Cellular FXIII in Human Macrophage-Derived Foam Cells
Abstract
1. Introduction
2. Results
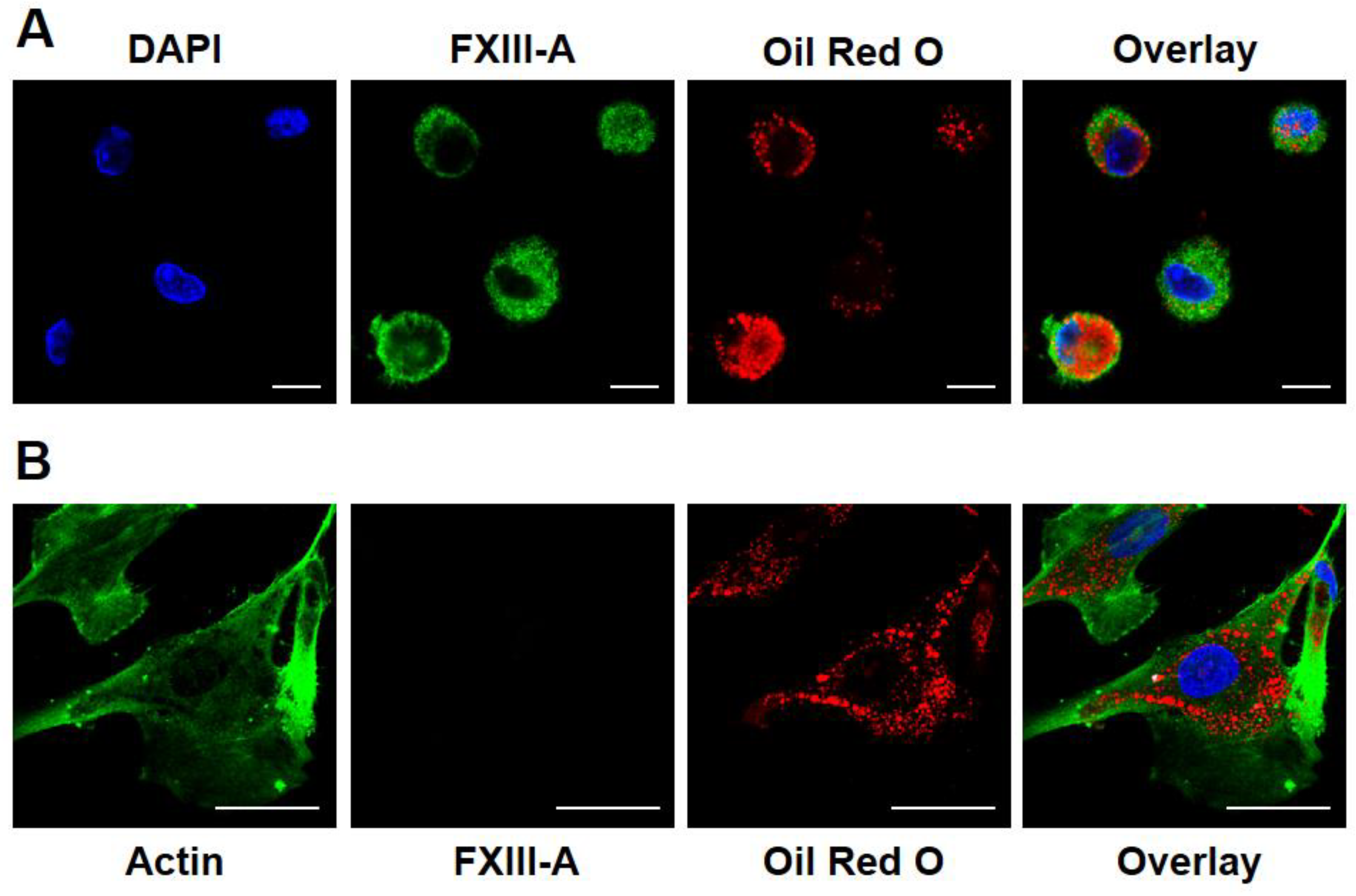
2.1. Investigation of Macrophage and HAoSMC-Derived Foam Cells by Immunofluorescence Microscopy for FXIII-A Expression and LDL Ingestion
2.2. Transformation of Macrophages into Foam Cells Results in Elevated Expression of Cellular FXIII
2.3. Macrophages and FXIII-A in the Atherosclerotic Plaque
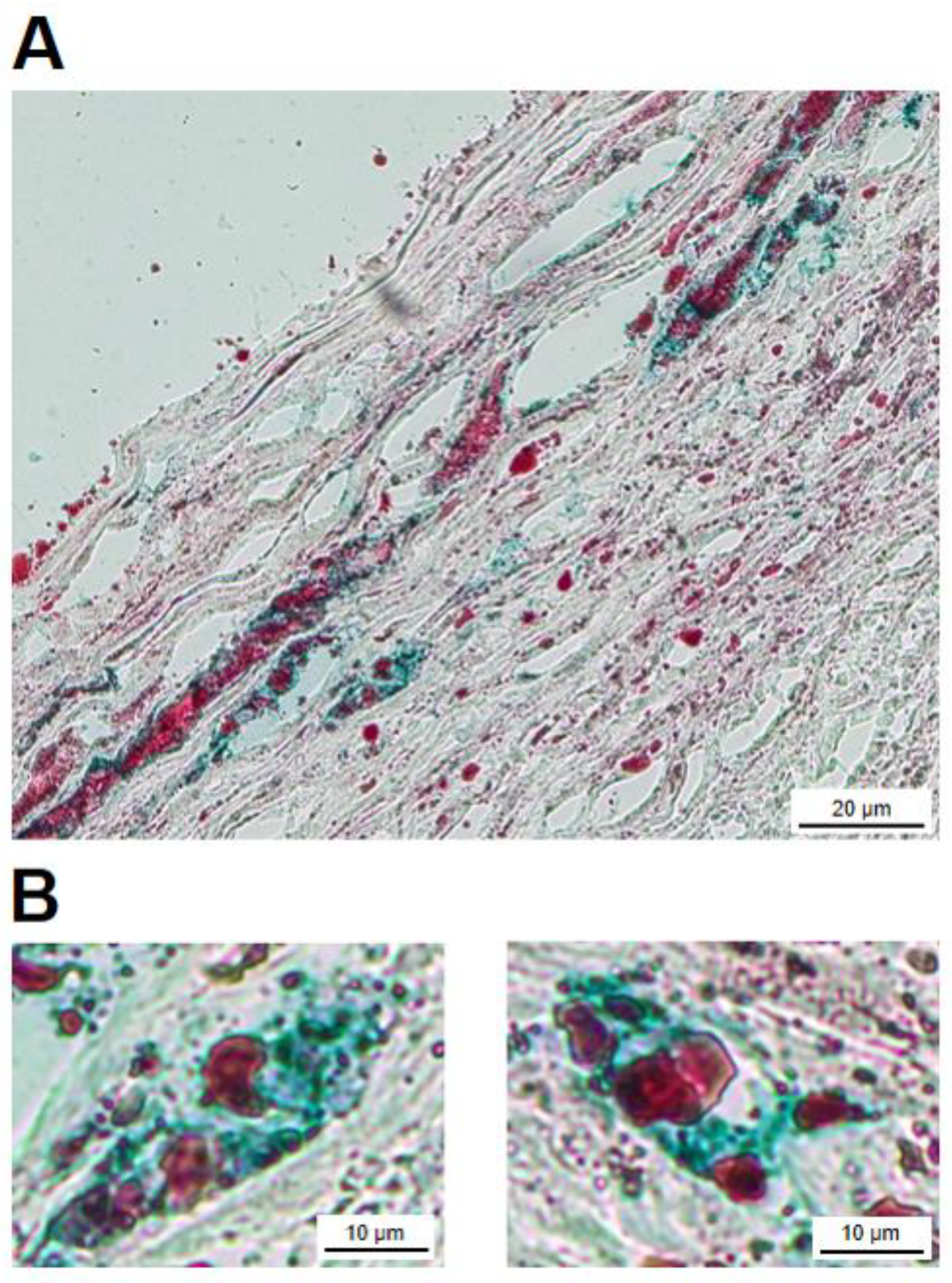
2.4. Visualization of FXIII-A and Isopeptide Cross-Links within the Atherosclerotic Plaque by Immunohistochemistry
2.5. FXIII-A-Containing Foam Cells within the Atherosclerotic Plaque
3. Discussion
4. Materials and Methods
4.1. Culturing of Macrophages and Induction of Foam Cell Formation
4.2. Immunofluorescent Analysis of Macrophage-Derived Foam Cells
4.3. Preparation of Enzyme-Modified LDL (eLDL)
4.4. Human Aortic Smooth Muscle Cell (HAoSMC)-Derived Foam Cell Formation
4.5. Immunofluorescent Analysis of HAoSMCs-Derived Foam Cells
4.6. Quantification of FXIII-A by ELISA in Macrophage-Derived Foam Cells
4.7. Western Blotting
4.8. Investigation of Atherosclerotic Plaque by Immunohistochemistry
4.9. Lipids in FXIII-A-Positive Cells of the Atherosclerotic Plaque
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komaromi, I.; Bagoly, Z.; Muszbek, L. Factor XIII: Novel structural and functional aspects. J. Thromb. Haemost. 2011, 9, 9–20. [Google Scholar] [CrossRef]
- Muszbek, L.; Bereczky, Z.; Bagoly, Z.; Komaromi, I.; Katona, E. Factor XIII: A coagulation factor with multiple plasmatic and cellular functions. Physiol. Rev. 2011, 91, 931–972. [Google Scholar] [CrossRef]
- Alshehri, F.S.M.; Whyte, C.S.; Mutch, N.J. Factor XIII-A: An Indispensable “Factor” in Haemostasis and Wound Healing. Int. J. Mol. Sci. 2021, 22, 3055. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.L.; Mutch, N.J. Let’s cross-link: Diverse functions of the promiscuous cellular transglutaminase factor XIII-A. J. Thromb. Haemost. 2019, 17, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, V.; Kohler, H.P. Factor XIII: Structure and Function. Semin. Thromb. Hemost. 2016, 42, 422–428. [Google Scholar]
- Byrnes, J.R.; Wolberg, A.S. Newly-Recognized Roles of Factor XIII in Thrombosis. Semin. Thromb. Hemost. 2016, 42, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, L.; Adany, R.; Szegedi, G.; Polgar, J.; Kavai, M. Factor XIII of blood coagulation in human monocytes. Thromb. Res. 1985, 37, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Adany, R.; Belkin, A.; Vasilevskaya, T.; Muszbek, L. Identification of blood coagulation factor XIII in human peritoneal macrophages. Eur. J. Cell. Biol. 1985, 38, 171–173. [Google Scholar]
- Henriksson, P.; Becker, S.; Lynch, G.; McDonagh, J. Identification of intracellular factor XIII in human monocytes and macrophages. J. Clin. Invest. 1985, 76, 528–534. [Google Scholar] [CrossRef]
- Sun, H.; Kaartinen, M.T. Transglutaminases in Monocytes and Macrophages. Med. Sci. (Basel) 2018, 6, 115. [Google Scholar] [CrossRef]
- Muszbek, L.; Adany, R.; Kavai, M.; Boda, Z.; Lopaciuk, S. Monocytes of patients congenitally deficient in plasma factor XIII lack factor XIII subunit a antigen and transglutaminase activity. Thromb. Haemost. 1988, 59, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Murtaugh, M.P.; Arend, W.P.; Davies, P.J. Induction of tissue transglutaminase in human peripheral blood monocytes. J. Exp. Med. 1984, 159, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Sarvary, A.; Szucs, S.; Balogh, I.; Becsky, A.; Bardos, H.; Kavai, M.; Seligsohn, U.; Egbring, R.; Lopaciuk, S.; Muszbek, L.; et al. Possible role of factor XIII subunit A in Fcgamma and complement receptor-mediated phagocytosis. Cell Immunol. 2004, 228, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Cordell, P.A.; Kile, B.T.; Standeven, K.F.; Josefsson, E.C.; Pease, R.J.; Grant, P.J. Association of coagulation factor XIII-A with Golgi proteins within monocyte-macrophages: Implications for subcellular trafficking and secretion. Blood 2010, 115, 2674–2681. [Google Scholar] [CrossRef]
- Alshehri, F.S.M.; Whyte, C.S.; Tuncay, A.; Williams, M.L.; Wilson, H.M.; Mutch, N.J. Monocytes Expose Factor XIII-A and Stabilize Thrombi against Fibrinolytic Degradation. Int. J. Mol. Sci. 2021, 22, 6591. [Google Scholar] [CrossRef]
- da Silva, R.F.; Lappalainen, J.; Lee-Rueckert, M.; Kovanen, P.T. Conversion of human M-CSF macrophages into foam cells reduces their proinflammatory responses to classical M1-polarizing activation. Atherosclerosis 2016, 248, 170–178. [Google Scholar] [CrossRef]
- Nagenborg, J.; Goossens, P.; Biessen, E.A.L.; Donners, M. Heterogeneity of atherosclerotic plaque macrophage origin, phenotype and functions: Implications for treatment. Eur. J. Pharmacol. 2017, 816, 14–24. [Google Scholar] [CrossRef]
- Gui, Y.; Zheng, H.; Cao, R.Y. Foam Cells in Atherosclerosis: Novel Insights Into Its Origins, Consequences, and Molecular Mechanisms. Front. Cardiovasc. Med. 2022, 9, 845942. [Google Scholar] [CrossRef]
- Lee-Rueckert, M.; Lappalainen, J.; Kovanen, P.T.; Escola-Gil, J.C. Lipid-Laden Macrophages and Inflammation in Atherosclerosis and Cancer: An Integrative View. Front. Cardiovasc. Med. 2022, 9, 777822. [Google Scholar] [CrossRef]
- Romanic, A.M.; Arleth, A.J.; Willette, R.N.; Ohlstein, E.H. Factor XIIIa cross-links lipoprotein(a) with fibrinogen and is present in human atherosclerotic lesions. Circ. Res. 1998, 83, 264–269. [Google Scholar] [CrossRef]
- Kapusta, P.; Wypasek, E.; Natorska, J.; Grudzien, G.; Sobczyk, D.; Sadowski, J.; Undas, A. Factor XIII expression within aortic valves and its plasma activity in patients with aortic stenosis: Association with severity of disease. Thromb. Haemost. 2012, 108, 1172–1179. [Google Scholar] [CrossRef]
- Gomez, D.; Owens, G.K. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc. Res. 2012, 95, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, H.; Huang, X.; Li, F.; Zhu, H.; Li, Y.; He, L.; Zhang, H.; Pu, W.; Liu, K.; et al. Arterial Sca1(+) Vascular Stem Cells Generate De Novo Smooth Muscle for Artery Repair and Regeneration. Cell. Stem. Cell. 2020, 26, 81–96.e4. [Google Scholar] [CrossRef] [PubMed]
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014, 129, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, J.; Corti, A.; Lerouge, L.; Pompella, A.; Gaucher, C. Phenotypic Modulation of Macrophages and Vascular Smooth Muscle Cells in Atherosclerosis-Nitro-Redox Interconnections. Antioxidants 2021, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Torocsik, D.; Bardos, H.; Nagy, L.; Adany, R. Identification of factor XIII-A as a marker of alternative macrophage activation. Cell Mol. Life Sci. 2005, 62, 2132–2139. [Google Scholar] [CrossRef]
- Torocsik, D.; Szeles, L.; Paragh, G., Jr.; Rakosy, Z.; Bardos, H.; Nagy, L.; Balazs, M.; Inbal, A.; Adany, R. Factor XIII-A is involved in the regulation of gene expression in alternatively activated human macrophages. Thromb. Haemost. 2010, 104, 709–717. [Google Scholar] [CrossRef]
- Rios, F.J.; Koga, M.M.; Pecenin, M.; Ferracini, M.; Gidlund, M.; Jancar, S. Oxidized LDL induces alternative macrophage phenotype through activation of CD36 and PAFR. Mediat. Inflamm 2013, 2013, 198193. [Google Scholar] [CrossRef]
- Adany, R.; Bardos, H.; Antal, M.; Modis, L.; Sarvary, A.; Szucs, S.; Balogh, I. Factor XIII of blood coagulation as a nuclear crosslinking enzyme. Thromb. Haemost. 2001, 85, 845–851. [Google Scholar]
- Bhakdi, S.; Torzewski, M.; Klouche, M.; Hemmes, M. Complement and atherogenesis: Binding of CRP to degraded, nonoxidized LDL enhances complement activation. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2348–2354. [Google Scholar] [CrossRef]
- Petho, D.; Gall, T.; Hendrik, Z.; Nagy, A.; Beke, L.; Gergely, A.P.; Mehes, G.; Toth, C.; Gram, M.; Akerstrom, B.; et al. Ferryl Hemoglobin and Heme Induce A(1)-Microglobulin in Hemorrhaged Atherosclerotic Lesions with Inhibitory Function against Hemoglobin and Lipid Oxidation. Int. J. Mol. Sci. 2021, 22, 6668. [Google Scholar] [CrossRef] [PubMed]
- Katona, E.E.; Ajzner, E.; Toth, K.; Karpati, L.; Muszbek, L. Enzyme-linked immunosorbent assay for the determination of blood coagulation factor XIII A-subunit in plasma and in cell lysates. J. Immunol. Methods 2001, 258, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W., Jr.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somodi, L.; Horváth, E.; Bárdos, H.; Baráth, B.; Pethő, D.; Katona, É.; Balla, J.; Mutch, N.J.; Muszbek, L. Cellular FXIII in Human Macrophage-Derived Foam Cells. Int. J. Mol. Sci. 2023, 24, 4802. https://doi.org/10.3390/ijms24054802
Somodi L, Horváth E, Bárdos H, Baráth B, Pethő D, Katona É, Balla J, Mutch NJ, Muszbek L. Cellular FXIII in Human Macrophage-Derived Foam Cells. International Journal of Molecular Sciences. 2023; 24(5):4802. https://doi.org/10.3390/ijms24054802
Chicago/Turabian StyleSomodi, Laura, Emőke Horváth, Helga Bárdos, Barbara Baráth, Dávid Pethő, Éva Katona, József Balla, Nicola J. Mutch, and László Muszbek. 2023. "Cellular FXIII in Human Macrophage-Derived Foam Cells" International Journal of Molecular Sciences 24, no. 5: 4802. https://doi.org/10.3390/ijms24054802
APA StyleSomodi, L., Horváth, E., Bárdos, H., Baráth, B., Pethő, D., Katona, É., Balla, J., Mutch, N. J., & Muszbek, L. (2023). Cellular FXIII in Human Macrophage-Derived Foam Cells. International Journal of Molecular Sciences, 24(5), 4802. https://doi.org/10.3390/ijms24054802






