Lipids in Mitochondrial Macroautophagy: Phase Behavior of Bilayers Containing Cardiolipin and Ceramide
Abstract
1. Introduction
2. Results
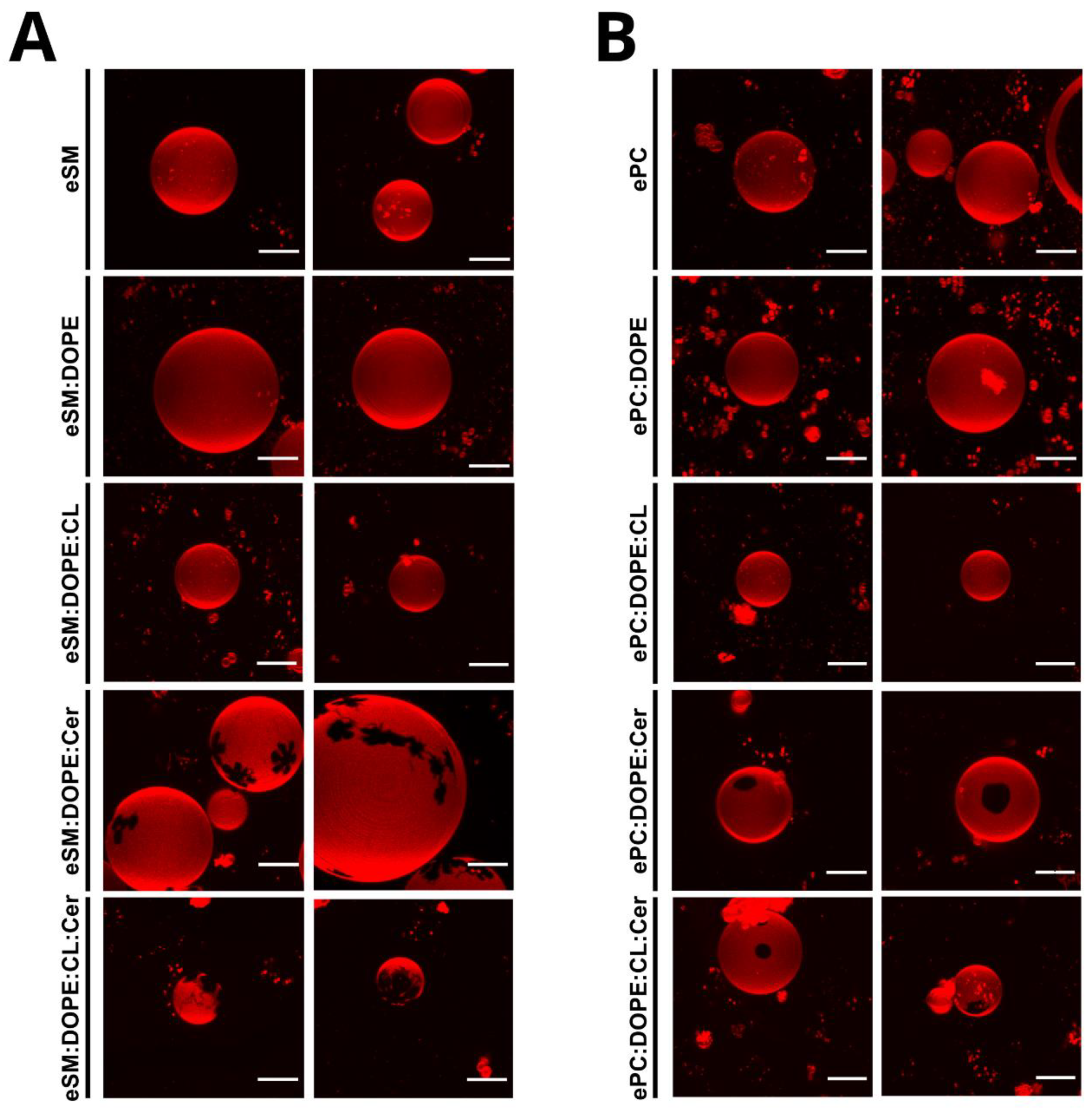
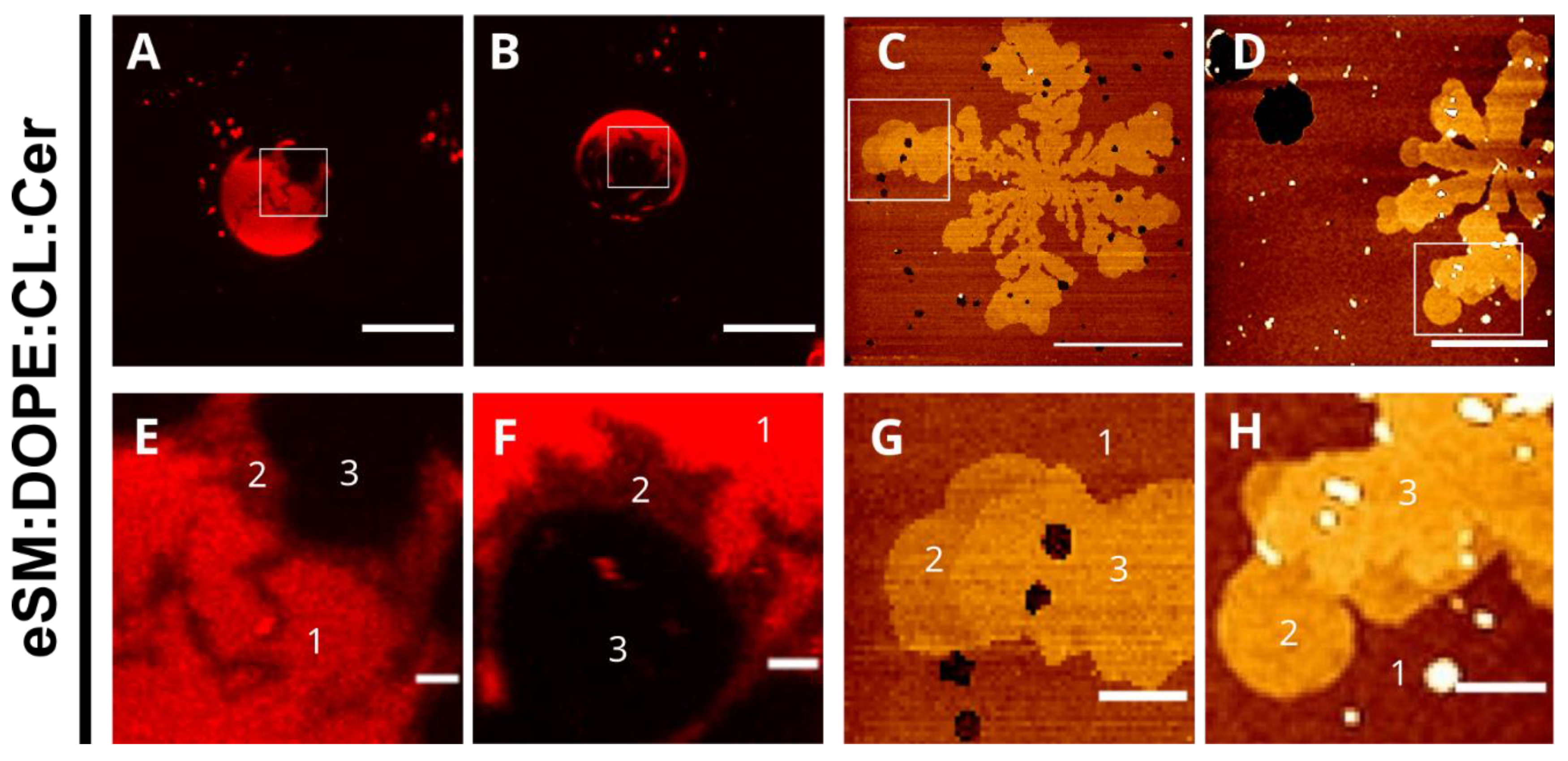
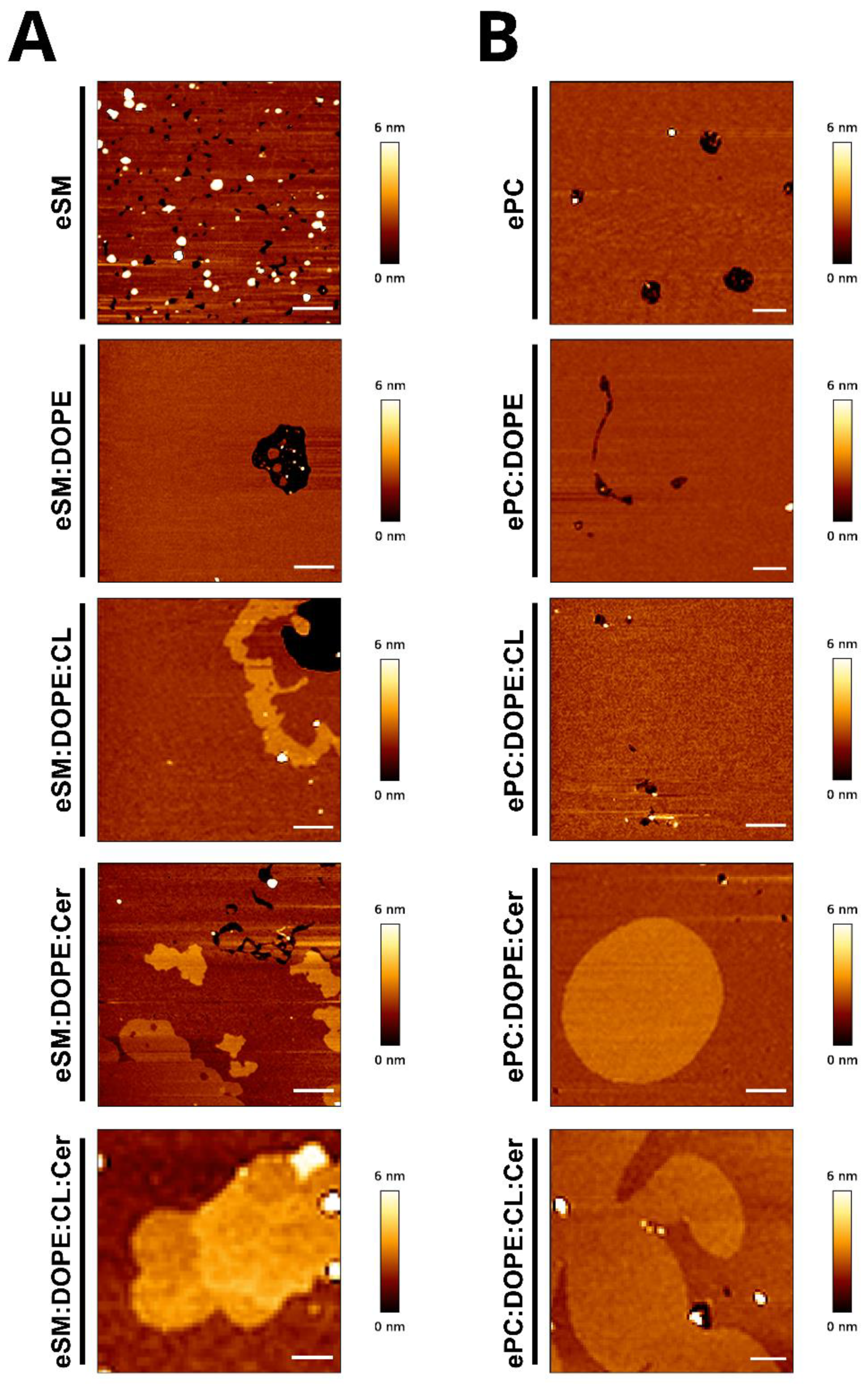
2.1. eSM-Based Samples
2.2. ePC-Based Samples
3. Discussion
3.1. Formation of CL-Containing Bilayers
3.2. Lipid Redistribution Caused by Ceramide in Cardiolipin-Containing Vesicles
3.3. The Relevance of Ceramide Miscibility for LC3/GABARAP Binding to Membranes
4. Materials and Methods
4.1. Chemicals
4.2. Multilamellar Vesicle (MLV) Preparation
4.3. Differential Scanning Calorimetry (DSC)
4.4. Confocal Microscopy of Giant Unilamellar Vesicles (GUV)
4.5. Supported Lipid Bilayer (SLB) Formation
4.6. Atomic Force Microscopy (AFM) Imaging
4.7. Force Spectroscopy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klionsky, D.J.; Baehrecke, E.H.; Brumell, J.H.; Chu, C.T.; Codogno, P.; Cuervo, A.M.; Debnath, J.; Deretic, V.; Elazar, Z.; Eskelinen, E.-L.; et al. A comprehensive glossary of autophagy-related molecules and processes (2nd edition). Autophagy 2011, 7, 1273–1294. [Google Scholar] [CrossRef]
- Montava-Garriga, L.; Ganley, I.G. Outstanding Questions in Mitophagy: What We Do and Do Not Know. J. Mol. Biol. 2020, 432, 206–230. [Google Scholar] [CrossRef]
- Nascimbeni, A.C.; Giordano, F.; Dupont, N.; Grasso, D.; I Vaccaro, M.; Codogno, P.; Morel, E. ER–plasma membrane contact sites contribute to autophagosome biogenesis by regulation of local PI 3P synthesis. EMBO J. 2017, 36, 2018–2033. [Google Scholar] [CrossRef]
- Nath, S.; Dancourt, J.; Shteyn, V.; Puente, G.; Fong, W.M.; Nag, S.; Bewersdorf, J.; Yamamoto, A.; Antonny, B.; Melia, T.J. Lipidation of the LC3/GABARAP family of autophagy proteins relies on a membrane-curvature-sensing domain in Atg3. Nature Cell Biol. 2014, 16, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Fracchiolla, D. Activation and targeting of ATG8 protein lipidation. Cell Discov. 2020, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Landajuela, A.; Hervás, J.H.; Antón, Z.; Montes, L.R.; Gil, D.; Valle, M.; Rodriguez, J.F.; Goñi, F.M.; Alonso, A. Lipid Geometry and Bilayer Curvature Modulate LC3/GABARAP-Mediated Model Autophagosomal Elongation. Biophys. J. 2016, 110, 411–422. [Google Scholar] [CrossRef]
- Hervás, J.H.; Landajuela, A.; Antón, Z.; Shnyrova, A.V.; Goñi, F.M.; Alonso, A. Human ATG3 binding to lipid bilayers: Role of lipid geometry, and electric charge. Sci. Rep. 2017, 7, 15614. [Google Scholar] [CrossRef] [PubMed]
- Iriondo, M.N.; Etxaniz, A.; Antón, Z.; Montes, L.R.; Alonso, A. Molecular and mesoscopic geometries in autophagosome generation. A review. Biochim. et Biophys. Acta Biomembr. 2021, 1863, 183731. [Google Scholar] [CrossRef]
- Anton, Z.; Landajuela, A.; Hervás, J.; Montes, L.R.; Hernández-Tiedra, S.; Velasco, G.; Goñi, F.M.; Alonso, A. Human Atg8-cardiolipin interactions in mitophagy: Specific properties of LC3B, GABARAPL2 and GABARAP. Autophagy 2016, 12, 2386–2403. [Google Scholar] [CrossRef]
- Iriondo, M.N.; Etxaniz, A.; Varela, Y.R.; Ballesteros, U.; Hervás, J.H.; Montes, L.R.; Goñi, F.M.; Alonso, A. LC3 subfamily in cardiolipin-mediated mitophagy: A comparison of the LC3A, LC3B and LC3C homologs. Autophagy 2022, 12, 2985–3003. [Google Scholar] [CrossRef]
- Varela, Y.R.; Iriondo, M.N.; Etxaniz, A.; Ballesteros, U.; Montes, L.R.; Goñi, F.M.; Alonso, A. Ceramide enhances binding of LC3/GABARAP autophagy proteins to cardiolipin-containing membranes. Int. J. Biol. Macromol. 2022, 217, 748–760. [Google Scholar] [CrossRef]
- Varela, Y.R.; Alonso, A. LC3/GABARAP binding to fluid membranes is potentiated by ceramide. Autophagy 2022, 1–3. [Google Scholar] [CrossRef]
- Ruiz-Argüello, M.B.; Basáñez, G.; Goñi, F.M.; Alonso, A. Different Effects of Enzyme-generated Ceramides and Diacylglycerols in Phospholipid Membrane Fusion and Leakage*. J. Biol. Chem. 1996, 271, 26616–26621. [Google Scholar] [CrossRef]
- Sentelle, R.D.; E Senkal, C.; Jiang, W.; Ponnusamy, S.; Gencer, S.; Selvam, S.P.; Ramshesh, V.K.; Peterson, Y.K.; Lemasters, J.J.; Szulc, Z.M.; et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat. Chem. Biol. 2012, 8, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.; Dulovic-Mahlow, M.; Mandik, F.; Frese, L.; Kanana, Y.; Diaw, S.H.; Depperschmidt, J.; Böhm, C.; Rohr, J.; Lohnau, T.; et al. Ceramide accumulation induces mitophagy and impairs β-oxidation in PINK1 deficiency. Proc. Natl. Acad. Sci. USA 2021, 118, e2025347118. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.N.; McElhaney, R.N. The physicochemical properties of cardiolipin bilayers and cardiolipin-containing lipid membranes. Biochim. Biophys. Acta Biomembr. 2009, 1788, 2069–2079. [Google Scholar] [CrossRef]
- Kaltenegger, M.; Kremser, J.; Frewein, M.P.; Ziherl, P.; Bonthuis, D.J.; Pabst, G. Intrinsic lipid curvatures of mammalian plasma membrane outer leaflet lipids and ceramides. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183709. [Google Scholar] [CrossRef]
- Alonso, A.; Goñi, F.M. The Physical Properties of Ceramides in Membranes. Annu. Rev. Biophys. 2018, 47, 633–654. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Kreder, R. Fluorescent Probes for Lipid Rafts: From Model Membranes to Living Cells. Chem. Biol. 2014, 21, 97–113. [Google Scholar] [CrossRef]
- Ruiz-Argüello, M.; Veiga, M.; Arrondo, J.L.; Goñi, F.M.; Alonso, A. Sphingomyelinase cleavage of sphingomyelin in pure and mixed lipid membranes. Influence of the physical state of the sphingolipid. Chem. Phys. Lipids 2002, 114, 11–20. [Google Scholar] [CrossRef]
- Jiménez-Rojo, N.; García-Arribas, A.B.; Sot, J.; Alonso, A.; Goñi, F.M. Lipid bilayers containing sphingomyelins and ceramides of varying N-acyl lengths: A glimpse into sphingolipid complexity. Biochim. Biophys. Acta Biomembr. 2014, 1838, 456–464. [Google Scholar] [CrossRef]
- Estes, D.J.; Mayer, M. Electroformation of giant liposomes from spin-coated films of lipids. Colloids Surf. B Biointerfaces 2005, 42, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.M.; de Almeida, R.F.; Silva, L.C.; Fedorov, A.; Prieto, M. Formation of Ceramide/Sphingomyelin Gel Domains in the Presence of an Unsaturated Phospholipid: A Quantitative Multiprobe Approach. Biophys. J. 2007, 93, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Goñi, F.M. The basic structure and dynamics of cell membranes: An update of the Singer–Nicolson model. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1467–1476. [Google Scholar] [CrossRef]
- Veiga, M.P.; Arrondo, J.L.R.; Goñi, F.M.; Alonso, A. Ceramides in Phospholipid Membranes: Effects on Bilayer Stability and Transition to Nonlamellar Phases. Biophys. J. 1999, 76, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N.; Marčelja, S.; Horn, R.G. Physical principles of membrane organization. Q. Rev. Biophys. 1980, 13, 121–200. [Google Scholar] [CrossRef]
- Goñi, F.M.; Alonso, A. Effects of ceramide and other simple sphingolipids on membrane lateral structure. Biochim. Biophys. Acta Biomembr. 2009, 1788, 169–177. [Google Scholar] [CrossRef]
- Unsay, J.D.; Cosentino, K.; Subburaj, Y.; García-Sáez, A.J. Cardiolipin Effects on Membrane Structure and Dynamics. Langmuir 2013, 29, 15878–15887. [Google Scholar] [CrossRef]
- Attwood, S.J.; Choi, Y.; Leonenko, Z. Preparation of DOPC and DPPC Supported Planar Lipid Bilayers for Atomic Force Microscopy and Atomic Force Spectroscopy. Int. J. Mol. Sci. 2013, 14, 3514–3539. [Google Scholar] [CrossRef] [PubMed]
- Powell, G.L.; Marsh, D. Polymorphic phase behavior of cardiolipin derivatives studied by phosphorus-31 NMR and x-ray diffraction. Biochemistry 1985, 24, 2902–2908. [Google Scholar] [CrossRef]
- García-Arribas, A.B.; Goñi, F.M.; Alonso, A. Lipid Self-Assemblies under the Atomic Force Microscope. Int. J. Mol. Sci. 2021, 22, 10085. [Google Scholar] [CrossRef]
- Domènech, A.; Morros, A.; Cabañas, M.E.; Montero, M.T.; Hernández-Borrell, J. Thermal response of domains in cardiolipin content bilayers. Ultramicroscopy 2007, 107, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, A.; Facci, P. Nanoscale mechanical properties of lipid bilayers and their relevance in biomembrane organization and function. Micron 2012, 43, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Seeger, H.; Marino, G.; Alessandrini, A.; Facci, P. Effect of Physical Parameters on the Main Phase Transition of Supported Lipid Bilayers. Biophys. J. 2009, 97, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Busto, J.V.; Fanani, M.L.; de Tullio, L.; Sot, J.; Maggio, B.; Goñi, F.M.; Alonso, A. Coexistence of Immiscible Mixtures of Palmitoylsphingomyelin and Palmitoylceramide in Monolayers and Bilayers. Biophys. J. 2009, 97, 2717–2726. [Google Scholar] [CrossRef]
- Sot, J.; Bagatolli, L.A.; Goñi, F.M.; Alonso, A. Detergent-Resistant, Ceramide-Enriched Domains in Sphingomyelin/Ceramide Bilayers. Biophys. J. 2006, 90, 903–914. [Google Scholar] [CrossRef]
- Perković, S.; McConnell, H.M. Cloverleaf Monolayer Domains. J. Phys. Chem. B 1997, 101, 381–388. [Google Scholar] [CrossRef]
- García-Arribas, A.B.; González-Ramírez, E.J.; Sot, J.; Areso, I.; Alonso, A.; Goñi, F.M. Complex Effects of 24:1 Sphingolipids in Membranes Containing Dioleoylphosphatidylcholine and Cholesterol. Langmuir 2017, 33, 5545–5554. [Google Scholar] [CrossRef]
- Dahlberg, M.; Maliniak, A. Molecular Dynamics Simulations of Cardiolipin Bilayers. J. Phys. Chem. B 2008, 112, 11655–11663. [Google Scholar] [CrossRef]
- Dahlberg, M.; Maliniak, A. Mechanical Properties of Coarse-Grained Bilayers Formed by Cardiolipin and Zwitterionic Lipids. J. Chem. Theory Comput. 2010, 6, 1638–1649. [Google Scholar] [CrossRef]
- De Tullio, L.; Maggio, B.; Fanani, M.L. Sphingomyelinase acts by an area-activated mechanism on the liquid-expanded phase of sphingomyelin monolayers. J. Lipid Res. 2008, 49, 2347–2355. [Google Scholar] [CrossRef]
- Goñi, F.M. “Rafts”: A nickname for putative transient nanodomains. Chem. Phys. Lipids 2019, 218, 34–39. [Google Scholar] [CrossRef]
- Goñi, F.M.; Alonso, A.; Contreras, F.X. Membrane nanodomains. In ELS; John Wiley & Sons, Ltd.: Chichester, UK, 2020. [Google Scholar] [CrossRef]
- Alonso, A.; Goñi, F.M.; Buckley, J.T. Lipids Favoring Inverted Phase Enhance the Ability of Aerolysin to Permeabilize Liposome Bilayers. Biochemistry 2000, 39, 14019–14024. [Google Scholar] [CrossRef] [PubMed]
- Feigenson, G.W. On the small size of liquid-disordered + liquid-ordered nanodomains. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183685. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.; Gomez-Fernandez, J.C.; Goñi, F. Intrinsic protein-lipid interactions. Physical and biochemical evidence. FEBS Lett. 1979, 98, 211–223. [Google Scholar] [CrossRef]
- Barlič, A.; Gutiérrez-Aguirre, I.; Caaveiro, J.M.M.; Cruz, A.; Ruiz-Argüello, M.-B.; Pérez-Gil, J.; González-Mañas, J.M. Lipid Phase Coexistence Favors Membrane Insertion of Equinatoxin-II, a Pore-forming Toxin from Actinia equina. J. Biol. Chem. 2004, 279, 34209–34216. [Google Scholar] [CrossRef] [PubMed]
- Sot, J.; Manni, M.M.; Viguera, A.R.; Castañeda, V.; Cano, A.; Alonso, C.; Gil, D.; Valle, M.; Alonso, A.; Goñi, F.M. High-Melting Lipid Mixtures and the Origin of Detergent-Resistant Membranes Studied with Temperature-Solubilization Diagrams. Biophys. J. 2014, 107, 2828–2837. [Google Scholar] [CrossRef]
- Larijani, B.; Rosser, C.A.; Woscholski, R. Chemical Biology: Applications and Techniques; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 1–258. [Google Scholar] [CrossRef]
- Jass, J.; Tjärnhage, T.; Puu, G. From Liposomes to Supported, Planar Bilayer Structures on Hydrophilic and Hydrophobic Surfaces: An Atomic Force Microscopy Study. Biophys. J. 2000, 79, 3153–3163. [Google Scholar] [CrossRef]
- McConnell, H.; Watts, T.; Weis, R.; Brian, A. Supported planar membranes in studies of cell-cell recognition in the immune system. Biochim. Biophys. Acta Rev. Biomembr. 1986, 864, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manyes, S.; Redondo-Morata, L.; Oncins, G.; Sanz, F. Nanomechanics of Lipid Bilayers: Heads or Tails? J. Am. Chem. Soc. 2010, 132, 12874–12886. [Google Scholar] [CrossRef]
- Oncins, G.; Garcia-Manyes, S.; Sanz, F. Study of Frictional Properties of a Phospholipid Bilayer in a Liquid Environment with Lateral Force Microscopy as a Function of NaCl Concentration. Langmuir 2005, 21, 7373–7379. [Google Scholar] [CrossRef] [PubMed]
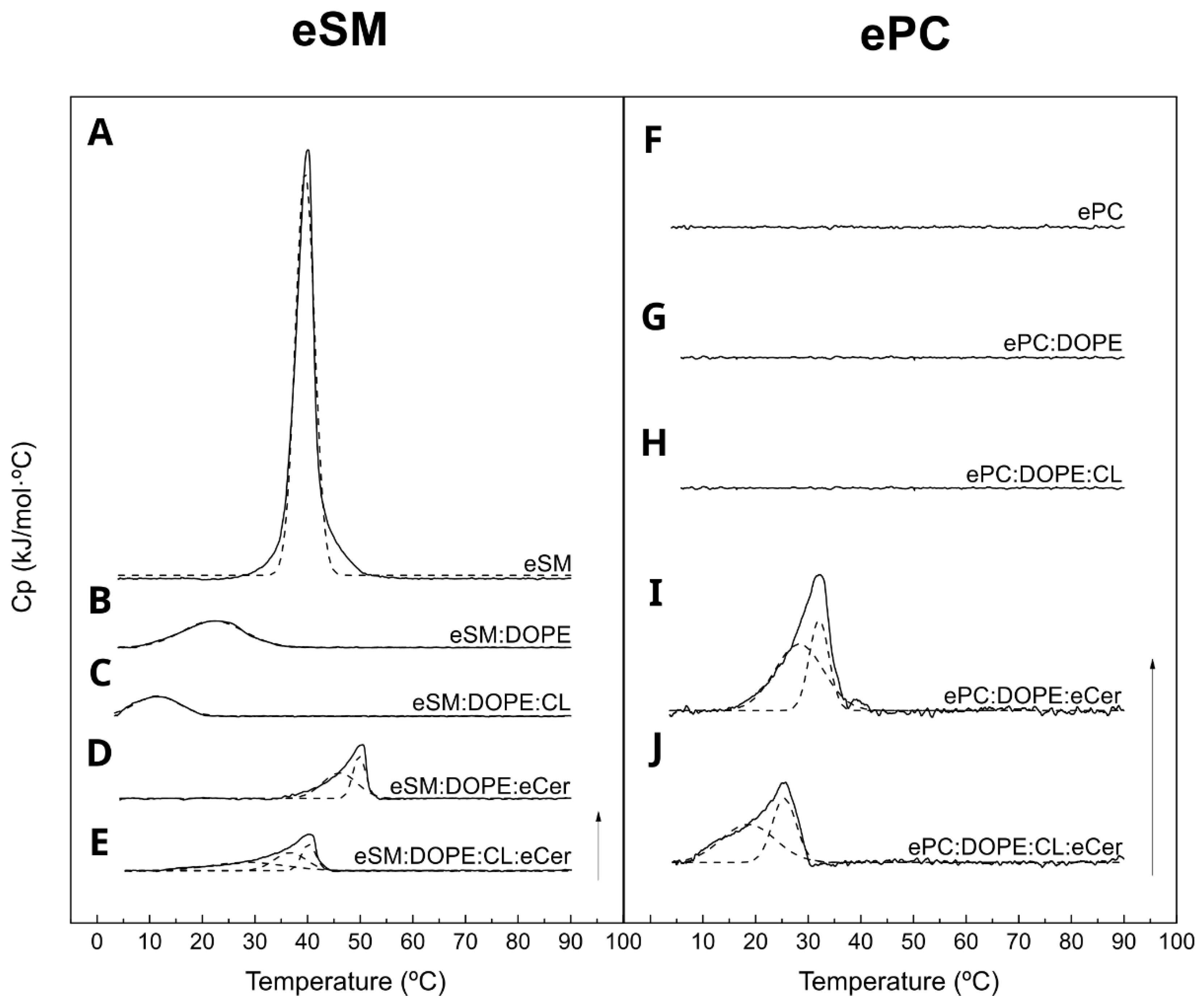
| Sample | Tm (°C) | T1/2 (°C) | ∆H (kJ/mol·°C) | ∆S (J/(mol·°C) | |
|---|---|---|---|---|---|
| eSM | 39.9 ± 0.1 | 4.1 ± 0.4 | 31.5 ± 0.3 | 100.7 ± 0.8 | |
| eSM:DOPE (50:50) | 21.8 ± 0.7 | 14.5 ± 0.8 | 5.4 ± 0.3 | 18.8 ± 0.1 | |
| eSM:DOPE:CL (33:33:33) | 11.6 ± 0.6 | 10.2 ± 0.7 | 2.9 ± 0.6 | 10.1 ± 2.3 | |
| eSM:DOPE:eCer (40:50:10) | total | 50.2 ± 0.6 | 4.9 ± 0.4 | 4.8 ± 0.5 | 14.9 ± 1.4 |
| a | 45.9 ± 0.7 | 9.2 ± 1.8 | 2.9 ± 0.2 | ||
| b | 50.0 ± 0.2 | 4.0 ± 1.7 | 2.1 ± 0.2 | ||
| eSM:DOPE:CL:eCer (23:33:33:10) | total | 41.2 ± 1.1 | 7.9 ± 0.6 | 5.1 ± 0.9 | 16.1 ± 2.7 |
| a | 28.9 ± 0.5 | 19.5 ± 4.2 | 2.2 ± 0.1 | ||
| b | 37.5 ± 0.9 | 8.5 ± 1.2 | 2.2 ± 0.2 | ||
| c | 41.3 ± 1.3 | 4.3 ± 1.0 | 1.5 ± 0.3 | ||
| ePC | - | - | - | - | |
| ePC:DOPE (50:50) | - | - | - | - | |
| ePC:DOPE:CL (33:33:33) | - | - | - | - | |
| ePC:DOPE:eCer (40:50:10) | total | 32.2 ± 0.3 | 12.8 ± 0.1 | 2.0 ± 0.1 | 12.2 ± 3.5 |
| a | 24.6 ± 3.3 | 11.8 ± 4.5 | 2.1 ± 1.0 | ||
| b | 31.8 ± 0.5 | 7.3 ± 3.3 | 2.1 ± 1.4 | ||
| ePC:DOPE:CL:eCer (23:33:33:10) | total | 26.2 ± 0.5 | 9.2 ± 0.2 | 3.7 ± 1.1 | 6.8 ± 0.4 |
| a | 19.9 ± 1.3 | 11.6 ± 1.7 | 1.3 ± 0.2 | ||
| b | 25.8 ± 0.5 | 5.3 ± 1.3 | 0.8 ± 0.0 |
| Sample | Continuous Phase (nm) | Segregated Phase (nm) | Student’s t-test |
|---|---|---|---|
| eSM | 4.3 ± 0.3 | - | |
| eSM:DOPE (50:50) | 5.0 ± 0.2 | - | |
| eSM:DOPE:CL (33:33:33) | 5.0 ± 0.4 | - | |
| eSM:DOPE:eCer (33:33:33) | 4.5 ± 0.4 | 5.6 ± 0.2 | * |
| eSM:DOPE:CL:eCer (23:33:33:10) | 5.4 ± 0.4 | 5.9 ± 0.1 6.2 ± 0.2 | - * |
| ePC | 4.9 ± 0.4 | - | |
| ePC:DOPE (50:50) | 5.0 ± 0.2 | - | |
| ePC:DOPE:CL (33:33:33) | 5.1 ± 0.2 | - | |
| ePC:DOPE:eCer (33:33:33) | 4.8 ± 0.2 | 5.8 ± 0.2 | ** |
| ePC:DOPE:CL:eCer (23:33:33:10) | 4.9 ± 0.2 | 5.9 ± 0.2 | ** |
| Sample | Continuous Phase (nN) | Segregated Phase (nN) | Student’s t-test |
|---|---|---|---|
| eSM | 11.1 ± 3.0 20.2 ± 3.2 | - | - |
| eSM:DOPE (50:50) | 5.5 ± 1.4 | - | - |
| eSM:DOPE:CL (33:33:33) | 3.1 ± 0.4 | - | - |
| eSM:DOPE:eCer (33:33:33) | 2.9 ± 1.0 | 11.3 ± 2.6 | ** |
| eSM:DOPE:CL:eCer (23:33:33:10) | 2.7 ± 0.6 | 5.8 ± 1.5 21.6 ± 1.3 | * *** |
| ePC | 2.7 ± 0.4 | - | - |
| ePC:DOPE (50:50) | 2.6 ± 0.7 | - | - |
| ePC:DOPE:CL (33:33:33) | 2.7 ± 0.6 | - | - |
| ePC:DOPE:eCer (33:33:33) | 2.0 ± 0.4 | 5.1 ± 0.7 | ** |
| ePC:DOPE:CL:eCer (23:33:33:10) | 2.4 ± 1.0 | 14.1 ± 0.9 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela, Y.R.; González-Ramírez, E.J.; Iriondo, M.N.; Ballesteros, U.; Etxaniz, A.; Montes, L.R.; Goñi, F.M.; Alonso, A. Lipids in Mitochondrial Macroautophagy: Phase Behavior of Bilayers Containing Cardiolipin and Ceramide. Int. J. Mol. Sci. 2023, 24, 5080. https://doi.org/10.3390/ijms24065080
Varela YR, González-Ramírez EJ, Iriondo MN, Ballesteros U, Etxaniz A, Montes LR, Goñi FM, Alonso A. Lipids in Mitochondrial Macroautophagy: Phase Behavior of Bilayers Containing Cardiolipin and Ceramide. International Journal of Molecular Sciences. 2023; 24(6):5080. https://doi.org/10.3390/ijms24065080
Chicago/Turabian StyleVarela, Yaiza R., Emilio J. González-Ramírez, Marina N. Iriondo, Uxue Ballesteros, Asier Etxaniz, Lidia Ruth Montes, Félix M. Goñi, and Alicia Alonso. 2023. "Lipids in Mitochondrial Macroautophagy: Phase Behavior of Bilayers Containing Cardiolipin and Ceramide" International Journal of Molecular Sciences 24, no. 6: 5080. https://doi.org/10.3390/ijms24065080
APA StyleVarela, Y. R., González-Ramírez, E. J., Iriondo, M. N., Ballesteros, U., Etxaniz, A., Montes, L. R., Goñi, F. M., & Alonso, A. (2023). Lipids in Mitochondrial Macroautophagy: Phase Behavior of Bilayers Containing Cardiolipin and Ceramide. International Journal of Molecular Sciences, 24(6), 5080. https://doi.org/10.3390/ijms24065080






