Sestrin2 as a Protective Shield against Cardiovascular Disease
Abstract
1. Introduction
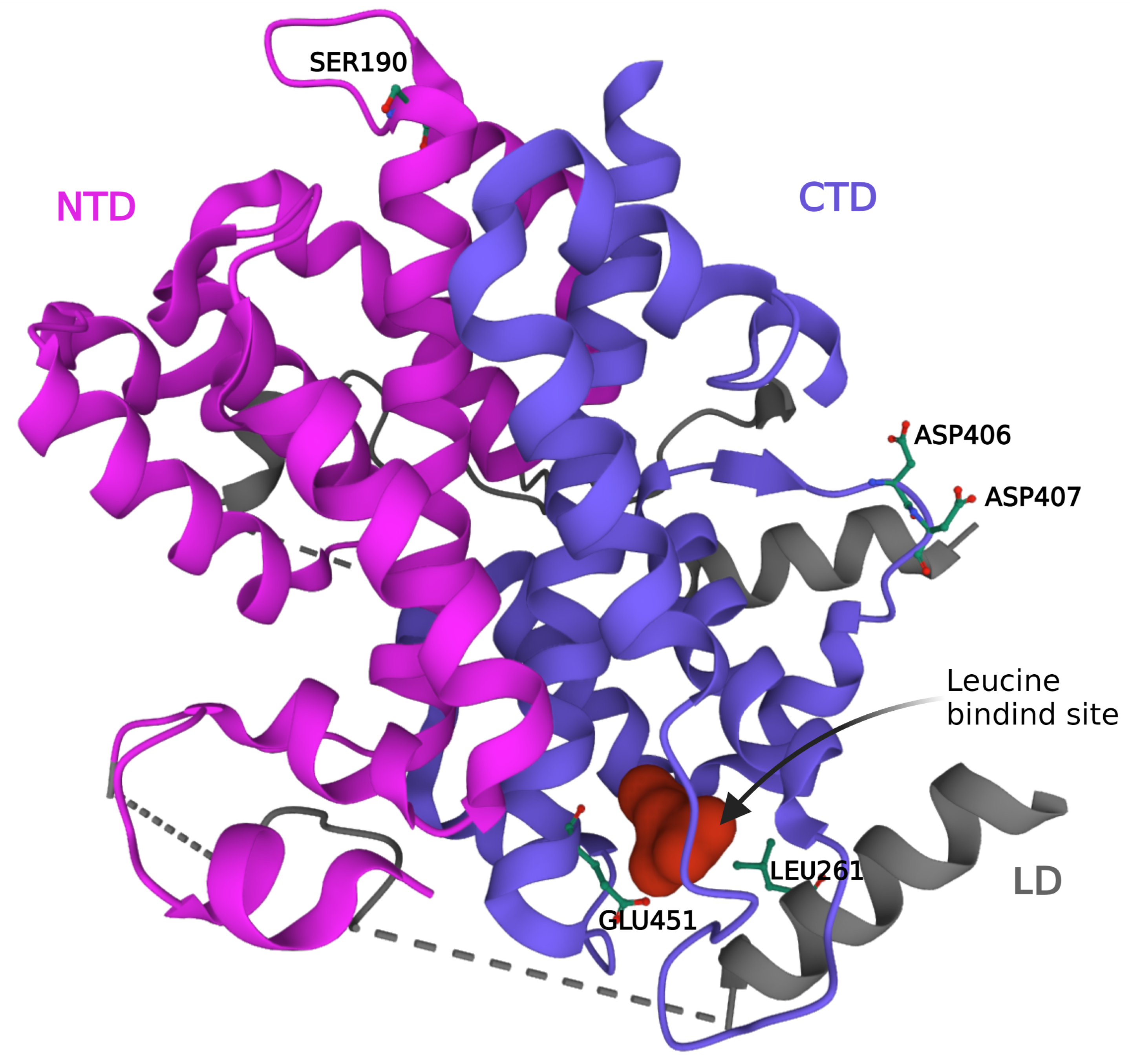
2. SESN2 Gene and Protein Structure
3. Upstream Regulators of SESN2 Expression in Response to Different Types of Cellular Stress
3.1. Hypoxia
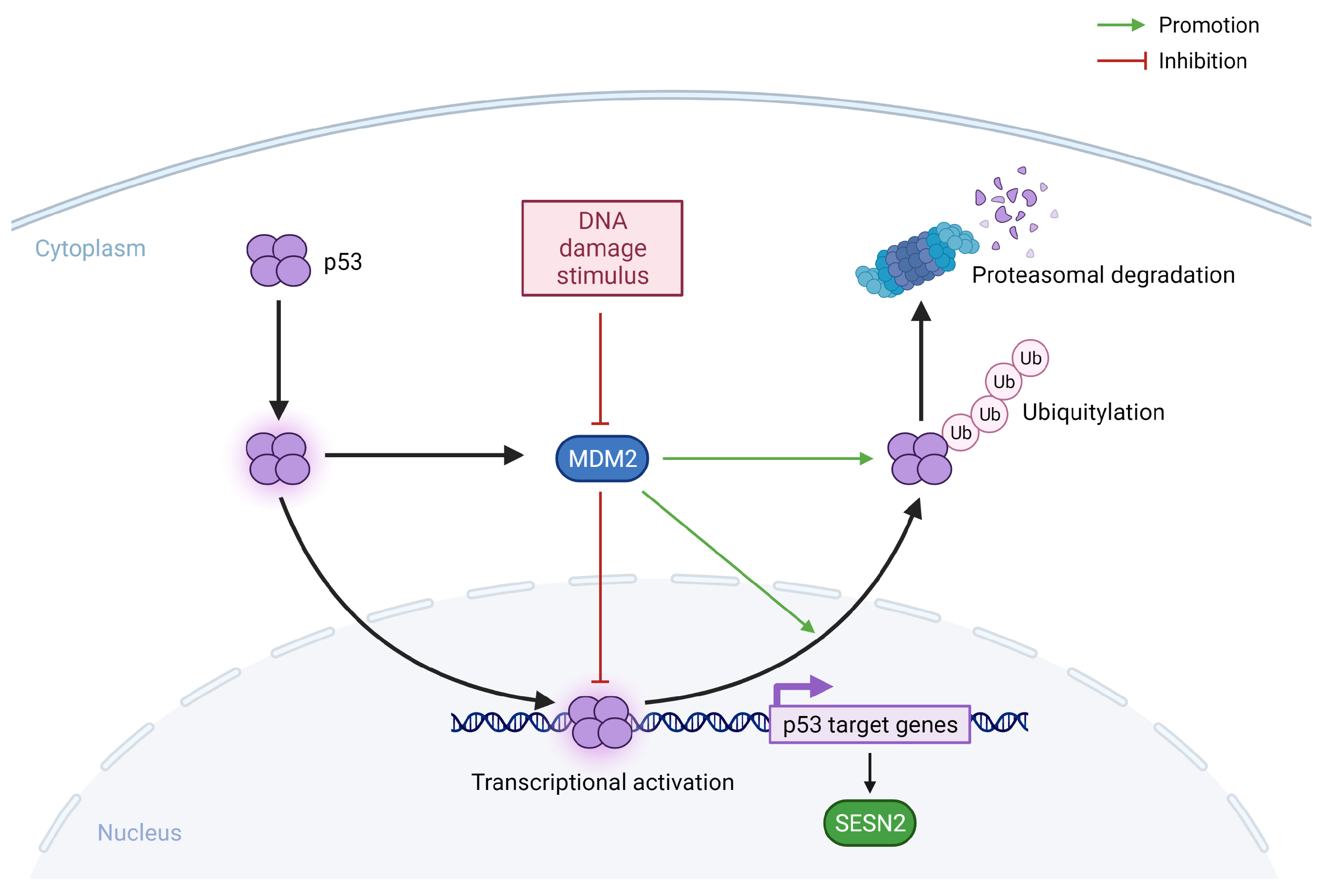
3.2. Genotoxic Stress
3.3. Endoplasmic Reticulum Stress
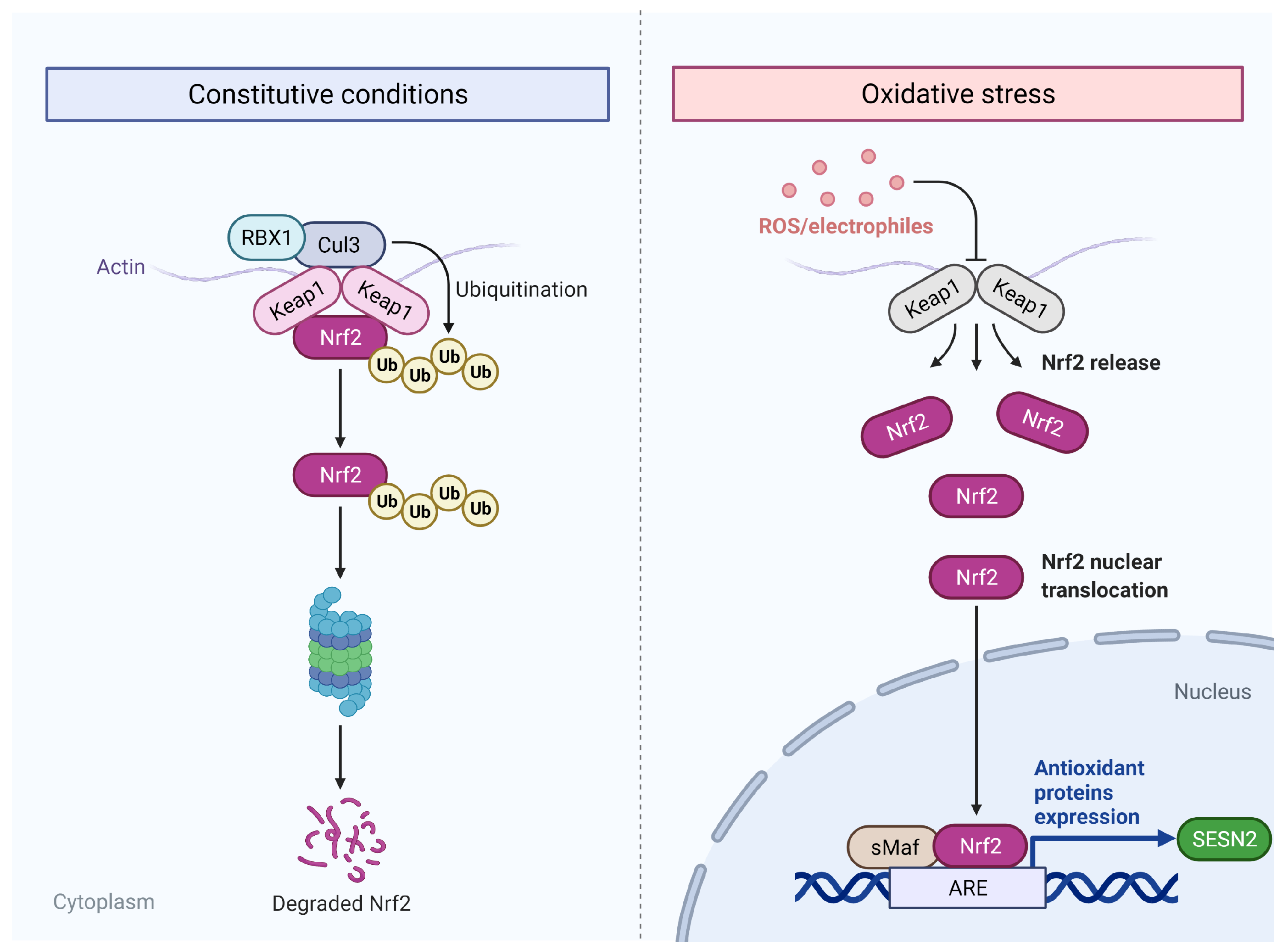
3.4. Oxidative Stress
4. Downstream Pathways Affected by SESN2
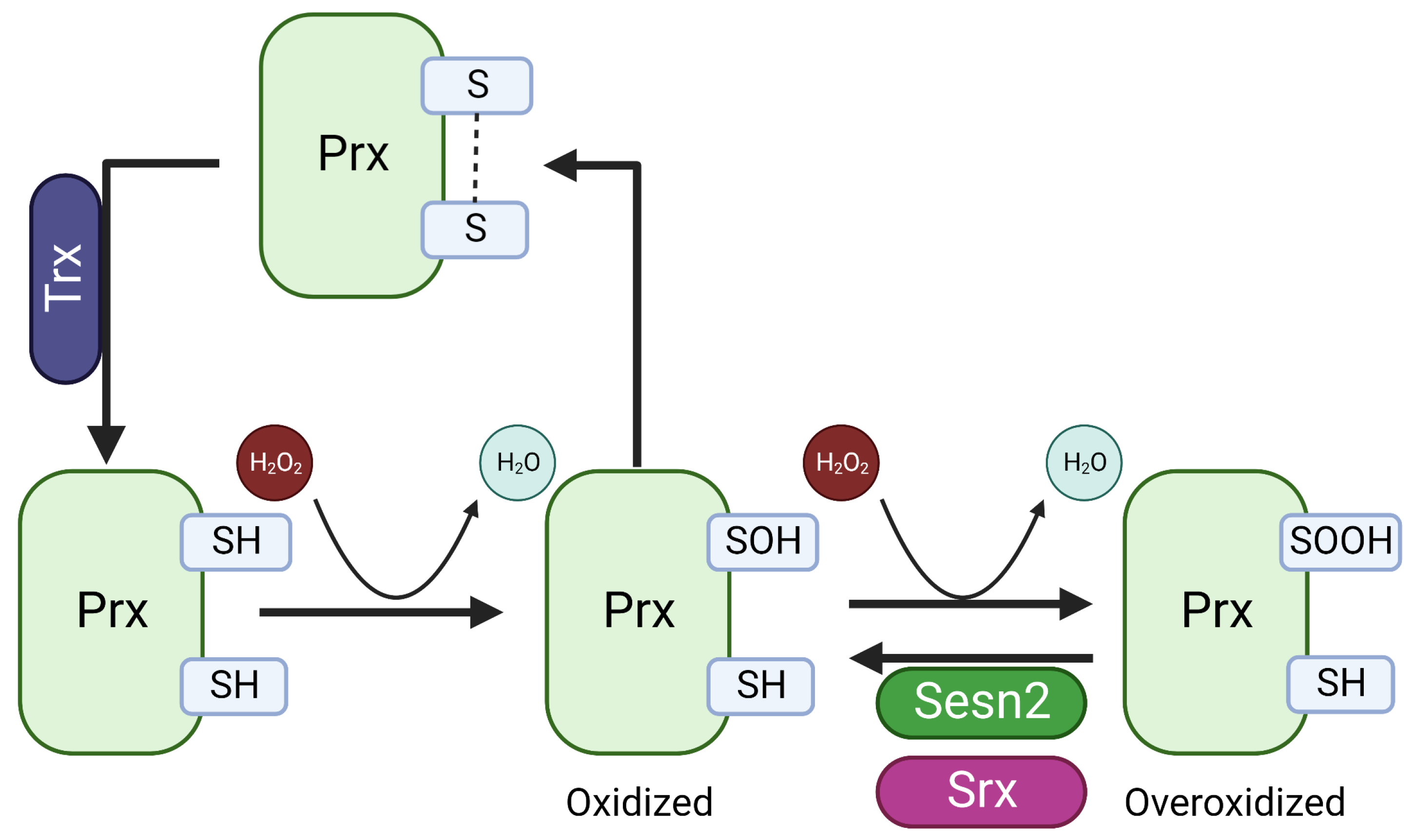
4.1. Intrinsic Antioxidant Enzyme Activity of SESN2
4.2. Inhibition of mTORC1
4.2.1. SESN2-AMPK-TSC2-RHEB-mTORC1 Axis
4.2.2. SESN2–GATOR2–GATOR1–RRAG–mTORC1 Axis
4.3. Activation of mTORC2
4.4. KEAP-1-NRF2 Axis
4.5. SESN2 and Autophagy
5. SESN2 and Cardiovascular Diseases
5.1. Hypertension
5.2. Atherosclerosis
5.3. Ischemia/Reperfusion Injury
5.4. Cardiac Hypertrophy and Heart Failure
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTD | C-terminal domain |
| LD | Linker domain |
| NTD | N-terminal domain |
| HRE | Hypoxia Response Element |
| VHL | Von Hippel–Lindau tumor suppressor |
| MDM2 | Mouse Double Minute homolog |
| Ub | Ubiquitin |
| ATF-4 | Activating Transcription Factor 4 |
| ATF-6 | Activating Transcription Factor 6 |
| IRE-1 | Inositol-Requiring Enzyme 1 |
| JNK | c-Jun N-terminal Kinase |
| PERK | Protein kinase RNA-like Endoplasmic Reticulum Kinase |
| RIDD | Regulated IRE1α-Dependent Decay |
| XBP-1s | spliced X-box binding protein 1 |
| Prx | Peroxiredoxin |
| Trx | Thioredoxin |
| Srx | Sulfiredoxin |
| SH | Reduced cysteine |
| SOH | Oxidized cysteine |
| SOOH | Overoxidized cysteine |
| Akt | Ak strain transforming |
| AMPK | AMP-activated Protein Kinase |
| ERK | Extracellular signal-Regulated Kinase |
| GATOR1 | Gap Activity TOward Rags 1 |
| GATOR2 | Gap Activity TOward Rags 2 |
| GDP | Guanosine DiPhosphate |
| GTP | Guanosine TriPhosphate |
| MEK | Mitogen-activated protein kinase |
| PI3K | Phosphoinositide 3-kinase |
| Raf | Rapidly accelerated fibrosarcoma kinase |
| Rag | Ras-related GTP-binding protein |
| Ras | Rat sarcoma kinase |
| Rheb | Ras Homolog Enriched in Brain |
| RSK | 90 kDa Ribosomal S6 Kinase |
| SESN2 | Sestrin2 |
| TSC1/2 | Tuberous Sclerosis Complex 1/2 |
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Gelino, S.; Hansen, M. Autophagy—An Emerging Anti-Aging Mechanism. J. Clin. Exp. Pathol. 2012, Suppl 4, 006. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Baur, J.A.; Boyd, A.R.; de Cabo, R.; Fernandez, E.; Flurkey, K.; Javors, M.A.; Nelson, J.F.; et al. Rapamycin, But Not Resveratrol or Simvastatin, Extends Life Span of Genetically Heterogeneous Mice. J. Gerontol. Ser. 2011, 66A, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.E.; Burmeister, L.; Brooks, S.V.; Chan, C.C.; Friedline, S.; Harrison, D.E.; Hejtmancik, J.F.; Nadon, N.; Strong, R.; Wood, L.K.; et al. Rapamycin slows aging in mice. Aging Cell 2012, 11, 675–682. [Google Scholar] [CrossRef]
- Morrison, A.; Chen, L.; Wang, J.; Zhang, M.; Yang, H.; Ma, Y.; Budanov, A.; Lee, J.H.; Karin, M.; Li, J. Sestrin2 promotes LKB1-mediated AMPK activation in the ischemic heart. FASEB J. 2015, 29, 408–417. [Google Scholar] [CrossRef]
- Cordani, M.; Sánchez-Álvarez, M.; Strippoli, R.; Bazhin, A.V.; Donadelli, M. Sestrins at the Interface of ROS Control and Autophagy Regulation in Health and Disease. Oxidative Med. Cell. Longev. 2019, 2019, e1283075. [Google Scholar] [CrossRef]
- Kim, J.S.; Ro, S.H.; Kim, M.; Park, H.W.; Semple, I.A.; Park, H.; Cho, U.S.; Wang, W.; Guan, K.L.; Karin, M.; et al. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Sci. Rep. 2015, 5, 9502. [Google Scholar] [CrossRef]
- Lee, J.H.; Budanov, A.V.; Park, E.J.; Birse, R.; Kim, T.E.; Perkins, G.A.; Ocorr, K.; Ellisman, M.H.; Bodmer, R.; Bier, E.; et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 2010, 327, 1223–1228. [Google Scholar] [CrossRef]
- Lee, J.; Budanov, A.; Talukdar, S.; Park, E.; Park, H.; Park, H.W.; Bandyopadhyay, G.; Li, N.; Aghajan, M.; Jang, I.; et al. Maintenance of Metabolic Homeostasis by Sestrin2 and Sestrin3. Cell Metab. 2012, 16, 311–321. [Google Scholar] [CrossRef]
- Quan, N.; Sun, W.; Wang, L.; Chen, X.; Bogan, J.S.; Zhou, X.; Cates, C.; Liu, Q.; Zheng, Y.; Li, J. Sestrin2 prevents age-related intolerance to ischemia and reperfusion injury by modulating substrate metabolism. FASEB J. 2017, 31, 4153–4167. [Google Scholar] [CrossRef]
- Quan, N.; Wang, L.; Wang, L.; Chen, X.; Luckett, C.; Cates, C.; Rousselle, T.; Zheng, Y.; Li, J. Sestrin2 prevents age-related intolerance to post myocardial infarction via AMPK/PGC-1α pathway. J. Mol. Cell. Cardiol. 2018, 115, 170–178. [Google Scholar] [CrossRef]
- Quan, N.; Li, X.; Zhang, J.; Han, Y.; Sun, W.; Ren, D.; Tong, Q.; Li, J. Substrate metabolism regulated by Sestrin2-mTORC1 alleviates pressure overload-induced cardiac hypertrophy in aged heart. Redox Biol. 2020, 36, 101637. [Google Scholar] [CrossRef]
- Dagenais, G.R.; Leong, D.P.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Gupta, R.; Diaz, R.; Avezum, A.; Oliveira, G.B.F.; Wielgosz, A.; et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): A prospective cohort study. Lancet 2020, 395, 785–794. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial Dysfunction in Cardiovascular Diseases: The Role of Oxidant Stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic Syndrome as a Precursor of Cardiovascular Disease and Type 2 Diabetes Mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef]
- Budanov, A.V.; Shoshani, T.; Faerman, A.; Zelin, E.; Kamer, I.; Kalinski, H.; Gorodin, S.; Fishman, A.; Chajut, A.; Einat, P.; et al. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene 2002, 21, 6017–6031. [Google Scholar] [CrossRef]
- Budanov, A.V.; Sablina, A.A.; Feinstein, E.; Koonin, E.V.; Chumakov, P.M. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 2004, 304, 596–600. [Google Scholar] [CrossRef]
- Budanov, A.V.; Karin, M. p53 Target Genes Sestrin1 and Sestrin2 Connect Genotoxic Stress and mTOR Signaling. Cell 2008, 134, 451–460. [Google Scholar] [CrossRef]
- Parmigiani, A.; Nourbakhsh, A.; Ding, B.; Wang, W.; Kim, Y.C.; Akopiants, K.; Guan, K.L.; Karin, M.; Budanov, A.V. Sestrins Inhibit mTORC1 Kinase Activation through the GATOR Complex. Cell Rep. 2014, 9, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Sung, S.H.; Oh, S.Y.; Lim, J.M.; Lee, S.K.; Park, Y.N.; Lee, H.E.; Kang, D.; Rhee, S.G. Sestrins Activate Nrf2 by Promoting p62-Dependent Autophagic Degradation of Keap1 and Prevent Oxidative Liver Damage. Cell Metab. 2013, 17, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kowalsky, A.H.; Namkoong, S.; Mettetal, E.; Park, H.W.; Kazyken, D.; Fingar, D.C.; Lee, J.H. The GATOR2–mTORC2 axis mediates Sestrin2-induced AKT Ser/Thr kinase activation. J. Biol. Chem. 2020, 295, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Malik, S.A.; Morselli, E.; Kepp, O.; Criollo, A.; Mouchel, P.L.; Carnuccio, R.; Kroemer, G. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle 2009, 8, 1571–1576. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, L.; Li, S.; Ferdous, M.; Zhao, P. ER stress induces myocardial dysfunction and cardiac autophagy in Sestrin2 knockout mice. Am. J. Transl. Res. 2022, 14, 5800–5811. [Google Scholar]
- Krause-Hauch, M.; Fedorova, J.; Zoungrana, L.I.; Wang, H.; Fatmi, M.K.; Li, Z.; Iglesias, M.; Slotabec, L.; Li, J. Targeting on Nrf2/Sesn2 Signaling to Rescue Cardiac Dysfunction during High-Fat Diet-Induced Obesity. Cells 2022, 11, 2614. [Google Scholar] [CrossRef]
- Yang, W.; Li, Y.; Bai, J.; You, T.; Yi, K.; Xie, D.; Zhang, X.; Xie, X. A Functional Variant Rs492554 Associated with Congenital Heart Defects Modulates SESN2 Expression Through POU2F1. Front. Cell Dev. Biol. 2021, 9, 668474. [Google Scholar] [CrossRef]
- Velasco-Miguel, S.; Buckbinder, L.; Jean, P.; Gelbert, L.; Talbott, R.; Laidlaw, J.; Seizinger, B.; Kley, N. PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene 1999, 18, 127–137. [Google Scholar] [CrossRef]
- Peeters, H.; Debeer, P.; Bairoch, A.; Wilquet, V.; Huysmans, C.; Parthoens, E.; Fryns, J.P.; Gewillig, M.; Nakamura, Y.; Niikawa, N.; et al. PA26 is a candidate gene for heterotaxia in humans: Identification of a novel PA26-related gene family in human and mouse. Hum. Genet. 2003, 112, 573–580. [Google Scholar] [CrossRef]
- Saxton, R.A.; Knockenhauer, K.E.; Wolfson, R.L.; Chantranupong, L.; Pacold, M.E.; Wang, T.; Schwartz, T.U.; Sabatini, D.M. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science 2016, 351, 53–58. [Google Scholar] [CrossRef]
- Wolfson, R.L.; Chantranupong, L.; Saxton, R.A.; Shen, K.; Scaria, S.M.; Cantor, J.R.; Sabatini, D.M. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016, 351, 43–48. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480. [Google Scholar] [CrossRef]
- Hashimoto, T.; Shibasaki, F. Hypoxia-Inducible Factor as an Angiogenic Master Switch. Front. Pediatr. 2015, 3, 33. [Google Scholar] [CrossRef]
- Olson, N.; Hristova, M.; Heintz, N.H.; Lounsbury, K.M.; van der Vliet, A. Activation of hypoxia-inducible factor-1 protects airway epithelium against oxidant-induced barrier dysfunction. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2011, 301, L993–L1002. [Google Scholar] [CrossRef]
- Shi, X.; Doycheva, D.M.; Xu, L.; Tang, J.; Yan, M.; Zhang, J.H. Sestrin2 induced by hypoxia inducible factor1 alpha protects the blood-brain barrier via inhibiting VEGF after severe hypoxic-ischemic injury in neonatal rats. Neurobiol. Dis. 2016, 95, 111–121. [Google Scholar] [CrossRef]
- Kumari, R.; Kohli, S.; Das, S. p53 regulation upon genotoxic stress: Intricacies and complexities. Mol. Cell. Oncol. 2014, 1, e969653. [Google Scholar] [CrossRef]
- Momand, J.; Zambetti, G.P.; Olson, D.C.; George, D.; Levine, A.J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 1992, 69, 1237–1245. [Google Scholar] [CrossRef]
- Lakin, N.D.; Jackson, S.P. Regulation of p53 in response to DNA damage. Oncogene 1999, 18, 7644–7655. [Google Scholar] [CrossRef]
- Daixing, Z.; Chengye, Z.; Qiang, Z.; Shusheng, L. Upregulation of Sestrin-2 Expression via P53 Protects Against 1-Methyl-4-Phenylpyridinium (MPP+) Neurotoxicity. J. Mol. Neurosci. 2013, 51, 967–975. [Google Scholar] [CrossRef]
- Jegal, K.H.; Park, S.M.; Cho, S.S.; Byun, S.H.; Ku, S.K.; Kim, S.C.; Ki, S.H.; Cho, I.J. Activating transcription factor 6-dependent sestrin 2 induction ameliorates ER stress-mediated liver injury. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1295–1307. [Google Scholar] [CrossRef]
- Park, H.W.; Park, H.; Ro, S.H.; Jang, I.; Semple, I.A.; Kim, D.N.; Kim, M.; Nam, M.; Zhang, D.; Yin, L.; et al. Hepatoprotective role of Sestrin2 against chronic ER stress. Nat. Commun. 2014, 5, 4233. [Google Scholar] [CrossRef] [PubMed]
- Brüning, A.; Rahmeh, M.; Friese, K. Nelfinavir and bortezomib inhibit mTOR activity via ATF4-mediated sestrin-2 regulation. Mol. Oncol. 2013, 7, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, X.A.; Hu, J.; Jiang, J.K.; Li, Y.; Chan-Salis, K.Y.; Gu, Y.; Chen, G.; Thomas, C.; Pugh, B.F.; et al. ATF4 Gene Network Mediates Cellular Response to the Anticancer PAD Inhibitor YW3-56 in Triple-Negative Breast Cancer Cells. Mol. Cancer Ther. 2015, 14, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Saveljeva, S.; Cleary, P.; Mnich, K.; Ayo, A.; Pakos-Zebrucka, K.; Patterson, J.B.; Logue, S.E.; Samali, A. Endoplasmic reticulum stress-mediated induction of SESTRIN 2 potentiates cell survival. Oncotarget 2016, 7, 12254–12266. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Shin, B.Y.; Jin, S.H.; Cho, I.J.; Ki, S.H. Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free Radic. Biol. Med. 2012, 53, 834–841. [Google Scholar] [CrossRef]
- Suzuki, T.; Motohashi, H.; Yamamoto, M. Toward clinical application of the Keap1–Nrf2 pathway. Trends Pharmacol. Sci. 2013, 34, 340–346. [Google Scholar] [CrossRef]
- Bryk, R.; Lima, C.D.; Erdjument-Bromage, H.; Tempst, P.; Nathan, C. Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science 2002, 295, 1073–1077. [Google Scholar] [CrossRef]
- Hillas, P.J.; del Alba, F.S.; Oyarzabal, J.; Wilks, A.; Ortiz De Montellano, P.R. The AhpC and AhpD antioxidant defense system of Mycobacterium tuberculosis. J. Biol. Chem. 2000, 275, 18801–18809. [Google Scholar] [CrossRef]
- Woo, H.A.; Bae, S.H.; Park, S.; Rhee, S.G. Sestrin 2 Is Not a Reductase for Cysteine Sulfinic Acid of Peroxiredoxins. Antioxid. Redox Signal. 2009, 11, 739–745. [Google Scholar] [CrossRef]
- Kim, H.; An, S.; Ro, S.H.; Teixeira, F.; Jin Park, G.; Kim, C.; Cho, C.S.; Kim, J.S.; Jakob, U.; Hee Lee, J.; et al. Janus-faced Sestrin2 controls ROS and mTOR signalling through two separate functional domains. Nat. Commun. 2015, 6, 10025. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef]
- Bar-Peled, L.; Schweitzer, L.D.; Zoncu, R.; Sabatini, D.M. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 2012, 150, 1196–1208. [Google Scholar] [CrossRef]
- Chantranupong, L.; Wolfson, R.L.; Orozco, J.M.; Saxton, R.A.; Scaria, S.M.; Bar-Peled, L.; Spooner, E.; Isasa, M.; Gygi, S.P.; Sabatini, D.M. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep. 2014, 9, 1–8. [Google Scholar] [CrossRef]
- Peng, M.; Yin, N.; Li, M.O. Sestrins Function as Guanine Nucleotide Dissociation Inhibitors for Rag GTPases to Control mTORC1 Signaling. Cell 2014, 159, 122–133. [Google Scholar] [CrossRef]
- Demetriades, C.; Doumpas, N.; Teleman, A.A. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell 2014, 156, 786–799. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Xu, T.; Guan, K.L. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003, 17, 1829–1834. [Google Scholar] [CrossRef]
- Fatima, M.T.; Hasan, M.; Abdelsalam, S.S.; Sivaraman, S.K.; El-Gamal, H.; Zahid, M.A.; Elrayess, M.A.; Korashy, H.M.; Zeidan, A.; Parray, A.S.; et al. Sestrin2 suppression aggravates oxidative stress and apoptosis in endothelial cells subjected to pharmacologically induced endoplasmic reticulum stress. Eur. J. Pharmacol. 2021, 907, 174247. [Google Scholar] [CrossRef]
- Bae, S.H.; Woo, H.A.; Sung, S.H.; Lee, H.E.; Lee, S.K.; Kil, I.S.; Rhee, S.G. Induction of sulfiredoxin via an Nrf2-dependent pathway and hyperoxidation of peroxiredoxin III in the lungs of mice exposed to hyperoxia. Antioxid. Redox Signal. 2009, 11, 937–948. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Ro, S.H.; Semple, I.A.; Park, H.; Park, H.; Park, H.W.; Kim, M.; Kim, J.S.; Lee, J.H. Sestrin2 promotes Unc-51-like kinase 1 mediated phosphorylation of p62/sequestosome-1. FEBS J. 2014, 281, 3816–3827. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cuevas, S.; Yang, S.; Villar, V.A.; Escano, C.; Asico, L.; Yu, P.; Jiang, X.; Weinman, E.J.; Armando, I.; et al. Sestrin2 Decreases Renal Oxidative Stress, Lowers Blood Pressure, and Mediates Dopamine D2 Receptor–Induced Inhibition of Reactive Oxygen Species Production. Hypertension 2014, 64, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Li, F.; Yong, Y.; Jianting, D.; Liting, Z.; Xuansheng, H.; Fei, L.; Jiewen, L. Upregulation of sestrin-2 expression protects against endothelial toxicity of angiotensin II. Cell Biol. Toxicol. 2014, 30, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Yang, Z.; Shi, L.; Zeng, T.; Shi, Y.; Liu, L.; Liu, H.; Lin, Y. Circulating Sestrin Levels Are Increased in Hypertension Patients. Disease Markers 2020, 2020, 3787295. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Saita, E.; Ohmori, R.; Kondo, K.; Momiyama, Y. Plasma sestrin2 concentrations and carotid atherosclerosis. Clin. Chim. Acta 2020, 504, 56–59. [Google Scholar] [CrossRef]
- Hwang, H.J.; Jung, T.W.; Choi, J.H.; Lee, H.J.; Chung, H.S.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, K.M.; Choi, D.S.; et al. Knockdown of sestrin2 increases pro-inflammatory reactions and ER stress in the endothelium via an AMPK dependent mechanism. Biochim. Biophys. Acta 2017, 1863, 1436–1444. [Google Scholar] [CrossRef]
- Sundararajan, S.; Jayachandran, I.; Balasubramanyam, M.; Mohan, V.; Venkatesan, B.; Manickam, N. Sestrin2 regulates monocyte activation through AMPK-mTOR nexus under high-glucose and dyslipidemic conditions. J. Cell. Biochem. 2019, 120, 8201–8213. [Google Scholar] [CrossRef]
- Hu, H.J.; Shi, Z.; Lin, X.L.; Chen, S.; Wang, Q.; Tang, S. Upregulation of Sestrin2 expression protects against macrophage apoptosis induced by oxidized low-density lipoprotein. DNA Cell Biol. 2015, 34, 296–302. [Google Scholar] [CrossRef]
- Lee, S.; Byun, J.K.; Park, M.; Kim, S.W.; Lee, S.; Kim, J.G.; Lee, I.-K.; Choi, Y.K.; Park, K.-G. Melatonin inhibits vascular smooth muscle cell proliferation and apoptosis through upregulation of Sestrin2. Exp. Ther. Med. 2020, 19, 3454–3460. [Google Scholar] [CrossRef]
- Verma, S.; Fedak, P.W.; Weisel, R.D.; Butany, J.; Rao, V.; Maitland, A.; Li, R.K.; Dhillon, B.; Yau, T.M. Fundamentals of Reperfusion Injury for the Clinical Cardiologist. Circulation 2002, 105, 2332–2336. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Li, M.; Sun, M.; Zhang, Y.; Li, X.; Sun, W.; Quan, N.; Quan, N. Sestrin2 is an endogenous antioxidant that improves contractile function in the heart during exposure to ischemia and reperfusion stress. Free Radic. Biol. Med. 2021, 165, 385–394. [Google Scholar] [CrossRef]
- Ren, D.; Quan, N.; Fedorova, J.; Zhang, J.; He, Z.; Li, J. Sestrin2 modulates cardiac inflammatory response through maintaining redox homeostasis during ischemia and reperfusion. Redox Biol. 2020, 34, 101556. [Google Scholar] [CrossRef]
- Ren, D.; He, Z.; Fedorova, J.; Zhang, J.; Wood, E.R.; Zhang, X.; Kang, D.E.; Li, J. Sestrin2 maintains OXPHOS integrity to modulate cardiac substrate metabolism during ischemia and reperfusion. Redox Biol. 2020, 38, 101824. [Google Scholar] [CrossRef]
- Li, L.; Xiao, L.; Hou, Y.; He, Q.; Zhu, J.; Li, Y.; Wu, J.; Zhao, J.; Yu, S.; Zhao, Y. Sestrin2 Silencing Exacerbates Cerebral Ischemia/Reperfusion Injury by Decreasing Mitochondrial Biogenesis through the AMPK/PGC-1α Pathway in Rats. Sci. Rep. 2016, 6, 30272. [Google Scholar] [CrossRef]
- Ye, J.; Wang, M.; Xu, Y.; Liu, J.; Jiang, H.; Wang, Z.; Lin, Y.; Wan, J. Sestrins increase in patients with coronary artery disease and associate with the severity of coronary stenosis. Clin. Chim. Acta 2017. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Aoyama, M.; Saita, E.; Ikegami, Y.; Ohmori, R.; Kondo, K.; Momiyama, Y. Association between Plasma Sestrin2 Levels and the Presence and Severity of Coronary Artery Disease. Disease Markers 2020, 2020, 7439574. [Google Scholar] [CrossRef]
- Tian, X.; Gao, Y.; Zhong, M.; Kong, M.; Zhao, L.; Feng, Z.; Sun, Q.; He, J.; Liu, X. The association between serum Sestrin2 and the risk of coronary heart disease in patients with type 2 diabetes mellitus. BMC Cardiovasc. Disord. 2022, 22, 281. [Google Scholar] [CrossRef]
- Dong, B.; Xue, R.; Sun, Y.; Dong, Y.; Dong, Y.; Liu, C. Sestrin 2 attenuates neonatal rat cardiomyocyte hypertrophy induced by phenylephrine via inhibiting ERK1/2. Mol. Cell. Biochem. 2017, 433, 113–123. [Google Scholar] [CrossRef]
- Kouzu, H.; Tatekoshi, Y.; Chang, H.C.; Shapiro, J.S.; McGee, W.A.; De Jesus, A.; Ben-Sahra, I.; Arany, Z.; Leor, J.; Chen, C.; et al. ZFP36L2 suppresses mTORc1 through a P53-dependent pathway to prevent peri-partum cardiomyopathy in mice. J. Clin. Investig. 2022, 1. [Google Scholar] [CrossRef]
- Gupta, A.; Shah, K.; Oza, M.J.; Behl, T. Reactivation of p53 gene by MDM2 inhibitors: A novel therapy for cancer treatment. Biomed. Pharmacother. 2019, 109, 484–492. [Google Scholar] [CrossRef]
- Dong, Z.; Dong, Z.; Lin, C.; Liu, Y.; Jin, H.; Wu, H.; Zhenjun, L.; Sun, L.; Zhang, L.; Hu, X.; et al. Upregulation of sestrins protect atriums against oxidative damage and fibrosis in human and experimental atrial fibrillation. Sci. Rep. 2017, 7, 46307. [Google Scholar] [CrossRef] [PubMed]
- Du, J.X.; Wu, J.Z.; Li, Z.; Zhang, C.; Shi, M.T.; Zhao, J.; Jin, M.W.; Liu, H. Pentamethylquercetin protects against cardiac remodeling via activation of Sestrin2. Biochem. Biophys. Res. Commun. 2019, 512, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Du, J.X.; He, W.; Zhang, C.; Wu, J.Z.; Li, Z.; Wang, M.; Feng, S.; Feng, S.Y.; Liang, G. Pentamethylquercetin Attenuates Cardiac Remodeling via Activation of the Sestrins/Keap1/Nrf2 Pathway in MSG-Induced Obese Mice. BioMed Res. Int. 2020, 2020, 3243906. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Han, F.; Lu, Q.; Li, X.; Ren, D.; Zhang, J.; Han, Y.; Xiang, Y.K.; Li, J. Empagliflozin Ameliorates Obesity-Related Cardiac Dysfunction by Regulating Sestrin2-Mediated AMPK-mTOR Signaling and Redox Homeostasis in High-Fat Diet-Induced Obese Mice. Diabetes 2020, 69, 1292–1305. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, G.; Kuang, Z.; Xu, Q.; Ye, T.; Li, X.; Qu, N.; Han, F.; Kan, C.; Sun, X. Empagliflozin activates Sestrin2-mediated AMPK/mTOR pathway and ameliorates lipid accumulation in obesity-related nonalcoholic fatty liver disease. Front. Pharmacol. 2022, 13, 944886. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, J.W.; Chung, H.S.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, K.M.; Baik, S.H.; Yoo, H.J. Knockdown of Sestrin2 Increases Lipopolysaccharide-Induced Oxidative Stress, Apoptosis, and Fibrotic Reactions in H9c2 Cells and Heart Tissues of Mice via an AMPK-Dependent Mechanism. Mediat. Inflamm. 2018, 2018, 6209140. [Google Scholar] [CrossRef]
- Huang, R.; Chen, F.; Zeng, A.; Ke, J.; Lin, S. Serum Sestrin2 Was Lower in Septic Shock Patients with Cardiomyopathy. Dis. Markers 2022, 2022, 1390373. [Google Scholar] [CrossRef]
- An, L.; Yang, T.; Zhong, Y.; Yin, Y.; Li, W.; Wang, Y. Molecular pathways in sepsis-induced cardiomyocyte pyroptosis: Novel finding on long non-coding RNA ZFAS1/miR-138-5p/SESN2 axis. Immunol. Lett. 2021, 238, 47–56. [Google Scholar] [CrossRef]
- Jegal, K.H.; Ko, H.L.; Park, S.M.; Byun, S.H.; Kang, K.W.; Cho, I.J.; Kim, S.C. Eupatilin induces Sestrin2-dependent autophagy to prevent oxidative stress. Apoptosis Int. J. Program. Cell Death 2016, 21, 642–656. [Google Scholar] [CrossRef]
- Jegal, K.H.; Kim, E.O.; Kim, J.K.; Park, S.M.; Jung, D.H.; Lee, G.H.; Ki, S.H.; Byun, S.H.; Ku, S.K.; Cho, I.J.; et al. Luteolin prevents liver from tunicamycin-induced endoplasmic reticulum stress via nuclear factor erythroid 2-related factor 2-dependent sestrin 2 induction. Toxicol. Appl. Pharmacol. 2020, 399, 115036. [Google Scholar] [CrossRef]
- Kim, G.T.; Lee, S.H.; Kim, J.I.; Kim, Y.M. Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner. Int. J. Mol. Med. 2014, 33, 863–869. [Google Scholar] [CrossRef]
- Seo, K.; Seo, S.; Han, J.Y.; Ki, S.H.; Shin, S.M. Resveratrol attenuates methylglyoxal-induced mitochondrial dysfunction and apoptosis by Sestrin2 induction. Toxicol. Appl. Pharmacol. 2014, 280, 314–322. [Google Scholar] [CrossRef]
- Sengupta, S.; Giaime, E.; Narayan, S.; Hahm, S.; Howell, J.; O’Neill, D.; Vlasuk, G.P.; Saiah, E. Discovery of NV-5138, the first selective Brain mTORC1 activator. Sci. Rep. 2019, 9, 4107. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahid, M.A.; Abdelsalam, S.S.; Raïq, H.; Parray, A.; Korashy, H.M.; Zeidan, A.; Elrayess, M.A.; Agouni, A. Sestrin2 as a Protective Shield against Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 4880. https://doi.org/10.3390/ijms24054880
Zahid MA, Abdelsalam SS, Raïq H, Parray A, Korashy HM, Zeidan A, Elrayess MA, Agouni A. Sestrin2 as a Protective Shield against Cardiovascular Disease. International Journal of Molecular Sciences. 2023; 24(5):4880. https://doi.org/10.3390/ijms24054880
Chicago/Turabian StyleZahid, Muhammad Ammar, Shahenda Salaheldin Abdelsalam, Hicham Raïq, Aijaz Parray, Hesham Mohamed Korashy, Asad Zeidan, Mohamed A. Elrayess, and Abdelali Agouni. 2023. "Sestrin2 as a Protective Shield against Cardiovascular Disease" International Journal of Molecular Sciences 24, no. 5: 4880. https://doi.org/10.3390/ijms24054880
APA StyleZahid, M. A., Abdelsalam, S. S., Raïq, H., Parray, A., Korashy, H. M., Zeidan, A., Elrayess, M. A., & Agouni, A. (2023). Sestrin2 as a Protective Shield against Cardiovascular Disease. International Journal of Molecular Sciences, 24(5), 4880. https://doi.org/10.3390/ijms24054880







