Dental Pulp Stem Cell-Derived Exosomes Alleviate Mice Knee Osteoarthritis by Inhibiting TRPV4-Mediated Osteoclast Activation
Abstract
1. Introduction
2. Results
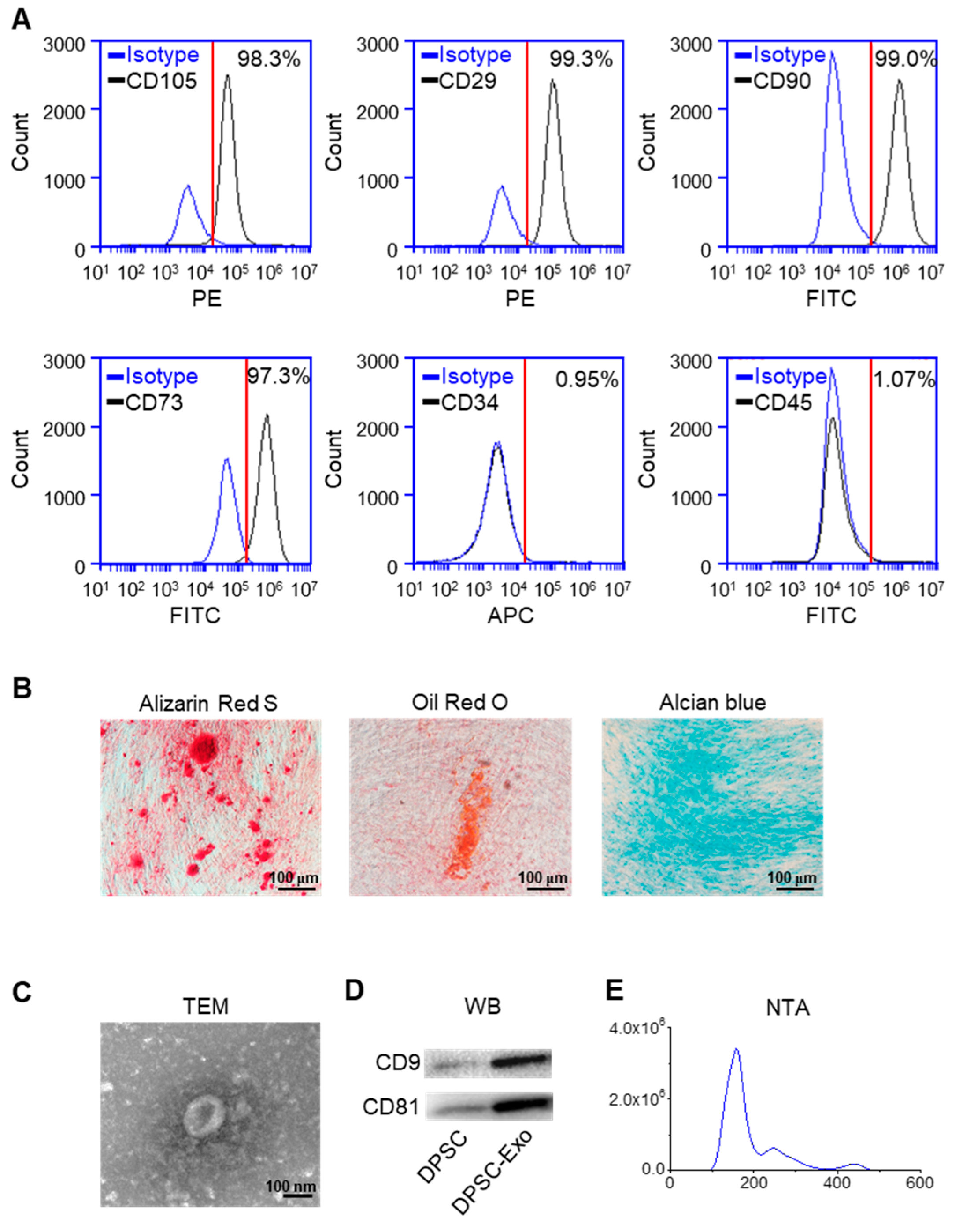
2.1. Characterization of DPSC-Derived Exosomes
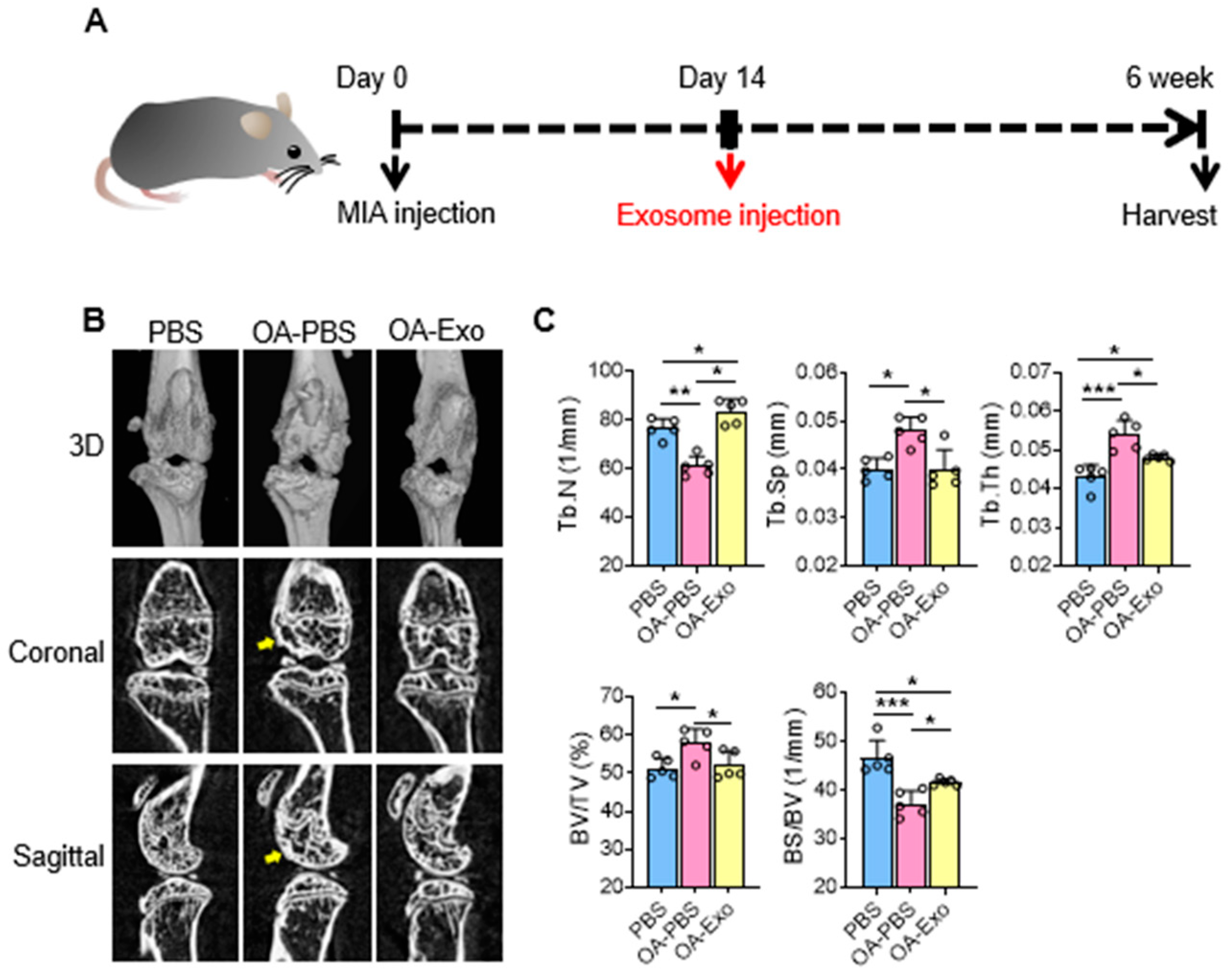
2.2. DPSC-Exosomes Alleviated Abnormal Subchondral Bone Remodeling of Knee OA
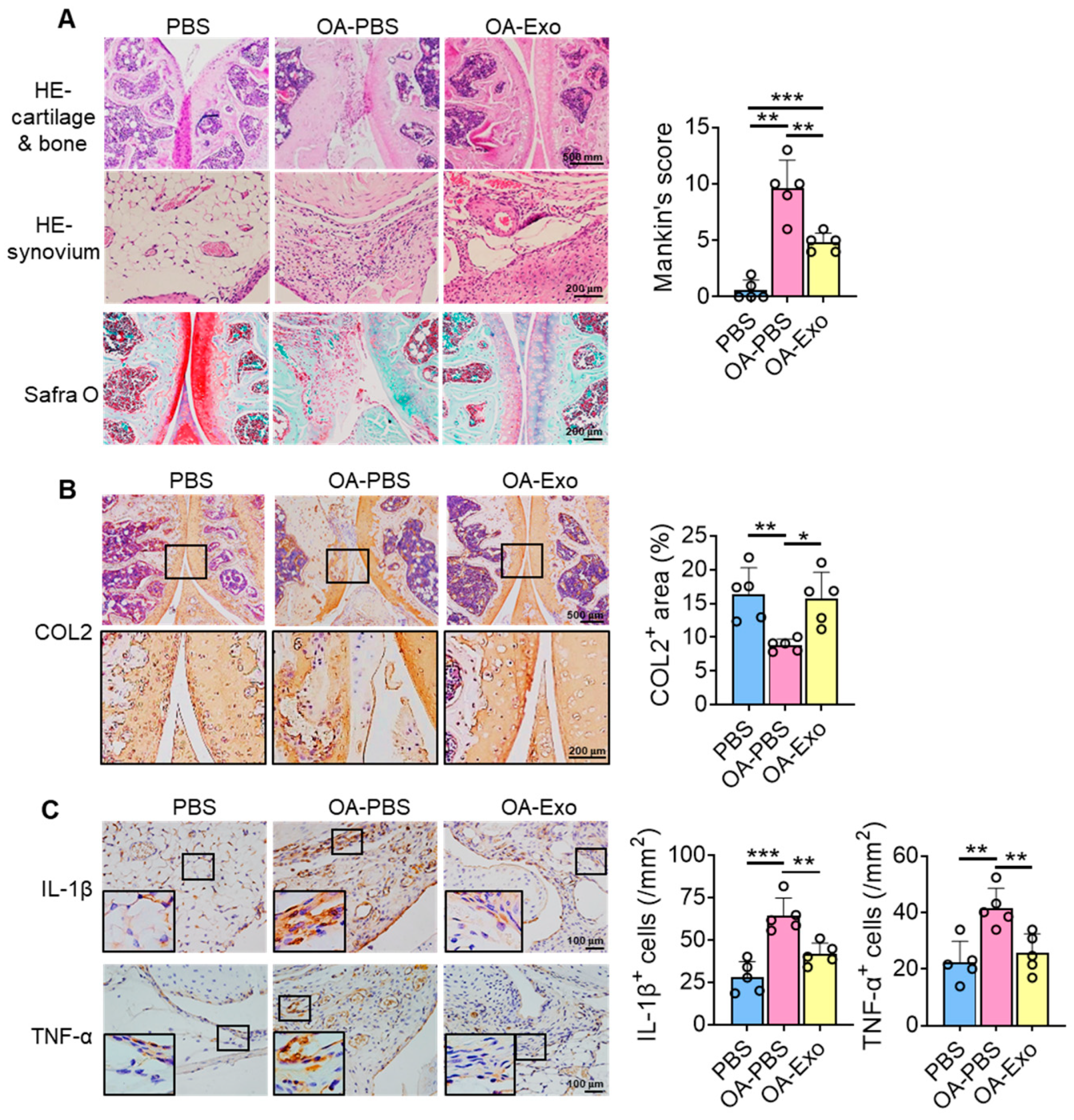
2.3. DPSC-Exosomes Improved Cartilage Degradation and Synovial Inflammation of Knee OA
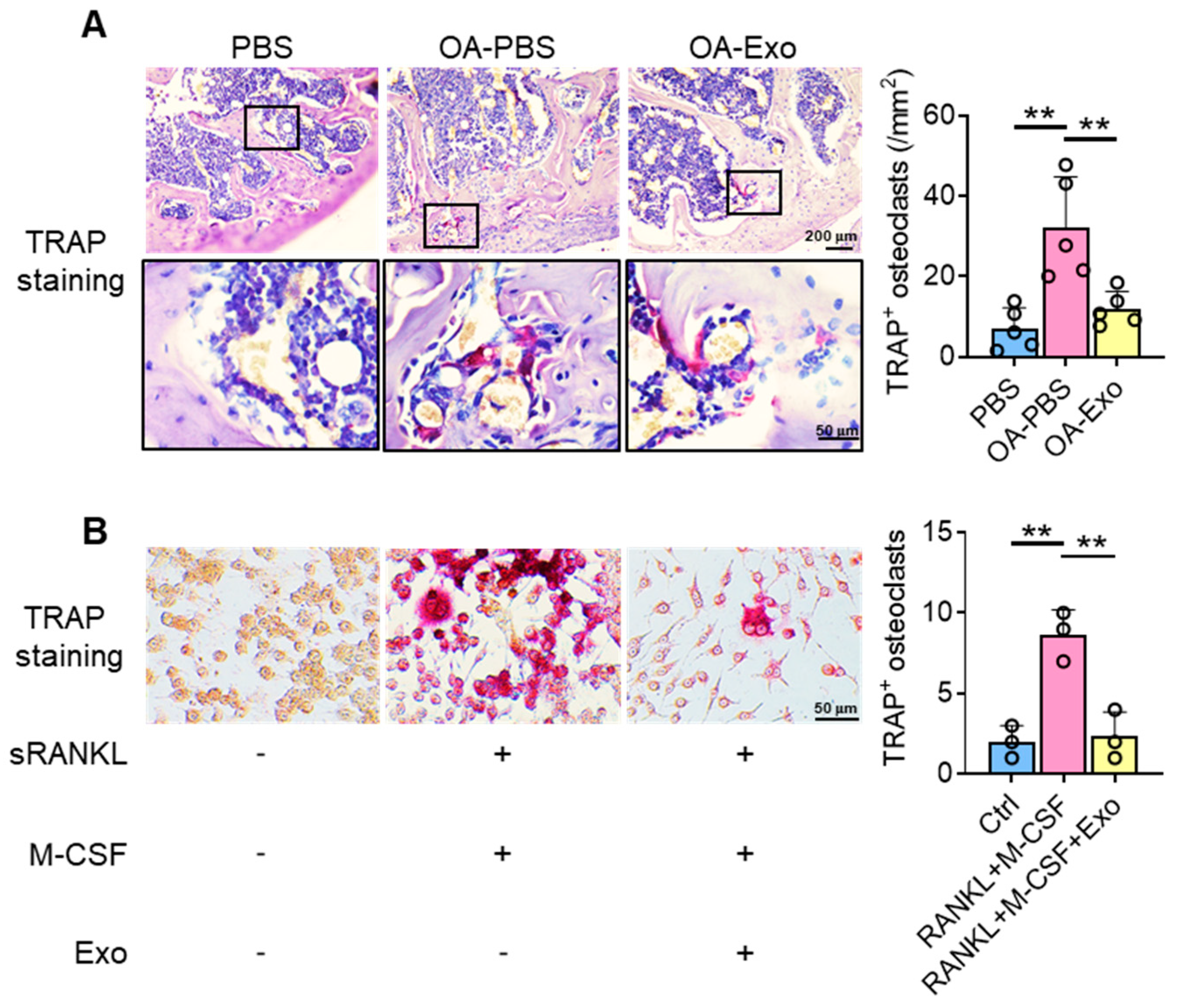
2.4. DPSC-Exosomes Inhibited the Activation of Osteoclasts
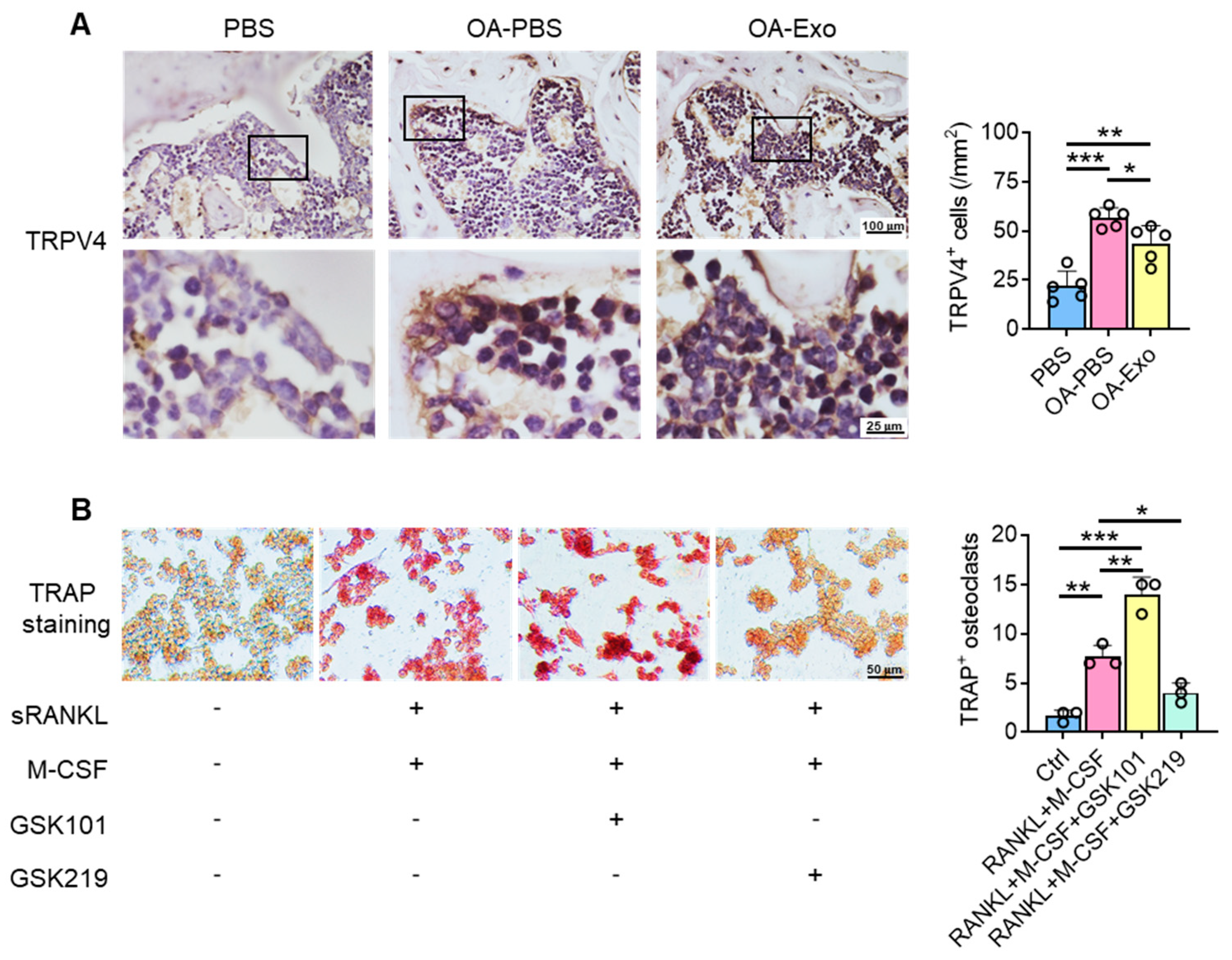
2.5. DPSC-Exosomes Regulated the Activation of Osteoclasts via TRPV4
3. Discussion
4. Materials and Methods
4.1. Isolation, Culture, and Identification of Human DPSCs
4.2. Isolation of DPSC-Derived Exosomes
4.3. Characterization of DPSC-Derived Exosomes
4.4. Knee Osteoarthritis Model
4.5. Micro-CT Examination
4.6. Tissue Preparation and Histological Staining
4.7. Immunohistochemistry (IHC) Staining
4.8. Cell Culture and Stimulation
4.9. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noel, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017, 7, 16214. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, S.; Chen, Q.; Xie, X. The Prevalence of Symptomatic Knee Osteoarthritis in Relation to Age, Sex, Area, Region, and Body Mass Index in China: A Systematic Review and Meta-Analysis. Front. Med. 2020, 7, 304. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Blanco, F.J.; Englund, M.; Karsdal, M.A.; Lohmander, L.S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Wen, C.; Jia, X.; Li, Y.; Crane, J.L.; Mears, S.C.; Askin, F.B.; Frassica, F.J.; Chang, W.; Yao, J.; et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013, 19, 704–712. [Google Scholar] [CrossRef]
- Zeng, C.Y.; Zhang, Z.R.; Tang, Z.M.; Hua, F.Z. Benefits and Mechanisms of Exercise Training for Knee Osteoarthritis. Front. Physiol. 2021, 12, 794062. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T., Jr. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 77–90. [Google Scholar] [CrossRef]
- Charlesworth, J.; Fitzpatrick, J.; Perera, N.K.P.; Orchard, J. Osteoarthritis- a systematic review of long-term safety implications for osteoarthritis of the knee. BMC Musculoskelet. Disord. 2019, 20, 151. [Google Scholar] [CrossRef]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef]
- Xiang, X.N.; Zhu, S.Y.; He, H.C.; Yu, X.; Xu, Y.; He, C.Q. Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Res. Ther. 2022, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Delle Monache, S.; Pulcini, F.; Santilli, F.; Martellucci, S.; Santacroce, C.; Fabrizi, J.; Angelucci, A.; Sorice, M.; Mattei, V. Hypoxia Induces DPSC Differentiation versus a Neurogenic Phenotype by the Paracrine Mechanism. Biomedicines 2022, 10, 1056. [Google Scholar] [CrossRef]
- Mattei, V.; Martellucci, S.; Pulcini, F.; Santilli, F.; Sorice, M.; Delle Monache, S. Regenerative Potential of DPSCs and Revascularization: Direct, Paracrine or Autocrine Effect? Stem Cell Rev. Rep. 2021, 17, 1635–1646. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Y.; Sun, X.; Xing, Y.; Wang, X.; Yang, Q. Immunomodulation of MSCs and MSC-Derived Extracellular Vesicles in Osteoarthritis. Front. Bioeng. Biotechnol. 2020, 8, 575057. [Google Scholar] [CrossRef]
- Al Madhoun, A.; Sindhu, S.; Haddad, D.; Atari, M.; Ahmad, R.; Al-Mulla, F. Dental Pulp Stem Cells Derived From Adult Human Third Molar Tooth: A Brief Review. Front. Cell Dev. Biol. 2021, 9, 717624. [Google Scholar] [CrossRef]
- Masuda, K.; Han, X.; Kato, H.; Sato, H.; Zhang, Y.; Sun, X.; Hirofuji, Y.; Yamaza, H.; Yamada, A.; Fukumoto, S. Dental Pulp-Derived Mesenchymal Stem Cells for Modeling Genetic Disorders. Int. J. Mol. Sci. 2021, 22, 2269. [Google Scholar] [CrossRef]
- Denadai-Souza, A.; Martin, L.; de Paula, M.A.; de Avellar, M.C.; Muscara, M.N.; Vergnolle, N.; Cenac, N. Role of transient receptor potential vanilloid 4 in rat joint inflammation. Arthritis Rheum. 2012, 64, 1848–1858. [Google Scholar] [CrossRef]
- Strotmann, R.; Harteneck, C.; Nunnenmacher, K.; Schultz, G.; Plant, T.D. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2000, 2, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, R.; Mizuno, A.; Komori, H.; Kajiya, H.; Uekawa, A.; Kitaura, H.; Okabe, K.; Ohyama, K.; Komori, T. Calcium/calmodulin-signaling supports TRPV4 activation in osteoclasts and regulates bone mass. J. Bone Miner. Res. 2012, 27, 1708–1721. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Dai, X.; Wang, W. Knockdown of TRPV4 suppresses osteoclast differentiation and osteoporosis by inhibiting autophagy through Ca(2+) -calcineurin-NFATc1 pathway. J. Cell Physiol. 2019, 234, 6831–6841. [Google Scholar] [CrossRef]
- Delle Monache, S.; Martellucci, S.; Clementi, L.; Pulcini, F.; Santilli, F.; Mei, C.; Piccoli, L.; Angelucci, A.; Mattei, V. In Vitro Conditioning Determines the Capacity of Dental Pulp Stem Cells to Function as Pericyte-Like Cells. Stem Cells Dev. 2019, 28, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Matas, J.; Orrego, M.; Amenabar, D.; Infante, C.; Tapia-Limonchi, R.; Cadiz, M.I.; Alcayaga-Miranda, F.; Gonzalez, P.L.; Muse, E.; Khoury, M.; et al. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl. Med. 2019, 8, 215–224. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, H.J.; Kim, K.I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef]
- Pigott, J.H.; Ishihara, A.; Wellman, M.L.; Russell, D.S.; Bertone, A.L. Inflammatory effects of autologous, genetically modified autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet. Comp. Orthop. Traumatol. 2013, 26, 453–460. [Google Scholar] [CrossRef]
- Cui, S.J.; Zhang, T.; Fu, Y.; Liu, Y.; Gan, Y.H.; Zhou, Y.H.; Yang, R.L.; Wang, X.D. DPSCs Attenuate Experimental Progressive TMJ Arthritis by Inhibiting the STAT1 Pathway. J. Dent. Res. 2020, 99, 446–455. [Google Scholar] [CrossRef]
- Xie, Q.X.; Liu, R.; Jiang, J.; Peng, J.; Yang, C.Y.; Zhang, W.; Wang, S.; Song, J. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res. Ther. 2020, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.H.; Paccou, J.; Ward, K.A.; Jameson, K.A.; Moss, C.; Woolston, J.; Javaid, M.K.; Cooper, C.; Dennison, E.M. The relationship of bone properties using high resolution peripheral quantitative computed tomography to radiographic components of hip osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Burchard, R.; Huflage, H.; Soost, C.; Richter, O.; Bouillon, B.; Graw, J.A. Efficiency of platelet-rich plasma therapy in knee osteoarthritis does not depend on level of cartilage damage. J. Orthop. Surg. Res. 2019, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Uritani, D.; Koda, H.; Yasuura, Y.; Kusumoto, A. Factors associated with subjective knee joint stiffness in people with knee osteoarthritis: A systematic review. Int. J. Rheum. Dis. 2022, 1–12. [Google Scholar] [CrossRef]
- Hinata, M.; Imai, S.; Sanaki, T.; Tsuchida, J.; Yoshioka, T.; Higashino, K.; Yamamoto, M.; Imai, M.; Soga, M.; Horita, N.; et al. Sensitization of transient receptor potential vanilloid 4 and increasing its endogenous ligand 5,6-epoxyeicosatrienoic acid in rats with monoiodoacetate-induced osteoarthritis. Pain 2018, 159, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Lee, H.P.; Smeriglio, P.; Grandi, F.; Goodman, S.; Chaudhuri, O.; Bhutani, N. A dysfunctional TRPV4-GSK3beta pathway prevents osteoarthritic chondrocytes from sensing changes in extracellular matrix viscoelasticity. Nat. Biomed. Eng. 2021, 5, 1472–1484. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Meng, H.; Inamdar, S.; Das, B.; Gupta, H.; Wang, W.; Thompson, C.L.; Knight, M.M. Activation of TRPV4 by mechanical, osmotic or pharmaceutical stimulation is anti-inflammatory blocking IL-1beta mediated articular cartilage matrix destruction. Osteoarthr. Cartil. 2021, 29, 89–99. [Google Scholar] [CrossRef]
- Xu, B.; Xing, R.; Huang, Z.; Yin, S.; Li, X.; Zhang, L.; Ding, L.; Wang, P. Excessive mechanical stress induces chondrocyte apoptosis through TRPV4 in an anterior cruciate ligament-transected rat osteoarthritis model. Life Sci. 2019, 228, 158–166. [Google Scholar] [CrossRef]
- Clark, A.L.; Votta, B.J.; Kumar, S.; Liedtke, W.; Guilak, F. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: Age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum. 2010, 62, 2973–2983. [Google Scholar] [CrossRef]
- Dutta, B.; Goswami, R.; Rahaman, S.O. TRPV4 Plays a Role in Matrix Stiffness-Induced Macrophage Polarization. Front. Immunol. 2020, 11, 570195. [Google Scholar] [CrossRef]
- Ji, L.; Bao, L.; Gu, Z.; Zhou, Q.; Liang, Y.; Zheng, Y.; Xu, Y.; Zhang, X.; Feng, X. Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunol. Res. 2019, 67, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Eitan, E.; Zhang, S.; Witwer, K.W.; Mattson, M.P. Extracellular vesicle-depleted fetal bovine and human sera have reduced capacity to support cell growth. J. Extracell. Vesicles 2015, 4, 26373. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yu, T.; Kou, X.; Gao, X.; Chen, C.; Liu, D.; Zhou, Y.; Shi, S. Tet1 and Tet2 maintain mesenchymal stem cell homeostasis via demethylation of the P2rX7 promoter. Nat. Commun. 2018, 9, 2143. [Google Scholar] [CrossRef]
- He, B.H.; Christin, M.; Mouchbahani-Constance, S.; Davidova, A.; Sharif-Naeini, R. Mechanosensitive ion channels in articular nociceptors drive mechanical allodynia in osteoarthritis. Osteoarthr. Cartil. 2017, 25, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Gui, T.; Wei, Y.; Luo, L.; Li, J.; Zhong, L.; Yao, L.; Beier, F.; Nelson, C.L.; Tsourkas, A.; Liu, X.S.; et al. Activating EGFR Signaling Attenuates Osteoarthritis Development Following Loading Injury in Mice. J. Bone Miner. Res. 2022, 37, 2498–2511. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.S.; He, D.Q.; Wang, Y.; Zhang, T.; Yu, H.J.; Li, Z.X.; Zhu, L.S.; Zhou, Y.H.; Liu, Y. Mechanical force modulates periodontal ligament stem cell characteristics during bone remodelling via TRPV4. Cell Prolif. 2020, 53, e12912. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Cui, S.; Zhou, Y.; Qiu, L. Dental Pulp Stem Cell-Derived Exosomes Alleviate Mice Knee Osteoarthritis by Inhibiting TRPV4-Mediated Osteoclast Activation. Int. J. Mol. Sci. 2023, 24, 4926. https://doi.org/10.3390/ijms24054926
Fu Y, Cui S, Zhou Y, Qiu L. Dental Pulp Stem Cell-Derived Exosomes Alleviate Mice Knee Osteoarthritis by Inhibiting TRPV4-Mediated Osteoclast Activation. International Journal of Molecular Sciences. 2023; 24(5):4926. https://doi.org/10.3390/ijms24054926
Chicago/Turabian StyleFu, Yu, Shengjie Cui, Yanheng Zhou, and Lixin Qiu. 2023. "Dental Pulp Stem Cell-Derived Exosomes Alleviate Mice Knee Osteoarthritis by Inhibiting TRPV4-Mediated Osteoclast Activation" International Journal of Molecular Sciences 24, no. 5: 4926. https://doi.org/10.3390/ijms24054926
APA StyleFu, Y., Cui, S., Zhou, Y., & Qiu, L. (2023). Dental Pulp Stem Cell-Derived Exosomes Alleviate Mice Knee Osteoarthritis by Inhibiting TRPV4-Mediated Osteoclast Activation. International Journal of Molecular Sciences, 24(5), 4926. https://doi.org/10.3390/ijms24054926







