The Role of Citicoline and Coenzyme Q10 in Retinal Pathology
Abstract
:1. Introduction
1.1. Citicoline
1.2. Coenzyme Q10
- (1)
- The synthesis of the benzoquinone structure, from tyrosine or phenylalanine. This forms the quinoid ring structure of the molecule;
- (2)
- The synthesis of the polyisoprenoid side chain (made up of 10 isoprenoid units) from acetyl coenzyme A (CoA) through the mevalonate pathway;
- (3)
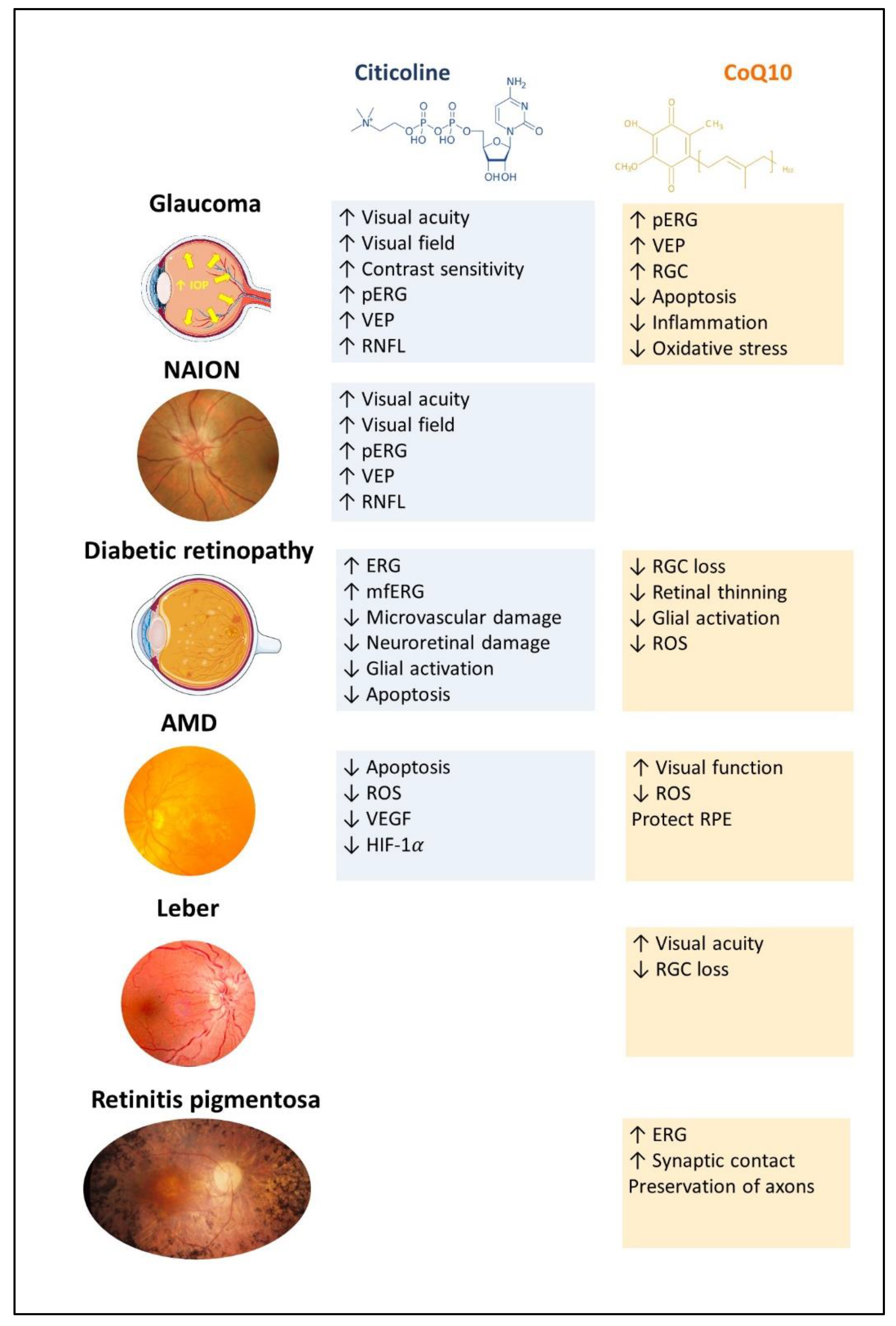
2. Citicoline and CoQ10: Relationship with Retinal Diseases
2.1. Treatments with Citicoline
2.1.1. Citicoline and Glaucoma
| Author | Study Population | Citicoline Dose | Results |
|---|---|---|---|
| Topical instillation | |||
| Rossetti et al. [28] | Humans: OAG | 0.2 g, 1 drop/day | ↑ VF ↑ RNFL thickness |
| Parisi et al. [29] | Humans: OAG | 2%, 3 drops/day | ↑ pERG ↑ VEP |
| Roberti et al. [30] | Humans: OAG | 2%, 3 drops/day | ↑ pERG ↑ VEP |
| Oral solution/tablet | |||
| van der Merwe et al. [27] | Rats: GM | 500 mg/day | ↑ VA ↓ Histological alterations of visual pathway |
| Marino et al. [31] | Humans: OAG | Neuprozin®: citicoline (500 mg), homotaurine (50 mg) and vitE (12 mg). | ↑ CS ↑ GQL-15 |
| Chitu et al. [32] | Humans: OAG | 600 mg/day | ↑ VEP |
| Lanza et al. [33] | Humans: OAG | 500 mg/day | ↑ SAP ↑ VF ↑ RNFL thickness |
| Ottobelli et al. [34] | Humans: OAG | 500 mg/day | ↑ VF |
2.1.2. Citicoline and Non-Arteritic Ischemic Optic Neuropathy
2.1.3. Citicoline and Diabetic Retinopathy
2.1.4. Citicoline and Age-Related Macular Degeneration
2.2. Treatments with CoQ10
2.2.1. CoQ10 and Glaucoma
| Author | Study Population | CoQ10 Type/Dose | Results |
|---|---|---|---|
| Topical instillation | |||
| Parisi et al. [59] | Humans: OAG | CoQ10 + Vitamin E; 2 drops/day | ↑ pERG ↑ VEP |
| Ozotes et al. [60] | Humans: PG | CoQ10 + Vitamin E; 2 drops/day | ↓ Oxidative stress |
| Acar et al. [58] | Rats: GM | CoQ10 + Vitamin E; 2 drops/day | ↓ RGC loss ↓ Gliosis |
| Davis et al. [45] | Cell culture | 20 μM CoQ10 with 57 μM TPGS (α-tocopherol) | ↓ RGC loss |
| Rats: GM | CoQ10 (0.1%) + TPGS (α-tocopherol) (0.5%) | ↑ neuroprotection | |
| Oral supplementation | |||
| Lee et al. [44] | Mice: GM | CoQ10 (1.600–2.000 mg/kg) | ↓ Oxidative stress and mitochondria alteration. |
| Lee et al. [50] | Mice: GM | CoQ10 (1.600–2.000 mg/kg) | ↑ RGC survival ↓ Apoptosis ↓ Bax ↑pBad ↓ Oxidative stress ↓ Glutamate excitotoxicity |
| Ju et al. [57] | Mice: GM | CoQ10 (1.600–2.000 mg/kg) | ↑ RGC survival |
| Edwards et al. [61] | Mice: GM | CoQ10 (1.600–2.000 mg/kg) | ↑ RGC survival ↓ Oxidative stress |
| Intravitreal injection | |||
| Arranz-Romera et al. [62] | Cell culture | CoQ10 (10–25 μM) | ↑ neuroprotection |
| Rats: GM | DMQ-MSs | ↑ RGC survival | |
2.2.2. CoQ10 and Age-Related Macular Degeneration
| Author | Study Population | CoQ10 Type/Dose | Results |
|---|---|---|---|
| Lulli et al. [48] | Cell culture | 10 μM CoQ10 | ↑ viability of ARPE-19 and RGC-5 cell lines |
| Rats: UV irradiation | 0.2% CoQ10 | ↓ apoptosis | |
| Feher et al. [66] | Humans: early AMD | Fatty acids (1320 mg/day) + acetyl-L-carnitine(500 mg/day) + CoQ10 (30 mg/day) + vitamin E (30 mg/day) | ↑ visual function |
| Feher et al. [67] | Humans: early AMD | Fatty acids (1320 mg/day) + acetyl-L-carnitine(500 mg/day) + CoQ10 (30 mg/day) + vitamin E (30 mg/day | ↑ visual function ↓ drusen area |
| Arend et al. [65] | Cell culture | Idebenone (1–100 μM) | ↓Bax/↑ Bcl-2 ↑ viability ARPE-19 cells ↓ ROS |
| López-Cano et al. [54] | Cell culture | Dexamethasone (0.2%)+ ketorolac (0.5%) + idebenone (1 μM) | ↓ Oxidative stress ↓ TNF-α |
2.2.3. CoQ10 and Leber’s Hereditary Optic Neuropathy
| Author | Study Population | CoQ10 Type/Dose | Results |
|---|---|---|---|
| Heitz et al. [55] | Cell culture Mouse: LM | Idebenone (1–100 μM) | Cytoprotective ↓ RGC death ↓ Gliosis |
| Klopstoc et al. [70] | Humans (LHON) | Raxone (900 mg/day) | ↑ VA ↑ Tritan color contrast |
| Catarino et al. [69] | Humans (LHON) Retrospective | Raxone (900 mg/day) | CRR 46% ↑ VA |
| Everdingen et al. [71] | Humans (LHON) Retrospective | Raxone (900 mg/day) | CRR 53% CRS 11% ↑ VA |
| Pemp et al. [73] | Humans LHON chronic | Raxone (900 mg/day) | ↑ VA |
2.2.4. CoQ10 and Retinitis Pigmentosa (Table 6)
| Author | Study Population | Coq10 Type/Dose | Results |
|---|---|---|---|
| Retinitis pigmentosa | |||
| Fernandez-Sanchez et al. [76] | Mouse: RPM | CoQ10 | ↑ ERG ↑ Photoreceptor preservation |
| Lodi R. et al. [77] | Human: RP | CoQ10 (100 mg/day) | ↑ Brain energy reserve |
| Diabetic retinopathy | |||
| Daniel et al. [78] | Rat: DRM | Idebenone (0.03 M) Elamipretide (0.03 M) SCQs (0.01 M #37, 0.02 M #77) 1 drop day | ↑ VA ↓ Oxidative damage ↓ RGC loss, ↓ Reactive gliosis ↓ Vascular leakage ↓ Retinal thinning |
| Rodríguez Carrizalez et al. [79] | Human: NPDR | CoQ10 (400 mg/day) | ↓ Oxidative stress |
2.2.5. CoQ10 and Diabetic Retinopathy (Table 6)
3. Citicoline and CoQ10: Concluding Remarks
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALA | α-lipoic acid |
| ARMD | age-related macular degeneration |
| ATP | adenosine triphosphate |
| CAT | combined antioxidant therapy |
| CRR | clinically relevant recovery |
| CRS | clinically relevant stabilization |
| CoQ10 | coenzyme Q10 |
| D | dexamethasone |
| DMSO | dimethylsulfoxid |
| DR | diabetic retinopathy |
| ERG | electroretinogram |
| ETDRS | Early Treatment Diabetic Retinopathy Studio |
| FDT | dual-frequency perimetry |
| GCL | ganglion cell layer |
| GQL-15 | Glaucoma Quality of Life-15 |
| HO-1 | heme oxygenase-1 |
| LASIK | keratomileusis, laser-assisted |
| LHON | Leber’s hereditary optic neuropathy |
| M | melatonin |
| MSs | microspheres |
| mfERG | multifocal electroretinogram |
| mmHg | millimeters of mercury |
| MNs | mitochondrial nutrients |
| mPTP | mitochondria permeability transition pores |
| NPRD | nonproliferative diabetic retinopathy |
| IOP | intraocular pressure |
| OAG | open-angle glaucoma |
| OCT | optical coherence tomography |
| PEMT | phosphatidylethanolamine methyltransferase |
| pERG | patterned electroretinogram |
| PLGA | polylactic-co-glycolic acid |
| PRK | photorefractive keratectomy |
| RNFL | retinal nerve fiber layer thickness |
| RGCs | retinal ganglion cells |
| ROS | reactive oxygen species |
| RP | retinitis pigmentosa |
| RPE | retinal pigment epithelium |
| SAM | S-adenosylmethionine |
| SAP | standard white-on-white perimetry |
| SCQs | short-chain quinones |
| SOD2 | superoxide dismutase 2 |
| TPGS | α-tocopherol polyethylene glycol succinate |
| VA | visual acuity |
| VEP | visually evoked potential |
| WHO | World Health Organization |
References
- Yorston, D. Retinal Diseases and VISION 2020. Community Eye Health 2003, 16, 19. [Google Scholar]
- Thapa, R.; Khanal, S.; Tan, H.S.; Thapa, S.S.; van Rens, G.H.M.B. Prevalence, Pattern and Risk Factors of Retinal Diseases Among an Elderly Population in Nepal: The Bhaktapur Retina Study. Clin. Ophthalmol. 2020, 14, 2109. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-Analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Report on Vision; World Health Organization: Geneva, Switzerland, 2019; Volume 214, Available online: https://www.who.Int/publications/i/item/9789241516570 (accessed on 28 November 2022).
- Dziedziak, J.; Kasarełło, K.; Cudnoch-Jędrzejewska, A. Dietary Antioxidants in Age-Related Macular Degeneration and Glaucoma. Antioxidants 2021, 10, 1743. [Google Scholar] [CrossRef]
- Secades, J.J. Citicoline: Pharmacological and Clinical Review, 2016 Update. Rev. Neurol. 2016, 63, S1–S73. [Google Scholar]
- Schauss, A.G.; Nakazaki, E. Citicoline (CDP-Choline). In Textbook of Natural Medicine, 5th ed.; Pizzorno, J.E., Murray, M.T., Eds.; Churchill Livingstone: St. Louis, MO, USA, 2020; pp. 515–525.e3. [Google Scholar]
- Oddone, F.; Rossetti, L.; Parravano, M.; Sbardella, D.; Coletta, M.; Ziccardi, L.; Roberti, G.; Carnevale, C.; Romano, D.; Manni, G.; et al. Citicoline in Ophthalmological Neurodegenerative Disease: A Comprehensive Review. Pharmaceuticals 2021, 14, 281. [Google Scholar] [CrossRef]
- Müller-Esterl, W. Bioquímica: Fundamentos Para Medicina y Ciencias de La Vida; Editorial Reverté: Barcelona, Spain, 2008. [Google Scholar]
- Sanders, L.M.; Zeisel, S.H. Choline: Dietary Requirements and Role in Brain Development. Nutr. Today 2007, 42, 181–186. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 1998; pp. 390–422. [Google Scholar] [CrossRef]
- Biasi, E. The Effects of Dietary Choline. Neurosci. Bull. 2011, 27, 330–342. [Google Scholar] [CrossRef] [Green Version]
- Bekdash, R.A. Neuroprotective Effects of Choline and Other Methyl Donors. Nutrients 2019, 11, 2995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauss, A.G.; Somfai-Relle, S.; Financsek, I.; Glavits, R.; Parent, S.C.; Endres, J.R.; Varga, T.; Szücs, Z.; Clewell, A. Single- and Repeated-Dose Oral Toxicity Studies of Citicoline Free-Base (Choline Cytidine 5′-Pyrophosphate) in Sprague-Dawley Rats. Int. J. Toxicol. 2009, 28, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Avila, M.; Fernandez Vega, A.; de La Mata, M.; Delgado Pavon, A.; Alcocer-Gomez, E.; Perez Calero, C.; Villanueva Paz, M.; Alanis, M.; et al. Clinical Applications of Coenzyme Q10. Front. Biosci. (Landmark Ed.) 2014, 19, 619–633. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, I.; Heaton, R.A.; Mantle, D. Disorders of Human Coenzyme Q10 Metabolism: An Overview. Int. J. Mol. Sci. 2020, 21, 6695. [Google Scholar] [CrossRef]
- Potgieter, M.; Pretorius, E.; Pepper, M.S. Primary and Secondary Coenzyme Q10 Deficiency: The Role of Therapeutic Supplementation. Nutr. Rev. 2013, 71, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Manzar, H.; Abdulhussein, D.; Yap, T.E.; Cordeiro, M.F. Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain. Int. J. Mol. Sci. 2020, 21, 9299. [Google Scholar] [CrossRef]
- Elena Díaz-Casado, M.; Quiles, J.L.; Barriocanal-Casado, E.; González-García, P.; Battino, M.; López, L.C.; Varela-López, A. The Paradox of Coenzyme Q10 in Aging. Nutrients 2019, 11, 2221. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tohari, A.M.; Marcheggiani, F.; Zhou, X.; Reilly, J.; Tiano, L.; Shu, X. Therapeutic Potential of Co-Enzyme Q10 in Retinal Diseases. Curr. Med. Chem. 2017, 24, 4329–4339. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Albarral, J.A.; Ramírez, A.I.; de Hoz, R.; Salazar, J.J. Retinal Microglial Activation in Glaucoma: Evolution over Time in a Unilateral Ocular Hypertension Model. Neural Regen. Res. 2022, 17, 797. [Google Scholar] [CrossRef]
- Faiq, M.A.; Wollstein, G.; Schuman, J.S.; Chan, K.C. Cholinergic Nervous System and Glaucoma: From Basic Science to Clinical Applications. Prog. Retin. Eye Res. 2019, 72, 100767. [Google Scholar] [CrossRef]
- Adornetto, A.; Rombolà, L.; Morrone, L.A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Russo, R. Natural Products: Evidence for Neuroprotection to Be Exploited in Glaucoma. Nutrients 2020, 12, 3158. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, S.; Marchini, G.; Caporossi, A.; Scuderi, G.; Tomasso, L.; Brunoro, A. Cytidine 5′-Diphosphocholine (Citicoline): Evidence for a Neuroprotective Role in Glaucoma. Nutrients 2020, 12, 793. [Google Scholar] [CrossRef] [Green Version]
- van der Merwe, Y.; Murphy, M.C.; Sims, J.R.; Faiq, M.A.; Yang, X.L.; Ho, L.C.; Conner, I.P.; Yu, Y.; Leung, C.K.; Wollstein, G.; et al. Citicoline Modulates Glaucomatous Neurodegeneration Through Intraocular Pressure-Independent Control. Neurotherapeutics 2021, 18, 1339–1359. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, L.; Iester, M.; Tranchina, L.; Ottobelli, L.; Coco, G.; Calcatelli, E.; Ancona, C.; Cirafici, P.; Manni, G. Can Treatment with Citicoline Eyedrops Reduce Progression in Glaucoma? The Results of a Randomized Placebo-Controlled Clinical Trial. J. Glaucoma 2020, 29, 513–520. [Google Scholar] [CrossRef]
- Parisi, V.; Centofanti, M.; Ziccardi, L.; Tanga, L.; Michelessi, M.; Roberti, G.; Manni, G. Treatment with Citicoline Eye Drops Enhances Retinal Function and Neural Conduction along the Visual Pathways in Open Angle Glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1327–1340. [Google Scholar] [CrossRef]
- Roberti, G.; Tanga, L.; Parisi, V.; Sampalmieri, M.; Centofanti, M.; Manni, G. A Preliminary Study of the Neuroprotective Role of Citicoline Eye Drops in Glaucomatous Optic Neuropathy. Indian J. Ophthalmol. 2014, 62, 549–553. [Google Scholar] [CrossRef]
- Marino, P.F.; Rossi, G.C.M.; Campagna, G.; Capobianco, D.; Costagliola, C. Effects of Citicoline, Homotaurine, and Vitamin E on Contrast Sensitivity and Visual-Related Quality of Life in Patients with Primary Open-Angle Glaucoma: A Preliminary Study. Molecules 2020, 25, 5614. [Google Scholar] [CrossRef]
- Chițu, I.; Voinea, L.-M.; Istrate, S.; Vrapciu, A.; Ciuluvică, R.C.; Tudosescu, R. The Neuroprotective Role of Citicoline Treatment in Glaucoma—6 Months Results of a Prospective Therapeutic Trial. Rom. J. Ophthalmol. 2019, 63, 222. [Google Scholar] [CrossRef]
- Lanza, M.; Carnevale, U.A.G.; Mele, L.; Sconocchia, M.B.; Bartollino, S.; Costagliola, C. Morphological and Functional Evaluation of Oral Citicoline Therapy in Chronic Open-Angle Glaucoma Patients: A Pilot Study with a 2-Year Follow-Up. Front. Pharmacol. 2019, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Ottobelli, L.; Manni, G.L.; Centofanti, M.; Iester, M.; Allevena, F.; Rossetti, L. Citicoline Oral Solution in Glaucoma: Is There a Role in Slowing Disease Progression? Ophthalmologica 2013, 229, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S. Ischemic Optic Neuropathy. Prog. Retin. Eye Res. 2009, 28, 34–62. [Google Scholar] [CrossRef]
- Parisi, V.; Barbano, L.; di Renzo, A.; Coppola, G.; Ziccardi, L. Neuroenhancement and Neuroprotection by Oral Solution Citicoline in Non-Arteritic Ischemic Optic Neuropathy as a Model of Neurodegeneration: A Randomized Pilot Study. PLoS ONE 2019, 14, e0220435. [Google Scholar] [CrossRef] [PubMed]
- Fragiotta, S.; Pinazo-Durán, M.D.; Scuderi, G. Understanding Neurodegeneration from a Clinical and Therapeutic Perspective in Early Diabetic Retinopathy. Nutrients 2022, 14, 792. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, P.; Sampedro, J.; Solà-Adell, C.; Simó-Servat, O.; Russo, C.; Varela-Sende, L.; Simó, R.; Hernández, C. Effects of Liposomal Formulation of Citicoline in Experimental Diabetes-Induced Retinal Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parravano, M.; Scarinci, F.; Parisi, V.; Giorno, P.; Giannini, D.; Oddone, F.; Varano, M. Citicoline and Vitamin B12 Eye Drops in Type 1 Diabetes: Results of a 3-Year Pilot Study Evaluating Morpho-Functional Retinal Changes. Adv. Ther. 2020, 37, 1646–1663. [Google Scholar] [CrossRef] [Green Version]
- Parisi, V.; Ziccardi, L.; Barbano, L.; Giorno, P.; Varano, M.; Parravano, M. Citicoline and Vitamin B12 Eye Drops in Type 1 Diabetes: Results of a 36-Month Pilot Study Evaluating Macular Electrophysiological Changes. Adv. Ther. 2021, 38, 3924–3936. [Google Scholar] [CrossRef]
- Abdelsalam, A.; Priore, L.d.; Zarbin, M.A. Drusen in Age-Related Macular Degeneration: Pathogenesis, Natural Course, and Laser Photocoagulation-Induced Regression. Surv. Ophthalmol. 1999, 44, 1–29. [Google Scholar] [CrossRef]
- Evans, J. Antioxidant Supplements to Prevent or Slow down the Progression of AMD: A Systematic Review and Meta-Analysis. Eye 2008, 22, 751–760. [Google Scholar] [CrossRef] [Green Version]
- Nashine, S.; Kenney, M.C. Role of Citicoline in an in Vitro AMD Model. Aging 2020, 12, 9031–9040. [Google Scholar] [CrossRef]
- Lee, D.; Kim, K.-Y.; Sup Shim, M.; Yeop Kim, S.; Ellisman, M.H.; Weinreb, R.N.; Ju, W.-K. Coenzyme Q10 Ameliorates Oxidative Stress and Prevents Mitochondrial Alteration in Ischemic Retinal Injury. Apoptosis 2014, 19, 603–614. [Google Scholar] [CrossRef] [Green Version]
- Davis, B.M.; Tian, K.; Pahlitzsch, M.; Brenton, J.; Ravindran, N.; Butt, G.; Malaguarnera, G.; Normando, E.M.; Guo, L.; Cordeiro, M.F. Topical Coenzyme Q10 Demonstrates Mitochondrial-Mediated Neuroprotection in a Rodent Model of Ocular Hypertension. Mitochondrion 2017, 36, 114–123. [Google Scholar] [CrossRef]
- Lulli, M.; Witort, E.; Papucci, L.; Torre, E.; Schipani, C.; Bergamini, C.; Monte, M.D.; Capaccioli, S. Coenzyme Q10 Instilled as Eye Drops on the Cornea Reaches the Retina and Protects Retinal Layers from Apoptosis in a Mouse Model of Kainate-Induced Retinal Damage. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8295–8302. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.S.; Jurkute, N.; Yu-Wai-Man, P. Leber Hereditary Optic Neuropathy-Light at the End of the Tunnel? Asia Pac. J. Ophthalmol. 2018, 7, 242–245. [Google Scholar] [CrossRef]
- Lulli, M.; Witort, E.; Papucci, L.; Torre, E.; Schiavone, N.; Dal Monte, M.; Capaccioli, S. Coenzyme Q10 Protects Retinal Cells from Apoptosis Induced by Radiation in Vitro and in Vivo. J. Radiat. Res. 2012, 53, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Nucci, C.; Russo, R.; Martucci, A.; Giannini, C.; Garaci, F.; Floris, R.; Bagetta, G.; Morrone, L.A. New Strategies for Neuroprotection in Glaucoma, a Disease That Affects the Central Nervous System. Eur. J. Pharmacol. 2016, 787, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Shim, M.S.; Kim, K.Y.; Noh, Y.H.; Kim, H.; Kim, S.Y.; Weinreb, R.N.; Ju, W.K. Coenzyme Q10 Inhibits Glutamate Excitotoxicity and Oxidative Stress-Mediated Mitochondrial Alteration in a Mouse Model of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 993–1005. [Google Scholar] [CrossRef] [Green Version]
- Martucci, A.; Nucci, C. Evidence on Neuroprotective Properties of Coenzyme Q10 in the Treatment of Glaucoma. Neural Regen. Res. 2019, 14, 197. [Google Scholar] [CrossRef]
- Noh, Y.; Kim, K.; Shim, M.; Choi, S.; Choi, S.; Ellisman, M.; Weinreb, R.; Perkins, G.; Ju, W. Inhibition of Oxidative Stress by Coenzyme Q10 Increases Mitochondrial Mass and Improves Bioenergetic Function in Optic Nerve Head Astrocytes. Cell Death Dis. 2013, 4, e820. [Google Scholar] [CrossRef] [Green Version]
- Varricchio, C.; Beirne, K.; Heard, C.; Newland, B.; Rozanowska, M.; Brancale, A.; Votruba, M. The Ying and Yang of Idebenone: Not Too Little, Not Too Much—Cell Death in NQO1 Deficient Cells and the Mouse Retina. Free Radic. Biol. Med. 2020, 152, 551–560. [Google Scholar] [CrossRef]
- López-Cano, J.J.; Sigen, A.; Andrés-Guerrero, V.; Tai, H.; Bravo-Osuna, I.; Molina-Martínez, I.T.; Wang, W.; Herrero-Vanrell, R. Thermo-Responsive PLGA-PEG-PLGA Hydrogels as Novel Injectable Platforms for Neuroprotective Combined Therapies in the Treatment of Retinal Degenerative Diseases. Pharmaceutics 2021, 13, 234. [Google Scholar] [CrossRef]
- Heitz, F.D.; Erb, M.; Anklin, C.; Robay, D.; Pernet, V.; Gueven, N. Idebenone Protects against Retinal Damage and Loss of Vision in a Mouse Model of Leber’s Hereditary Optic Neuropathy. PLoS ONE 2012, 7, e45182. [Google Scholar] [CrossRef] [Green Version]
- Kernt, M.; Arend, N.; Buerger, A.; Mann, T.; Haritoglou, C.; Ulbig, M.W.; Kampik, A.; Hirneiss, C. Idebenone Prevents Human Optic Nerve Head Astrocytes from Oxidative Stress, Apoptosis, and Senescence by Stabilizing BAX/Bcl-2 Ratio. J. Glaucoma 2013, 22, 404–412. [Google Scholar] [CrossRef]
- Ju, W.K.; Shim, M.S.; Kim, K.Y.; Bu, J.H.; Park, T.L.; Ahn, S.; Weinreb, R.N. Ubiquinol Promotes Retinal Ganglion Cell Survival and Blocks the Apoptotic Pathway in Ischemic Retinal Degeneration. Biochem. Biophys. Res. Commun. 2018, 503, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Ekicier Acar, S.; Sarıcaoğlu, M.S.; Çolak, A.; Aktaş, Z.; Sepici Dinçel, A. Neuroprotective Effects of Topical Coenzyme Q10 + Vitamin E in Mechanic Optic Nerve Injury Model. Eur. J. Ophthalmol. 2020, 30, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Centofanti, M.; Gandolfi, S.; Marangoni, D.; Rossetti, L.; Tanga, L.; Tardini, M.; Traina, S.; Ungaro, N.; Vetrugno, M.; et al. Effects of Coenzyme Q10 in Conjunction with Vitamin E on Retinal-Evoked and Cortical-Evoked Responses in Patients with Open-Angle Glaucoma. J. Glaucoma 2014, 23, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Ozates, S.; Elgin, K.U.; Yilmaz, N.S.; Demirel, O.O.; Sen, E.; Yilmazbas, P. Evaluation of Oxidative Stress in Pseudo-Exfoliative Glaucoma Patients Treated with and without Topical Coenzyme Q10 and Vitamin E. Eur. J. Ophthalmol. 2019, 29, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Lee, Y.; Kim, M.; Bhanvadia, S.; Kim, K.Y.; Ju, W.K. Effect of Ubiquinol on Glaucomatous Neurodegeneration and Oxidative Stress: Studies for Retinal Ganglion Cell Survival and/or Visual Function. Antioxidants 2020, 9, 952. [Google Scholar] [CrossRef]
- Arranz-Romera, A.; Davis, B.M.; Bravo-Osuna, I.; Esteban-Pérez, S.; Molina-Martínez, I.T.; Shamsher, E.; Ravindran, N.; Guo, L.; Cordeiro, M.F.; Herrero-Vanrell, R. Simultaneous Co-Delivery of Neuroprotective Drugs from Multi-Loaded PLGA Microspheres for the Treatment of Glaucoma. J. Control. Release 2019, 297, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Bilbao-Malavé, V.; González-Zamora, J.; de la Puente, M.; Recalde, S.; Fernandez-Robredo, P.; Hernandez, M.; Layana, A.G.; de Viteri, M.S. Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in Age Related Macular Degeneration, Role in Pathophysiology, and Possible New Therapeutic Strategies. Antioxidants 2021, 10, 1170. [Google Scholar] [CrossRef]
- Blasi, M.A.; Bovina, C.; Carella, G.; Genova, M.L.; Jansen, A.M.A.; Lenaz, G.; Brancato, R. Does Coenzyme Q10 Play a Role in Opposing Oxidative Stress in Patients with Age-Related Macular Degeneration? Ophthalmologica 2001, 215, 51–54. [Google Scholar] [CrossRef]
- Arend, N.; Wertheimer, C.; Laubichler, P.; Wolf, A.; Kampik, A.; Kernt, M. Idebenone Prevents Oxidative Stress, Cell Death and Senescence of Retinal Pigment Epithelium Cells by Stabilizing BAX/Bcl-2 Ratio. Ophthalmologica 2015, 234, 73–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feher, J.; Papale, A.; Mannino, G.; Gualdi, L.; Balacco Gabrieli, C. Mitotropic Compounds for the Treatment of Age-Related Macular Degeneration. The Metabolic Approach and a Pilot Study. Ophthalmologica 2003, 217, 351–357. [Google Scholar] [CrossRef]
- Feher, J.; Kovacs, B.; Kovacs, I.; Schvöller, M.; Papale, A.; Balacco Gabrieli, C. Improvement of Visual Functions and Fundus Alterations in Early Age-Related Macular Degeneration Treated with a Combination of Acetyl-L-Carnitine, n-3 Fatty Acids, and Coenzyme Q10. Ophthalmologica 2005, 219, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Meyerson, C.; van Stavern, G.; McClelland, C. Leber Hereditary Optic Neuropathy: Current Perspectives. Clin. Ophthalmol. 2015, 9, 1165. [Google Scholar] [CrossRef] [Green Version]
- Catarino, C.B.; von Livonius, B.; Priglinger, C.; Banik, R.; Matloob, S.; Tamhankar, M.A.; Castillo, L.; Friedburg, C.; Halfpenny, C.A.; Lincoln, J.A.; et al. Real-World Clinical Experience with Idebenone in the Treatment of Leber Hereditary Optic Neuropathy. J. Neuroophthalmol. 2020, 40, 558–565. [Google Scholar] [CrossRef]
- Klopstock, T.; Yu-Wai-Man, P.; Dimitriadis, K.; Rouleau, J.; Heck, S.; Bailie, M.; Atawan, A.; Chattopadhyay, S.; Schubert, M.; Garip, A.; et al. A Randomized Placebo-Controlled Trial of Idebenone in Leber’s Hereditary Optic Neuropathy. Brain 2011, 134, 2677–2686. [Google Scholar] [CrossRef]
- van Everdingen, J.A.M.; Pott, J.W.R.; Bauer, N.J.C.; Krijnen, A.M.; Lushchyk, T.; Wubbels, R.J. Clinical Outcomes of Treatment with Idebenone in Leber’s Hereditary Optic Neuropathy in the Netherlands: A National Cohort Study. Acta Ophthalmol. 2022, 100, 700–706. [Google Scholar] [CrossRef]
- Vignal-Clermont, C.; Girmens, J.F.; Audo, I.; Said, S.M.; Errera, M.H.; Plaine, L.; O’Shaughnessy, D.; Taiel, M.; Sahel, J.A. Safety of Intravitreal Gene Therapy for Treatment of Subjects with Leber Hereditary Optic Neuropathy Due to Mutations in the Mitochondrial ND4 Gene: The REVEAL Study. BioDrugs 2021, 35, 201–214. [Google Scholar] [CrossRef]
- Pemp, B.; Kircher, K.; Reitner, A. Visual Function in Chronic Leber’s Hereditary Optic Neuropathy during Idebenone Treatment Initiated 5 to 50 Years after Onset. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2751–2757. [Google Scholar] [CrossRef] [Green Version]
- Carelli, V.; Carbonelli, M.; de Coo, I.F.; Kawasaki, A.; Klopstock, T.; Lagrèze, W.A.; la Morgia, C.; Newman, N.J.; Orssaud, C.; Pott, J.W.R.; et al. International Consensus Statement on the Clinical and Therapeutic Management of Leber Hereditary Optic Neuropathy. J. Neuroophthalmol. 2017, 37, 371–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagano, G.; Pallardó, F.v.; Lyakhovich, A.; Tiano, L.; Trifuoggi, M. Mitigating the Pro-Oxidant State and Melanogenesis of Retinitis Pigmentosa: By Counteracting Mitochondrial Dysfunction. Cell. Mol. Life Sci. 2021, 78, 7491–7503. [Google Scholar] [CrossRef]
- Fernandez-Sanchez, L.; Pedrero-Prieto, C.; Kutsyr, O.; Rabanal-Ruiz, Y.; Martínez-Gil, N.; Sánchez-Sáez, X.; Noailles, A.; Lax, P.; Alcain, F.J.; Cuenca, N. Dietary Intake of Coenzyme Q10 Is Able to Slow down Retinal Degeneration in a Model of Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4883. [Google Scholar]
- Lodi, R.; Iotti, S.; Scorolli, L.; Scorolli, L.; Bargossi, A.M.; Sprovieri, C.; Zaniol, P.; Meduri, R.; Barbiroli, B. The Use of Phosphorus Magnetic Resonance Spectroscopy to Study in Vivo the Effect of Coenzyme Q10 Treatment in Retinitis Pigmentosa. Mol. Asp. Med. 1994, 15, s221–s230. [Google Scholar] [CrossRef]
- Daniel, A.; Premilovac, D.; Foa, L.; Feng, Z.; Shah, K.; Zhang, Q.; Woolley, K.L.; Bye, N.; Smith, J.A.; Gueven, N. Novel Short-Chain Quinones to Treat Vision Loss in a Rat Model of Diabetic Retinopathy. Int. J. Mol. Sci. 2021, 22, 1016. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrizalez, A.D.; Castellanos-González, J.A.; Martínez-Romero, E.C.; Miller-Arrevillaga, G.; Pacheco-Moisés, F.P.; Román-Pintos, L.M.; Miranda-Díaz, A.G. The Effect of Ubiquinone and Combined Antioxidant Therapy on Oxidative Stress Markers in Non-Proliferative Diabetic Retinopathy: A Phase IIa, Randomized, Double-Blind, and Placebo-Controlled Study. Redox Rep. 2016, 21, 155–163. [Google Scholar] [CrossRef]
- Pawar, P.; Mumbare, S.; Patil, M.; Ramakrishnan, S. Effectiveness of the Addition of Citicoline to Patching in the Treatment of Amblyopia around Visual Maturity: A Randomized Controlled Trial. Indian J. Ophthalmol. 2014, 62, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Sabetti, L.; Masedu, F.; Tresca, C.; Bianchi, F.; Valenti, M. The Use of Choline in Association with the Bangerter Filters for the Treatment of Amblyopia. Int. J. Ophthalmol. 2017, 10, 1777–1778. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Liu, S.; Fu, C. Citicoline Retards Myopia Progression Following Form Deprivation in Guinea Pigs. Exp. Biol. Med. 2016, 241, 1258–1263. [Google Scholar] [CrossRef] [Green Version]
- Cinar, E.; Yuce, B.; Aslan, F.; Erbakan, G. Neuroprotective Effect of Citicoline Eye Drops on Corneal Sensitivity After LASIK. J. Refract. Surg. 2019, 35, 764–770. [Google Scholar] [CrossRef]
- Cialdai, F.; Bolognini, D.; Vignali, L.; Iannotti, N.; Cacchione, S.; Magi, A.; Balsamo, M.; Vukich, M.; Neri, G.; Donati, A.; et al. Effect of Space Flight on the Behavior of Human Retinal Pigment Epithelial ARPE-19 Cells and Evaluation of Coenzyme Q10 Treatment. Cell. Mol. Life Sci. 2021, 78, 7795–7812. [Google Scholar] [CrossRef]
- Brancato, R.; Schiavone, N.; Siano, S.; Lapucci, A.; Papucci, L.; Donnini, M.; Formigli, L.; Zecchi Orlandini, S.; Carella, G.; Carones, F.; et al. Prevention of Corneal Keratocyte Apoptosis after Argon Fluoride Excimer Laser Irradiation with the Free Radical Scavenger Ubiquinone Q10. Eur. J. Ophthalmol. 2000, 10, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Brancato, R.; Fiore, T.; Papucci, L.; Schiavone, N.; Formigli, L.; Zecchi Orlandini, S.; Gobbi, P.G.; Carones, F.; Donnini, M.; Lapucci, A.; et al. Concomitant Effect of Topical Ubiquinone Q10 and Vitamin E to Prevent Keratocyte Apoptosis after Excimer Laser Photoablation in Rabbits. J. Refract. Surg. 2002, 18, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.G.; Seto, S.; Ganne, P.; Votruba, M. A Randomized, Placebo-Controlled Trial of the Benzoquinone Idebenone in a Mouse Model of OPA1-Related Dominant Optic Atrophy Reveals a Limited Therapeutic Effect on Retinal Ganglion Cell Dendropathy and Visual Function. Neuroscience 2016, 319, 92–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giorgio, V.; Petronilli, V.; Ghelli, A.; Carelli, V.; Rugolo, M.; Lenaz, G.; Bernardi, P. The Effects of Idebenone on Mitochondrial Bioenergetics. Biochim. Biophys. Acta 2012, 1817, 363–369. [Google Scholar] [CrossRef]
- Seo, Y.; Park, J.; Kim, M.; Lee, H.K.; Kim, J.H.; Jeong, J.H.; Namkung, W. Inhibition of ANO1/TMEM16A Chloride Channel by Idebenone and Its Cytotoxicity to Cancer Cell Lines. PLoS ONE 2015, 10, e0133656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gueven, N.; Woolley, K.; Smith, J. Border between Natural Product and Drug: Comparison of the Related Benzoquinones Idebenone and Coenzyme Q10. Redox Biol. 2015, 4, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quaranta, L.; Riva, I.; Biagioli, E.; Rulli, E.; Rulli, E.; Poli, D.; Legramandi, L.; Fossarello, M.; Uva, M.; Carmassi, L.; et al. Evaluating the Effects of an Ophthalmic Solution of Coenzyme Q10 and Vitamin E in Open-Angle Glaucoma Patients: A Study Protocol. Adv. Ther. 2019, 36, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, L. CoQun Study—(COQUN): A Study to Evaluate the Effects of CoQun in Patients Affected by Open-Angle Glaucoma. Clinical Trial Identifier NCT03611530 2017, September 4-2021, February, 28. Available online: https://ichgcp.net/clinical-trials-registry/NCT03611530 (accessed on 28 November 2022).
- Wallace, T.C.; Blusztajn, J.K.; Caudill, M.A.; Klatt, K.C.; Natker, E.; Zeisel, S.H.; Zelman, K.M. Choline: The Underconsumed and Underappreciated Essential Nutrient. Nutr. Today 2018, 53, 240–253. [Google Scholar] [CrossRef]
| Author | Study Population | Citicoline Dose | Results |
|---|---|---|---|
| Topical instillation | |||
| Bogdanov et al. [38] | Mice: DM | 2%, 2 drops/day | ↑ ERG |
| Prevent glial activation and apoptosis | |||
| Parravano et al. [39] | Humans: NPDR | 2% (+VitB12), | ↓ Microvascular damage |
| 3 drops/day | ↓ Neuroretinal degeneration | ||
| Parisi et al. [40] | Humans: NPDR | 2% (+VitB12) 3 drops/day | ↑ mfERG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-López, C.; García-López, V.; Matamoros, J.A.; Fernández-Albarral, J.A.; Salobrar-García, E.; de Hoz, R.; López-Cuenca, I.; Sánchez-Puebla, L.; Ramírez, J.M.; Ramírez, A.I.; et al. The Role of Citicoline and Coenzyme Q10 in Retinal Pathology. Int. J. Mol. Sci. 2023, 24, 5072. https://doi.org/10.3390/ijms24065072
García-López C, García-López V, Matamoros JA, Fernández-Albarral JA, Salobrar-García E, de Hoz R, López-Cuenca I, Sánchez-Puebla L, Ramírez JM, Ramírez AI, et al. The Role of Citicoline and Coenzyme Q10 in Retinal Pathology. International Journal of Molecular Sciences. 2023; 24(6):5072. https://doi.org/10.3390/ijms24065072
Chicago/Turabian StyleGarcía-López, Claudia, Verónica García-López, José A. Matamoros, José A. Fernández-Albarral, Elena Salobrar-García, Rosa de Hoz, Inés López-Cuenca, Lidia Sánchez-Puebla, José M. Ramírez, Ana I. Ramírez, and et al. 2023. "The Role of Citicoline and Coenzyme Q10 in Retinal Pathology" International Journal of Molecular Sciences 24, no. 6: 5072. https://doi.org/10.3390/ijms24065072
APA StyleGarcía-López, C., García-López, V., Matamoros, J. A., Fernández-Albarral, J. A., Salobrar-García, E., de Hoz, R., López-Cuenca, I., Sánchez-Puebla, L., Ramírez, J. M., Ramírez, A. I., & Salazar, J. J. (2023). The Role of Citicoline and Coenzyme Q10 in Retinal Pathology. International Journal of Molecular Sciences, 24(6), 5072. https://doi.org/10.3390/ijms24065072














