Diamine Oxidase as a Therapeutic Enzyme: Study of Germination from Vegetal Sources and Investigation of the Presence of β-N-Oxalyl-L-α,β-diaminopropionic Acid (β-ODAP) Using LC-MS/MS
Abstract
1. Introduction
2. Results
2.1. vDAO Activity in Different Seed Varieties
| Seedlings | vDAO Activity (U/mL) | Total Volume (mL) | vDAO Total Activity (**U) | Protein Concentration (mg/mL) | vDAO Specific Activity (U/mg Protein) |
|---|---|---|---|---|---|
| Lathyrus sativus | 2.21 ± 0.09 | 45 | 99.45 ± 4.05 | 1.57 ± 0.47 | 1.41 ± 0.06 |
| CDC Amarillo | 1.31 ± 0.01 | 45 | 58.95 ± 0.45 | 1.71 ± 0.17 | 0.77 ± 0.01 |
| CDC Meadow | 0.82 ± 0.01 | 45 | 36.97 ± 0.61 | 1.30 ± 0.04 | 0.63 ± 0.01 |
| CDC Limerick | 0.75 ± 0.02 | 45 | 33.82 ± 0.99 | 1.84 ± 0.15 | 0.41 ± 0.02 |
| CDC Inca | 0.70 ± 0.01 | 45 | 31.52 ± 0.73 | 1.86 ± 0.21 | 0.38 ± 0.02 |
| * Yellow pea | 0.50 ± 0.03 | 45 | 22.50 ± 1.52 | 1.35 ± 0.09 | 0.36 ± 0.02 |
| CDC Dakota | 0.40 ± 0.01 | 45 | 18.40 ± 0.68 | 1.48 ± 0.08 | 0.27 ± 0.01 |
2.2. vDAO Activity during Germination of CDC Amarillo Seedlings
2.3. vDAO Activity in Seedling Organs
| DAYS | vDAO Activity (U/mL) | Total Volume (mL) | vDAO Total Activity (U) | Protein Concentration (mg/mL) | vDAO Specific Activity (U/mg Protein) |
|---|---|---|---|---|---|
| 1 | * NA | NA | NA | NA | NA |
| 2 | NA | NA | NA | NA | NA |
| 3 | NA | NA | NA | NA | NA |
| 4 | 0.44 ± 0.02 | 4.1 | 1.80 ± 0.08 | 2.37 ± 0.18 | 0.19 ± 0.01 |
| 5 | 0.59 ± 0.05 | 7 | 4.13 ± 0.35 | 2.74 ± 0.18 | 0.22 ± 0.02 |
| 6 | 0.68 ± 0.08 | 7 | 4.76 ± 0.56 | 1.29 ± 0.11 | 0.53 ± 0.06 |
| 7 | 0.84 ± 0.11 | 20 | 16.82 ± 2.2 | 1.39 ± 0.01 | 0.61 ± 0.07 |
| 8 | 1.08 ± 0.06 | 29 | 31.32 ± 1.74 | 1.34 ± 0.25 | 0.81 ± 0.18 |
| 9 | 0.91 ± 0.02 | 32 | 29.12 ± 0.64 | 1.52 ± 0.16 | 0.59 ± 0.04 |
| 10 | 0.66 ± 0.04 | 17 | 11.22 ± 0.68 | 1.41 ± 0.01 | 0.47 ± 0.02 |
| CDC Amarillo | vDAO Activity (U/mL) | Volume (mL) | vDAO Total Activity (U) | Protein Concentration (mg/mL) | vDAO Specific Activity (U/mg Protein) |
|---|---|---|---|---|---|
| Cotyledons | 0.44 ± 0.04 | 5 | 2.22 ± 0.24 | 13.54 ± 0.38 | 0.03 ± 0.01 |
| Roots | 0.12 ± 0.006 | 5 | 0.59 ± 0.01 | 1.78 ± 0.23 | 0.06 ± 0.01 |
| Shoots | 0.84 ± 0.05 | 5 | 4.21 ± 0.22 | 2.74 ± 0.09 | 0.30 ± 0.03 |
2.4. LC-MRM Method Development and β-ODAP in Seedling Extracts
2.5. β-ODAP Quantitation in DAO Extracts
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Germination of Seeds
4.3. vDAO Extraction
4.4. Spectrometric Assay of vDAO Activity
4.5. Dialysis of Raw Extracts
4.6. ODAP Calibration Curve Preparation and Validation of Quantitative Analysis
4.7. Sample Preparation for β-ODAP Analysis
4.8. LC-MRM Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.D.C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Nuutinen, S.; Panula, P. Histamine in neurotransmission and brain diseases. Adv. Exp. Med. Biol. 2010, 709, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Schnedl, W.J.; Enko, D. Histamine Intolerance Originates in the Gut. Nutrients 2021, 13, 1262. [Google Scholar] [CrossRef] [PubMed]
- Hungerford, J.M. Histamine and Scombrotoxins. Toxicon 2021, 201, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Reese, I.; Ballmer-Weber, B.; Beyer, K.; Fuchs, T.; Kleine-Tebbe, J.; Klimek, L.; Lepp, U.; Niggemann, B.; Saloga, J.; Schäfer, C.; et al. German guideline for the management of adverse reactions to ingested histamine. Allergo J. Int. 2017, 26, 72–79. [Google Scholar] [CrossRef]
- Mušič, E.; Korošec, P.; Šilar, M.; Adamič, K.; Košnik, M.; Rijavec, M. Serum diamine oxidase activity as a diagnostic test for histamine intolerance. Wien. Klin. Wochenschr. 2013, 125, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Sattler, J.; Häfner, D.; Klotter, H.J.; Lorenz, W.; Wagner, P.K. Food-induced histaminosis as an epidemiological problem: Plasma histamine elevation and haemodynamic alterations after oral histamine administration and blockade of diamine oxidase (DAO). Agents Act. 1988, 23, 361–365. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- Raithel, M.; Matek, M.; Baenkler, H.W.; Jorde, W.; Hahn, E.G. Mucosal histamine content and histamine secretion in Crohn’s disease, ulcerative colitis and allergic enteropathy. Int. Arch. Allergy Immunol. 1995, 108, 127–133. [Google Scholar] [CrossRef]
- Elmore, B.O.; Bollinger, J.A.; Dooley, D.M. Human kidney diamine oxidase: Heterologous expression, purification, and characterization. J. Biol. Inorg. Chem. 2002, 7, 565–579. [Google Scholar] [CrossRef]
- San Mauro Martin, I.; Brachero, S.; Garicano Vilar, E. Histamine intolerance and dietary management: A complete review. Allergol. Immunopathol. 2016, 44, 475–483. [Google Scholar] [CrossRef]
- Comas-Basté, O.; Latorre-Moratalla, M.L.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. In vitro determination of diamine oxidase activity in food matrices by an enzymatic assay coupled to UHPLC-FL. Anal. Bioanal. Chem. 2019, 411, 7595–7602. [Google Scholar] [CrossRef] [PubMed]
- Neree, A.T.; Soret, R.; Marcocci, L.; Pietrangeli, P.; Pilon, N.; Mateescu, M.A. Vegetal diamine oxidase alleviates histamine-induced contraction of colonic muscles. Sci. Rep. 2020, 10, 21563. [Google Scholar] [CrossRef] [PubMed]
- Medda, R.; Padiglia, A.; Floris, G. Plant copper-amine oxidases. Phytochemistry 1995, 39, 1–9. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055. [Google Scholar] [CrossRef]
- Ehrenshaft, M.; Brambl, R. Respiration and mitochondrial biogenesis in germinating embryos of maize. Plant Physiol. 1990, 93, 295–304. [Google Scholar] [CrossRef]
- Fincher, G.B. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu. Rev. Plant Biol. 1989, 40, 305–346. [Google Scholar] [CrossRef]
- Welbaum, G.; Bradford, K.; Yim, K.-O.; Booth, D.; Oluoch, M. Biophysical, physiological and biochemical processes regulating seed germination. Seed Sci. Res. 1998, 8, 161–172. [Google Scholar] [CrossRef]
- Matilla, A.; Garcia, S.; Bueno, M. Diamine oxidase activity during the germinative and post-germinative growth of the embryonic axis in chickpea seeds. Biol. Plant. 2002, 45, 551–556. [Google Scholar] [CrossRef]
- Choudhary, A.; Singh, R.P. Cadmium-induced changes in diamine oxidase activity and polyamine levels in Vigna radiata Wilczek seedlings. J. Plant Physiol. 2000, 156, 704–710. [Google Scholar] [CrossRef]
- Maccarrone, M.; Rossi, A.; Avigliano, L.; Agro, A.F. Activity and expression of diamine oxidase in lentil seedling under different growth conditions. Plant Sci. 1991, 79, 51–55. [Google Scholar] [CrossRef]
- Luhova, L.; Lebeda, A.; Hedererová, D.; Pec, P. Activities of amine oxidase, peroxidase and catalase in seedlings of Pisum sativum L. under different light conditions. Plant Soil Environ. 2003, 49, 151–157. [Google Scholar] [CrossRef]
- Kivirand, K.; Rinken, T. Purification and properties of amine oxidase from pea seedlings. Proc. Est. Acad. Sci. 2007, 56, 164–171. [Google Scholar]
- Masini, E.; Bani, D.; Marzocca, C.; Mateescu, M.A.; Mannaioni, P.F.; Federico, R.; Mondovì, B. Pea seedling histaminase as a novel therapeutic approach to anaphylactic and inflammatory disorders. Sci. World J. 2007, 7, 888–902. [Google Scholar] [CrossRef] [PubMed]
- Hanbury, C.D.; White, C.L.; Mullan, B.P.; Siddique, K.H.M. A review of the potential of Lathyrus sativus L.; L. cicera L. grain for use as animal feed. Anim. Feed Sci. Technol. 2000, 87, 1–27. [Google Scholar] [CrossRef]
- Kumar, S.; Bejiga, G.; Ahmed, S.; Nakkoul, H.; Sarker, A. Genetic improvement of grass pea for low neurotoxin (β-ODAP) content. Food Chem. Toxicol. 2011, 49, 589–600. [Google Scholar] [CrossRef]
- Woldeamanuel, Y.W.; Hassan, A.; Zenebe, G. Neurolathyrism: Two Ethiopian case reports and review of the literature. J. Neurol. 2012, 259, 1263–1268. [Google Scholar] [CrossRef]
- Singh, M.; Singh, G. Lathyrism; a neurological and economic problem in India. J. Nerv. Ment. Dis. 1952, 116, 923–932. [Google Scholar] [CrossRef]
- Fikre, A.; Korbu, L.; Kuo, Y.-H.; Lambein, F. The contents of the neuro-excitatory amino acid β-ODAP (β-N-oxalyl-l-α,β-diaminopropionic acid), and other free and protein amino acids in the seeds of different genotypes of grass pea (Lathyrus sativus L.). Food Chem. 2008, 110, 422–427. [Google Scholar] [CrossRef]
- Rao, S.L.; Adiga, P.R.; Sarma, P.S. The isolation and characterization of beta-N-oxalyl-L-alpha, beta-diaminopropionic acid: A neurotoxin from the seeds of Lathyrus sativus. Biochemistry 1964, 3, 432–436. [Google Scholar] [CrossRef]
- Buta, M.B.; Emire, S.A.; Posten, C.; Andrée, S.; Greiner, R. Reduction of β-ODAP and IP6 contents in Lathyrus sativus L. seed by high hydrostatic pressure. Food Res. Int. 2019, 120, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Shinomol, G.K.; Muralidhara. Differential induction of oxidative impairments in brain regions of male mice following subchronic consumption of Khesari dhal (Lathyrus sativus) and detoxified Khesari dhal. Neurotoxicology 2007, 28, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.G.; Jun, Y.B.; Hau, Z.W.; Liang, L.Y. Higher accumulation of γ-aminobutyric acid induced by salt stress through stimulating the activity of diamine oxidases in Glycine max (L.) Merr. roots. Plant Physiol. Biochem. 2007, 45, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Federico, R.; Angelini, R. Distribution of polyamines and their related catabolic enzyme in etiolated and light-grown leguminosae seedlings. Planta 1988, 173, 317–321. [Google Scholar] [CrossRef]
- Yang, R.; Chen, H.; Gu, Z. Factors influencing diamine oxidase activity and γ-aminobutyric acid content of fava bean (Vicia faba L.) during germination. J. Agric. Food Chem. 2011, 59, 11616–11620. [Google Scholar] [CrossRef]
- Tarade, K.M.; Singhal, R.S.; Jayram, R.V.; Pandit, A.B. Kinetics of degradation of ODAP in Lathyrus sativus L. flour during food processing. Food Chem. 2007, 104, 643–649. [Google Scholar] [CrossRef]
- Ghosh, B.; Mitra, J.; Chakraborty, S.; Bhattacharyya, J.; Chakraborty, A.; Sen, S.K.; Neerathilingam, M. Simple detection methods for antinutritive factor β-ODAP present in Lathyrus sativus L. by high pressure liquid chromatography and thin layer chromatography. PLoS ONE 2015, 10, e0140649. [Google Scholar] [CrossRef]
- He, K.; Blaney, L. Systematic optimization of an SPE with HPLC-FLD method for fluoroquinolone detection in wastewater. J. Hazard. Mater. 2015, 282, 96–105. [Google Scholar] [CrossRef]
- Calinescu, C.; Mondovi, B.; Federico, R.; Ispas-Szabo, P.; Mateescu, M.A. Carboxymethyl starch: Chitosan monolithic matrices containing diamine oxidase and catalase for intestinal delivery. Int. J. Pharm. 2012, 428, 48–56. [Google Scholar] [CrossRef]
- Del Cura, M.I.; Huertas, R. Describing neurolathyrism. Physicians and the lathyrism epidemic in post-civil war Spain. Rev. Neurol. 2009, 48, 265–270. [Google Scholar]
- Lim, J.Y.; Kim, N.A.; Lim, D.G.; Kim, K.H.; Choi, D.H.; Jeong, S.H. Process cycle development of freeze drying for therapeutic proteins with stability evaluation. J. Pharm. Investig. 2016, 46, 519–536. [Google Scholar] [CrossRef]
- Pieters, S.; De Beer, T.; Kasper, J.C.; Boulpaep, D.; Waszkiewicz, O.; Goodarzi, M.; Tistaert, C.; Friess, W.; Remon, J.-P.; Vervaet, C. Near-infrared spectroscopy for in-line monitoring of protein unfolding and its interactions with lyoprotectants during freeze-drying. Anal. Chem. 2012, 84, 947–955. [Google Scholar] [CrossRef]
- Mehta, T.; Zarghami, N.S.; Parker, A.J.; Cusick, P.K.; Haskell, B.E. Neurotoxicity of orally or intraperitoneally administered L-3-oxalylamino-2-aminopropionic acid in the mouse. Toxicol. Appl. Pharmacol. 1979, 48, 1–9. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Spencer, P.S.; Li, Z.X.; Liang, Y.M.; Wang, Y.F.; Wang, C.Y.; Li, F.M. Lathyrus sativus (grass pea) and its neurotoxin ODAP. Phytochemistry 2006, 67, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, B. Seed morphology as an indicator of low neurotoxin in Lathyrus sativus L. Qual. Plant. 1976, 25, 391–394. [Google Scholar] [CrossRef]
- Pietrangeli, P.; Bellelli, A.; Fattibene, P.; Mondovì, B.; Morpurgo, L. Lathyrus cicera copper amine oxidase reactions with tryptamine. J. Inorg. Biochem. 2012, 109, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Warkentin, T.; Vandenberg, A.; Tar’an, B.; Banniza, S.; Arganosa, G.; Barlow, B.; Ife, S.; Horner, J.; de Silva, D.; Thompson, M. CDC Amarillo yellow field pea. Can. J. Plant Sci. 2014, 94, 1539–1541. [Google Scholar] [CrossRef]
- Warkentin, T.; Vandenberg, A.; Tar’an, B.; Barlow, S.; Ife, S. CDC Meadow field pea. Can. J. Plant Sci. 2007, 87, 909–910. [Google Scholar] [CrossRef]
- Warkentin, T.; Vandenberg, A.; Tar’An, B.; Banniza, S.; Arganosa, G.; Barlow, B.; Ife, S.; Horner, J.; De Silva, D.; Thompson, M. CDC Limerick green field pea. Can. J. Plant Sci. 2014, 94, 1547–1549. [Google Scholar] [CrossRef]
- Warkentin, T.; Tar’an, B.; Banniza, S.; Vandenberg, A.; Bett, K.; Arganosa, G.; Barlow, B.; Ife, S.; Horner, J.; de Silva, D. CDC Inca yellow field pea. Can. J. Plant Sci. 2017, 98, 218–220. [Google Scholar] [CrossRef]
- Dahiya, B.S.; Jeswani, L.M. Genotype x environment interaction for neurotoxic principle (BOAA) in grass-pea. Indian J. Agric. Sci. 1975, 45, 437–439. [Google Scholar]
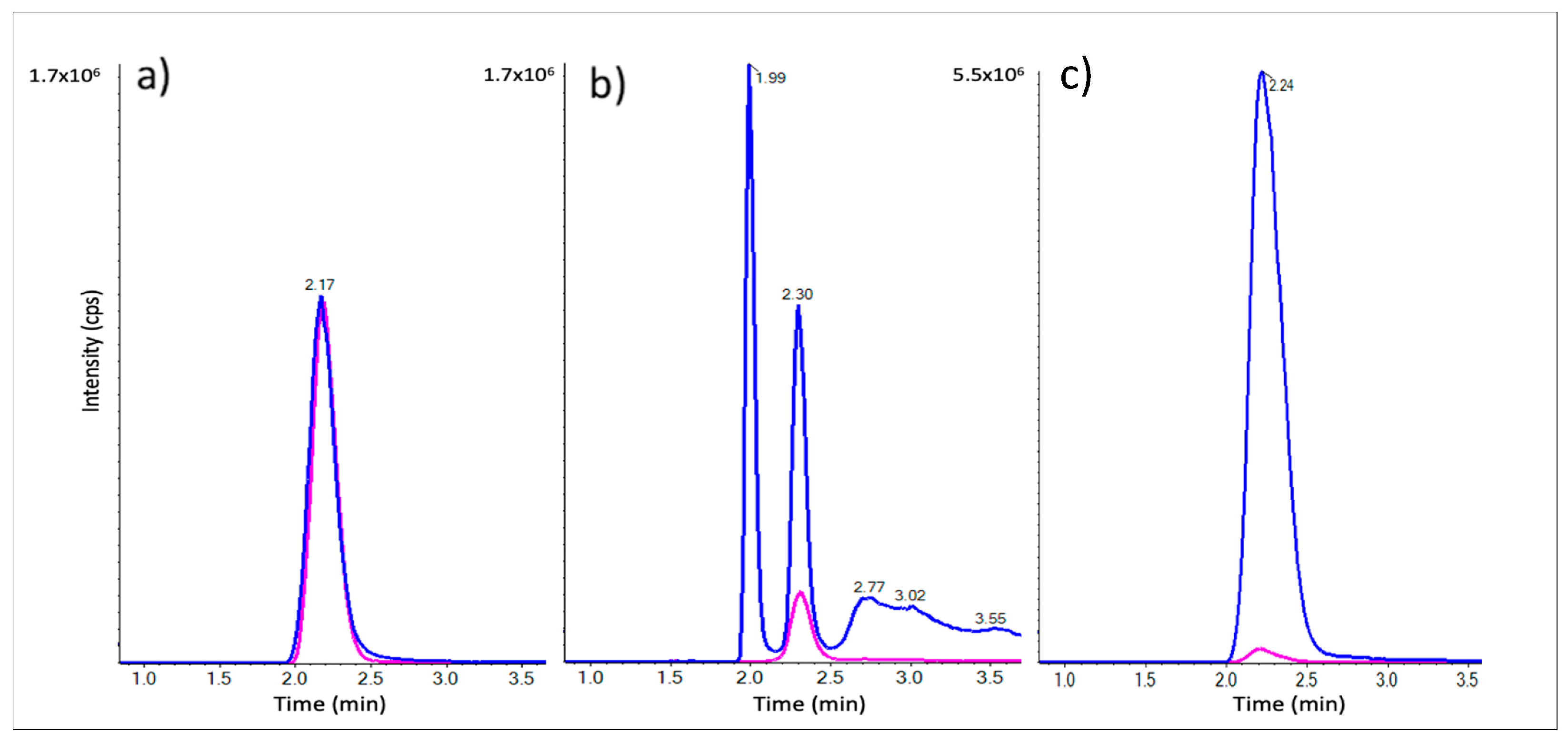
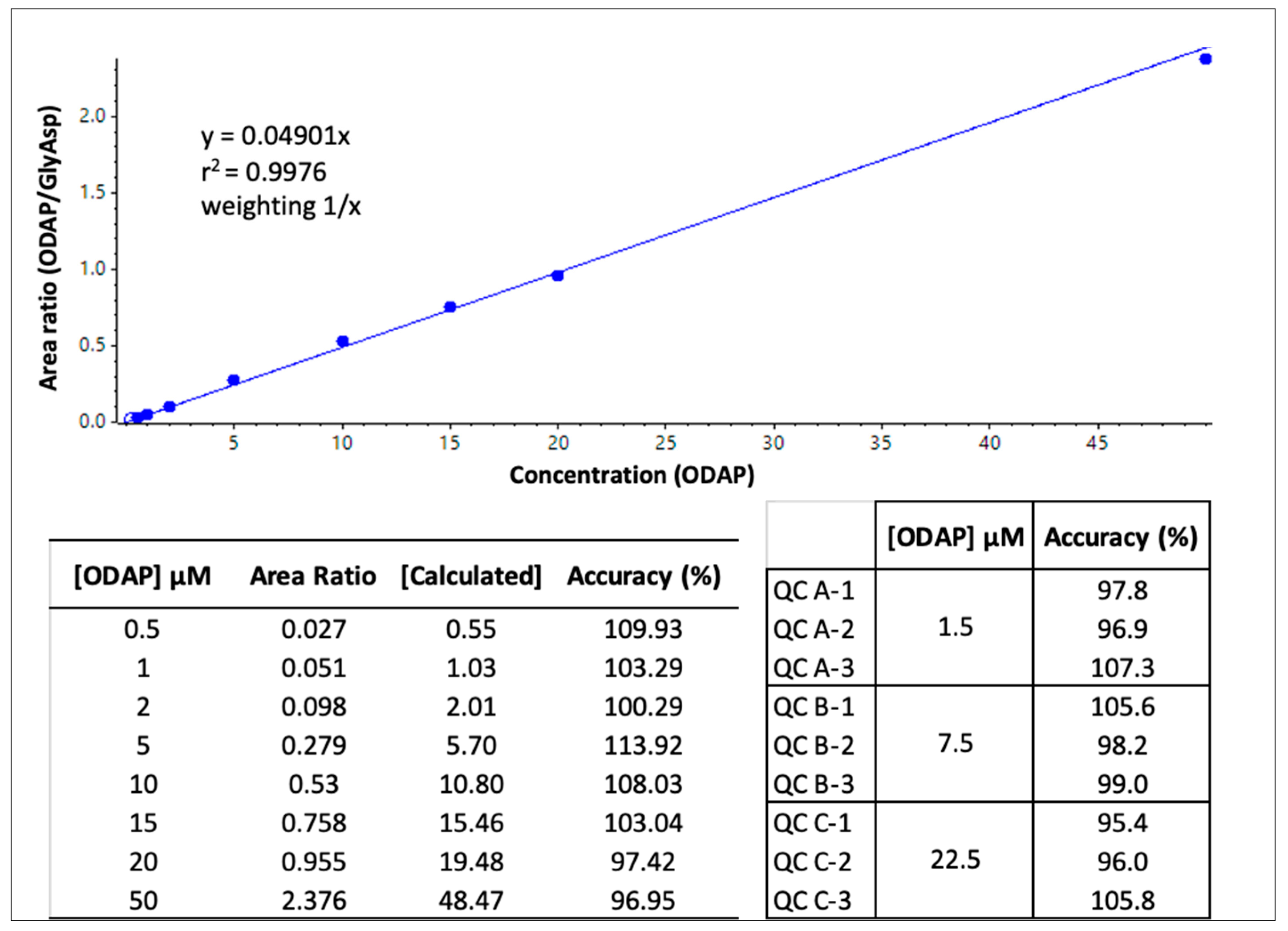
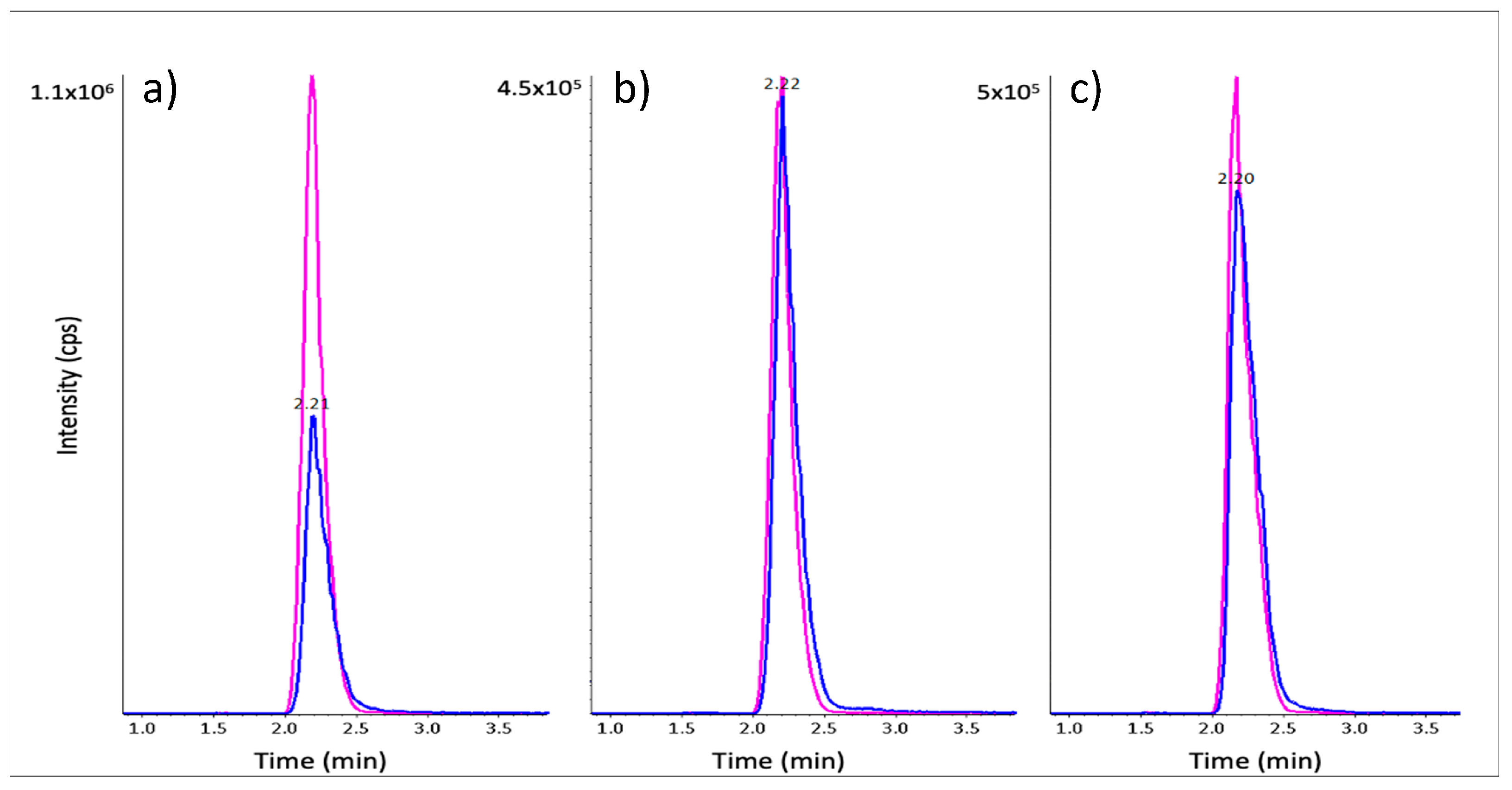
| Samples | Protein Content (µg/mL) | [β-ODAP] (µmol/L) | [β-ODAP] (µmol/mg Protein) |
|---|---|---|---|
| L. sativus crude extract | 600 ± 120 | 4722 ± 2116 | 7.65 ± 2.02 |
| L. sativus dialysed extract | 876 ± 219 | 158 ± 35 | 0.18 ± 0.02 |
| CDC Amarillo crude extract | 624 ± 115 | 0.99 ± 0.03 | 0.0016 ± 0.0003 |
| * Yellow pea crude extract | 615 ± 181 | 0.58 ± 0.16 | 0.0011 ± 0.0006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boulfekhar, R.; Ohlund, L.; Kumaresan, K.M.; Megoura, M.; Warkentin, T.D.; Ispas-Szabo, P.; Sleno, L.; Mateescu, M.A. Diamine Oxidase as a Therapeutic Enzyme: Study of Germination from Vegetal Sources and Investigation of the Presence of β-N-Oxalyl-L-α,β-diaminopropionic Acid (β-ODAP) Using LC-MS/MS. Int. J. Mol. Sci. 2023, 24, 4625. https://doi.org/10.3390/ijms24054625
Boulfekhar R, Ohlund L, Kumaresan KM, Megoura M, Warkentin TD, Ispas-Szabo P, Sleno L, Mateescu MA. Diamine Oxidase as a Therapeutic Enzyme: Study of Germination from Vegetal Sources and Investigation of the Presence of β-N-Oxalyl-L-α,β-diaminopropionic Acid (β-ODAP) Using LC-MS/MS. International Journal of Molecular Sciences. 2023; 24(5):4625. https://doi.org/10.3390/ijms24054625
Chicago/Turabian StyleBoulfekhar, Rym, Leanne Ohlund, Kathrina Mae Kumaresan, Meriem Megoura, Thomas D. Warkentin, Pompilia Ispas-Szabo, Lekha Sleno, and Mircea Alexandru Mateescu. 2023. "Diamine Oxidase as a Therapeutic Enzyme: Study of Germination from Vegetal Sources and Investigation of the Presence of β-N-Oxalyl-L-α,β-diaminopropionic Acid (β-ODAP) Using LC-MS/MS" International Journal of Molecular Sciences 24, no. 5: 4625. https://doi.org/10.3390/ijms24054625
APA StyleBoulfekhar, R., Ohlund, L., Kumaresan, K. M., Megoura, M., Warkentin, T. D., Ispas-Szabo, P., Sleno, L., & Mateescu, M. A. (2023). Diamine Oxidase as a Therapeutic Enzyme: Study of Germination from Vegetal Sources and Investigation of the Presence of β-N-Oxalyl-L-α,β-diaminopropionic Acid (β-ODAP) Using LC-MS/MS. International Journal of Molecular Sciences, 24(5), 4625. https://doi.org/10.3390/ijms24054625









