Acid Increases the Phosphorylation of AMPK, ACC, and Raptor
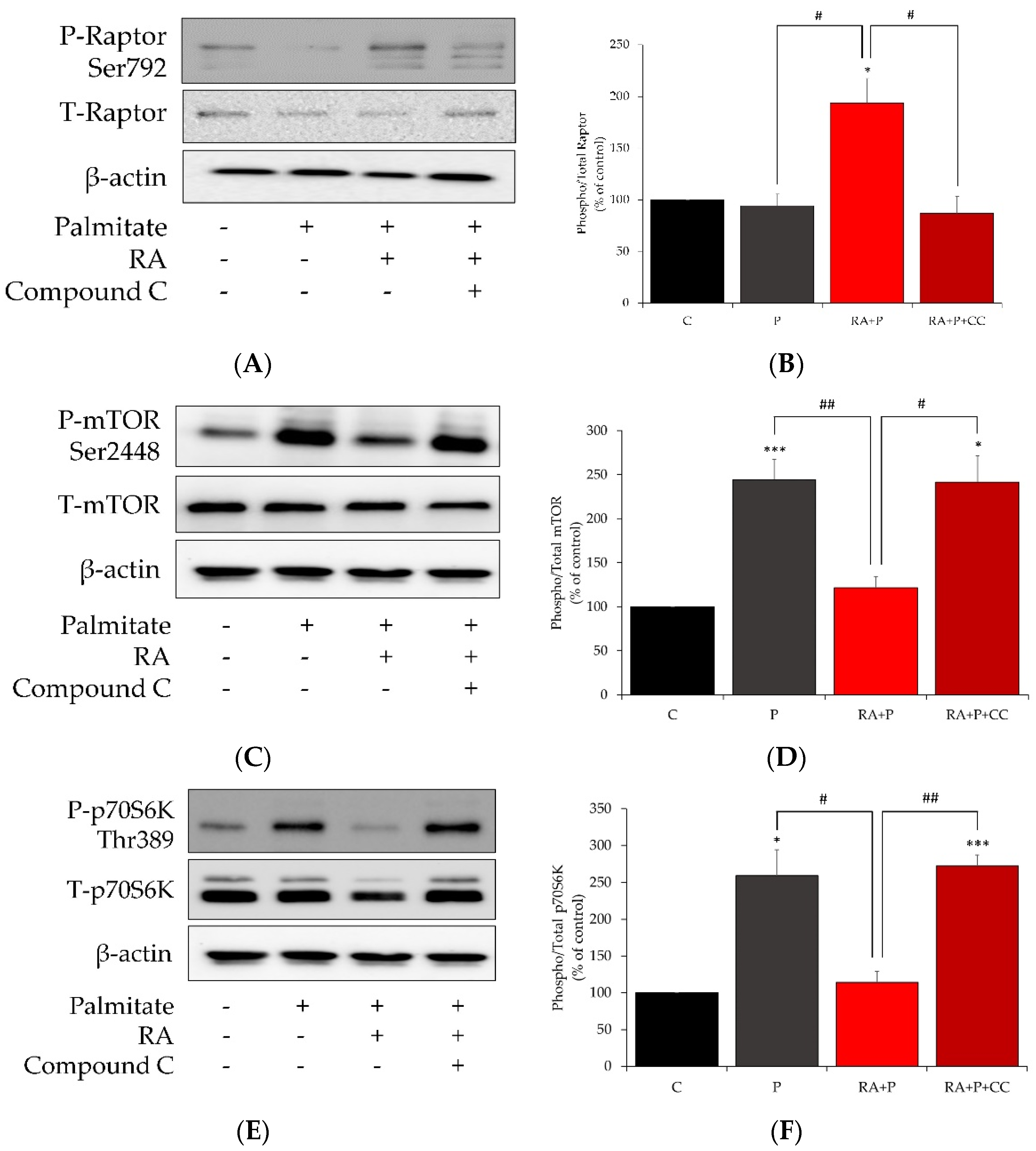
2.7. AMPK Inhibition Reverses the Effects of Rosmarinic Acid on Palmitate-Induced Phosphorylation of Raptor, mTOR, and p70S6K
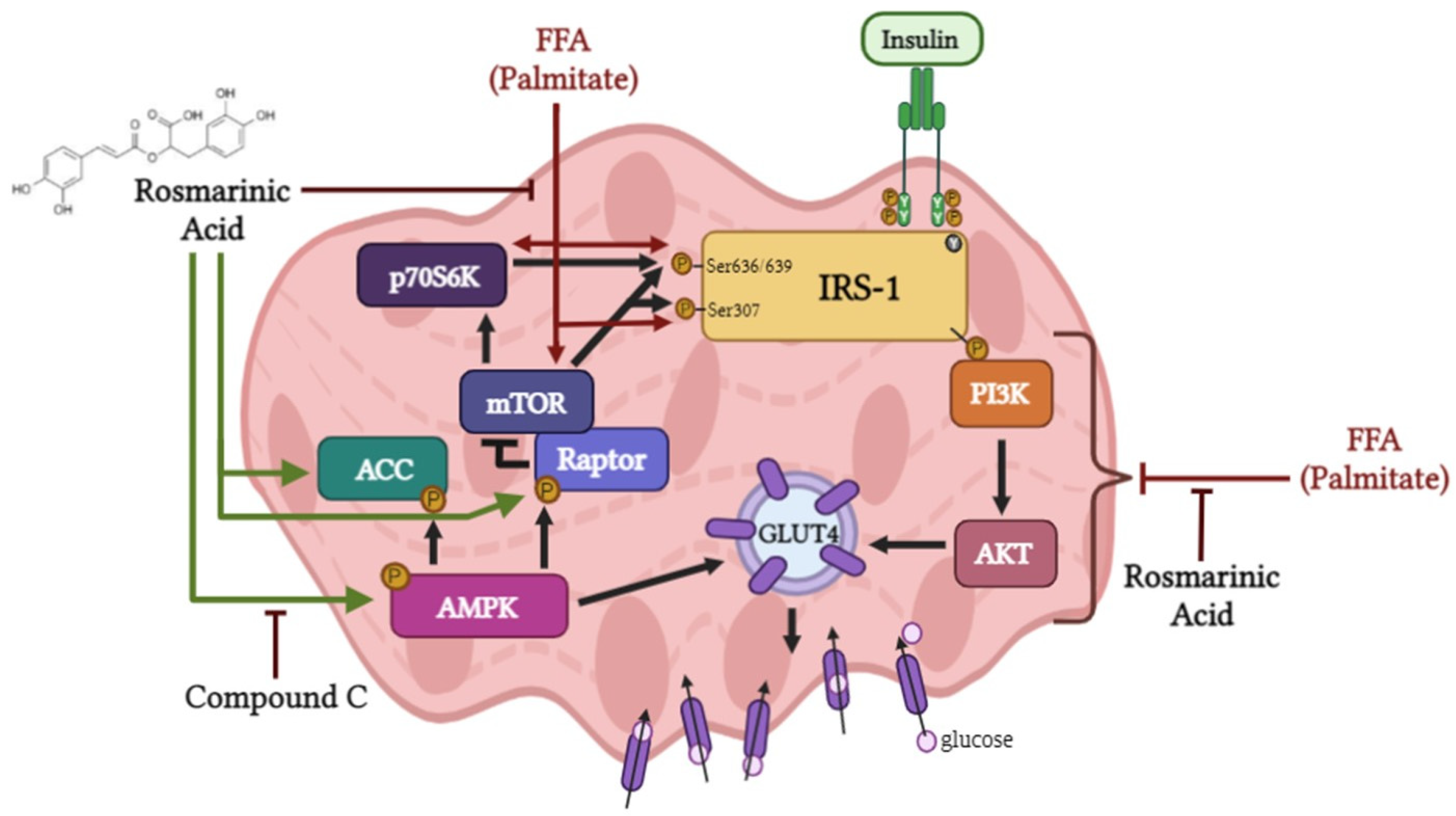
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Palmitate Stock Solution
4.3. Cell Culture, Treatment, and Glucose Uptake
4.4. GLUT4myc Translocation Assay
4.5. Immunoprecipitation
4.6. Immunoblotting
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahn, B.B.; Flier, J.S. Obesity and Insulin Resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef]
- Guo, S. Molecular Basis of Insulin Resistance: The Role of IRS and Foxo1 in the Control of Diabetes Mellitus and Its Complications. Drug Discov. Today Dis. Mech. 2013, 10, e27–e33. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical Nodes in Signalling Pathways: Insights into Insulin Action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Chavez, A.O. Defects in Insulin Secretion and Action in the Pathogenesis of Type 2 Diabetes Mellitus. Curr. Diab. Rep. 2010, 10, 184–191. [Google Scholar] [CrossRef]
- Sinha, S.; Perdomo, G.; Brown, N.F.; O’Doherty, R.M. Fatty Acid-Induced Insulin Resistance in L6 Myotubes Is Prevented by Inhibition of Activation and Nuclear Localization of Nuclear Factor Kappa B. J. Biol. Chem. 2004, 279, 41294–41301. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Petersen, K.F.; Shulman, G.I. Lipid-Induced Insulin Resistance: Unravelling the Mechanism. Lancet 2010, 375, 2267–2277. [Google Scholar] [CrossRef]
- Pereira, S.; Park, E.; Moore, J.; Faubert, B.; Breen, D.M.; Oprescu, A.I.; Nahle, A.; Kwan, D.; Giacca, A.; Tsiani, E. Resveratrol Prevents Insulin Resistance Caused by Short-Term Elevation of Free Fatty Acids In Vivo. Appl. Physiol. Nutr. Metab. 2015, 40, 1129–1136. [Google Scholar] [CrossRef]
- Hancock, C.R.; Han, D.-H.; Chen, M.; Terada, S.; Yasuda, T.; Wright, D.C.; Holloszy, J.O. High-Fat Diets Cause Insulin Resistance despite an Increase in Muscle Mitochondria. Proc. Natl. Acad. Sci. USA 2008, 105, 7815–7820. [Google Scholar] [CrossRef]
- Feinstein, R.; Kanety, H.; Papa, M.Z.; Lunenfeld, B.; Karasik, A. Tumor Necrosis Factor-Alpha Suppresses Insulin-Induced Tyrosine Phosphorylation of Insulin Receptor and Its Substrates. J. Biol. Chem. 1993, 268, 26055–26058. [Google Scholar] [CrossRef]
- Ueno, M.; Carvalheira, J.B.C.; Tambascia, R.C.; Bezerra, R.M.N.; Amaral, M.E.; Carneiro, E.M.; Folli, F.; Franchini, K.G.; Saad, M.J.A. Regulation of Insulin Signalling by Hyperinsulinaemia: Role of IRS-1/2 Serine Phosphorylation and the MTOR/P70 S6K Pathway. Diabetologia 2005, 48, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.J.; White, M.F.; Rondinone, C.M. Mammalian Target of Rapamycin Regulates IRS-1 Serine 307 Phosphorylation. Biochem. Biophys. Res. Commun. 2004, 316, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Mordier, S.; Iynedjian, P.B. Activation of Mammalian Target of Rapamycin Complex 1 and Insulin Resistance Induced by Palmitate in Hepatocytes. Biochem. Biophys. Res. Commun. 2007, 362, 206–211. [Google Scholar] [CrossRef]
- Manning, B.D. Balancing Akt with S6K: Implications for Both Metabolic Diseases and Tumorigenesis. J. Cell Biol. 2004, 167, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; Frigerio, F.; Watanabe, M.; Picard, F.; Joaquin, M.; Sticker, M.; Fumagalli, S.; Allegrini, P.R.; Kozma, S.C.; Auwerx, J.; et al. Absence of S6K1 Protects against Age- and Diet-Induced Obesity While Enhancing Insulin Sensitivity. Nature 2004, 431, 200–205. [Google Scholar] [CrossRef]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A Central Role for JNK in Obesity and Insulin Resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Li, Y.; Soos, T.J.; Li, X.; Wu, J.; Degennaro, M.; Sun, X.; Littman, D.R.; Birnbaum, M.J.; Polakiewicz, R.D. Protein Kinase C Theta Inhibits Insulin Signaling by Phosphorylating IRS1 at Ser(1101). J. Biol. Chem. 2004, 279, 45304–45307. [Google Scholar] [CrossRef]
- Hulver, M.W.; Dohm, G.L. The Molecular Mechanism Linking Muscle Fat Accumulation to Insulin Resistance. Proc. Nutr. Soc. 2004, 63, 375–380. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, D.; Dyck, J.R.B.; Li, Y.; Zhang, H.; Morishima, M.; Mann, D.L.; Taffet, G.E.; Baldini, A.; Khoury, D.S.; et al. A Pivotal Role for Endogenous TGF-Beta-Activated Kinase-1 in the LKB1/AMP-Activated Protein Kinase Energy-Sensor Pathway. Proc. Natl. Acad. Sci. USA 2006, 103, 17378–17383. [Google Scholar] [CrossRef]
- Towler, M.C.; Hardie, D.G. AMP-Activated Protein Kinase in Metabolic Control and Insulin Signaling. Circ. Res. 2007, 100, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-Activated Protein Kinase in Mechanism of Metformin Action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Fryer, L.G.D.; Parbu-Patel, A.; Carling, D. The Anti-Diabetic Drugs Rosiglitazone and Metformin Stimulate AMP-Activated Protein Kinase through Distinct Signaling Pathways. J. Biol. Chem. 2002, 277, 25226–25232. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lai, X.; Xiang, L.; Li, Q.; Sun, L.; Lai, Z.; Li, Z.; Zhang, W.; Wen, S.; Cao, J.; et al. Aged Green Tea Reduces High-Fat Diet-Induced Fat Accumulation and Inflammation via Activating the AMP-Activated Protein Kinase Signaling Pathway. Food Nutr. Res. 2022, 66, 7923. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.A.; Goya, L.; Ramos, S. Protective Effects of Tea, Red Wine and Cocoa in Diabetes. Evidences from Human Studies. Food Chem. Toxicol. 2017, 109, 302–314. [Google Scholar] [CrossRef]
- Breen, D.M.; Sanli, T.; Giacca, A.; Tsiani, E. Stimulation of Muscle Cell Glucose Uptake by Resveratrol through Sirtuins and AMPK. Biochem. Biophys. Res. Commun. 2008, 374, 117–122. [Google Scholar] [CrossRef]
- Zygmunt, K.; Faubert, B.; MacNeil, J.; Tsiani, E. Naringenin, a Citrus Flavonoid, Increases Muscle Cell Glucose Uptake via AMPK. Biochem. Biophys. Res. Commun. 2010, 398, 178–183. [Google Scholar] [CrossRef]
- Martin, M.Á.; Goya, L.; Ramos, S. Antidiabetic Actions of Cocoa Flavanols. Mol. Nutr. Food Res. 2016, 60, 1756–1769. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-Activated Protein Kinase—An Energy Sensor That Regulates All Aspects of Cell Function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A Nutrient and Energy Sensor That Maintains Energy Homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Gasparrini, M.; Giampieri, F.; Alvarez Suarez, J.; Mazzoni, L.; Y Forbes Hernandez, T.; Quiles, J.L.; Bullon, P.; Battino, M. AMPK as a New Attractive Therapeutic Target for Disease Prevention: The Role of Dietary Compounds AMPK and Disease Prevention. Curr. Drug Targets 2016, 17, 865–889. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.; Tai, J. Anti-Proliferative and Antioxidant Properties of Rosemary Rosmarinus Officinalis. Oncol. Rep. 2007, 17, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- Bakirel, T.; Bakirel, U.; Keleş, O.U.; Ulgen, S.G.; Yardibi, H. In Vivo Assessment of Antidiabetic and Antioxidant Activities of Rosemary (Rosmarinus Officinalis) in Alloxan-Diabetic Rabbits. J. Ethnopharmacol. 2008, 116, 64–73. [Google Scholar] [CrossRef]
- Emam, M. Comparative Evaluation of Antidiabetic Activity of Rosmarinus officinalis L. and Chamomile Recutita in Streptozotocin Induced Diabetic Rats. ABJNA 2012, 3, 247–252. [Google Scholar] [CrossRef]
- Ramadan, K.S.; Khalil, O.A.; Danial, E.N.; Alnahdi, H.S.; Ayaz, N.O. Hypoglycemic and Hepatoprotective Activity of Rosmarinus Officinalis Extract in Diabetic Rats. J. Physiol. Biochem. 2013, 69, 779–783. [Google Scholar] [CrossRef]
- Romo Vaquero, M.; Yáñez-Gascón, M.-J.; García Villalba, R.; Larrosa, M.; Fromentin, E.; Ibarra, A.; Roller, M.; Tomás-Barberán, F.; Espín de Gea, J.C.; García-Conesa, M.-T. Inhibition of Gastric Lipase as a Mechanism for Body Weight and Plasma Lipids Reduction in Zucker Rats Fed a Rosemary Extract Rich in Carnosic Acid. PLoS ONE 2012, 7, e39773. [Google Scholar] [CrossRef]
- Naimi, M.; Vlavcheski, F.; Shamshoum, H.; Tsiani, E. Rosemary Extract as a Potential Anti-Hyperglycemic Agent: Current Evidence and Future Perspectives. Nutrients 2017, 9, 968. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical Profiling of Flavonoids, Phenolic Acids, Terpenoids, and Volatile Fraction of a Rosemary (Rosmarinus officinalis L.) Extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef]
- Naimi, M.; Tsakiridis, T.; Stamatatos, T.C.; Alexandropoulos, D.I.; Tsiani, E. Increased Skeletal Muscle Glucose Uptake by Rosemary Extract through AMPK Activation. Appl. Physiol. Nutr. Metab. 2015, 40, 407–413. [Google Scholar] [CrossRef]
- Naimi, M.; Vlavcheski, F.; Murphy, B.; Hudlicky, T.; Tsiani, E. Carnosic Acid as a Component of Rosemary Extract Stimulates Skeletal Muscle Cell Glucose Uptake via AMPK Activation. Clin. Exp. Pharmacol. Physiol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Vlavcheski, F.; Naimi, M.; Murphy, B.; Hudlicky, T.; Tsiani, E. Rosmarinic Acid, a Rosemary Extract Polyphenol, Increases Skeletal Muscle Cell Glucose Uptake and Activates AMPK. Molecules 2017, 22, 1669. [Google Scholar] [CrossRef] [PubMed]
- Vlavcheski, F.; Baron, D.; Vlachogiannis, I.A.; MacPherson, R.E.K.; Tsiani, E. Carnosol Increases Skeletal Muscle Cell Glucose Uptake via AMPK-Dependent GLUT4 Glucose Transporter Translocation. Int. J. Mol. Sci. 2018, 19, 1321. [Google Scholar] [CrossRef] [PubMed]
- Vlavcheski, F.; Tsiani, E. Attenuation of Free Fatty Acid-Induced Muscle Insulin Resistance by Rosemary Extract. Nutrients 2018, 10, 1623. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Vlavcheski, F.; Giacca, A.; MacPherson, R.E.K.; Tsiani, E. Carnosic Acid Attenuates the Free Fatty Acid-Induced Insulin Resistance in Muscle Cells and Adipocytes. Cells 2022, 11, 167. [Google Scholar] [CrossRef]
- Runtuwene, J.; Cheng, K.-C.; Asakawa, A.; Amitani, H.; Amitani, M.; Morinaga, A.; Takimoto, Y.; Kairupan, B.H.R.; Inui, A. Rosmarinic Acid Ameliorates Hyperglycemia and Insulin Sensitivity in Diabetic Rats, Potentially by Modulating the Expression of PEPCK and GLUT4. Drug Des. Dev. Ther. 2016, 10, 2193–2202. [Google Scholar] [CrossRef]
- Jayanthy, G.; Roshana Devi, V.; Ilango, K.; Subramanian, S.P. Rosmarinic Acid Mediates Mitochondrial Biogenesis in Insulin Resistant Skeletal Muscle through Activation of AMPK. J. Cell. Biochem. 2017, 118, 1839–1848. [Google Scholar] [CrossRef]
- Bao, T.-Q.; Li, Y.; Qu, C.; Zheng, Z.-G.; Yang, H.; Li, P. Antidiabetic Effects and Mechanisms of Rosemary (Rosmarinus officinalis L.) and Its Phenolic Components. Am. J. Chin. Med. 2020, 48, 1353–1368. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Randhawa, V.K.; Bilan, P.J.; Khayat, Z.A.; Daneman, N.; Liu, Z.; Ramlal, T.; Volchuk, A.; Peng, X.R.; Coppola, T.; Regazzi, R.; et al. VAMP2, but Not VAMP3/Cellubrevin, Mediates Insulin-Dependent Incorporation of GLUT4 into the Plasma Membrane of L6 Myoblasts. Mol. Biol. Cell 2000, 11, 2403–2417. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.; Siegel, N.; Valli, A.; Fuchs, C.; Hengstschläger, M. MTOR Phosphorylated at S2448 Binds to Raptor and Rictor. Amino Acids 2010, 38, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Wei, L.; Huang, J. MTOR Signaling, Function, Novel Inhibitors, and Therapeutic Targets. J. Nucl. Med. 2011, 52, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Vlavcheski, F.; Giacca, A.; Tsiani, E. Attenuation of Free Fatty Acid (FFA)-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol Is Linked to Activation of AMPK and Inhibition of MTOR and P70 S6K. Int. J. Mol. Sci. 2020, 21, 4900. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, G.; Commerford, S.R.; Richard, A.-M.T.; Adams, S.H.; Corkey, B.E.; O’Doherty, R.M.; Brown, N.F. Increased Beta-Oxidation in Muscle Cells Enhances Insulin-Stimulated Glucose Metabolism and Protects against Fatty Acid-Induced Insulin Resistance Despite Intramyocellular Lipid Accumulation. J. Biol. Chem. 2004, 279, 27177–27186. [Google Scholar] [CrossRef]
- Dimopoulos, N.; Watson, M.; Sakamoto, K.; Hundal, H.S. Differential Effects of Palmitate and Palmitoleate on Insulin Action and Glucose Utilization in Rat L6 Skeletal Muscle Cells. Biochem. J. 2006, 399, 473–481. [Google Scholar] [CrossRef]
- Powell, D.J.; Turban, S.; Gray, A.; Hajduch, E.; Hundal, H.S. Intracellular Ceramide Synthesis and Protein Kinase Czeta Activation Play an Essential Role in Palmitate-Induced Insulin Resistance in Rat L6 Skeletal Muscle Cells. Biochem. J. 2004, 382, 619–629. [Google Scholar] [CrossRef]
- Capel, F.; Cheraiti, N.; Acquaviva, C.; Hénique, C.; Bertrand-Michel, J.; Vianey-Saban, C.; Prip-Buus, C.; Morio, B. Oleate Dose-Dependently Regulates Palmitate Metabolism and Insulin Signaling in C2C12 Myotubes. Biochim. Biophys. Acta 2016, 1861, 2000–2010. [Google Scholar] [CrossRef]
- Jung, T.W.; Kim, H.-C.; Abd El-Aty, A.M.; Jeong, J.H. Protectin DX Ameliorates Palmitate- or High-Fat Diet-Induced Insulin Resistance and Inflammation through an AMPK-PPARα-Dependent Pathway in Mice. Sci. Rep. 2017, 7, 1397. [Google Scholar] [CrossRef]
- Wu, W.; Tang, S.; Shi, J.; Yin, W.; Cao, S.; Bu, R.; Zhu, D.; Bi, Y. Metformin Attenuates Palmitic Acid-Induced Insulin Resistance in L6 Cells through the AMP-Activated Protein Kinase/Sterol Regulatory Element-Binding Protein-1c Pathway. Int. J. Mol. Med. 2015, 35, 1734–1740. [Google Scholar] [CrossRef]
- Deng, Y.-T.; Chang, T.-W.; Lee, M.-S.; Lin, J.-K. Suppression of Free Fatty Acid-Induced Insulin Resistance by Phytopolyphenols in C2C12 Mouse Skeletal Muscle Cells. J. Agric. Food Chem. 2012, 60, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Gual, P.; Le Marchand-Brustel, Y.; Tanti, J.-F. Positive and Negative Regulation of Insulin Signaling through IRS-1 Phosphorylation. Biochimie 2005, 87, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Copps, K.D.; White, M.F. Regulation of Insulin Sensitivity by Serine/Threonine Phosphorylation of Insulin Receptor Substrate Proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef] [PubMed]
- Bogachus, L.D.; Turcotte, L.P. Genetic Downregulation of AMPK-Alpha Isoforms Uncovers the Mechanism by Which Metformin Decreases FA Uptake and Oxidation in Skeletal Muscle Cells. Am. J. Physiol. Cell Physiol. 2010, 299, C1549–C1561. [Google Scholar] [CrossRef]
- Rivas, D.A.; Yaspelkis, B.B.; Hawley, J.A.; Lessard, S.J. Lipid-Induced MTOR Activation in Rat Skeletal Muscle Reversed by Exercise and 5’-Aminoimidazole-4-Carboxamide-1-Beta-D-Ribofuranoside. J. Endocrinol. 2009, 202, 441–451. [Google Scholar] [CrossRef]
- Wang, X.; Yu, W.; Nawaz, A.; Guan, F.; Sun, S.; Wang, C. Palmitate Induced Insulin Resistance by PKCtheta-Dependent Activation of MTOR/S6K Pathway in C2C12 Myotubes. Exp. Clin. Endocrinol. Diabetes 2010, 118, 657–661. [Google Scholar] [CrossRef]
- Woo, J.H.; Shin, K.O.; Lee, Y.H.; Jang, K.S.; Bae, J.Y.; Roh, H.T. Effects of Treadmill Exercise on Skeletal Muscle MTOR Signaling Pathway in High-Fat Diet-Induced Obese Mice. J. Phys. Ther. Sci. 2016, 28, 1260–1265. [Google Scholar] [CrossRef]
- Le Bacquer, O.; Petroulakis, E.; Paglialunga, S.; Poulin, F.; Richard, D.; Cianflone, K.; Sonenberg, N. Elevated Sensitivity to Diet-Induced Obesity and Insulin Resistance in Mice Lacking 4E-BP1 and 4E-BP2. J. Clin. Investig. 2007, 117, 387–396. [Google Scholar] [CrossRef]
- Kwon, B.; Querfurth, H.W. Palmitate Activates MTOR/P70S6K through AMPK Inhibition and Hypophosphorylation of Raptor in Skeletal Muscle Cells: Reversal by Oleate Is Similar to Metformin. Biochimie 2015, 118, 141–150. [Google Scholar] [CrossRef]
- Tzatsos, A. Raptor Binds the SAIN (Shc and IRS-1 NPXY Binding) Domain of Insulin Receptor Substrate-1 (IRS-1) and Regulates the Phosphorylation of IRS-1 at Ser-636/639 by MTOR. J. Biol. Chem. 2009, 284, 22525–22534. [Google Scholar] [CrossRef]
- Shah, O.J.; Wang, Z.; Hunter, T. Inappropriate Activation of the TSC/Rheb/MTOR/S6K Cassette Induces IRS1/2 Depletion, Insulin Resistance, and Cell Survival Deficiencies. Curr. Biol. 2004, 14, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Marette, A. Amino Acid and Insulin Signaling via the MTOR/P70 S6 Kinase Pathway. A Negative Feedback Mechanism Leading to Insulin Resistance in Skeletal Muscle Cells. J. Biol. Chem. 2001, 276, 38052–38060. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.S.; Findlay, G.M.; Gray, A.; Tolkacheva, T.; Wigfield, S.; Rebholz, H.; Barnett, J.; Leslie, N.R.; Cheng, S.; Shepherd, P.R.; et al. The TSC1-2 Tumor Suppressor Controls Insulin-PI3K Signaling via Regulation of IRS Proteins. J. Cell Biol. 2004, 166, 213–223. [Google Scholar] [CrossRef]
- Guillén, C.; Benito, M. MTORC1 Overactivation as a Key Aging Factor in the Progression to Type 2 Diabetes Mellitus. Front. Endocrinol. 2018, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Bhattacharya, S.; Swamy, O.R.; Tandon, R.; Wang, Y.; Janda, R.; Riedel, H. Growth Factor Receptor-Binding Protein 10 (Grb10) as a Partner of Phosphatidylinositol 3-Kinase in Metabolic Insulin Action. J. Biol. Chem. 2003, 278, 39311–39322. [Google Scholar] [CrossRef]
- Yu, Y.; Yoon, S.-O.; Poulogiannis, G.; Yang, Q.; Ma, X.M.; Villén, J.; Kubica, N.; Hoffman, G.R.; Cantley, L.C.; Gygi, S.P.; et al. Quantitative Phosphoproteomic Analysis Identifies the Adaptor Protein Grb10 as an MTORC1 Substrate That Negatively Regulates Insulin Signaling. Science 2011, 332, 1322–1326. [Google Scholar] [CrossRef]
- Hsu, P.P.; Kang, S.A.; Rameseder, J.; Zhang, Y.; Ottina, K.A.; Lim, D.; Peterson, T.R.; Choi, Y.; Gray, N.S.; Yaffe, M.B.; et al. The MTOR-Regulated Phosphoproteome Reveals a Mechanism of MTORC1-Mediated Inhibition of Growth Factor Signaling. Science 2011, 332, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. AMP-Activated Protein Kinase and Its Downstream Transcriptional Pathways. Cell. Mol. Life Sci. 2010, 67, 3407–3423. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK Signalling Pathway Coordinates Cell Growth, Autophagy and Metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Johnson, J.J. Carnosol: A Promising Anti-Cancer and Anti-Inflammatory Agent. Cancer Lett. 2011, 305, 1–7. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Den Hartogh, D.J.; Vlavcheski, F.; Tsiani, E. Muscle Cell Insulin Resistance Is Attenuated by Rosmarinic Acid: Elucidating the Mechanisms Involved. Int. J. Mol. Sci. 2023, 24, 5094. https://doi.org/10.3390/ijms24065094
Den Hartogh DJ, Vlavcheski F, Tsiani E. Muscle Cell Insulin Resistance Is Attenuated by Rosmarinic Acid: Elucidating the Mechanisms Involved. International Journal of Molecular Sciences. 2023; 24(6):5094. https://doi.org/10.3390/ijms24065094
Chicago/Turabian StyleDen Hartogh, Danja J., Filip Vlavcheski, and Evangelia Tsiani. 2023. "Muscle Cell Insulin Resistance Is Attenuated by Rosmarinic Acid: Elucidating the Mechanisms Involved" International Journal of Molecular Sciences 24, no. 6: 5094. https://doi.org/10.3390/ijms24065094
APA StyleDen Hartogh, D. J., Vlavcheski, F., & Tsiani, E. (2023). Muscle Cell Insulin Resistance Is Attenuated by Rosmarinic Acid: Elucidating the Mechanisms Involved. International Journal of Molecular Sciences, 24(6), 5094. https://doi.org/10.3390/ijms24065094






