Citral Modulates MMP-2 and MMP-9 Activities on Healing of Gastric Ulcers Associated with High-Fat Diet-Induced Obesity
Abstract
1. Introduction
2. Results
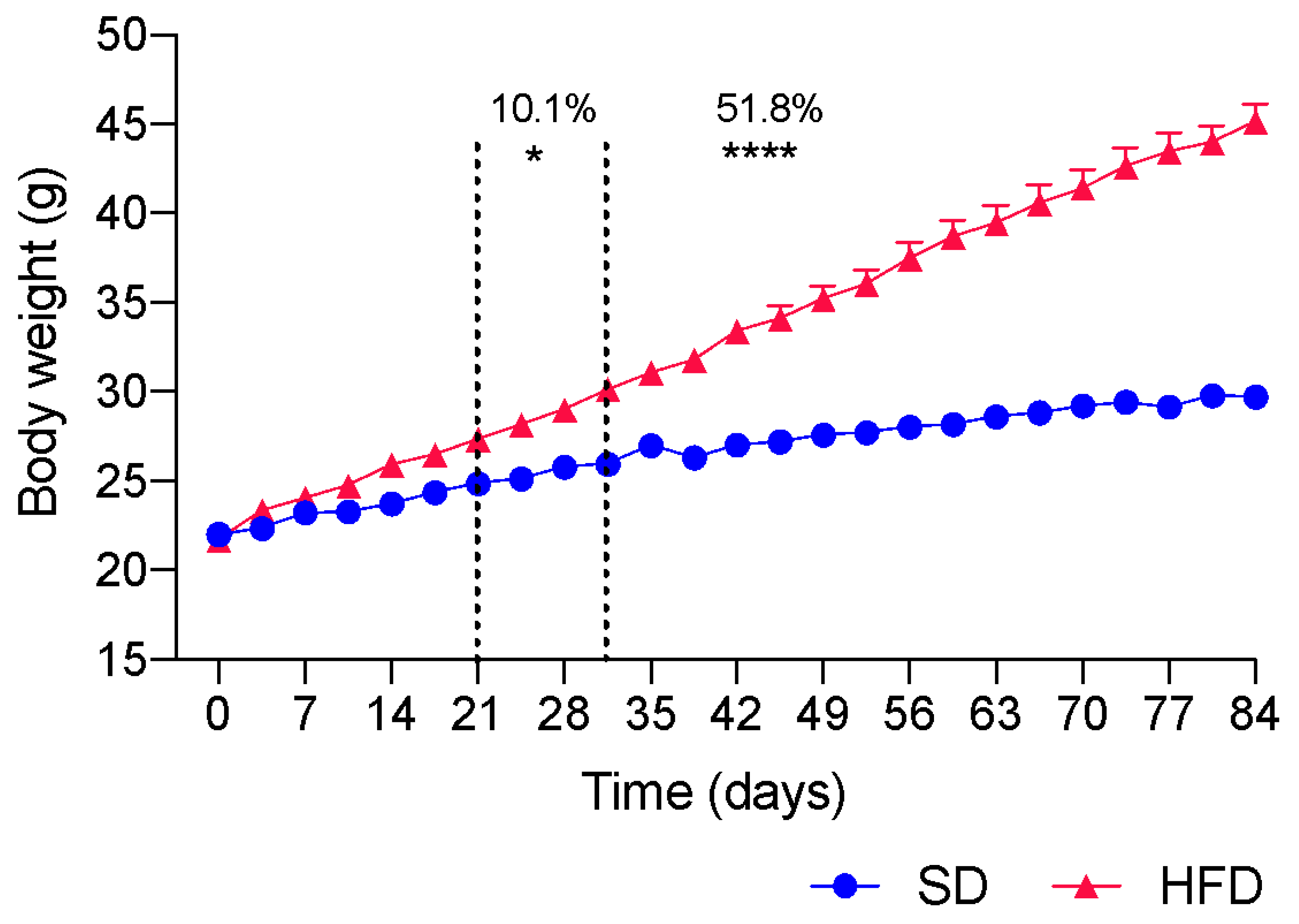
2.1. Induction of Obesity by HFD Ingestion
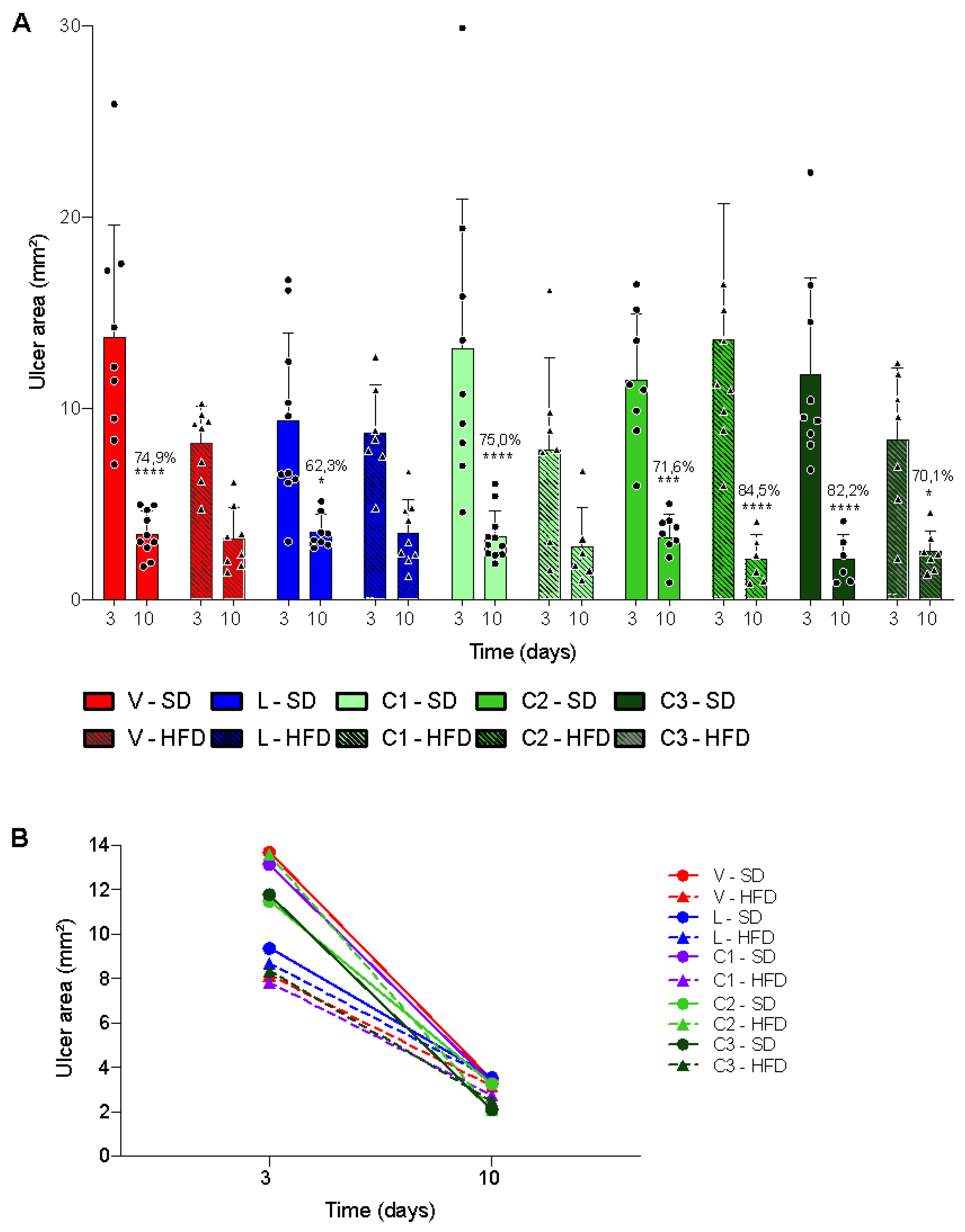
2.2. Acetic Acid-Induced Gastric Ulcer
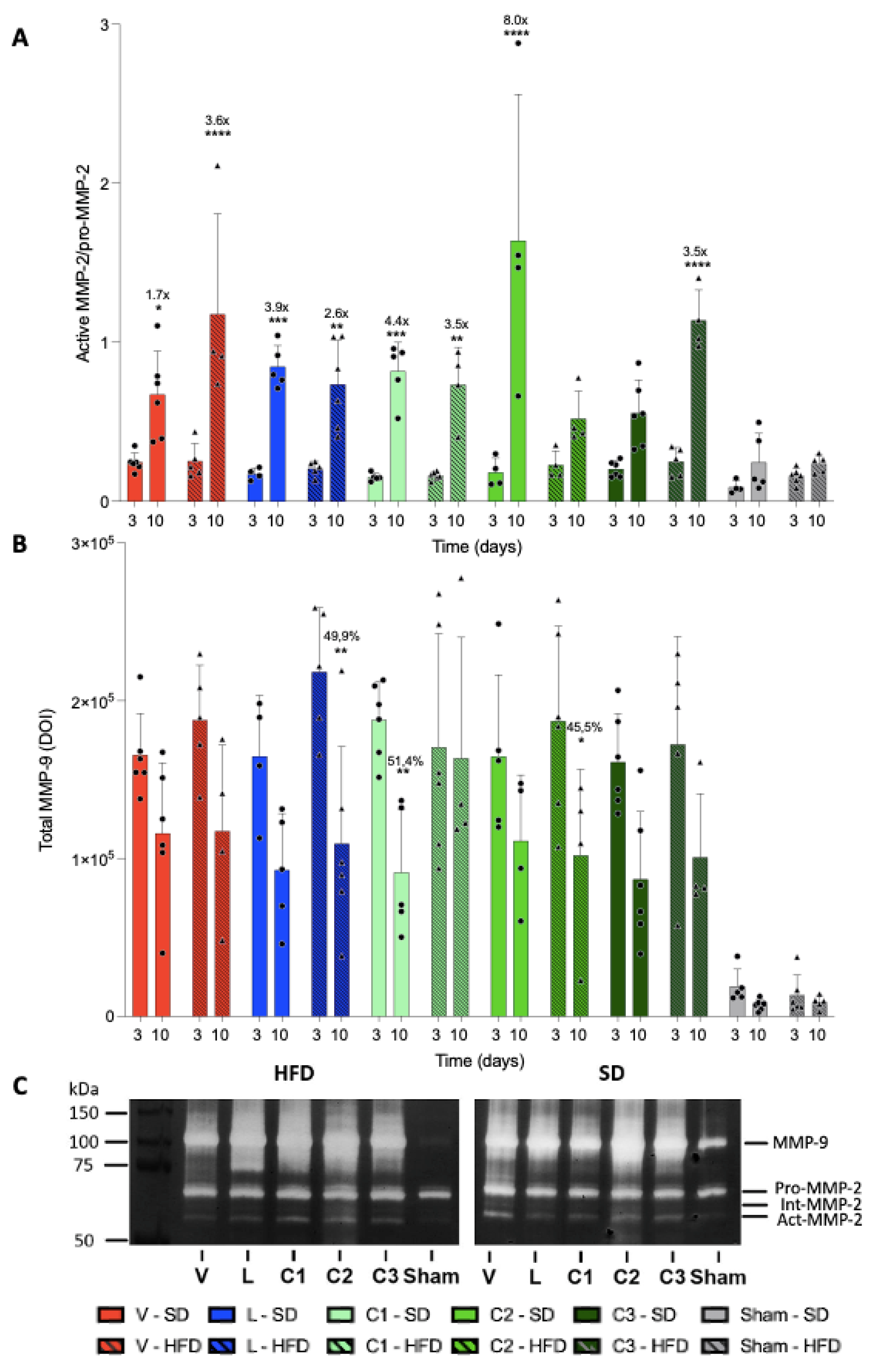
2.3. MMP Activity
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Obesity Induction
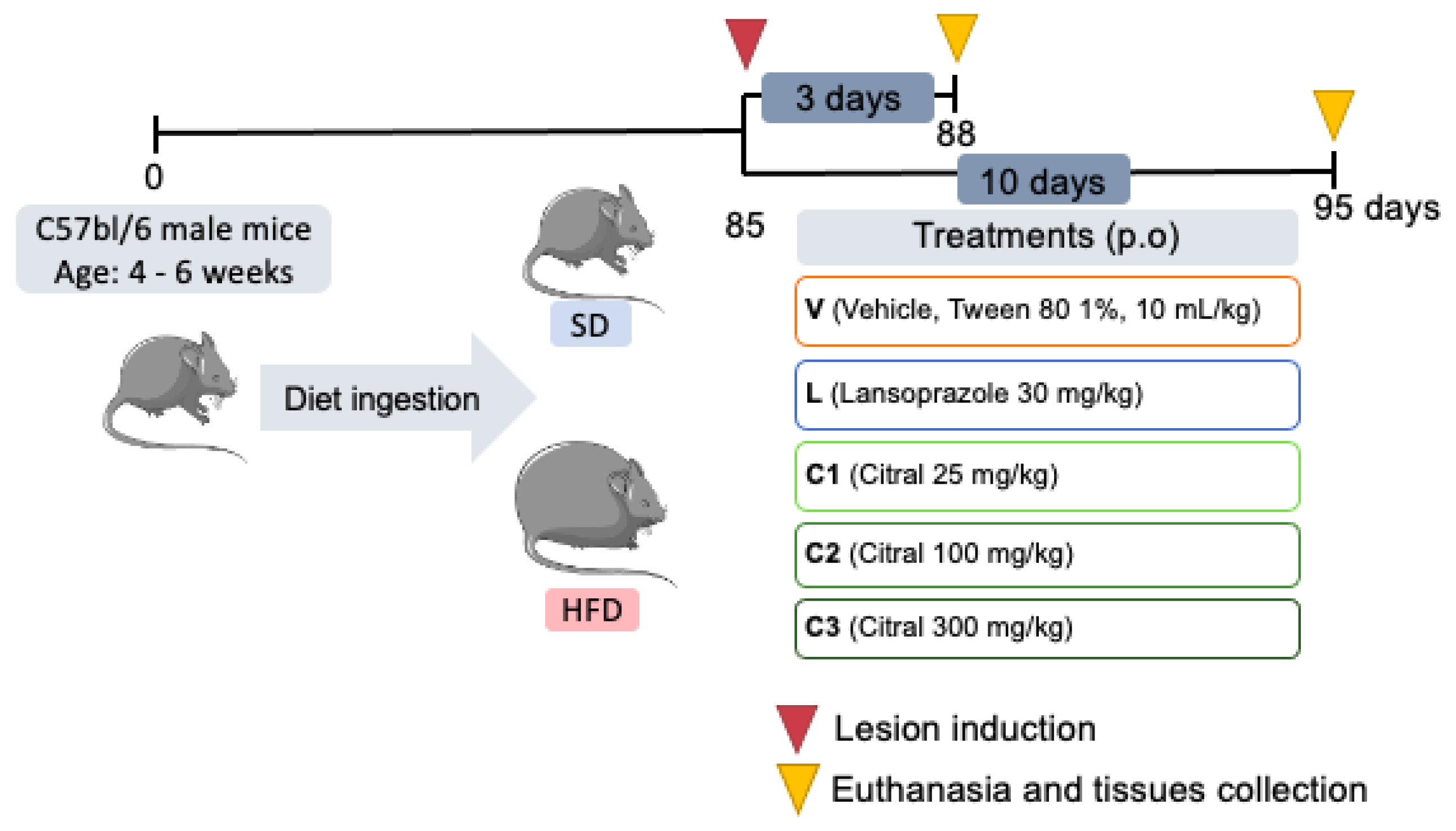
4.3. Experimental Design
4.4. Acetic Acid-Induced Gastric Ulcer
4.5. Biochemical Parameter
4.6. Macroscopic Analysis
4.7. Quantification of MMP-2 and MMP-9 by Zymography
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO|Overweight and Obesity. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 January 2022).
- Hall, K.D.; Guo, J. Obesity Energetics: Body Weight Regulation and the Effects of Diet Composition. Gastroenterology 2017, 152, 1718–1727.e3. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Jo, M.; Cho, E.; Ahn, S.; Kwon, Y.; Ko, G. The Roles and Associated Mechanisms of Adipokines in Development of Metabolic Syndrome. Molecules 2022, 27, 334. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Hariri, N.; Thibault, L. High-Fat Diet-Induced Obesity in Animal Models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Sampath, C.; Wilus, D.; Tabatabai, M.; Freeman, M.L.; Gangula, P.R. Mechanistic Role of Antioxidants in Rescuing Delayed Gastric Emptying in High Fat Diet Induced Diabetic Female Mice. Biomed. Pharmacother. 2021, 137, 111370. [Google Scholar] [CrossRef] [PubMed]
- Sogabe, M.; Okahisa, T.; Kimura, T.; Okamoto, K.; Miyamoto, H.; Muguruma, N.; Takayama, T. Influence of Metabolic Syndrome on Upper Gastrointestinal Disease. Clin. J. Gastroenterol. 2016, 9, 191–202. [Google Scholar] [CrossRef]
- Fukuhara, H.; Kadowaki, Y.; Ose, T.; Monowar, A.; Imaoka, H.; Ishihara, S.; Takasawa, S.; Kinoshita, Y. In Vivo Evidence for the Role of RegI in Gastric Regeneration: Transgenic Overexpression of RegI Accelerates the Healing of Experimental Gastric Ulcers. Lab. Investig. 2010, 90, 556–565. [Google Scholar] [CrossRef]
- Li, Z.; Chen, H.; Chen, T. Genetic Liability to Obesity and Peptic Ulcer Disease: A Mendelian Randomization Study. BMC Med. Genom. 2022, 15, 209. [Google Scholar] [CrossRef]
- Anderson, K.; Hamm, R.L. Factors That Impair Wound Healing. J. Am. Coll. Clin. Wound Spec. 2014, 4, 84–91. [Google Scholar] [CrossRef]
- Takeuchi, K.; Amagase, K. Roles of Cyclooxygenase, Prostaglandin E2 and EP Receptors in Mucosal Protection and Ulcer Healing in the Gastrointestinal Tract. Curr. Pharm. Des. 2018, 24, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, J.J.; Patterson, S. Peptic Ulcer Disease. Gastroenterol. Nurs. 2019, 42, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Tarnawski, A.S.; Ahluwalia, A. The Critical Role of Growth Factors in Gastric Ulcer Healing: The Cellular and Molecular Mechanisms and Potential Clinical Implications. Cells 2021, 10, 1964. [Google Scholar] [CrossRef] [PubMed]
- Fossmark, R.; Martinsen, T.C.; Waldum, H.L. Adverse Effects of Proton Pump Inhibitors—Evidence and Plausibility. Int. J. Mol. Sci. 2019, 20, 5203. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Fu, R.; Liu, Y.; Wang, P.; Ma, P.; Zhu, C.; Fan, D. Human-like Collagen Promotes the Healing of Acetic Acid-Induced Gastric Ulcers in Rats by Regulating NOS and Growth Factors. Food Funct. 2020, 11, 4123–4137. [Google Scholar] [CrossRef] [PubMed]
- Kangwan, N. Quality of Healing of Gastric Ulcers: Natural Products beyond Acid Suppression. World J. Gastrointest. Pathophysiol. 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Barchuk, M.; Miksztowicz, V. Behavior of Metalloproteinases in Adipose Tissue, Liver and Arterial Wall: An Update of Extracellular Matrix Remodeling. Cells 2019, 8, 158. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Do, L.D.; Hritz, B.G. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells 2020, 9, 1313. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Li, S.L.; Zhao, J.R.; Ren, X.Y.; Xie, J.P.; Ma, Q.Z.; Rong, Q.H. Increased Expression of Matrix Metalloproteinase-9 Associated with Gastric Ulcer Recurrence. World J. Gastroenterol. 2013, 19, 4590–4595. [Google Scholar] [CrossRef]
- Kanno, E.; Tanno, H.; Masaki, A.; Sasaki, A.; Sato, N.; Goto, M.; Shisai, M.; Yamaguchi, K.; Takagi, N.; Shoji, M.; et al. Defect of Interferon γ Leads to Impaired Wound Healing through Prolonged Neutrophilic Inflammatory Response and Enhanced MMP-2 Activation. Int. J. Mol. Sci. 2019, 20, 5657. [Google Scholar] [CrossRef]
- Derosa, G.; Ferrari, I.; D’Angelo, A.; Tinelli, C.; Salvadeo, S.A.T.; Ciccarelli, L.; Piccinni, M.N.; Gravina, A.; Ramondetti, F.; Maffioli, P.; et al. Matrix Metalloproteinase-2 and -9 Levels in Obese Patients. Endothel. J. Endothel. Cell Res. 2008, 15, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, H.; Wang, S.; Wang, W.; Chen, Q.; Ma, Y.; Zhang, Y.; Wang, T. Natural Products, an Important Resource for Discovery of Multitarget Drugs and Functional Food for Regulation of Hepatic Glucose Metabolism. Drug Des. Devel. Ther. 2018, 12, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Targets and Pathways Involved in the Antitumor Activity of Citral and Its Stereo-Isomers. Eur. J. Pharmacol. 2020, 871, 172945. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Behbahani, B.; Shahidi, F. Melissa Officinalis Essential Oil: Chemical Compositions, Antioxidant Potential, Total Phenolic Content and Antimicrobial Activity. Nutr. Food Sci. Res. 2019, 6, 17–25. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Remadevi, R.; Naebe, M. Lemongrass (Cymbopogon): A Review on Its Structure, Properties, Applications and Recent Developments. Cellulose 2018, 25, 5455–5477. [Google Scholar] [CrossRef]
- Lai, Y.-S.; Lee, W.-C.; Lin, Y.-E.; Ho, C.-T.; Lu, K.-H.; Lin, S.-H.; Panyod, S.; Chu, Y.-L.; Sheen, L.-Y. Ginger Essential Oil Ameliorates Hepatic Injury and Lipid Accumulation in High Fat Diet-Induced Nonalcoholic Fatty Liver Disease. J. Agric. Food Chem. 2016, 64, 2062–2071. [Google Scholar] [CrossRef]
- Emílio-Silva, M.T.; Rodrigues, V.P.; Bueno, G.; Ohara, R.; Martins, M.G.; Horta-Júnior, J.A.C.; Branco, L.G.S.; Rocha, L.R.M.; Hiruma-Lima, C.A. Hypothermic Effect of Acute Citral Treatment during LPS-Induced Systemic Inflammation in Obese Mice: Reduction of Serum TNF-α and Leptin Levels. Biomolecules 2020, 10, 1454. [Google Scholar] [CrossRef]
- Périco, L.L.; Emílio-Silva, M.T.; Ohara, R.; Rodrigues, V.P.; Bueno, G.; Barbosa-Filho, J.M.; da Rocha, L.R.M.; Batista, L.M.; Hiruma-Lima, C.A. Systematic Analysis of Monoterpenes: Advances and Challenges in the Treatment of Peptic Ulcer Diseases. Biomolecules 2020, 10, 265. [Google Scholar] [CrossRef]
- Nishijima, C.M.; Ganev, E.G.; Mazzardo-Martins, L.; Martins, D.F.; Rocha, L.R.M.; Santos, A.R.S.; Hiruma-Lima, C.A. Citral: A Monoterpene with Prophylactic and Therapeutic Anti-Nociceptive Effects in Experimental Models of Acute and Chronic Pain. Eur. J. Pharmacol. 2014, 736, 16–25. [Google Scholar] [CrossRef]
- Preguiça, I.; Alves, A.; Nunes, S.; Fernandes, R.; Gomes, P.; Viana, S.D.; Reis, F. Diet-Induced Rodent Models of Obesity-Related Metabolic Disorders—A Guide to a Translational Perspective. Obes. Rev. 2020, 21, e13081. [Google Scholar] [CrossRef]
- Boylan, M.R.; Khalili, H.; Huang, E.S.; Chan, A.T. Measures of Adiposity Are Associated with Increased Risk of Peptic Ulcer. Clin. Gastroenterol. Hepatol. 2014, 12, 1688–1694. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; De Angelis, M.H.; Schürmann, A.; et al. Animal Models of Obesity and Diabetes Mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Bueno, G.; Chavez Rico, S.L.; Périco, L.L.; Ohara, R.; Rodrigues, V.P.; Emílio-Silva, M.T.; Assunção, R.; Machado da Rocha, L.R.; Nunes, D.S.; Besten, M.A.; et al. The Essential Oil from Baccharis trimera (Less.) DC Improves Gastric Ulcer Healing in Rats through Modulation of VEGF and MMP-2 Activity. J. Ethnopharmacol. 2021, 271, 113832. [Google Scholar] [CrossRef]
- Magierowska, K.; Brzozowski, T.; Magierowski, M. Emerging Role of Carbon Monoxide in Regulation of Cellular Pathways and in the Maintenance of Gastric Mucosal Integrity. Pharmacol. Res. 2018, 129, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A Guide to the Composition and Functions of the Extracellular Matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Niederberger, E.; Parnham, M.J. The Impact of Diet and Exercise on Drug Responses. Int. J. Mol. Sci. 2021, 22, 7692. [Google Scholar] [CrossRef]
- AGREN, M.S. Gelatinase Activity during Wound Healing. Br. J. Dermatol. 1994, 131, 634–640. [Google Scholar] [CrossRef]
- Baragi, V.M.; Qiu, L.; Gunja-Smith, Z.; Woessner, J.F.; Lesch, C.A.; Guglietta, A. Role of Metalloproteinases in the Development and Healing of Acetic Acid-Induced Gastric Ulcer in Rats. Scand. J. Gastroenterol. 1997, 32, 419–426. [Google Scholar] [CrossRef]
- Lempinen, M.; Inkinen, K.; Wolff, H.; Ahonen, J. Matrix Metalloproteinases 2 and 9 in Indomethacin-Lnduced Rat Gastric Ulcer. Eur. Surg. Res. 2000, 32, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Mu, H.; Pang, Y.; Liu, L.; Li, F.; Wang, J. Citral Promotes the Cell Proliferation, Differentiation, and Calcium Mineralization in Human Osteoblast-like MG-63 Cells. Pharmacogn. Mag. 2021, 17, 250. [Google Scholar] [CrossRef]
- Ahmadi, S.; Farahpour, M.R.; Tabatabaei, Z.G. Fabrication, Characterization and Application of Novel Nanoemulsion Polyvinyl Alcohol/Chitosan Hybrid Incorporated with Citral for Healing of Infected Full-Thickness Wound. J. Drug Deliv. Sci. Technol. 2022, 74, 103589. [Google Scholar] [CrossRef]
- Gotardo, É.M.F.; dos Santos, A.N.; Miyashiro, R.A.; Gambero, S.; Rocha, T.; Ribeiro, M.L.; Gambero, A. Mice That Are Fed a High-Fat Diet Display Increased Hepcidin Expression in Adipose Tissue. J. Nutr. Sci. Vitaminol. 2013, 59, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Okabe, S.; Roth, J.L.A.; Pfeiffer, C.J. A Method for Experimental, Penetrating Gastric and Duodenal Ulcers in Rats—Observations on Normal Healing. Am. J. Dig. Dis. 1971, 16, 277–284. [Google Scholar] [CrossRef]
- Steffensen, B.; Häkkinen, L.; Larjava, H. Proteolytic Events of Wound-Healing—Coordinated Interactions among Matrix Metalloproteinases (MMPs), Integrins, and Extracellular Matrix Molecules. Crit. Rev. Oral Biol. Med. 2001, 12, 373–398. [Google Scholar] [CrossRef]



| SD | ||||||
|---|---|---|---|---|---|---|
| Vehicle 10 mL/kg | Lansoprazole 30 mg/kg | Citral 25 mg/kg | Citral 100 mg/kg | Citral 300 mg/kg | Sham | |
| Adiposity index (%) | 2.34 ± 0.09 a | 2.03 ± 0.16 a | 2.48 ± 0.33 a | 2.22 ± 0.19 a | 1.99 ± 0.20 a | 2.76 ± 0.15 a |
| Glucose (mg/dL) | 204.5 ± 12.85 | 203.2 ± 15.96 | 184.50 ± 5.69 | 205.66 ± 7.67 | 180.33 ± 15.93 | 167.4 ± 9.89 |
| Total cholesterol (mg/dL) | 136.7 ± 5.59 a | 133.00 ± 6.53 a | 140.84 ± 4.63 a | 135.17 ± 5.31 a | 128.00 ± 7.49 a | 138.80 ± 5.57 a |
| HDL (mg/dL) | 78.0 ± 5.08 a | 83.34 ± 3.92 a | 77.17 ± 3.14 a | 78.34 ± 3.28 a | 80.67 ± 3.04 a | 75.40 ±5.50 a |
| LDL (mg/dL) | 28.50 ± 8.17 | 22.84 ± 5.21 | 35.67 ± 6.07 | 34.34 ± 6.60 | 22.34 ± 4.78 | 35.20 ± 8.96 |
| Triglycerides (mg/dL) | 151.0 ± 16.95 | 134.00 ± 14.89 | 142.30 ± 20.12 | 112.00 ± 12.70 | 124.67 ± 17.13 | 142.40 ± 15.86 |
| HFD | ||||||
| Adiposity index (%) | 7.51 ± 0.62 b | 8.64 ± 0.35 b | 6.92 ± 0.92 b | 7.33 ± 0.52 b | 7.24± 0.66 b | 8.84 ± 0.47 b |
| Glucose (mg/dL) | 196.83 ± 30.10 | 280.30 ± 21.27 | 179.20 ± 42.91 | 205.20 ± 22.17 | 178.33 ± 22.23 | 242.40 ± 27.00 |
| Total cholesterol (mg/dL) | 184.00 ± 27.59 b | 221.80 ± 25.32 b | 156.20 ± 19.12 b | 150.30 ± 20.32 b | 185.70 ± 16.47 b | 202.80 ± 12.15 b |
| HDL (mg/dL) | 75.00 ± 5.30 b | 76.34 ± 4.64 b | 65.60 ± 11.42 b | 72.17 ± 3.12 b | 75.67 ± 3.56 b | 73.00 ± 2.55 b |
| LDL (mg/dL) | 87.67 ± 24.42 | 125.30 ± 23.55 | 75.00 ± 13.75 | 60.34 ± 17.13 | 91.50 ± 15.65 | 91.80 ± 23.52 |
| Triglycerides (mg/dL) | 107.00 ± 22.12 | 100.80 ± 15.37 | 78.00 ± 7.18 | 88.67 ± 10.80 | 92.34 ± 8.29 | 90.80 ± 4.77 |
| Component | g/kg | kcal/kg |
|---|---|---|
| Corn starch (q.s.) | 115.5 | 462 |
| Casein | 200 | 800 |
| Sucrose | 100 | 400 |
| Maltodextrin | 132 | 528 |
| Lard | 312 | 2808 |
| Soy oil | 40 | 360 |
| Cellulose | 50 | - |
| Mineral mix | 35 | - |
| Vitamin mix | 10 | - |
| L-Cystine | 3 | - |
| Choline | 2.5 | - |
| Total | 1000 | 5358 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohara, R.; Dario, F.L.; Emílio-Silva, M.T.; Assunção, R.; Rodrigues, V.P.; Bueno, G.; Raimundo, P.R.; da Rocha, L.R.M.; Hiruma-Lima, C.A. Citral Modulates MMP-2 and MMP-9 Activities on Healing of Gastric Ulcers Associated with High-Fat Diet-Induced Obesity. Int. J. Mol. Sci. 2023, 24, 4888. https://doi.org/10.3390/ijms24054888
Ohara R, Dario FL, Emílio-Silva MT, Assunção R, Rodrigues VP, Bueno G, Raimundo PR, da Rocha LRM, Hiruma-Lima CA. Citral Modulates MMP-2 and MMP-9 Activities on Healing of Gastric Ulcers Associated with High-Fat Diet-Induced Obesity. International Journal of Molecular Sciences. 2023; 24(5):4888. https://doi.org/10.3390/ijms24054888
Chicago/Turabian StyleOhara, Rie, Felipe Lima Dario, Maycon Tavares Emílio-Silva, Renata Assunção, Vinícius Peixoto Rodrigues, Gabriela Bueno, Priscila Romano Raimundo, Lúcia Regina Machado da Rocha, and Clelia Akiko Hiruma-Lima. 2023. "Citral Modulates MMP-2 and MMP-9 Activities on Healing of Gastric Ulcers Associated with High-Fat Diet-Induced Obesity" International Journal of Molecular Sciences 24, no. 5: 4888. https://doi.org/10.3390/ijms24054888
APA StyleOhara, R., Dario, F. L., Emílio-Silva, M. T., Assunção, R., Rodrigues, V. P., Bueno, G., Raimundo, P. R., da Rocha, L. R. M., & Hiruma-Lima, C. A. (2023). Citral Modulates MMP-2 and MMP-9 Activities on Healing of Gastric Ulcers Associated with High-Fat Diet-Induced Obesity. International Journal of Molecular Sciences, 24(5), 4888. https://doi.org/10.3390/ijms24054888







