1. Introduction
Digital stress has been identified as a new source of stress by the cosmetic industry; it has become a subject of increasing interest along with the increase in daily exposure to screens. Moreover, several papers have demonstrated that screen exposure induces skin damage; however, the related mechanisms remain to be elucidated [
1,
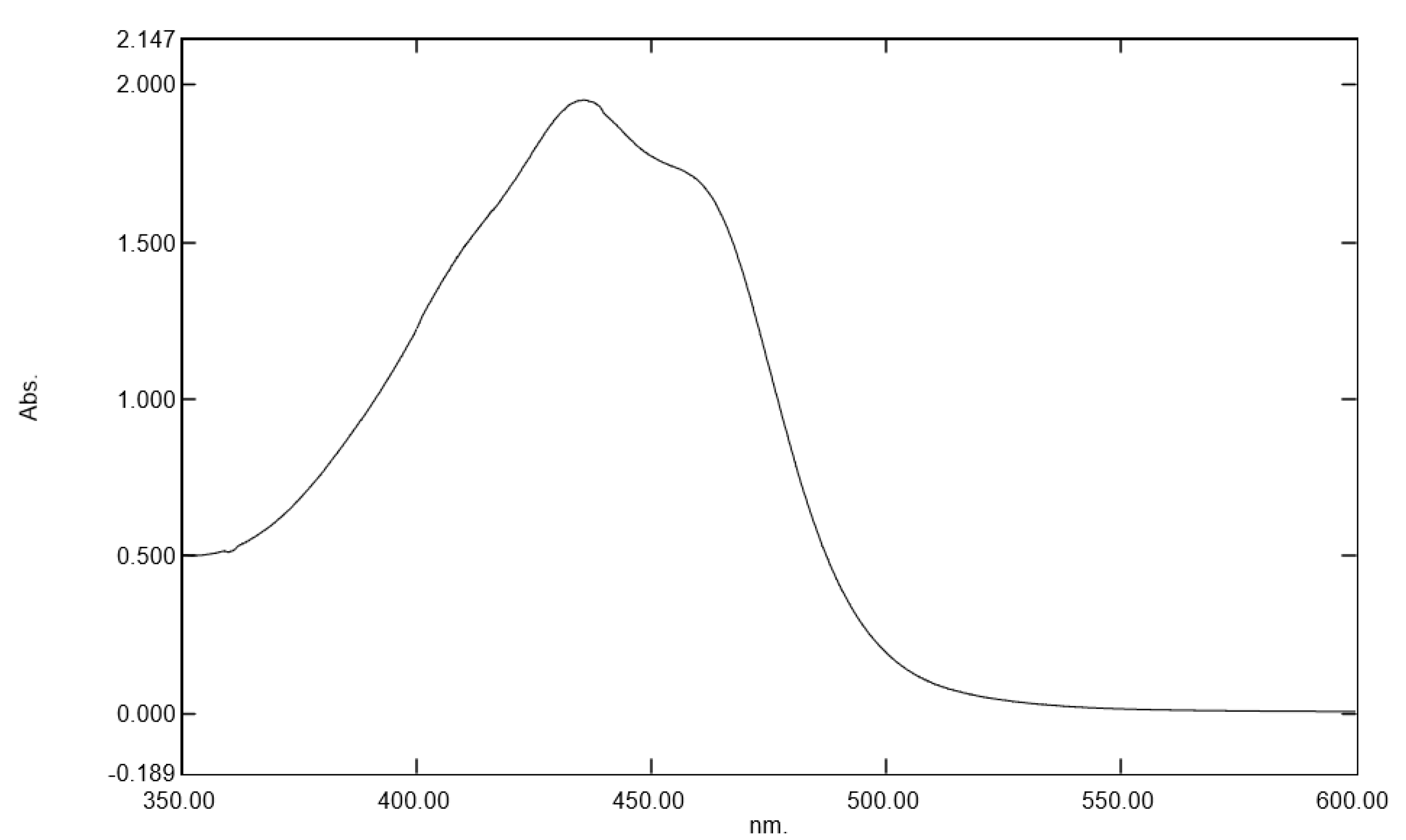
2]. This stress is mainly represented by the blue light from light-emitting diodes in digital electronic devices such as smartphones, computer screens, tablets, and televisions. Indeed, the maximal absorption from these digital devices was at 450 nm, corresponding to the blue light range [
3]. To be precise, blue light is characterized as high-energy visible light with a short wavelength within the range of 400 to 490 nm [
4]. Blue light is close to the UVA spectrum and results in similar cutaneous disorders to those observed in photo-aged skin [
5]. These disorders are now well described, and numerous studies have reported that blue light generates reactive oxygen species and thus causes oxidative damage, DNA damage, mitochondrial stress, hyperpigmentation in darker skin types, inflammation, and barrier disruption [
1,
2,
3,
4,
5], all of which lead to premature aging.
Blue light is part of the visible light spectrum, and its negative effects on the body have been well documented [
6]. Sleep disorders are the main negative consequence of exposure because visible light impairs the body’s internal clock. Various studies have demonstrated that visible light, especially blue light, has deleterious effects on the levels of melatonin, a crucial hormone secreted by the pineal gland and involved in day/night rhythms and thermoregulation [
7,
8]. Indeed, studies have reported a delay in, and partial suppression of, melatonin levels after long exposure to blue light (or visible light) [
8,
9,
10,
11,
12].
Melatonin’s internal clock activity affects various tissues, including the skin. Some skin cells express a melatonin receptor (mainly MT1) and can synthesize melatonin, thus suggesting that skin has its own melatoninergic system [
13,
14,
15,
16,
17]. Melatonin is an amphiphilic molecule with numerous beneficial effects on the skin. This pleiotropic molecule is a strong antioxidant that scavenges reactive oxygen species and stimulates the gene expression of enzymes involved in antioxidant defense. Through this antioxidant activity, melatonin decreases UV and DNA damage and protects the mitochondria [
18]. Melatonin can stimulate wound healing, keratinocyte proliferation, and hair growth, and can inhibit melanogenesis and apoptosis [
13,
15]. Moreover, melatonin can slow cancer development. On the basis of these activities, melatonin is considered to be an anti-aging molecule [
17].
Melatonin levels decrease with aging, and the amplitude of the difference between day and night levels decreases [
19,
20]. Therefore, blue light has a greater influence on younger than on older people [
8]. Moreover, sleep deprivation is a factor affecting the skin and contributing to premature aging [
21,
22,
23].
Thus, melatonin appears to be strongly correlated with blue light, and both play a role in skin aging.
The aim of this study was to evaluate the beneficial effects of Gardenia jasminoides fruit extract when stabilized in Natural Deep Eutectic Solvent (NaDES), which may act as a blue light filter and as a melatonin-like ingredient. Firstly, several experiments were conducted to prove the deleterious effects of digital stress on the skin and the protective effect of the plant extract. Secondly, an innovative co-culture model was designed to quantify and follow melatonin release and demonstrate its suppression by blue light.
In this work, we demonstrated that preserving the natural melatonin cycle is an innovative way to combat skin aging. Overall, these data explain how blue light affects skin aging in a process involving melatonin.
3. Discussion
Digital stress is not a cosmetic trend but is instead an external factor that has become increasingly present in human lives through increasing exposure to technology. Digital stress is mainly represented by exposure to blue light through radiation close to UVA that is emitted by the screens of electronic devices such as mobile phones, computers, or televisions [
4] The consequences for the skin are well known, some of which are similar to those of UVA exposure. Numerous studies have related premature photo-aging to an increase in oxidative stress, DNA damage, and mitochondrial stress, among other conditions [
1,
2,
5]. The aim of our work was to reveal the beneficial properties of the
Gardenia jasminoides extract, regarding its ability to protect the skin against these direct deleterious effects of blue light irradiation. We selected a botanical extract that had specific absorption in the range of blue light (400 to 490 nm) and that could be used as a blue light filter to protect the skin against digital stress [
24]. Moreover, this plant extract was traditionally used in folk medicine and in traditional Chinese medicine to treat inflammation, headache, edema, fever, hepatic disorders, and hypertension, evidencing its skin benefits [
25,
26]. Its anti-aging properties due to crocin content have been previously demonstrated but without there being a scientific link to digital stress and melatonin cycle preservation [
27]. Rascalou and co-workers [
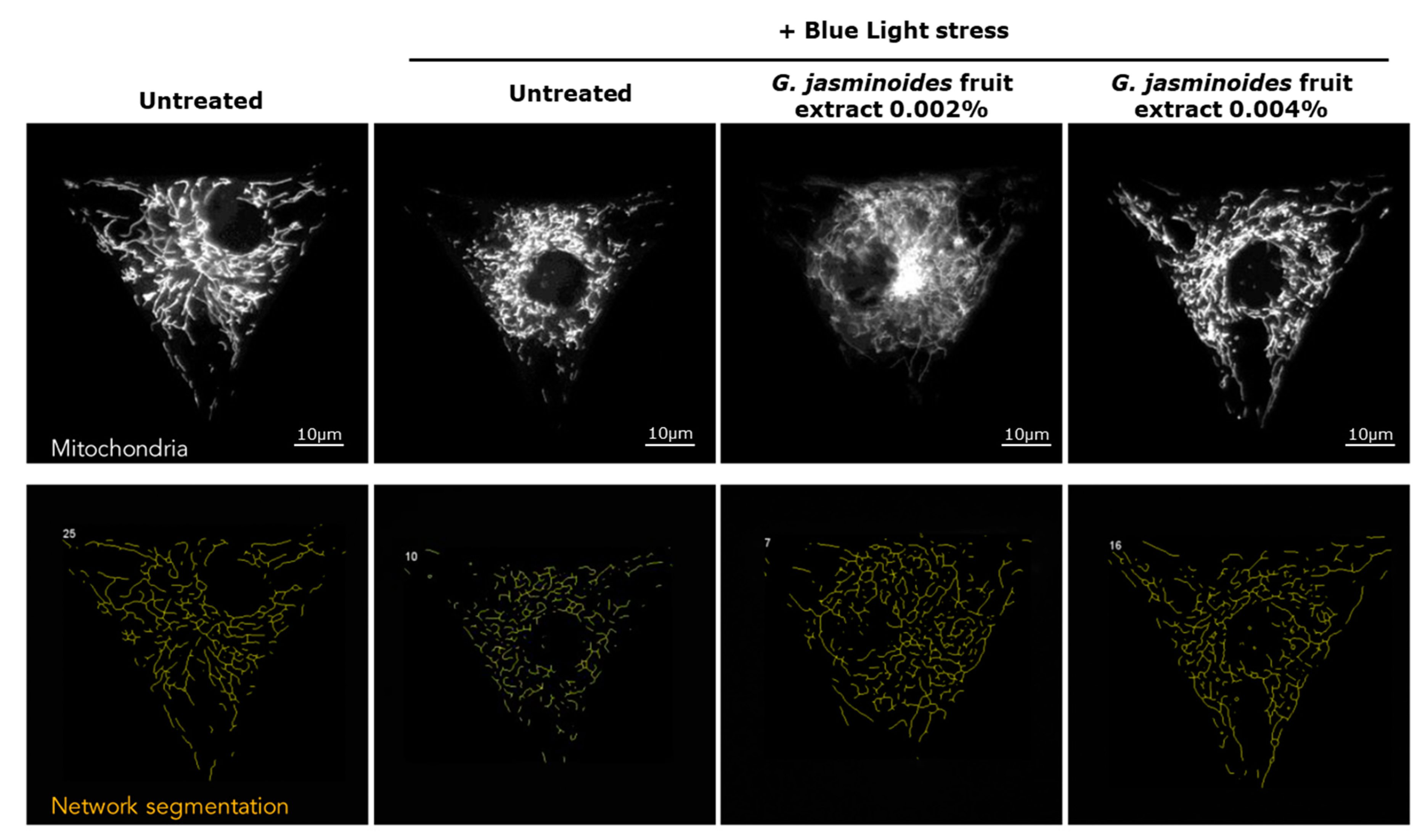
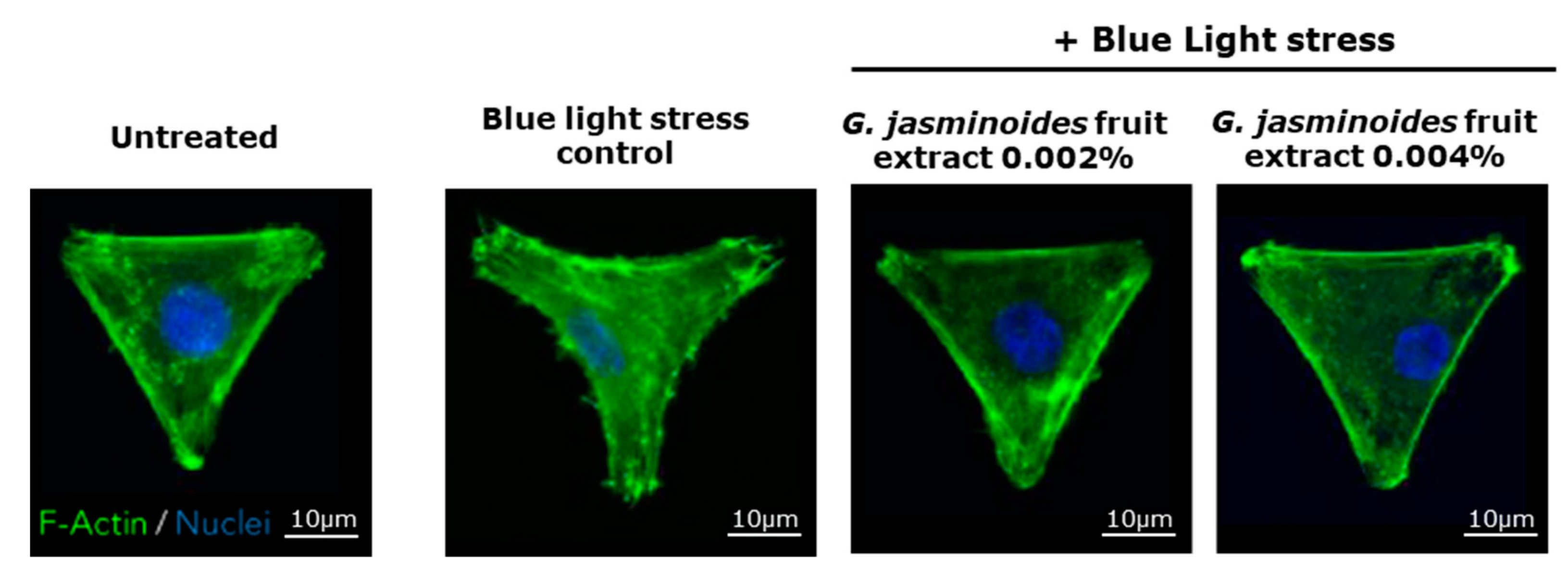
3] have demonstrated that digital stress has dramatic effects on mitochondrial networks. Consequently, we evaluated the protective effects of our extract in an in vitro model of normal human dermal fibroblasts and observed the effects of blue light on the mitochondrial network. We demonstrated that the damage caused by blue light irradiation was significantly reversed in the presence of the extract. Indeed, the mitochondrial network was significantly less fragmented, and the cell spreading was significantly restored, thus indicating decreased cell stress. Therefore, the extract protected the mitochondria against digital stress.
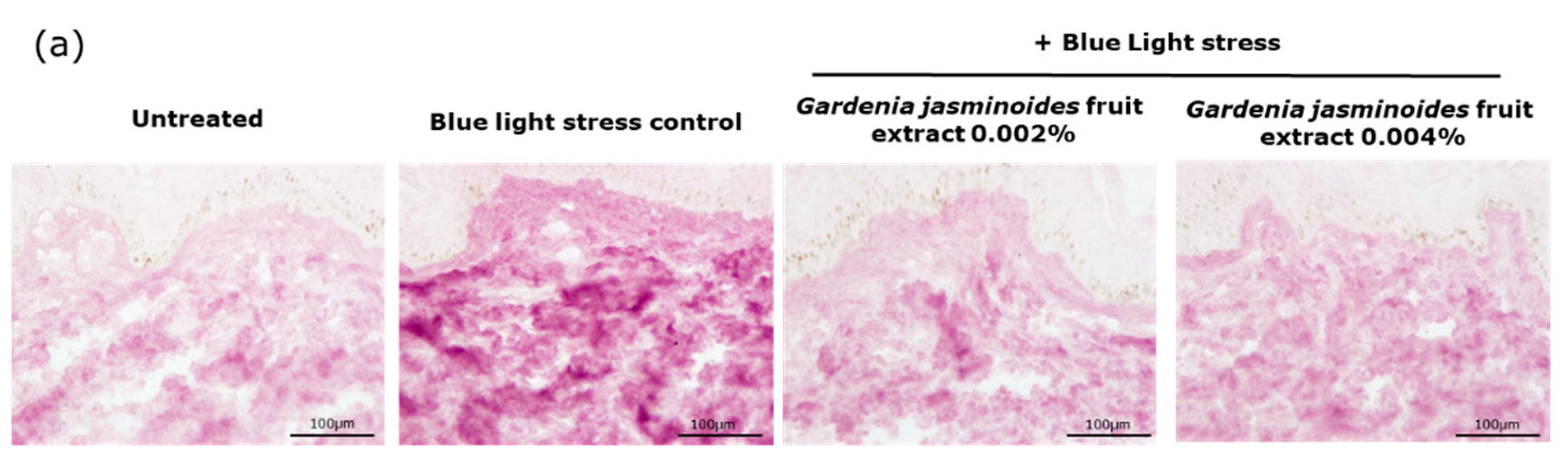
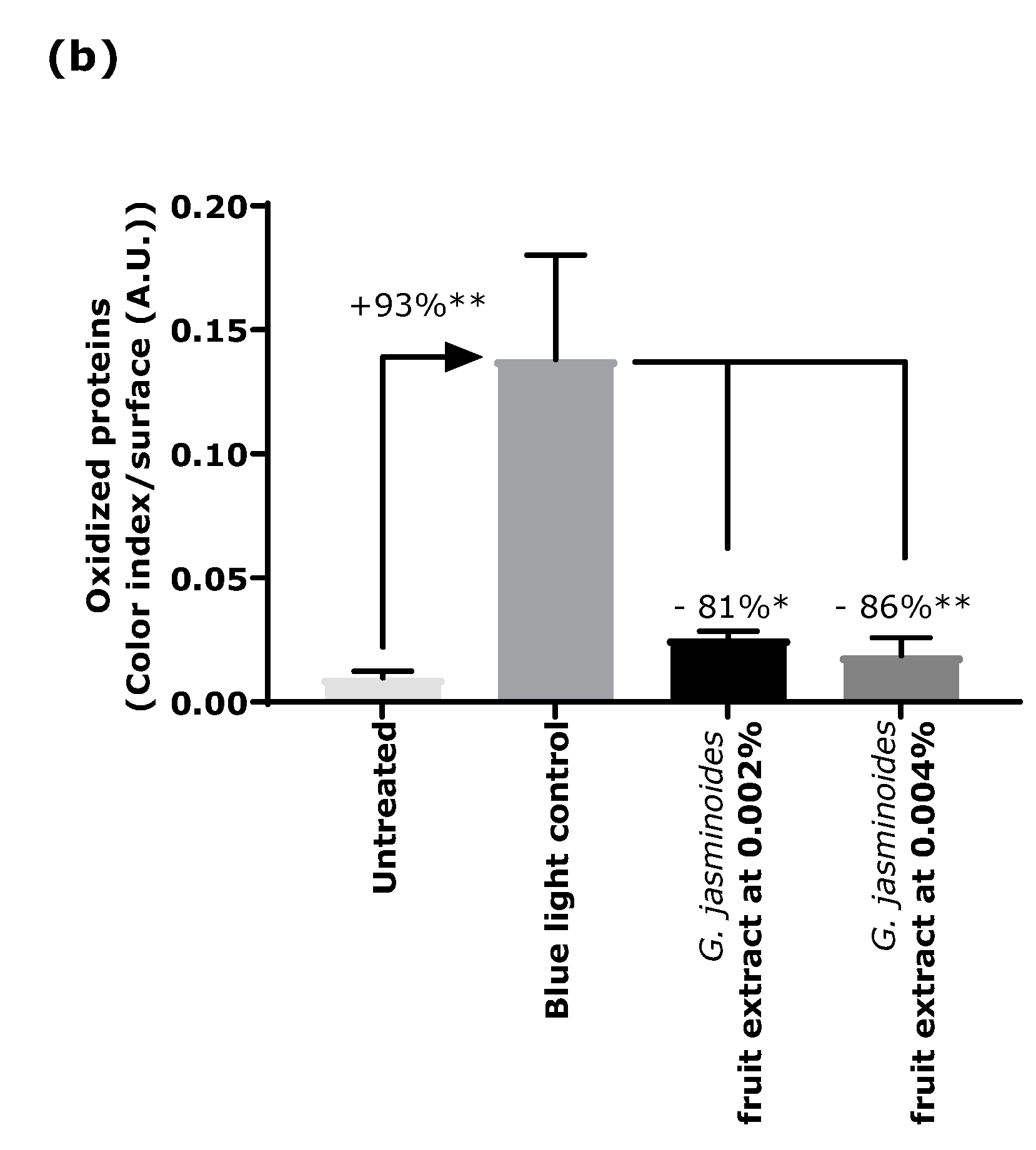
Oxidation is another major consequence of digital stress; therefore, we evaluated the levels of oxidized proteins on skin explants after blue light irradiation. Oxidized proteins significantly increased after irradiation, as demonstrated by staining. In the presence of the extract when topically applied on skin explants, we observed a significant decrease by 81% and 86% with 0.002% and 0.004% extract, respectively. These results confirmed that the filtering effect of the extract provided strong protection against blue light.
Beyond the direct damage associated with irradiation, blue light perturbs the natural cycle of melatonin [
8,
9,
10,
11,
12]. Melatonin is a powerful hormone involved in numerous biological functions and is a widely recognized anti-aging molecule [
17]. Melatonin is also involved in day/night rhythms and the control of sleep [
7,
8]. Its perturbation, when triggered by blue light exposure, affects not only the overall body health and mental health but also the skin. Indeed, skin cells express melatonin receptors and, thus, can be affected by melatonin dysregulation. Because melatonin has numerous benefits when inside the cells [
13,
15,
18], its natural cycle must be preserved. We designed an innovative co-culture model to visualize the effects of blue light on the melatonin cycle. As described in the literature [
8,
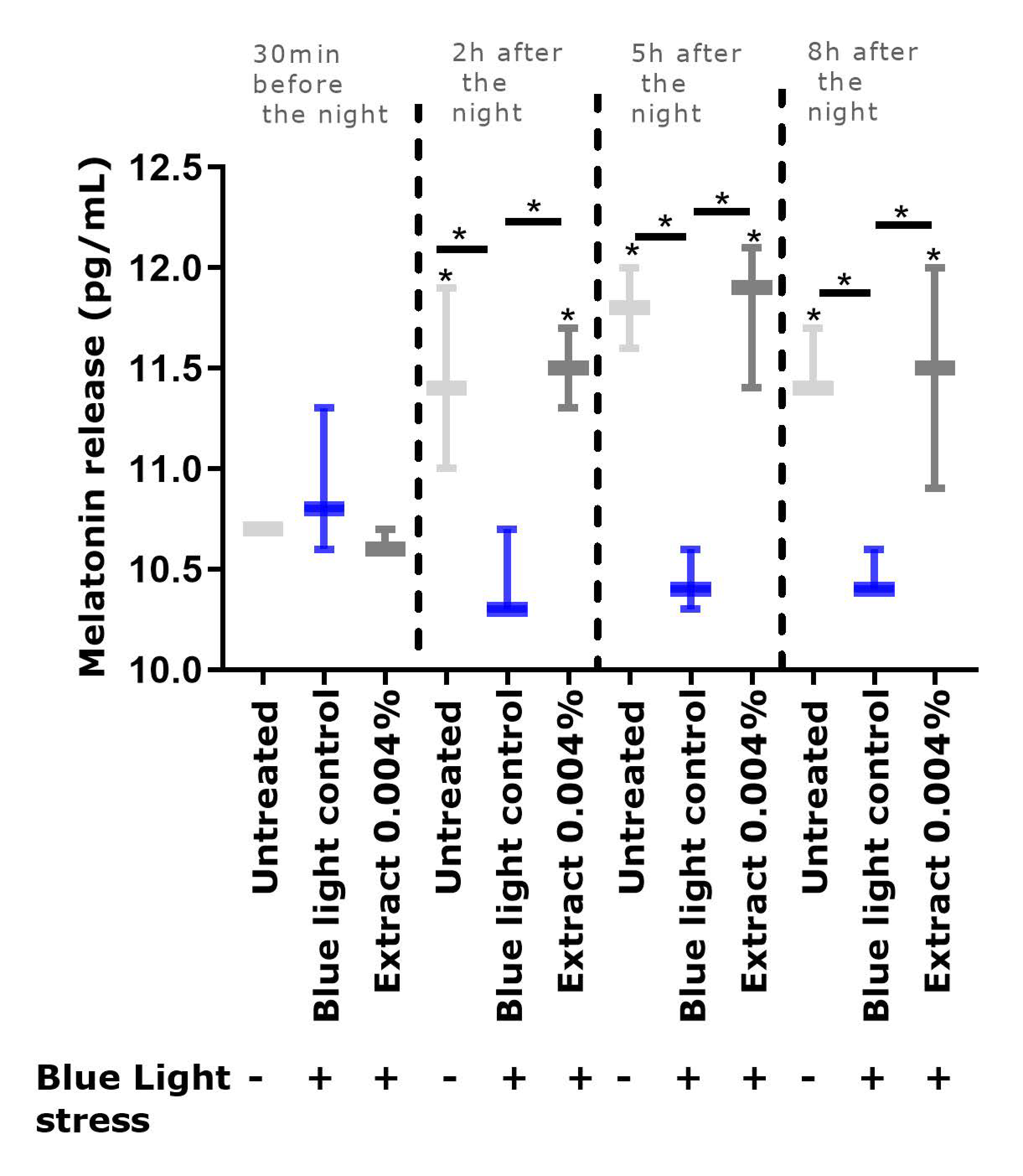
9], we observed that repeated exposure to blue light significantly depleted the level of melatonin, in comparison to the un-exposed condition. In the presence of the extract, the melatonin cycle was preserved, and its level was significantly higher than that in the blue light control.
Thus, we clearly demonstrated that the extract, through its filtering activity against blue light, protected the skin from oxidative damage and mitochondrial stress, and preserved the natural melatonin cycle.
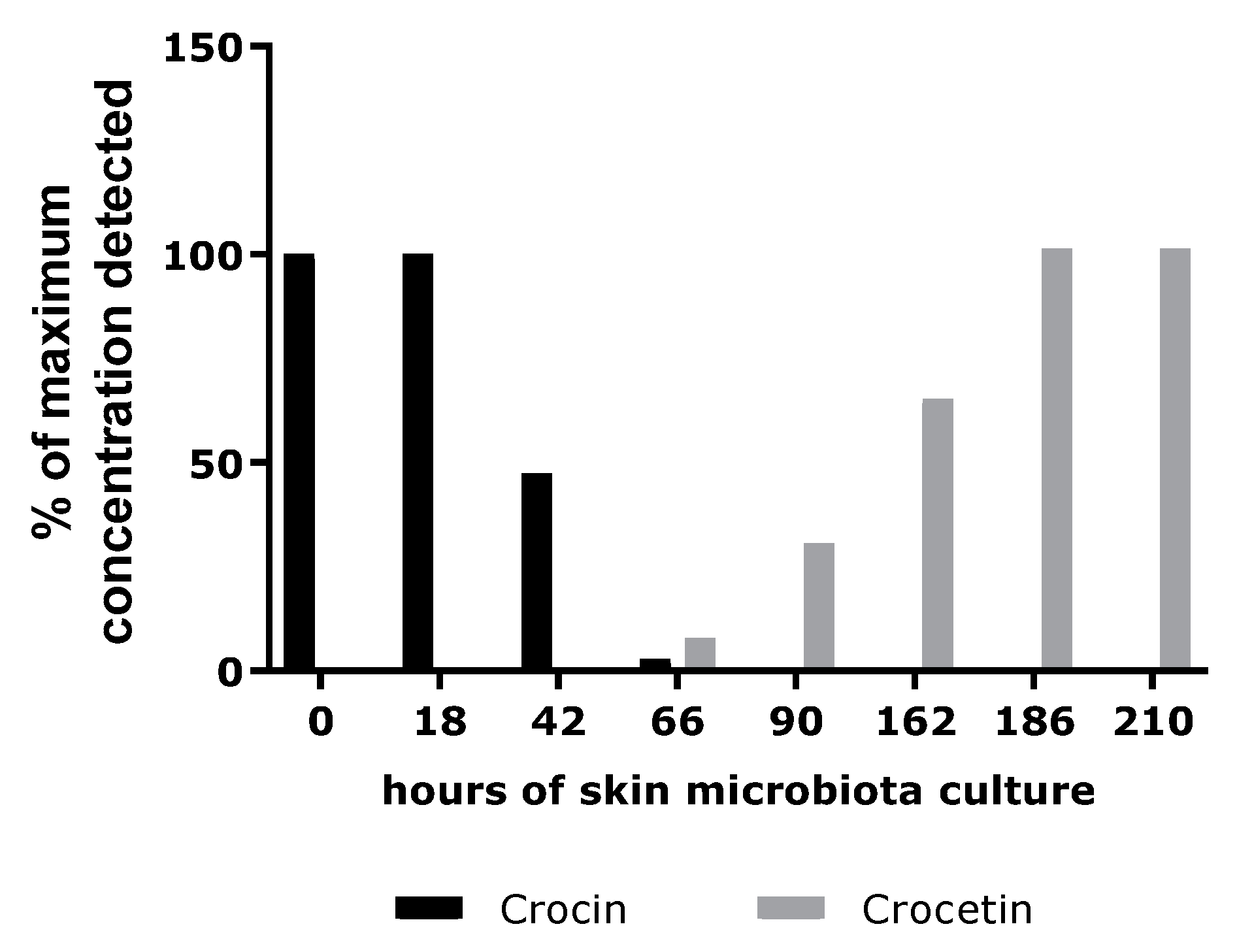
In the second part of our investigation, we investigated the hypothesis that the extract might act as a melatonin-like molecule. Because skin microbiota have been widely purported to hydrolyze glycosylated compounds, we performed a study to verify this hypothesis [
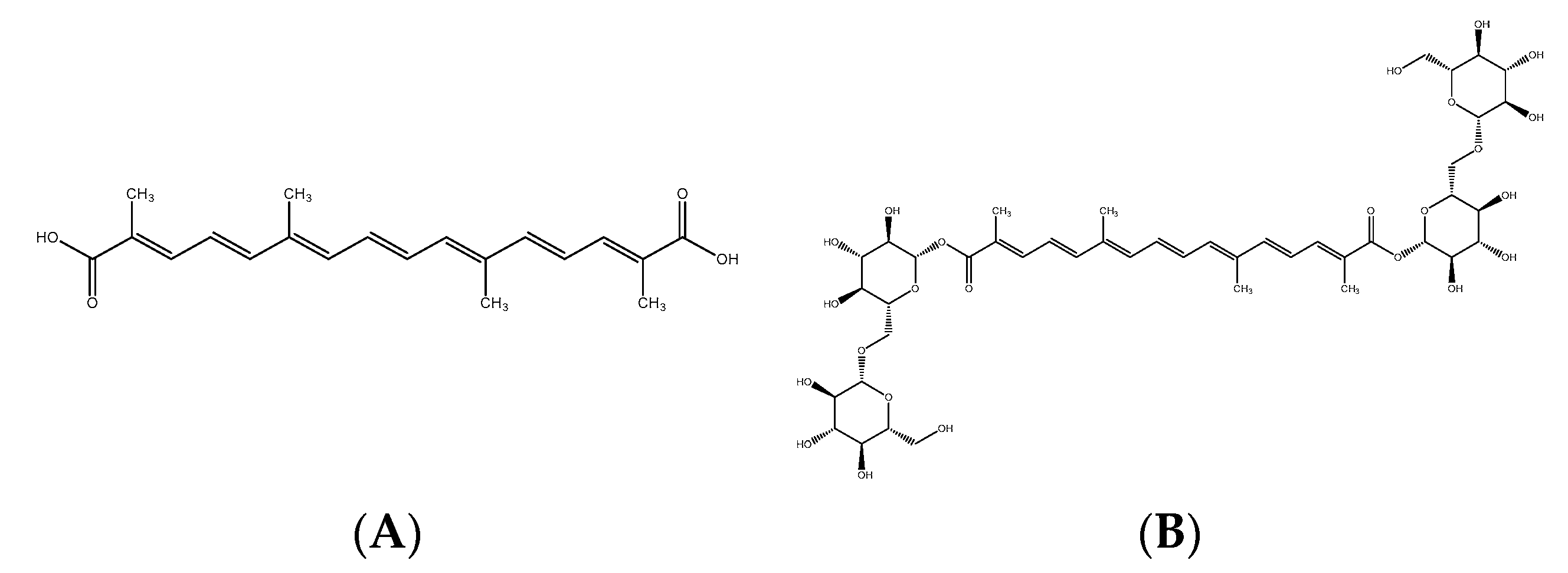
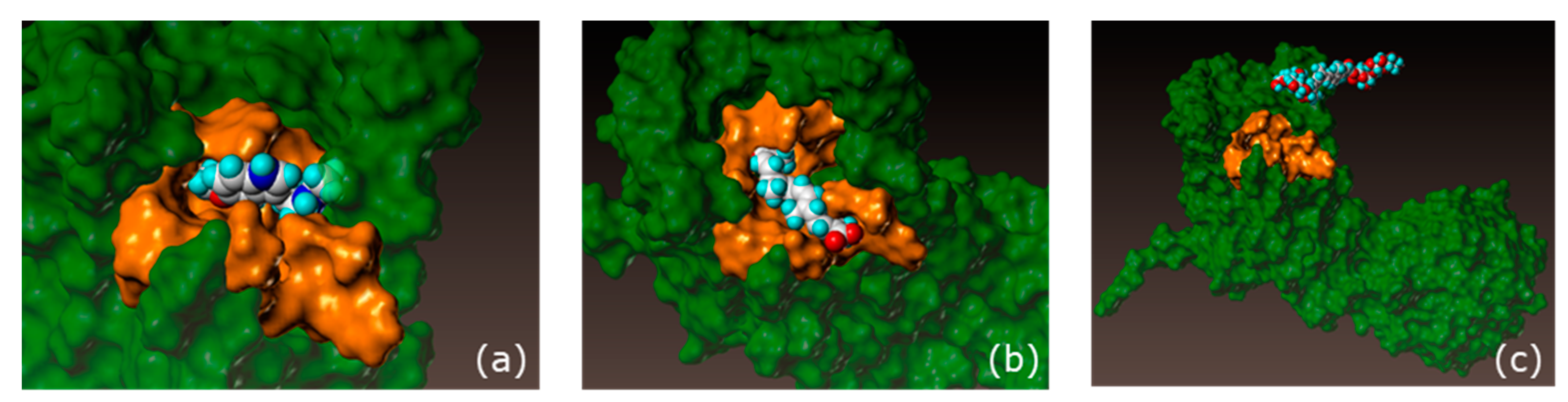
28]. Skin microbiota sampled from healthy donors were incubated in the presence of crocin. An analytical method was designed to follow the consumption of crocin and the appearance of crocetin, its deglycosylated form. According to the results, the skin microbiota was able to convert the crocin into crocetin. We then sought to identify the biological function of crocetin in the skin. To do this, we evaluated the potential docking of crocin and its deglycosylated form, crocetin, with the melatonin receptor, MT1 [
16]. We demonstrated that crocetin had an affinity score close to that of melatonin, thereby indicating the potential melatonin-like activity of the extract.
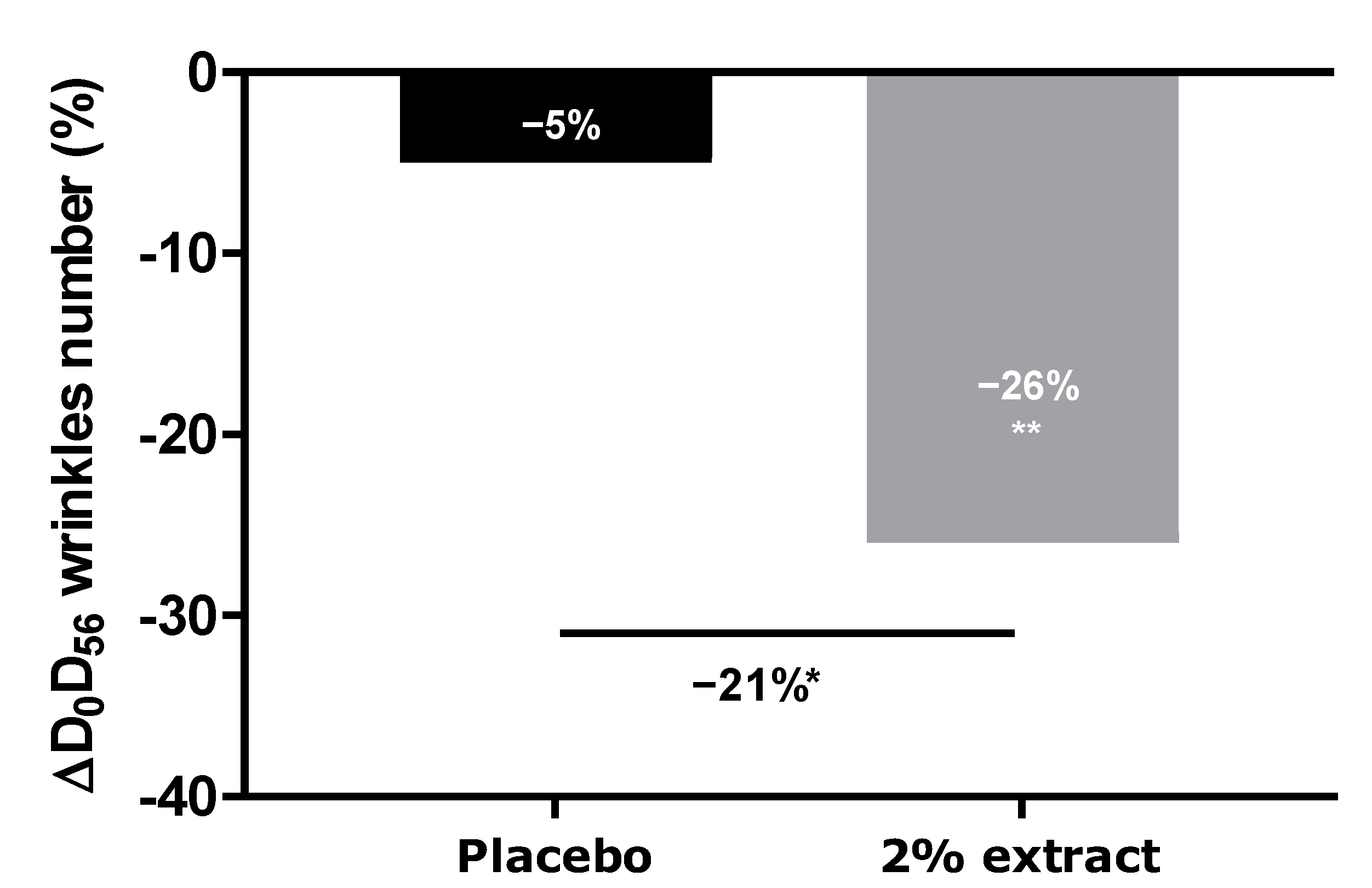
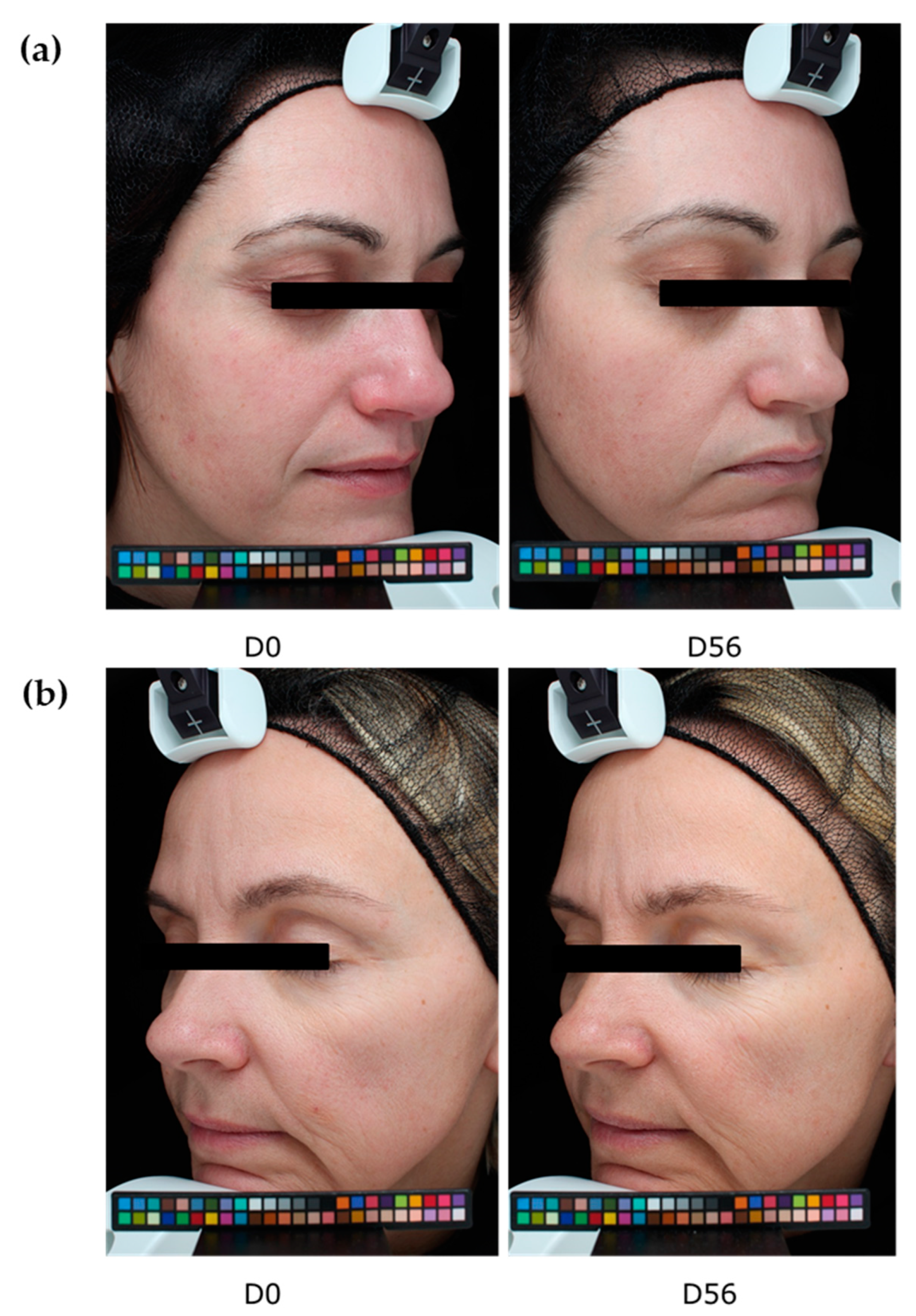
We thus demonstrated that our extract protects against digital stress not only by acting as a filter protecting the skin against oxidative stress but also by preserving the natural melatonin cycle. We further demonstrated that this extract acts as a melatonin-like molecule after transformation by the skin microbiota. All effects led to anti-aging activity. We then extended our investigation to an in vivo study by designing an innovative experimental protocol in which volunteers were exposed for at least 4 h per day to digital devices (with 100% luminosity). After 56 days of a twice-daily application of the extract, we observed a significant decrease in the number of crow’s feet, whereas the placebo did not show any effect. The difference between the active and placebo groups was significant.
Thus, we conclude that the extract is a 2-in-1 cosmetic ingredient that can protect against digital stress, preserve the natural melatonin cycle, and act as a melatonin-like molecule that decreases the signs of aging.
4. Materials and Methods
4.1. Gardenia jasminoides Extract Description
The extract is a botanical cosmetic ingredient from crocin-rich Gardenia jasminoides J Ellis extract stabilized by a natural deep eutectic solvent (NaDES) composed of glycerin/betaine/water. Its composition is a mixture of crocin isomers.
4.1.1. Extract Preparation
Gardenia jasminoides J Ellis (Rubiaceae) is cultivated in South China in Guangxi Province. For this study, the fruits were harvested in November. After drying and grinding, the plant was extracted in water (extract ratio 25–60:1). After filtration, to remove solids, the water extract was purified by column chromatography on a resin column with ethanol/water (70/30, w/w). The extract was then concentrated (to remove ethanol) and dried with maltodextrin as a carrier using a spray dryer to obtain a powder extract. The composition was Gardenia jasminoides extract/maltodextrin (95:5, w/w). The powder was titrated in crocin to 40% (w/w).
4.1.2. Preparation of the NaDES Mixture
To produce 100 g of NaDES mixture, we mixed 40.4 g of glycerin, purchased from Cremer OLEO (UK) Ltd., Hamburg, Germany, 34.6 g of betaine purchased from Evonik (Tego Natural Betaine), and 25 g of water at 50 °C under stirring until homogenization and the complete dissolution of betaine.
4.1.3. Final Preparation and Composition
To produce 100 g of the final product, 0.1 g of Gardenia jasminoides fruit extract (prepared as detailed above) was dissolved in 99.9 g of NaDES mixture (prepared as detailed above) at 50 °C until the complete dissolution of the powder extract; the mixture was then heated to 80 °C for 1 h.
The final extract is prepared with a specification of between 0.05 to 0.15% of the powder extract in a mixture of betaine, glycerin, and water.
4.2. Mitochondrial Network and Cell Spreading Analyses after Digital Stress
Normal human dermal fibroblasts were incubated at 37 °C under 5% CO2 in CNTPRF medium (CELLnTEC, Bern, Switzerland) until reaching confluence. The cells were then treated for 24 h with two concentrations of Gardenia jasminoides fruit extract (0.002% and 0.004%, v/v) or were left untreated. Then the cells were loaded with MitoTracker Green dye (Thermo Fisher, Waltham, MA, USA) for 15 min. The cells were washed with PBS (Gibco, Thermo Fisher), detached, and seeded into a CYTOOplate™ with extra-large Y micropatterns at 2000 cells per well in 10% serum medium. After 1 h 30 min of incubation at 37 °C under 5% CO2 to allow the cells to spread, the medium was exchanged with a medium containing 1% serum and the extract at the two concentrations. After 2 h of treatment at 37 °C under 5% CO2, cells were irradiated with light-emitting diodes (reference: Kingbright KA-3529AQB25Z4S) at 447 nm for 1 h at 20 J/cm2, corresponding to a dose of 1 month (28 days) of digital screen exposure at 10 cm distance.
A Hoechst solution was added for 15 min in each well to stain the nuclei. The medium was replaced to wash off the Hoechst solution, then the cells were incubated in a medium with the extract. Live imaging was performed with a Leica microscope (Wetzlar, Germany) to observe the mitochondrial network on the basis of MitoTracker Green dye. At the end of the live imaging, the cells were fixed, and F-actin (Thermo Fisher) was stained with phalloidin 555 (Thermo Fisher). Images were acquired on the Operetta HCS platform (Perkin Elmer, Waltham, MA, USA).
The mitochondrial network was analyzed according to the sum of the lengths of all filaments of a single-cell network to generate the network total length, which was averaged across all single cells from the same well. The mitochondrial network was divided into groups of continuously linked filaments: this basal unit was called a tree. The number of trees per network, as well as their total lengths, were averaged across all single cells detected in each well. Each tree was divided into branches, defined at each end by either a junction or an endpoint. These branches were characterized on the basis of the measurement of their average and maximum lengths in the whole network of each single cell (average branch length). Image analysis was used to detect single cells on the micropattern and measure their area and spread. Correctly spread cells with an area exceeding 1800 µm² were counted.
4.3. Immunostaining of Oxidized Proteins on Cyclized Skin Explants after Digital Stress
Human skin explants prepared from an abdominal plasty coming from a 35-year-old Caucasian woman were stored in a survival BEM culture medium (BIO-EC’s Explant Medium) at 37 °C under 5% CO2. The skin explants were exposed every day to a dose of 63.75 J/cm² of blue light (λmax = 455 nm), in 1 mL of HBSS medium, by using the Solarbox® device to mimic digital stress for 4 days, resulting in 4 iterations of blue light exposure and a 255 J/cm² cumulative dose of blue light. The unirradiated skin explants were kept in 1 mL HBSS in the dark for the entire blue-light exposure time. At the end of the exposure, the medium was exchanged for BEM medium for all explants (2 mL). The extract diluted in water at 0.002% (v/v) and 0.004% (v/v) was topically applied every day before blue light exposure, and the medium was then renewed. After 4 days of incubation, immediately after the last blue light irradiation, then the skin explants were collected and frozen at −80 °C. The oxidized proteins were stained on frozen sections after pre-incubation with 2.4-dinitrophenylhydrazine (DNPH, Millipore, Burlington, MA, USA 90448) and incubation with anti-DNP antibody (Millipore 90451), diluted at 1:250 in PBS (Gibco, Thermo Fisher) and 0.3% (w/v) BSA (Sigma-Aldrich, Saint-Louis, MI, USA) for 1 h at 37 °C, with a biotin/streptavidin amplifying system (Thermo Fisher). The results were visualized with VIP, a violet substrate of peroxidase (Vector SK-4600, Vector Laboratories, Burlingame, CA, USA). The immunostaining was assessed via microscopic observation and pictures were taken using a Leica DMLB or Olympus BX43 microscope. Pictures were digitized with a numeric DP72 Olympus camera utilizing Cell D storing software.
Oxidized protein staining intensity was quantified with two open-source optical imaging software programs. Pictures (in .jpeg format) were opened using the GIMP-GNU image manipulation program. The strong- to light-pink color signals corresponding to the staining were selected, copied, and pasted into a new image and were saved as a .jpeg file, consisting solely of masks with the selected staining. This image was then opened in the ImageJ program. An area of the surface of interest was selected (in this instance, the dermis). Then, a histogram of the section was created that separated the total number of pixels in the image into 255 color categories spanning the visible spectrum. The peak corresponding to the strong- to light-pink color was determined by cutting and summing the appropriate counts from each picture. Then, the numbers corresponding to the peak were pasted into an Excel spreadsheet and summed.
The color index was then calculated by dividing the sum count by the surface area. The measurements were then expressed as a percentage of the blue light-stressed control condition.
All explants used in this study were obtained from surgical residues after written informed consent was obtained from the donors. The use of surgical waste did not require any prior authorization by an ethics committee.
4.4. Evaluation of Melatonin Release in a Cyclized Co-Culture of Sensory Neurons and Keratinocytes Exposed to Digital Stress
Sensory neurons derived from hiPS (human induced Pluripotent Stem cells) were seeded on six-well plates coated with Matrigel® (Corning 354277, Boulogne-Billancourt, France, batch 72005017) at 250,000 cells per well with a differentiation medium. The differentiation medium consisted of DMEM-F12 (Panbiotech P04-41450, Aidenbach, Germany, batch 2730618) supplemented with 10% Knockout Serum Replacement (KSR)( Life Technologies, Thermo Fisher, 10828028, batch 1896527), 0.1 μM of retinoic acid (Sigma-Aldrich, St. Louis, MO, USA, R4643, batch SLBF3638V), a cocktail of central differentiation pathway inhibitors and 1% penicillin-streptomycin antibiotics (PS, Panbiotech P06-07100, batch 7631018). The cells were incubated for 6 days at 37 °C under 5% CO2, and the medium was changed every other day. The cells were then collected and seeded on 24-well plates coated with Matrigel® at 100,000 cells per well in a differentiation medium. The cells were incubated for 3 more days at 37 °C under 5% CO2 in a differentiation medium. Then the medium was replaced by a maturation medium for sensory neurons, which was composed of DMEM-F12 supplemented with 1% N2 (Life Technologies 11520536, batch 2004543), BDNF at 10 ng/mL (PanBiotech CB-1115002, batch 051861), GDNF at 10 ng/mL (PeproTech, Thermo Fisher, 450-10, batch H170806), NT3 at 10 ng/mL (PeproTech 450-03, batch H171010), NGF at 10 ng/mL (Sigma N1408. batch SLBW7063) and 1% PS antibiotics. Cells were maintained in culture at 37 °C under 5% CO2. The culture medium was changed every other day. After 5 days of incubation, normal human epidermal keratinocytes were added to the plates above the differentiated hiPS cell layer and were seeded at 30,000 cells per well in culture medium consisting of 2:3 of medium for sensory neurons and 1:3 growth medium for keratinocytes (Lonza, Bâle, Switzerland, 192152, batch 723883). Cells were maintained in culture for 3 days at 37 °C under 5% CO2. The culture medium was changed every other day.
A cyclization protocol based on the use of glutamate and an increase in temperature (day phase) was then followed. In addition, two shocks with medium containing 50% FCS were performed for 2 h to synchronize the cell cycles [
29,
30,
31,
32,
33]. The cultures were subsequently subjected for 8 h per day to conditions mimicking a day phase (glutamate 10 nM and temperature of 39.5 °C). The untreated culture with no cyclization was left in the dark at 37 °C. After 3 days of cyclization, the
Gardenia jasminoides fruit extract at 0.004% (
v/
v) was added during the day phase. On the same day, 30 min before the night phase, the cells were exposed to blue light (λ = 450 nm; 20 mJ/cm²) to mimic exposure to digital stress. The extract was subsequently incubated again with each change in medium. After 24 h and 48 h, the culture supernatants were collected 30 min before the night phase, then 2 h, 5 h, and 8 h after the shift to the night phase, and were stored at −80 °C. At the end of the culture, the cells were washed once with PBS, and an MTT test was performed to validate cell viability.
ELISA was performed to determine the amount of melatonin released in the cell medium collected (ABIN511419, BlueGene, Shanghai, China).
4.5. In Vitro Study of Conversion of Crocin to Crocetin by the Skin Microbiota
4.5.1. Microbiota Collection and Culture
Seven volunteers (three Caucasian women and four Caucasian men) showing no symptoms of skin disease were subjected to skin microbiota sampling on five body parts (forehead, cheek, nose, neck, and forearm). Ethics approval had been obtained and the study was approved by the Comité de Protection des Personnes (CPP), No. 2022-A02047-36, and No. SI 22.04050.000139.
Samples of skin microbiota were collected by non-invasive swabbing with sheets of 5 × 5 cm sterile gauze that had previously been soaked in 3 mL of 0.8% sodium chloride. All suspensions obtained by centrifugation of the impregnated gauze were pooled, filtered through a 40 µm filter, and centrifuged to remove large skin sample materials. The optical density at 600 nm (1 cm) was measured, and a value of 12.9 was obtained.
A 50 g·L−1 solution of Gardenia jasminoides fruit extract (batch 01A047-1EAA8869; Naturex, Avignon, France) was prepared in demineralized water, then filter-sterilized through a 0.22 µm filter. Its crocetin content (molar mass 976.97 g.mole−1) was 35% (molar content 358 mmoles.kg−1).
Flasks containing an appropriate culture medium (starch 10 g·L−1, yeast extract 1 g·L−1, meat extract 1 g·L−1 and bacto tryptone 2 g·L−1, pH 5.22) and 10% Gardenia jasminoides fruit extract (assay) or no extract (growth control) were inoculated with a volume of microbial suspension corresponding to 3% of the growth medium volume. A chemical stability control was also performed (with a non-inoculated mixture of culture medium and Gardenia jasminoides fruit extract).
The flasks were incubated for several days in a shaker incubator (30 °C, 115 rpm). The optical density was regularly measured at 600 nm. The carotenoid content was regularly determined via HPLC-UV analysis (cell removal by centrifugation; two-times dilution with 0.5 N sulfuric acid for stabilization and, finally, appropriate dilution with a 75% aqueous solution of DMSO).
4.5.2. Stability and Crocin Conversion Study; HPLC Analysis
Chemical, Reagents, and Plant Materials
Trans-crocetin (purity 99.8% by HPLC; molar mass, 328.41 g·mole−1) was supplied by MedChemExpress (Sollentuna, Sweden). HPLC grade solvents and other reagents of analytical grade were from Merck KGaA.
Preparation of Standard Solutions
Trans-crocetin was solubilized in a mixture of 7.5 mL of DMSO and 2.5 mL of demineralized water, whereas the Gardenia jasminoides fruit extract solution was obtained with a DMSO, methanol, and water mixture (20/20/60, respectively).
Preparation of Sample Solutions
Acid-stabilized and conveniently diluted conversion samples were directly used for the HPLC-UV-MS analysis.
HPLC Instrumentation and Analysis
UltiMate 3000® system from Thermo Scientific (Courtaboeuf, France) and an X-Bridge C18 column (150 mm × 4.6 mm; particle size, 5 µm; Waters, Guyancourt, France) with a standard guard cartridge for X-Bridge C18.
The elution gradient profile used for HPLC-UV and MS was as follows:
Mobile phase: solvent A, ultrapure water; solvent B, methanol;
Gradient: time, minutes/% A, 0/70, 20/20, 25/20, 26/70, and 30/70.
The injection volume was 10 µL. The column was equilibrated at 40 °C and the mobile phase flow rate was maintained at 1.0 mL.min−1.
Molecules were detected using an UltiMate 3000® diode array detector with the wavelength set at 440 nm. Data were analyzed using Chromeleon software (Thermo Scientific; version 7.2).
Mass Spectrometric Conditions
An ISQ™EC Single Quadrupole Mass Spectrometer from Thermo Scientific was connected to the previously described UltiMate 3000® system. Full-scan mass spectra were measured for mass-to-charge ratios (m/z) between 100 and 1000 in negative ion mode.
4.6. Molecular Docking Study of Crocin and Crocetin to the Melatonin Receptor MT1R
The crystallographic structures of crocin, crocetin, and melatonin were retrieved from the Protein Data Bank (
www.rcsb.org, accessed on 21 November 2019). They were crystallized with four different agonists. The four models were derived for the docking studies after structure clean-up, consisting of the removal of solvent molecules and cations and the addition of hydrogen atoms, along with structure minimization. For each model, three sets of parameters for the docking (with increasing precision) were studied—Screen, Geom, and GeomX—corresponding to screening, accuracy, and exhaustive accuracy parameters. The software started with more conformations and a more accurate and detailed conformation search for Geom and GeomX. The best model was required to reproduce the experimental poses of the four reference agonists, and their docking score range was required to be consistent with their Ki ranges.
4.7. Analysis of Number of Crow’s Feet Wrinkles in Volunteers Exposed to Digital Stress
4.7.1. INCI Formula
AQUA/WATER, CETYL ALCOHOL, GLYCERYL STEARATE, PEG-75 STERATE, CETEH-20, STEARETH-20, ISODECYL NEOPENTANOATE, ±GLYCERIN (and) BETAINE (and) WATER (and) GARDENIA JASMINOIDES FRUIT EXTRACT (and) MALTODEXTRIN, PHENOXYETHANOL, METHYL PARABEN, PROPYL PARABEN, ETHYL PARABEN, DIMETHICONE, FRAGRANCE, BENZYL SALICYLATE, LINALOOL, and D-LIMONENE.
4.7.2. Study Design
A double-blind, inter-individual, and placebo-controlled clinical evaluation was performed on female volunteers, comprising two groups of 20 participants between 18 and 50 years of age. The volunteers met the inclusion and exclusion criteria, including having wrinkles on their faces and being in front of a screen (digital devices) for at least 4 h per day, including 2 consecutive hours during the evening at 100% of the digital devices’ luminosity. They were informed of the possible adverse effects of using the product and the technical conditions in which the assessment was performed. They willingly signed an informed consent form written in compliance with the Declaration of Helsinki and the act of 20 December 1988 of the Code de la Santé Publique.
One group of 20 volunteers tested the placebo cream, and a second group of 20 volunteers tested the cream containing 2% (v/v) of the extract. The treatment was applied twice daily for 56 days.
The anti-aging properties of the product were analyzed through the quantification of wrinkle numbers, using a VISIA® 6th generation system. Digital photographs of the face were performed on days 0, 28, and 56. The control of the repositioning occurred directly on the data-processing screen, using an overlay visualization of the images at each time of acquisition. VISIA® allows pictures to be taken with different types of illumination and very rapid image capture. A series of photos taken under multi-spectral imaging and analysis allowed for the capture of visual information affecting the appearance of the skin.
In this study, we analyzed crow’s feet wrinkles after 56 days of product application.
4.8. Statistical Analysis
All results are presented as mean ± standard error of the means of three independent triplicates. A Shapiro–Wilk test was performed to verify the normal distribution of data, following the Gaussian law. In a case where normality was passed, confirming parametric data, the mean values were compared with either an unpaired t-test (for two or fewer groups) or a one-way ANOVA, followed by a post hoc test (for more than two groups). In the case of non-parametric data, either a Kruskal–Wallis test, followed by a Mann–Whitney U test, was used for unpaired data, or a Wilcoxon test was used for non-parametric-paired data.
In all cases, results were considered significant, where p < 0.1 with #, p < 0.05 with *, p < 0.01 with ** and p < 0.001 with ***.