Mycobacterial Heat Shock Proteins in Sarcoidosis and Tuberculosis
Abstract
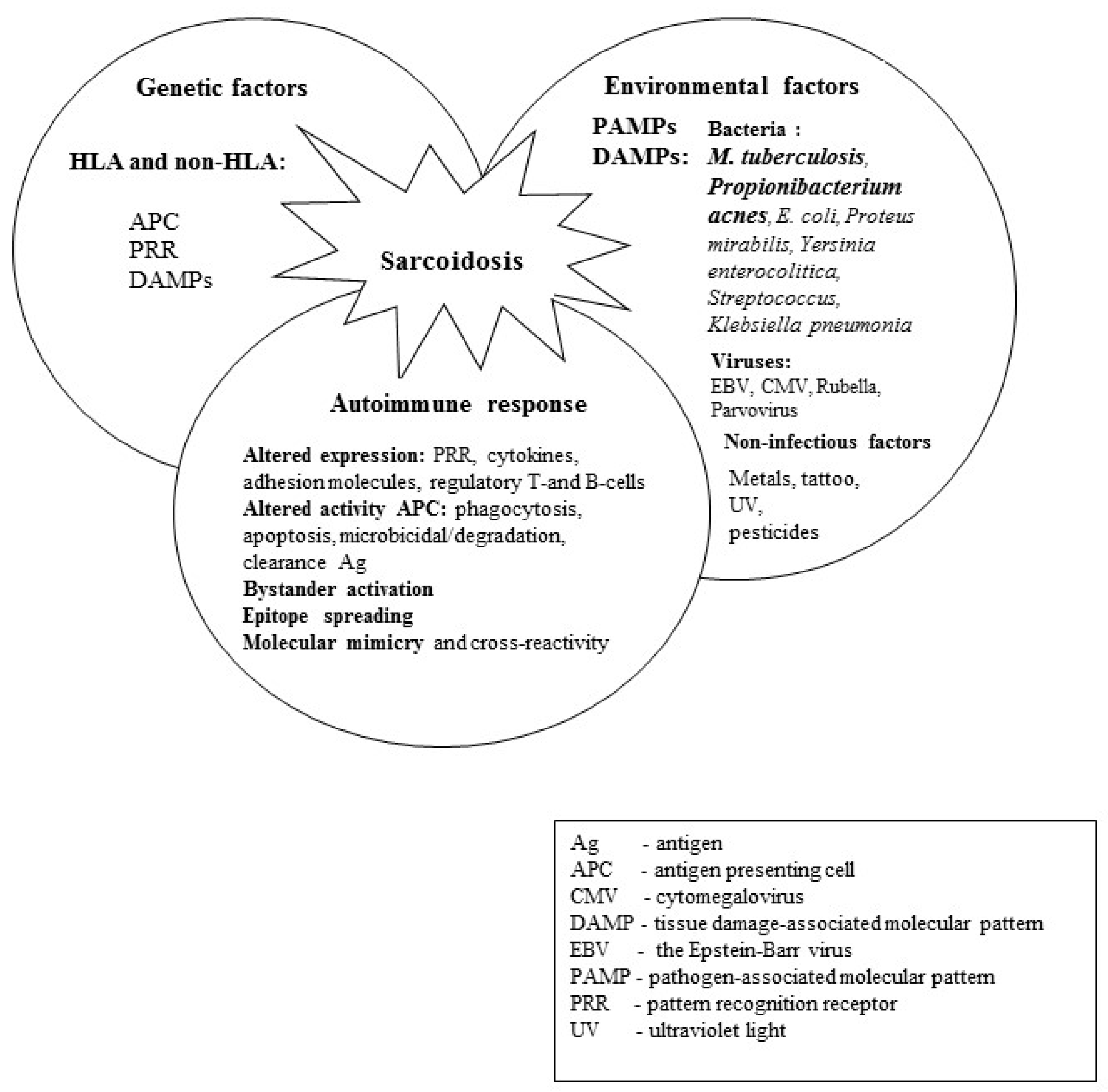
1. Introduction
2. Heat Shock Proteins as ‘Danger Signals’
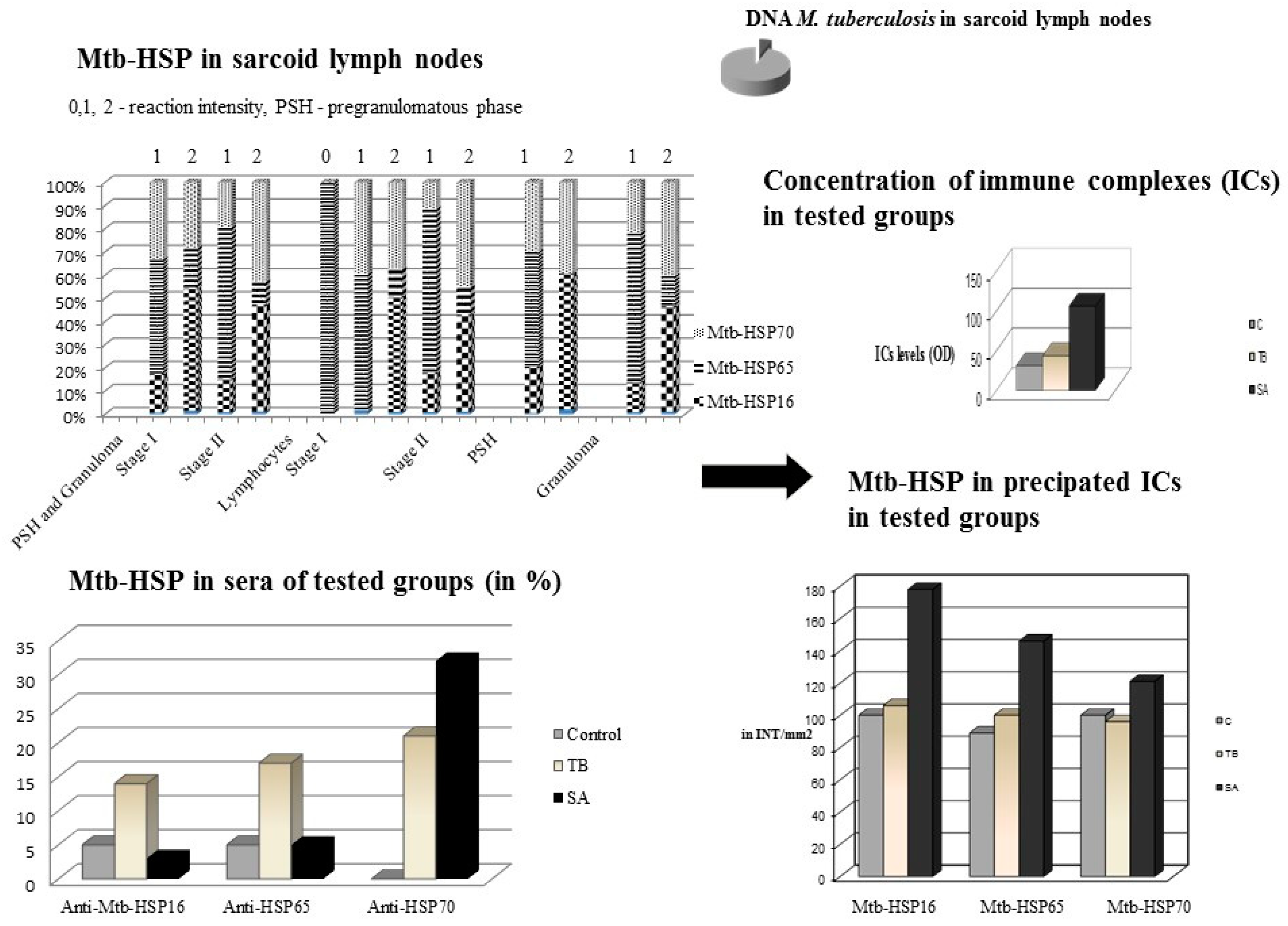
3. Mycobacterium tuberculosis Complex and Mycobacterial Heat Shock Proteins in Lymph Nodes from Patients with SA, Patients with TB as Positive Controls, and Patients with Metastatic Non-Small-Cell Lung Cancer and Patients with Nonspecific Lymph Nodes as Negative Controls
4. Mycobacterial Heat Shock Proteins in Sera of Patients with SA, Patients with TB and in Healthy Individuals as Controls
5. The Level of Circulating Immune Complexes and the Concentration of Mycobacterial Heat Shock Proteins in Precipitated ICs in the Sera of Patients with SA, Patients with TB and Healthy Individuals
6. Concentration of Nitrates/Nitrites (NOx) in the Blood Serum of Patients with SA, Patients with TB and Healthy People
7. Peripheral Blood Mononuclear Cell (PBMC) Apoptosis Induced by Mycobacterial Heat Shock Proteins in Sarcoidosis, Tuberculosis and Healthy Controls
8. Mtb-HSP-Stimulated T Cell Subsets and Th1/Th2 Cytokine Patterns in Peripheral Blood Mononuclear Cell Culture Supernatant from Patients with SA, Patients with TB and Healthy Individuals as Controls
9. Concentration of Peroxynitrite (ONOO−) in Supernatants of PBMC Cultures Treated with Mtb-HSP in Patients with SA, Patients with TB and Healthy Individuals
10. Microbial Heat Shock Proteins and Autoimmunity in Sarcoidosis
11. Genetic Predispositions (HLA Class I and II, SLC11A1, FCGR) of Patients with SA, Patients with TB and Healthy Controls (Figure 3)
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Thoracic Society: Statement on sarcoidosis. Am. J. Respir. Crit. Care Med. 1999, 160, 736–755. [CrossRef]
- Dubaniewicz, A. Microbial and human heat shock proteins as ‘danger signals’ in sarcoidosis. Hum. Immunol. 2013, 74, 1550–1558. [Google Scholar] [CrossRef]
- Danila, E.; Zurauskas, E. Diagnostic value of epithelioid cell granulomas in bronchoscopic biopsies. Intern. Med. 2008, 47, 2121–2126. [Google Scholar] [CrossRef]
- Dubaniewicz, A.; Szczerkowska, Z.; Hoppe, A. Comparative analysis of HLA class I antigens in pulmonary sarcoidosis and tuberculosis in the same ethnic group. Mayo Clin. Proc. 2003, 78, 436–442. [Google Scholar] [CrossRef]
- Dubaniewicz, A.; Zimmermann, A.; Smigielska, M.; Dubaniewicz-Wybieralska, M.; Moszkowska, G.; Wysocka, J.; Adamczyk-Bak, K.; Slominski, J.M.; Deeg, P. Sarcoidosis and tuberculosis: A connection to the HLA system. Adv. Exp. Med. Biol. 2013, 756, 229–237. [Google Scholar]
- Dubaniewicz, A.; Dubaniewicz-Wybieralska, M.; Moszkowska, G.; Sternau, A.; Dubaniewicz, A. Comparative analysis of DR and DQ alleles occurrence in sarcoidosis and tuberculosis in the same ethnic group: Preliminary study. Sarcoidosis Vasc. Diffuse Lung Dis. 2006, 23, 180–189. [Google Scholar]
- Dubaniewicz, A.; Jamieson, S.E.; Dubaniewicz-Wybieralska, M.; Fakiola, M.; Miller, N.E.; Blackwell, J.M. Association between SLC11A1 (formerly NRAMP1) and the risk of sarcoidosis in Poland. Eur. J. Hum. Genet. 2005, 13, 829–834. [Google Scholar] [CrossRef]
- Typiak, M.; Rębała, K.; Dudziak, M.; Słomiński, J.M.; Dubaniewicz, A. Polymorphism of FCGR2A, FCGR2C, and FCGR3B genes in the pathogenesis of sarcoidosis. Adv. Exp. Med. Biol. 2016, 905, 57–68. [Google Scholar]
- Fischer, A.; Rybicki, B.A. Granuloma genes in sarcoidosis: What is new? Curr. Opin. Pulm. Med. 2015, 21, 510–516. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nunez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Song, M.; Manansala, M.; Parmar, P.J.; Ascoli, C.; Rubinstein, I.; Sweiss, N.J. Sarcoidosis and autoimmunity. Curr. Opin. Pulm. Med. 2021, 27, 448–454. [Google Scholar] [CrossRef]
- Dubaniewicz, A. Mycobacterium tuberculosis heat shock proteins and autoimmunity in sarcoidosis. Autoimmun. Rev. 2010, 9, 419–424. [Google Scholar] [CrossRef]
- Oswald-Richter, K.A.; Beachboard, D.C.; Zhan, X.; Gaskill, C.F.; Abraham, S.; Jenkins, C.; Culver, D.A.; Drake, W. Multiple mycobacterial antigens are targets of the adaptive immune response in pulmonary sarcoidosis. Respir. Res. 2010, 11, 161. [Google Scholar] [CrossRef]
- Dubaniewicz, A.; Dubaniewicz-Wybieralska, M.; Sternau, A.; Zwolska, Z.; Izycka-Swieszewska, E.; Augustynowicz-Kopec, E.; Skokowski, J.; Singh, M.; Zimnoch, L. Mycobacterium tuberculosis complex and mycobacterial heat shock proteins in lymph node tissue from patients with pulmonary sarcoidosis. J. Clin. Microbiol. 2006, 44, 3448–3451. [Google Scholar] [CrossRef]
- Dubaniewicz, A.; Kämpfer, S.; Singh, M. Serum anti-mycobacterial heat shock proteins antibodies in sarcoidosis and tuberculosis. Tuberculosis 2006, 86, 60–67. [Google Scholar] [CrossRef]
- Dubaniewicz, A.; Holownia, A.; Kalinowski, L.; Wybieralska, M.; Dobrucki, I.T.; Singh, M. Is mycobacterial heat shock protein 16 kDa, a marker of the dormant stage of Mycobacterium tuberculosis, a sarcoid antigen? Hum. Immunol. 2013, 74, 45–51. [Google Scholar] [CrossRef]
- Dubaniewicz, A.; Trzonkowski, P.; Dubaniewicz-Wybieralska, M.; Dubaniewicz, A.; Singh, M.; Myśliwski, A. Mycobacterial heat shock protein-induced blood T lymphocytes subsets and cytokine pattern: Comparison of sarcoidosis with tuberculosis and healthy controls. Respirology 2007, 12, 346–354. [Google Scholar] [CrossRef]
- Dubaniewicz, A.; Trzonkowski, P.; Dubaniewicz-Wybieralska, M.; Dubaniewicz, A.; Singh, M.; Myśliwski, A. Comparative analysis of mycobacterial heat shock proteins-induced apoptosis of peripheral blood mononuclear cells in sarcoidosis and tuberculosis. J. Clin. Immunol. 2006, 26, 243–250. [Google Scholar] [CrossRef]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef]
- Zhang, X.; Mosser, D.M. Macrophage activation by endogenous danger signals. J. Pathol. 2008, 214, 161–178. [Google Scholar] [CrossRef]
- Tamura, Y.; Torigoe, T.; Kutomi, G.; Hirata, K.; Sato, N. New paradigm for intrinsic function of heat shock proteins as endogenous ligands in inflammation and innate immunity. Curr. Mol. Med. 2012, 12, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, A.M.; Midwood, K.S. DAMPening inflammation by modulating TLR signaling. Mediators Inflamm. 2010, 2010, 672395. [Google Scholar] [CrossRef]
- Sims, G.P.; Rowe, D.C.; Rietdijk, S.T. HMGB1 and RAGE in inflammation and cancer. Ann. Rev. Immunol. 2010, 28, 367–388. [Google Scholar] [CrossRef]
- Marshak-Rothstein, A. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 2006, 6, 823–835. [Google Scholar] [CrossRef]
- Bulut, Y.; Michelsen, K.S.; Hayrapetian, L.; Naiki, Y.; Spallek, R.; Singh, M.; Arditi, M. Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate proinflammatory signals. J. Biol. Chem. 2005, 280, 20961–20967. [Google Scholar] [CrossRef] [PubMed]
- Negi, M.; Takemura, T.; Guzman, J.; Uchida, K.; Furukawa, A.; Suzuki, Y.; Iida, T.; Ishige, I.; Minami, J.; Yamada, T.; et al. Localization of Propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium. Mod. Pathol. 2012, 25, 1284–1297. [Google Scholar] [CrossRef]
- Riviere, E.; Neau, D.; Roux, X.; Lippa, N.; Roger-Schmeltz, J.; Mercie, P.; Longy-Boursier, M. Pulmonary streptomyces infection in patient with sarcoidosis, France, 2012. Emerg. Infect. Dis. 2012, 18, 1907–1909. [Google Scholar] [CrossRef] [PubMed]
- Yi, E.S.; Lee, H.; Suh, Y.K.; Tang, W.; Qi, M.; Yin, S.; Remick, D.G.; Ulich, T.R. Experimental extrinsic allergic alveolitis and pulmonary angiitis induced by intratracheal or intravenous challenge with Corynebacterium parvum in sensitized rats. Am. J. Pathol. 1996, 149, 1303–1312. [Google Scholar]
- Post, J.; Hull, P. Tattoo reactions as a sign of sarcoidosis. CMAJ 2012, 184, 43. [Google Scholar]
- Izbicki, G.; Chavko, R.; Banauch, G.I.; Weiden, M.D.; Berger, K.I.; Aldrich, T.K.; Hall, C.; Kelly, K.J.; Prezant, D.J. World Trade Center ‘‘sarcoid-like’’ granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest 2007, 131, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Ang, S.C.; Moscovitz, E.A. Cross-react and species specific Mycobacterium tuberculosis antigens in the immunoprofile of Schaumann bodies: A major clue to the etiology sarcoidosis. Histol. Histopathol. 1996, 11, 125–134. [Google Scholar]
- Zügel, U.; Kaufmann, S.H.E. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin. Microbiol. Rev. 1999, 12, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Valdez, M.M.; Clark, J.I.; Wu, G.J.S.; Muchowski, P.J. Functional similarities between the small heat shock proteins Mycobacterium tuberculosis HSP 16.3 and human a B-crystallin. Eur. J. Biochem. 2002, 269, 1806–1813. [Google Scholar] [CrossRef]
- Blank, M.; Barzilai, O.; Shoenfeld, Y. Molecular mimicry and autoimmunity. Clin. Rev. Allergy Immunol. 2007, 32, 111–118. [Google Scholar] [CrossRef]
- Wendling, U.; Liesbeth, P.; van der Zee, R.; Prakken, B.; Singh, M.; van Eden, W. A conserved mycobacterial heat shock protein (HSP) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-HSP70 homologue. J. Immunol. 2000, 164, 2711–2717. [Google Scholar] [CrossRef]
- Kivity, S.; Agmon-Levin, N.; Blank, M.; Shoenfeld, Y. Infections and autoimmunity–friends or foes? Trends Immunol. 2009, 30, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Rajaiah, R.; Moudgil, K.D. Heat shock proteins can promote as well as regulate autoimmunity. Autoimmun. Rev. 2009, 8, 388–393. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Gilburd, B.; Abu-Shakra, M.; Amital, H.; Barzilai, O.; Berkun, Y.; Blank, M.; Zandman-Goddard, G.; Katz, U.; Krause, I.; et al. The mosaic of autoimmunity: Genetic factors involved in autoimmune diseases2008. Isr. Med. Assoc. J. 2008, 10, 3–7. [Google Scholar] [PubMed]
- Popper, H.; Winter, E.; Hofler, G. DNA of Mycobacterium tuberculosis in paraffin-embedded tissue in tuberculosis and sarcoidosis detected by polymerase chain reaction. Am. J. Clin. Pathol. 1994, 101, 738–741. [Google Scholar] [CrossRef]
- Saboor, S.A.; Johnson, N.I.; McFadden, J.J. Detection of mycobacterial DNA in sarcoidosis and tuberculosis with polymerase chain reaction. Lancet 1992, 339, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Oswald-Richter, K.A.; Culver, D.A.; Hawkins, C.; Hajizadeh, R.; Abraham, S.; Shepherd, B.E.; Jenkins, C.A.; Judson, M.A.; Drake, W.P. Cellular responses to mycobacterial antigens are present in bronchoalveolar lavage fluid used in the diagnosis of sarcoidosis. Infect. Immun. 2009, 77, 3740–3748. [Google Scholar] [CrossRef] [PubMed]
- Jee, B.; Katoch, V.M.; Awasthi, S.K. Dissection of relationship between small heat shock proteins and mycobacterial diseases. Indian J. Lepr. 2008, 80, 231–245. [Google Scholar] [PubMed]
- Cunningham, A.F.; Spreadbury, C.L. Mycobacterial stationary phase induced by low oxygen tension: Cell wall thickening and localization of the 16 kDa alpha crystallin homolog. J. Bacteriol. 1998, 180, 801–808. [Google Scholar] [CrossRef]
- Wayne, L.G. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur. J. Clin. Microbiol. Infect. Dis. 1994, 13, 908–914. [Google Scholar] [CrossRef]
- Simpson, R.M.; Zhu, Y.Q.; Hickey, M.J.; Sherman, D.R.; Barry, C.E., 3rd. The 16 kDa alpha-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc. Natl. Acad. Sci. USA 1998, 95, 9578–9583. [Google Scholar]
- Voskuil, M.I.; Schnappinger, D.; Visconti, K.C.; Harrell, M.I.; Dolganov, G.M.; Sherman, D.R.; Schoolnik, G.K. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 2003, 198, 705–713. [Google Scholar] [CrossRef]
- Garbe, T.R.; Hibler, N.S.; Deretic, V. Response to reactive nitrogen intermediates in Mycobacterium tuberculosis: Induction of the 16 kDa a-crystallin homolog by exposure to nitric oxide donors. Infect. Immun. 1999, 67, 460–465. [Google Scholar] [CrossRef]
- Mulvey, M.R.; Switala, J.; Borys, A.; Loewen, P.C. Regulation of transcription of katE and katF in Escherichia coli. J. Bacteriol. 1990, 172, 6713–6720. [Google Scholar] [CrossRef]
- Arrigo, A.P. Small stress proteins: Chaperones that act as regulators of intracellular redox state and programmed cell death. Biol. Chem. 1998, 379, 19–26. [Google Scholar] [PubMed]
- Mehlen, P.; Briolay, J.; Smith, I.; Diaz-Latoud, C.; Fabre, N.; Pauli, D.; Arrigo, A.P. Analysis of the resistance to heat and hydrogen peroxide stresses in COS cells transiently expressing wild type or deletion mutants of the Drosophila 27 kDa heat-shock protein. Eur. J. Biochem. 1993, 215, 277–284. [Google Scholar] [CrossRef]
- Rojas, M.; Barrera, L.F.; Puzo, G.; Garcia, L.F. Differential induction of apoptosis by virulent Mycobacterium tuberculosis in resistant and susceptible murine macrophages: Role of nitric oxide and mycobacterial products. J. Immunol. 1997, 159, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, H.; Henne, A.; Höster, F.; Liesegang, H.; Wiezer, A.; Strittmatter, A.; Hujer, S.; Durre, P.; Gottschalk, G. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science 2004, 305, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.D.; Ingham, E.; Holland, K.T. Heat shock proteins and inflammatory acne vulgaris: Molecular cloning, overlevel and purification of a Propionibacterium acnes, GroEL and DnaK homologue. FEMS Microbiol. Lett. 2009, 1, 183–186. [Google Scholar]
- Dubaniewicz, A.; Kalinowski, L.; Dudziak, M.; Kalinowska, A.; Singh, M. Peroxynitrite in Sarcoidosis: Relation to Mycobacterium Stationary Phase. Adv. Exp. Med. Biol. 2015, 866, 41–49. [Google Scholar] [PubMed]
- Rutherford, R.M.; Kehren, J.; Staedtler, F.; Chibout, S.D.; Egan, J.J.; Tamm, M.; Gilmartin, J.J.; Brutsche, M.H. Functional genomics in sarcoidosis- reduced or increased apoptosis? Swiss Med. Wkly. 2001, 131, 459–470. [Google Scholar]
- Xaus, J.; Besalduch, N.; Comalada, M.; Marcoval, J.; Pujol, R.; Mañá, J.; Celada, A. High level of p21 Waf1 in sarcoid granulomas: A putative role for long-lasting inflammation. J. Leukoc. Biol. 2003, 74, 295–301. [Google Scholar] [CrossRef]
- Sly, L.M.; Hingley-Wilson, S.M.; Reiner, N.E.; McMaster, W.R. Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mc1-1. J. Immunol. 2003, 170, 430–437. [Google Scholar] [CrossRef]
- Beagley, K.W.; Fujihashi, K.; Black, C.A.; Lagoo, A.S.; Yamamoto, M.; McGhee, J.R.; Kiyono, H. The Mycobacterium tuberculosis 71-kDa heat –shock protein induces proliferation and cytokine secretion by murine gut intraepithelial lymphocytes. Eur. J. Immunol. 1993, 23, 2049–2059. [Google Scholar] [CrossRef]
- Friendland, J.S.; Shattock, R.; Remick, D.G.; Griffin, G.E. Mycobacterial 65-kD heat shock protein induces relase of proinflammatory cytokines from huamn monocytic cells. Clin. Exp. Immunol. 1993, 91, 58–62. [Google Scholar] [CrossRef]
- Tsan, M.F.; Gao, B. Cytokine function of heat shock proteins. Am. J. Physiol. Cell Physiol. 2004, 286, 739–744. [Google Scholar] [CrossRef]
- Peetermans, W.E.; Raats, C.J.; Langermans, J.A.; van Furth, R. Mycobacterial heat-shock protein 65 induces proinflammatory cytokines but does not activate human mononuclear phagocytes. Scand. J. Immunol. 1994, 39, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Retzlaff, C.; Yamamoto, Y.; Hofman, P.S.; Friedman, H.; Klein, T.W. Bacterial heat shock proteins directly induce cytokine mRNA and interleukin-1 secretion in macrophage cultures. Infect. Immun. 1994, 62, 5689–5693. [Google Scholar] [CrossRef] [PubMed]
- Detanico, T.; Rodrigues, L.; Sabritto, A.C.; Keisermann, M.; Bauer, M.E.; Zwickey, H.; Bonorino, C. Mycobacterial heat shock protein 70 induces interleukin-10 production: Immunomodulation of synovial cell cytokine profile and dendritic cell maturation. Clin. Exp. Immunol. 2004, 135, 336–342. [Google Scholar] [CrossRef]
- Wang, Y.; Kelly, C.G.; Singh, M.; McGowan, E.G.; Carrara, A.S.; Bergmeier, L.A.; Lehner, T. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J. Immunol. 2002, 169, 2422–2429. [Google Scholar] [CrossRef]
- Beth, K.; Staib, F.; Distler, M.; Schmitt, U.; Jonuleit, H.; Enk, A.H.; Galle, P.R.; Heike, M. Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: Superiority of HSP60. J. Immunol. 2002, 169, 6141–6148. [Google Scholar] [CrossRef] [PubMed]
- Beltan, E.; Horgen, L.; Rastogi, N. Secretion of cytokines by human macrophages upon infection by pathogenic and non-pathogenic mycobacteria. Microbiol. Patholog. 2000, 28, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Manca, C.; Tsenova, L.; Barry, C., 3rd; Bergtold, A.; Freeman, S.; Haslett, P.A.; Musser, J.M.; Freedman, V.H.; Kaplan, G. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J. Immunol. 1999, 162, 6740–6746. [Google Scholar] [CrossRef] [PubMed]
- Hisaeda, H.; Sakai, T.; Ishikawa, H.; Maekawa, Y.; Yasutomo, K.; Good, R.A.; Himeno, K. Heat shock protein 65 induced by γδ T cells prevents apoptosis of macrophages and contributes to host defense in mice infected with Toxoplasma gondii. J. Immunol. 1997, 159, 2375–2381. [Google Scholar] [CrossRef]
- Marchetti, P.; Hirsch, T.; Zamzami, N.; Zamzami, N.; Hirsch, T.; Macho, A.; Haeffner, A.; Hirsch, F.; Geuskens, M.; Kroemer, G. Mitochondrial permability transition is a central coordinating event of apoptosis. J. Exp. Med. 1996, 184, 1155–1160. [Google Scholar] [CrossRef]
- Stuart, J.K.; Myszka, D.G.; Joss, L.; Mitchell, R.S.; McDonald, S.M.; Xie, Z.; Takayama, S.; Reed, J.C.; Ely, K.R. Characterization of interactions between the anti-apoptotic protein BAG-1 and Hsc70 molecular chaperones. J. Biol. Chem. 1998, 273, 22506–22514. [Google Scholar] [CrossRef]
- Lang, D.; Hubrich, A.; Dohle, F.; Terstesse, M.; Saleh, H.; Schmidt, M.; Pauels, H.G.; Heidenreich, S. Differential level of heat shock protein 70 (HSP70) in human monocytes rendered apoptotic by IL-4 or serum deprivation. J. Leukoc. Biol. 2000, 68, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Thoma-Uszynski, S.; Stenger, S.; Modlin, R.L. CTL-mediated killing of intracellular Mycobacterium tuberculosis is independent of target cell nuclear apoptosis. J. Immunol. 2000, 165, 5773–5779. [Google Scholar] [CrossRef]
- de Waal, M.; Haanen, R.J.; Spits, H.; Roncarolo, M.G.; te Velde, A.; Figdor, C.; Johnson, K.; Kastelein, R.; Yssel, H.; de Vries, J.E.I. Interleukin-1- (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex level. J. Exp. Med. 1991, 174, 915–924. [Google Scholar] [CrossRef]
- Prasse, A.; Georges, C.G.; Biller, H.; Hamm, H.; Matthys, H.; Luttmann, W.; Virchow, J.C., Jr. Th1 cytokine pattern in sarcoidosis is expressed by bronchoalveolar CD4+ and CD8+T cells. Clin. Exp. Immunol. 2000, 122, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Setoguchi, Y.; Nukiwa, T.; Crystal, R.G. Soluble interleukin-2 receptor in sera of patients with pulmonary tuberculosis. Chest 1991, 99, 310–314. [Google Scholar] [CrossRef]
- Lawrence, E.C.; Berger, M.B.; Brousseau, K.P.; Rodriguez, T.M.; Siegel, S.J.; Kurman, C.C.; Nelson, D.L. Elevated serum levels of soluble interleukin-2 receptors in active pulmonary sarcoidosis: Relative specificity and association with hypercalcemia. Sarcoidosis 1987, 4, 87–93. [Google Scholar]
- Müller-Quernheim, J.; Saltini, C.; Sondermeyer, P.; Crystal, R.G. Compartmentalized activation of the interleukin-2 gene by lung T-lymphocytes in active pulmonary sarcoidosis. J. Immunol. 1986, 137, 3475–3483. [Google Scholar] [CrossRef] [PubMed]
- Miyara, M.; Amoura, Z.; Parizot, C.; Badoual, C.; Dorgham, K.; Trad, S.; Kambouchner, M.; Valeyre, D.; Chapelon-Abric, C.; Debré, P.; et al. The immune paradox of sarcoidosis and regulatory T cells. J. Exp. Med. 2006, 203, 359–370. [Google Scholar] [CrossRef]
- Planck, A.; Katchar, K.; Eklund, A.; Gripenbäck, S.; Grunewald, J. T-lymphocyte activity in HLA-DR17 positive patients with active and clinically recovered sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2003, 20, 110–117. [Google Scholar] [PubMed]
- Trinchieri, G. Regulatory role of T cells producing both interferon γ and interleukin 10 in persistent infection. J. Exp. Med. 2001, 194, 53–57. [Google Scholar] [CrossRef]
- Flad, H.D.; Grage-Griebenow, E.; Petersen, F.; Scheuerer, B.; Brandt, E.; Baran, J.; Pryjma, J.; Ernst, M. The role cytokines in monocyte apoptosis. Pathobiology 1999, 67, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Dobrucki, L.W.; Marsh, B.J.; Kalinowski, L. Elucidating structure-function relationships from molecule-to-cell-to-tissue: From research modalities to clinical realities. J. Physiol. Pharmacol. 2009, 60, 83–93. [Google Scholar] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Tishler, M.; Shoenfeld, Y. Anti-heat-shock protein antibodies in rheumatic and autoimmune diseases. Semin. Arthritis. Rheum. 1996, 26, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Pockley, A.G.; Muthana, M.; Calderwood, S.K. The dual immunoregulatory roles of stress proteins. Trends Biochem. Sci 2008, 33, 71–79. [Google Scholar] [CrossRef]
- Salvetti, M.; Ristori, G.; Buttinelli, C.; Fiori, P.; Falcone, M.; Britton, W.; Adams, E.; Paone, G.; Grasso, M.G.; Pozzilli, C. The immune response to mycobacterial 70-kDa heat shock proteins frequently involves autoreactive T cells and is quantitatively disregulated in multiple sclerosis. J. Neuroimmunol. 1996, 65, 143–153. [Google Scholar] [CrossRef]
- Osterloh, A.; Breloer, M. Heat shock proteins: Linking danger and pathogen recognition. Med. Microbiol. Immunol. 2008, 197, 1–8. [Google Scholar] [CrossRef]
- Shapira, Y.; Agmon-Levin, N.; Shoenfeld, Y. Mycobacterium tuberculosis, autoimmunity, and vitamin D. Clin. Rev. Allergy Immunol. 2010, 38, 169–177. [Google Scholar] [CrossRef]
- Ercolini, A.M.; Miller, S.D. The role of infections in autoimmune disease. Clin. Exp. Immunol. 2009, 155, 1–15. [Google Scholar] [CrossRef]
- Cossu, D.; Masala, S.; Frau, J.; Mameli, G.; Marrosu, M.G.; Cocco, E.; Sechi, L.A. Antigenic epitopes of MAP2694 homologous to T-cell receptor gamma-chain are highly recognized in multiple sclerosis Sardinian patients. Mol. Immunol. 2014, 57, 138–140. [Google Scholar] [CrossRef]
- Esaguy, N.; Aguas, A.P.; van Embden, J.D.; Silva, M.T. Mycobacteria and human autoimmune disease: Direct evidence of cross-reactivity between human lactoferrin and the 65-kilodalton protein of tubercle and leprosy bacilli. Infect. Immun. 1991, 59, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Czelusta, A.; Moore, A.Y. Cutaneous Mycobacterium kansasii infection in a patient with systemic lupus erythematosus: Case report and review. J. Am. Acad. Dermatol. 1999, 40, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.Y.; Yang, Y.H.; Hsiao, C.H.; Lee, P.I.; Chiang, B.L. Mycobacterium kansasii infection presenting as cellulitis in a patient with systemic lupus erythematosus. J. Formos. Med. Assoc. 2002, 101, 581–584. [Google Scholar] [PubMed]
- Tasneem, S.; Islam, N.; Ali, R. Crossreactivity of SLE autoantibodies with 70 kDa heat shock proteins of Mycobacterium tuberculosis. Microbiol. Immunol. 2001, 45, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Chodisetti, B.S.; Rai, P.K.; Gowthaman, U.; Pahari, S.; Agrewala, J.N. Potential T cell epitopes of Mycobacterium tuberculosis that can instigate molecular mimicry against host: Implications in autoimmune pathogenesis. BMC Immunol. 2012, 13, 13. [Google Scholar] [CrossRef]
- Haregowoin, A.; Singh, B.; Gupta, R.S.; Finberg, R.W. A mycobacterial heat-shock protein - responsive gamma delta T cell clone also responds to the homologous human heat-shock protein: A possible link between infection and autoimmunity. J. Infect. Dis. 1991, 163, 156–160. [Google Scholar] [CrossRef]
- Ventura, M.; Canchaya, C.; Zhang, Z.; Bernini, V.; Fitzgerald, G.F.; van Sinderen, D. How high G+C Gram-positive bacteria and in particular bifidobacteria cope with heat stress: Protein players and regulators. FEMS Microbiol. Rev. 2006, 30, 734–759. [Google Scholar] [CrossRef]
- Brzuszkiewicz, E.; Weiner, J.; Wollherr, A.; Thürmer, A.; Hüpeden, J.; Lomholt, H.B.; Kilian, M.; Gottschalk, G.; Daniel, R.; Mollenkopf, H.J.; et al. Comparative genomics and transcriptomics of Propionibacterium acnes. PLoS ONE 2011, 6, e21581. [Google Scholar] [CrossRef]
- Yamazaki-Nakashimada, M.A.; Unzueta, A.; Berenise Gámez-González, L.; González-Saldaña, N.; Sorensen, R.U. BCG: A vaccine with multiple faces. Hum. Vaccin. Immunother. 2020, 16, 1841–1850. [Google Scholar] [CrossRef]
- Hong, J.; Leung, E.; Fraser, A.G.; Merriman, T.R.; Vishnu, P.; Krissansen, G.W. TLR2, TLR4 and TLR9 polymorphisms and Crohn’s disease in a New Zealand Caucasian cohort. J. Gastroenterol. Hepatol. 2007, 22, 1760–1766. [Google Scholar] [CrossRef]
- Goh, F.G.; Midwood, K.S. Intrinsic danger: Activation of Toll-like receptors in rheumatoid arthritis. Rheumatology 2012, 51, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Dai, Y.; Lin, Y.; Chen, K. Association between serum amyloid A and rheumatoid arthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2021, 30, 151943. [Google Scholar] [CrossRef]
- Beijer, E.; Roodenburg-Benschop, C.; Schimmelpennink, M.C.; Grutters, J.C.; Meek, B.; Veltkamp, M. Elevated serum amyloid A levels are not specific for sarcoidosis but associate with a fibrotic pulmonary phenotype. Cells 2021, 10, 585. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, L.; Tükel, Ç. Bacterial Amyloids: The Link between Bacterial Infections and Autoimmunity. Trends Microbiol. 2019, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Rizo, V.; Martinez-Guzman, M.A.; Iniguez-Gutierrez, L.; Garcia-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front. Immunol. 2017, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.C.; Caricchio, R.; Gallucci, S. Triggers of Autoimmunity: The Role of Bacterial Infections in the Extracellular Exposure of Lupus Nuclear Autoantigens. Front. Immunol. 2019, 10, 2608. [Google Scholar] [CrossRef]
- Iba, T.; Hashiguchi, N.; Nagaoka, I.; Tabe, Y.; Murai, M. Neutrophil cell death in response to infection and its relation to coagulation. J. Intensive Care 2013, 1, 13. [Google Scholar] [CrossRef]
- Persson, Y.A.; Blomgran-Julinder, R.; Rahman, S.; Zheng, L.; Stendahl, O. Mycobacterium tuberculosis-induced apoptotic neutrophils trigger a pro-inflammatory response in macrophages through release of heat shock protein 72, acting in synergy with the bacteria. Microbes Infect. 2008, 10, 233–240. [Google Scholar] [CrossRef]
- Braian, C.; Hogea, V.; Stendahl, O. Mycobacterium tuberculosis-induced neutrophil extracellular traps activate human macrophages. J. Innate. Immun. 2013, 5, 591–602. [Google Scholar] [CrossRef]
- Grosser, M.; Luther, T.; Fuessel, M.; Bickhardt, J.; Magdolen, V.; Baretton, G. Clinical course of sarcoidosis in dependence on HLA-DRB1 allele frequencies, inflammatory markers, and the presence of M. tuberculosis DNA fragments. Sarcoidosis Vasc. Diffuse Lung Dis. 2005, 22, 66–74. [Google Scholar] [PubMed]
- Maliarik, M.J.; Chen, K.M.; Sheffer, R.G.; Roberta, G.; Sheffer, B.; Rybicki, M.; Major, M.; Popovich, J.; Iannuzzi, M.C. Natural resistance-associated macrophage protein gene in African Americans with sarcoidosis. Am. J. Respir. Cell Mol. Biol. 2000, 22, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Typiak, M.J.; Rębała, K.; Dudziak, M.; Dubaniewicz, A. Polymorphism of FCGR3A gene in sarcoidosis. Hum. Immunol. 2014, 75, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Dubaniewicz, A.; Typiak, M.; Wybieralska, M.; Szadurska, M.; Nowakowski, S.; Staniewicz-Panasik, A.; Rogoza, K.; Sternau, A.; Deeg, P.; Trzonkowski, P. Changed phagocytic activity and pattern of Fcγ and complement receptors on blood monocytes in sarcoidosis. Hum. Immunol. 2012, 73, 788–794. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubaniewicz, A. Mycobacterial Heat Shock Proteins in Sarcoidosis and Tuberculosis. Int. J. Mol. Sci. 2023, 24, 5084. https://doi.org/10.3390/ijms24065084
Dubaniewicz A. Mycobacterial Heat Shock Proteins in Sarcoidosis and Tuberculosis. International Journal of Molecular Sciences. 2023; 24(6):5084. https://doi.org/10.3390/ijms24065084
Chicago/Turabian StyleDubaniewicz, Anna. 2023. "Mycobacterial Heat Shock Proteins in Sarcoidosis and Tuberculosis" International Journal of Molecular Sciences 24, no. 6: 5084. https://doi.org/10.3390/ijms24065084
APA StyleDubaniewicz, A. (2023). Mycobacterial Heat Shock Proteins in Sarcoidosis and Tuberculosis. International Journal of Molecular Sciences, 24(6), 5084. https://doi.org/10.3390/ijms24065084




