Fluorescence Analysis of Biocide Efficiency in Antifouling Coatings against Cyanobacteria
Abstract
1. Introduction
2. Results
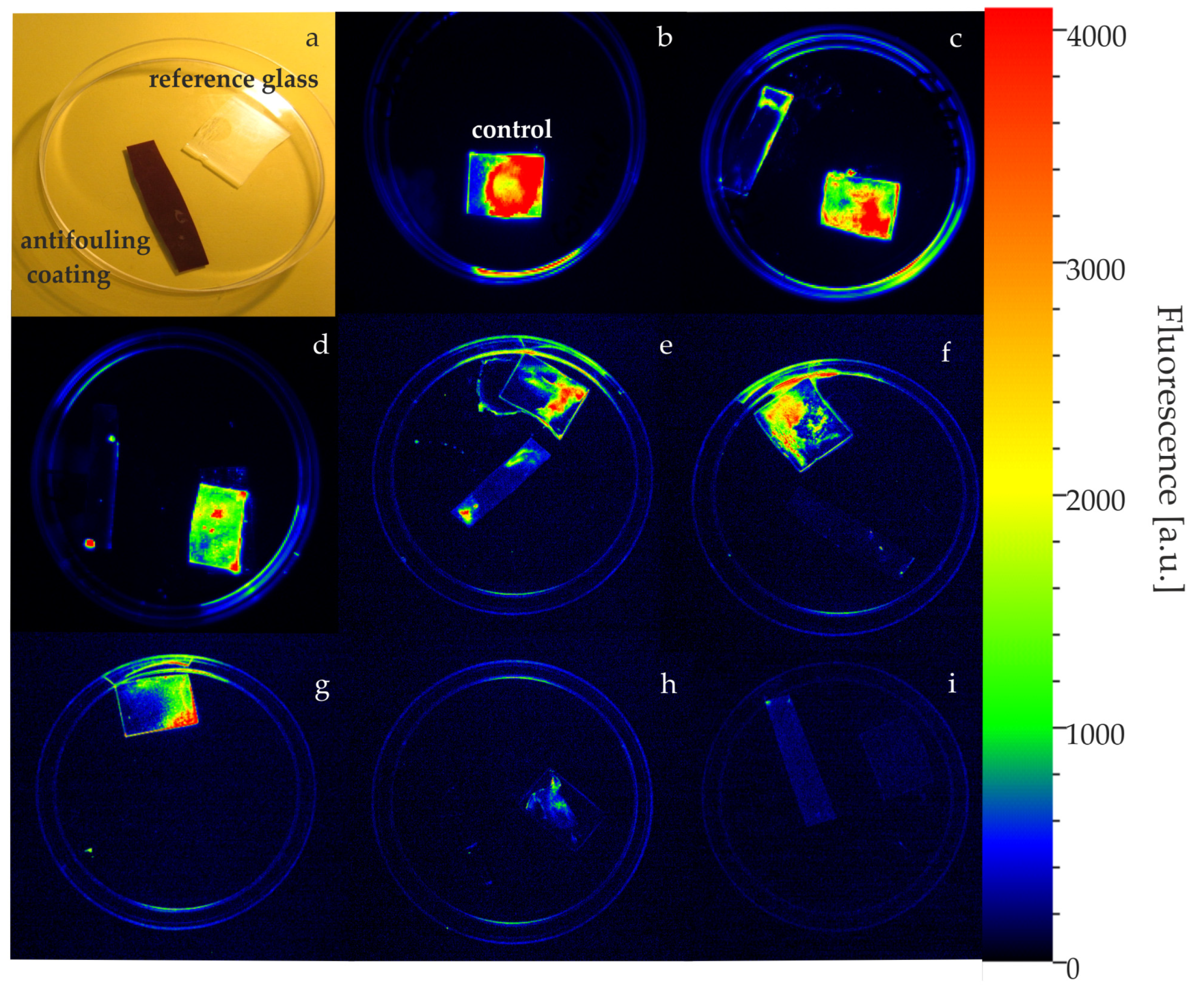
2.1. Bioaccumulation of Elements in Cyanobacterial Cells
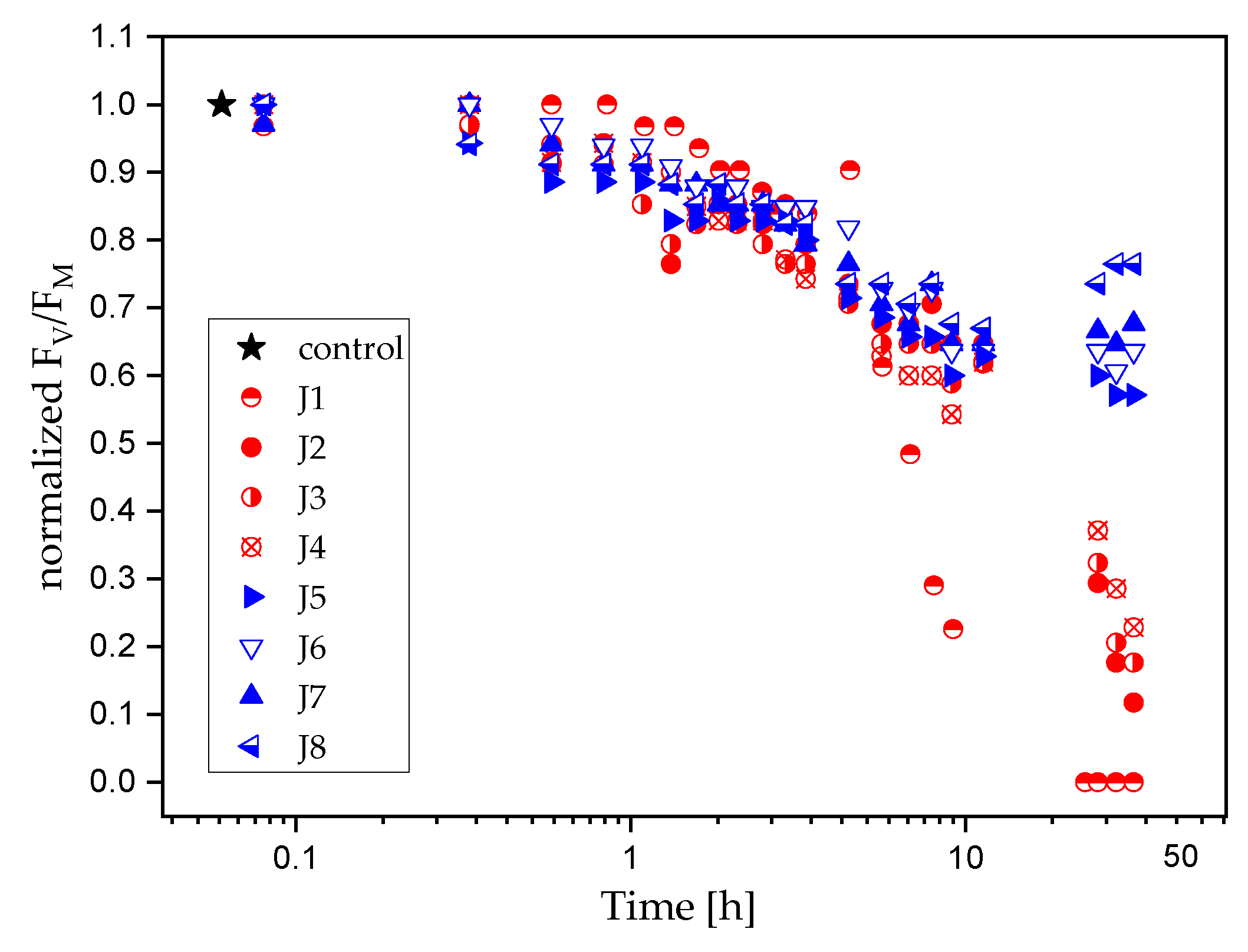
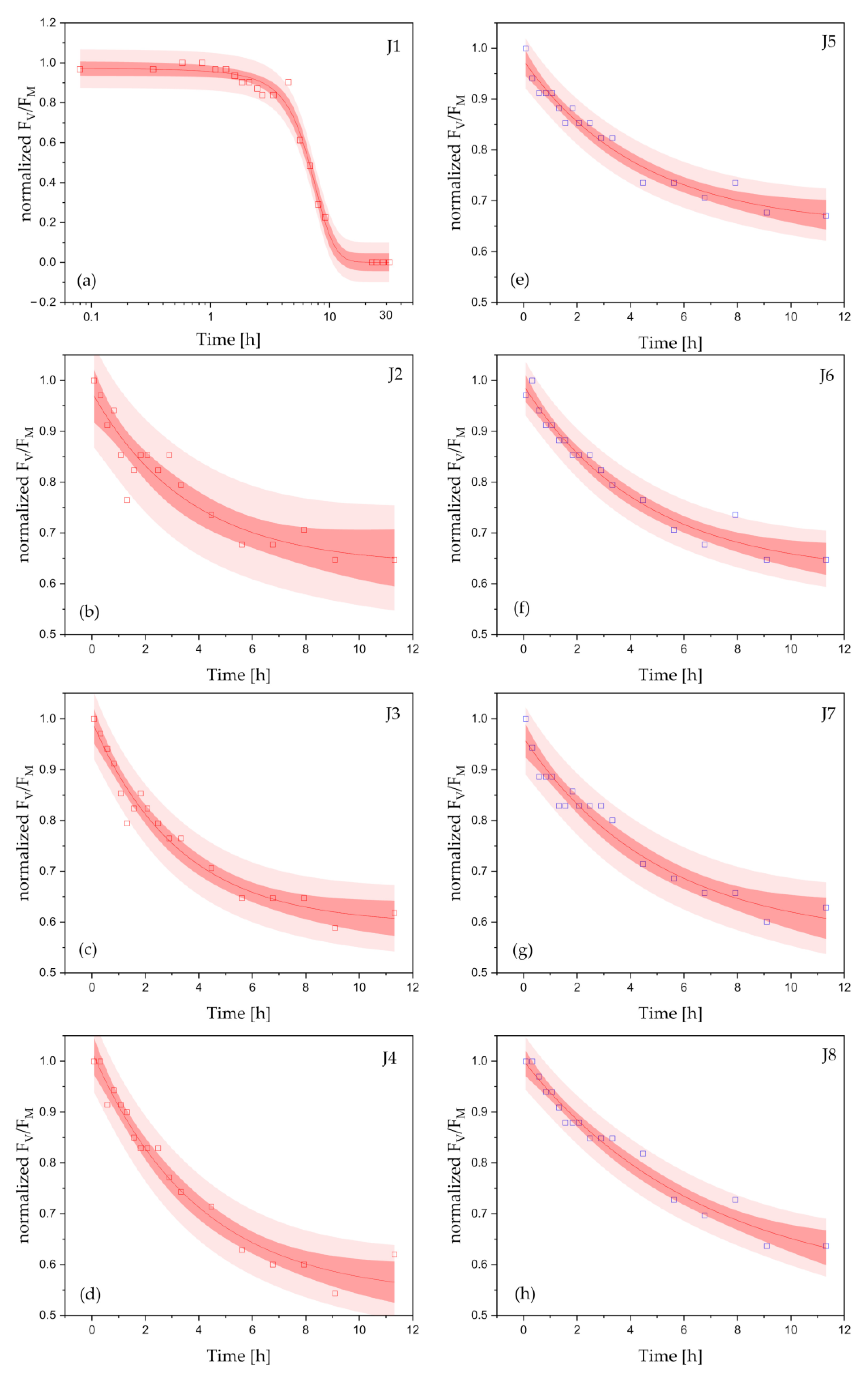
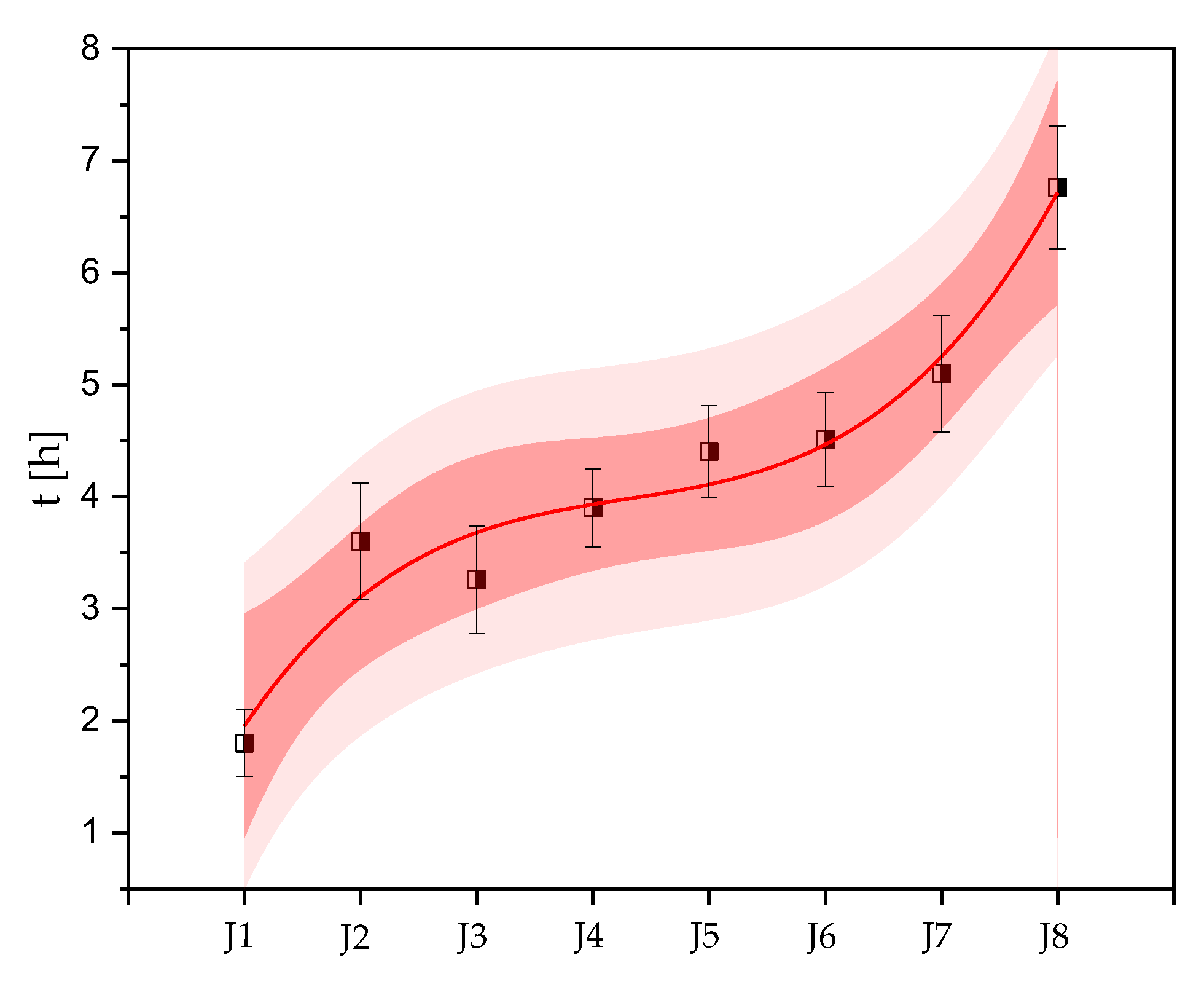
2.2. Response of the Photosynthetic Activity to Antifouling Coatings in Cyanothece sp. ATCC 51142
3. Discussion
4. Materials and Methods
4.1. Cyanobacterial Culture Exposed to Antifouling Coatings
4.2. Element Analysis
4.3. Measure of Photosynthesis
4.4. Imaging of a Biofilm on Antifouling Coatings
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bixler, G.D.; Bhushan, B. Biofouling: Lessons from nature. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2012, 370, 2381–2417. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Erol, E.; Cansoy, C.E.; Aybar, O.Ö. Assessment of the impact of fouling on vessel energy efficiency by analyzing ship automation data. Appl. Ocean Res. 2020, 105, 102418. [Google Scholar] [CrossRef]
- Farkas, A.; Degiuli, N.; Martić, I.; Dejhalla, R. Impact of Hard Fouling on the Ship Performance of Different Ship Forms. J. Mar. Sci. Eng. 2020, 8, 748. [Google Scholar] [CrossRef]
- Jin, H.C.; Tian, L.M.; Bing, W.; Zhao, J.; Ren, L.Q. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Oliveira, D.R.; Granhag, L. Ship hull in-water cleaning and its effects on fouling-control coatings. Biofouling 2020, 36, 332–350. [Google Scholar] [CrossRef]
- Tian, L.; Yin, Y.; Jin, H.; Bing, W.; Jin, E.; Zhao, J.; Ren, L. Novel marine antifouling coatings inspired by corals. Mater. Today Chem. 2020, 17, 100294. [Google Scholar] [CrossRef]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef]
- Dobretsov, S.; Coutinho, R.; Rittschof, D.; Salta, M.; Ragazzola, F.; Hellio, C. The oceans are changing: Impact of ocean warming and acidification on biofouling communities. Biofouling 2019, 35, 585–595. [Google Scholar] [CrossRef]
- Chan, F.T.; Ogilvie, D.; Sylvester, F.; Baliey, S.A. Ship biofouling as a vector for non-indigenous aquatic species to canadian arctic coastal ecosystems: A survey and modeling-based assessment. Front. Mar. Sci. 2022, 9, 808055. [Google Scholar] [CrossRef]
- Jin, H.C.; Wang, J.F.; Tian, L.M.; Gao, M.Y.; Zhao, J.; Ren, L.Q. Recent advances in emerging integrated antifouling and anticorrosion coatings. Mater. Des. 2022, 213, 110307. [Google Scholar] [CrossRef]
- Panchal, C.B. Review of fouling mechanisms. In Proceedings of the International Conference on Mitigation of Heat Exchanger Fouling and Its Economic and Environmental Implications, Banff, AB, Canada, 18–23 July 1999; pp. 8–15. [Google Scholar]
- Lindgren, J.F.; Ytreberg, E.; Holmqvist, A.; Dahlström, M.; Dahl, P.; Berglin, M.; Wrange, A.-L.; Dahlström, M. Copper release rate needed to inhibit fouling on the west coast of Sweden and control of copper release using zinc oxide. Biofouling 2018, 34, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.; Davis, C.I.; Brady, D.C. Copper biology. Curr. Biol. 2021, 31, R421–R427. [Google Scholar] [CrossRef]
- Jin, M.F.; You, M.X.; Lan, Q.Q.; Cai, L.Y.; Lin, M.Z. Effect of copper on the photosynthesis and growth of Eichhornia crassipes. Plant Biol. 2021, 23, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Kiaune, L.; Singhasemanon, N. Pesticidal copper (I) oxide: Environmental fate and aquatic toxicity. Rev Env. Contam. Toxicol. 2011, 213, 1–26. [Google Scholar] [CrossRef]
- Smolyakov, B.S.; Zhigula, M.V.; Ryzhikh, A.P.; Sinitsyna, E.V.; Ermolaeva, N.I.; Fedotova, A.A. Copper (II) Speciation in a Freshwater Ecosystem. Water Resour. 2004, 31, 55–63. [Google Scholar] [CrossRef]
- Burkhead, J.L.; Reynolds, K.A.G.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper homeostasis. New Phytol. 2009, 182, 799–816. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Han, H.; Du, R.; Wang, X. Physiological and Molecular Mechanisms of Plant Responses to Copper Stress. Int. J. Mol. Sci. 2022, 23, 12950. [Google Scholar] [CrossRef]
- Huertas, M.J.; López-Maury, L.; Giner-Lamia, J.; Sánchez-Riego, A.M.; Florencio, F.J. Metals in Cyanobacteria: Analysis of the Copper, Nickel, Cobalt and Arsenic Homeostasis Mechanisms. Life 2014, 4, 865–886. [Google Scholar] [CrossRef]
- Hadjoudja, S.; Vignoles, C.; Deluchat, V.; Lenain, J.F.; Le Jeune, A.H.; Baudu, M. Short term copper toxicity on Microcystis aeruginosa and Chlorella vulgaris using flow cytometry. Aquat. Toxicol. 2009, 94, 255–264. [Google Scholar] [CrossRef]
- Mota, R.; Pereira, S.B.; Meazzini, M.; Fernandes, R.; Santos, A.; Evans, C.A.; De Philippis, R.; Wright, P.C.; Tamagnini, P. Effects of heavy metals on Cyanothece sp. CCY 0110 growth, extracellular polymeric substances (EPS) production, ultrastructure and protein profiles. J. Proteom. 2015, 120, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Cavalletti, E.; Romano, G.; Palma Esposito, F.; Barra, L.; Chiaiese, P.; Balzano, S.; Sardo, A. Copper Effect on Microalgae: Toxicity and Bioremediation Strategies. Toxics 2022, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. Biometals 2021, 34, 737–759. [Google Scholar] [CrossRef] [PubMed]
- EU. Evaluation of active substances, Zineb Product-Type 21 (Anti-fouling products). In Regulation (EU) n°528/2012 Concerning the Making Available on the Market and Use of Biocidal Products; European Union: Dublin, Ireland, 2012; p. 112. [Google Scholar]
- PubChem Compound Summary for CID 5284484, Zineb; National Library of Medicine (US), National Center for Biotechnology Information: Bethesda, MD, USA, 2004.
- Gaysina, L.A.; Saraf, A.; Singh, P. Chapter 1—Cyanobacteria in Diverse Habitats. In Cyanobacteria; Mishra, A.K., Tiwari, D.N., Rai, A.N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–28. [Google Scholar] [CrossRef]
- Burnat, M.; Diestra, E.; Esteve, I.; Solé, A. In Situ Determination of the Effects of Lead and Copper on Cyanobacterial Populations in Microcosms. PLoS ONE 2009, 4, e6204. [Google Scholar] [CrossRef]
- Le Jeune, A.H.; Charpin, M.; Sargos, D.; Lenain, J.F.; Deluchat, V.; Ngayila, N.; Baudu, M.; Amblard, C. Planktonic microbial community responses to added copper. Aquat. Toxicol. 2007, 83, 223–237. [Google Scholar] [CrossRef]
- Micheletti, E.; Colica, G.; Viti, C.; Tamagnini, P.; De Philippis, R. Selectivity in the heavy metal removal by exopolysaccharide-producing cyanobacteria. J. Appl. Microbiol. 2008, 105, 88–94. [Google Scholar] [CrossRef]
- Orzechowska, A.; Trtílek, M.; Tokarz, K.; Rozpądek, P. A study of light-induced stomatal response in Arabidopsis using thermal imaging. Biochem. Biophys. Res. Commun. 2020, 533, 1129–1134. [Google Scholar] [CrossRef]
- Orzechowska, A.; Trtílek, M.; Tokarz, K.M.; Szymańska, R.; Niewiadomska, E.; Rozpądek, P.; Wątor, K. Thermal Analysis of Stomatal Response under Salinity and High Light. Int. J. Mol. Sci. 2021, 22, 4663. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F. Detection of photosynthetic activity and water stress by imaging the red chlorophyll fluorescence. Plant Physiol. Biochem. 2000, 38, 889–895. [Google Scholar] [CrossRef]
- Miszalski, Z.; Skoczowski, A.; Silina, E.; Dymova, O.; Golovko, T.; Kornas, A.; Strzalka, K. Photosynthetic activity of vascular bundles in Plantago media leaves. J. Plant Physiol. 2016, 204, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, A.; Beardall, J.; Kromkamp, J.C.; Kopecky, J.; Masojidek, J.; van Bergeijk, S.; Gabai, S.; Shaham, E.; Yamshon, A. Photosynthetic performance of outdoor Nannochloropsis mass cultures under a wide range of environmental conditions. Aquat. Microb. Ecol. 2009, 56, 297–308. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Kong, L. Copper Requirement and Acquisition by Marine Microalgae. Microorganisms 2022, 10, 1853. [Google Scholar] [CrossRef]
- Lopez, J.S.; Lee, L.; Mackey, K.R.M. The toxicity of copper to Crocosphaera watsonii and other marine phytoplankton: A systematic review. Front. Mar. Sci. 2019, 5, 511. [Google Scholar] [CrossRef]
- Bhargava, P.; Mishra, Y.; Srivastava, A.K.; Narayan, O.P.; Rai, L.C. Excess copper induces anoxygenic photosynthesis in Anabaena doliolum: A homology based proteomic assessment of its survival strategy. Photosynth. Res. 2008, 96, 61–74. [Google Scholar] [CrossRef]
- Dudkowiak, A.; Olejarz, B.; Łukasiewicz, J.; Banaszek, J.; Sikora, J.; Wiktorowicz, K. Heavy Metals Effect on Cyanobacteria Synechocystis aquatilis Study Using Absorption, Fluorescence, Flow Cytometry, and Photothermal Measurements. Int. J. Thermophys. 2011, 32, 762–773. [Google Scholar] [CrossRef]
- Löschau, M.; Krätke, R. Efficacy and toxicity of self-polishing biocide-free antifouling paints. Environ. Pollut. 2005, 138, 260–267. [Google Scholar] [CrossRef]
- Ytreberg, E.; Karlsson, J.; Eklund, B. Comparison of toxicity and release rates of Cu and Zn from anti-fouling paints leached in natural and artificial brackish seawater. Sci. Total Environ. 2010, 408, 2459–2466. [Google Scholar] [CrossRef]
- Latifi, A.; Ruiz, M.; Zhang, C.-C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef]
- Jellali, R.; Kromkamp, J.C.; Campistron, I.; Laguerre, A.; Lefebvre, S.; Perkins, R.G.; Pilard, J.-F.; Mouget, J.-L. Antifouling Action of Polyisoprene-Based Coatings by Inhibition of Photosynthesis in Microalgae. Environ. Sci. Technol. 2013, 47, 6573–6581. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Depuydt, S.; Choi, S.; Han, T.; Park, J. Rapid toxicity assessment of six antifouling booster biocides using a microplate-based chlorophyll fluorescence in Undaria pinnatifida gametophytes. Ecotoxicology 2020, 29, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Papadatou, M.; Knight, M.; Salta, M. High-throughput method development for in-situ quantification of aquatic phototrophic biofilms. Biofouling 2022, 38, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S.; Rittschof, D. Love at First Taste: Induction of Larval Settlement by Marine Microbes. Int. J. Mol. Sci. 2020, 21, 731. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Cuesta, A.; Meseguer, J.; Esteban, M.A. Risks of Using Antifouling Biocides in Aquaculture. Int. J. Mol. Sci. 2012, 13, 1541–1560. [Google Scholar] [CrossRef]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Chau, N.D.; Wątor, K.; Rusiniak, P.; Gorczyca, Z.; Van Hao, D. Chemical composition, radioactive and stable isotopes in several selected thermal waters in North Vietnam. Ecol. Indic. 2022, 138, 108856. [Google Scholar] [CrossRef]
- Wątor, K.; Dobrzyński, D. Towards a better practice in water sampling: Case studies on used in practice geothermal waters. Chemosphere 2022, 303, 134913. [Google Scholar] [CrossRef]
| Paint Label | Cu2O [%] | Zineb [%] |
|---|---|---|
| J1 | 21.4 | 12.8 |
| J2 | 21.4 | 8.6 |
| J3 | 21.4 | 4.3 |
| J4 | 21.4 | 0 |
| J5 | 0 | 12.8 |
| J6 | 0 | 8.6 |
| J7 | 0 | 4.3 |
| J8 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orzechowska, A.; Czaderna-Lekka, A.; Trtílek, M.; Rusiniak, P. Fluorescence Analysis of Biocide Efficiency in Antifouling Coatings against Cyanobacteria. Int. J. Mol. Sci. 2023, 24, 4972. https://doi.org/10.3390/ijms24054972
Orzechowska A, Czaderna-Lekka A, Trtílek M, Rusiniak P. Fluorescence Analysis of Biocide Efficiency in Antifouling Coatings against Cyanobacteria. International Journal of Molecular Sciences. 2023; 24(5):4972. https://doi.org/10.3390/ijms24054972
Chicago/Turabian StyleOrzechowska, Aleksandra, Anna Czaderna-Lekka, Martin Trtílek, and Piotr Rusiniak. 2023. "Fluorescence Analysis of Biocide Efficiency in Antifouling Coatings against Cyanobacteria" International Journal of Molecular Sciences 24, no. 5: 4972. https://doi.org/10.3390/ijms24054972
APA StyleOrzechowska, A., Czaderna-Lekka, A., Trtílek, M., & Rusiniak, P. (2023). Fluorescence Analysis of Biocide Efficiency in Antifouling Coatings against Cyanobacteria. International Journal of Molecular Sciences, 24(5), 4972. https://doi.org/10.3390/ijms24054972






