1. Introduction
Mayaro virus (MAYV) is a mosquito-borne virus of the Togaviridae family, Alphavirus genus, with other important human pathogens such as Chikungunya, Ross River, Sindbis and Venezuelan Equine Encephalitis viruses [
1]. The MAYV genome is a positive-sense, single-stranded RNA of approximately 11.5 kb comprising four nonstructural proteins (nsPs) involved in viral replication and five structural proteins that participate in the generation and maturation of viral particles [
2,
3]. First isolated in Trinidad in 1954, MAYV has been detected in several countries in South and Central America. Human cases of Mayaro Fever were reported in Brazil, Bolivia, Costa Rica, French Guiana, Guatemala, Haiti, Panama, Peru, Suriname and Venezuela [
4,
5]. Although Mayaro Fever occurrence is largely underreported or misdiagnosed as other arboviral diseases, recent changes in MAYV distribution in Brazil indicate that Mayaro Fever cases may be increasing in number and size [
6,
7]. There are no specific treatments or licensed vaccines against MAYV infections, despite several pre-clinical studies and ongoing phase 2/3 vaccine clinical trials [
5,
8]. Mayaro Fever consists of a mild, self-limited febrile disease with symptoms such as headache, skin rash, myalgia and arthralgia, but it may evolve into a long-lasting arthritogenic disease with joint inflammation and intense pain, leading to significant morbidity. The inflammasome and neutrophil extracellular traps were shown to participate in alphaviral arthritogenic diseases, but the pathogenesis of Mayaro Fever remains incompletely understood [
9,
10]. The currently used techniques to characterize and study Mayaro Fever often result in sample destruction or consumption. The use of non-destructive advanced imaging techniques may represent an important advantage, allowing multiple analyses in tissues that are hard to obtain, such as from joints.
X-ray tomography is a well-established technique used in medical radiography to view tissues in a living body using X-rays with a typical resolution of 1 to 2 mm. The achievement of X-ray tomography was recognized in the Nobel prize in Physiology or Medicine in 1979, and since then, it has been widely used in biological and material sciences for non-invasive cross-sectional imaging. Flannery and colleagues developed microtomography in 1987, producing three-dimensional (3D) images of internal structures of small samples with a micrometer resolution. Since then, X-ray microtomography (µCT) can be used to non-destructively create 3D images of internal sections of a sample with a resolution limit comparable to that of a light microscope.
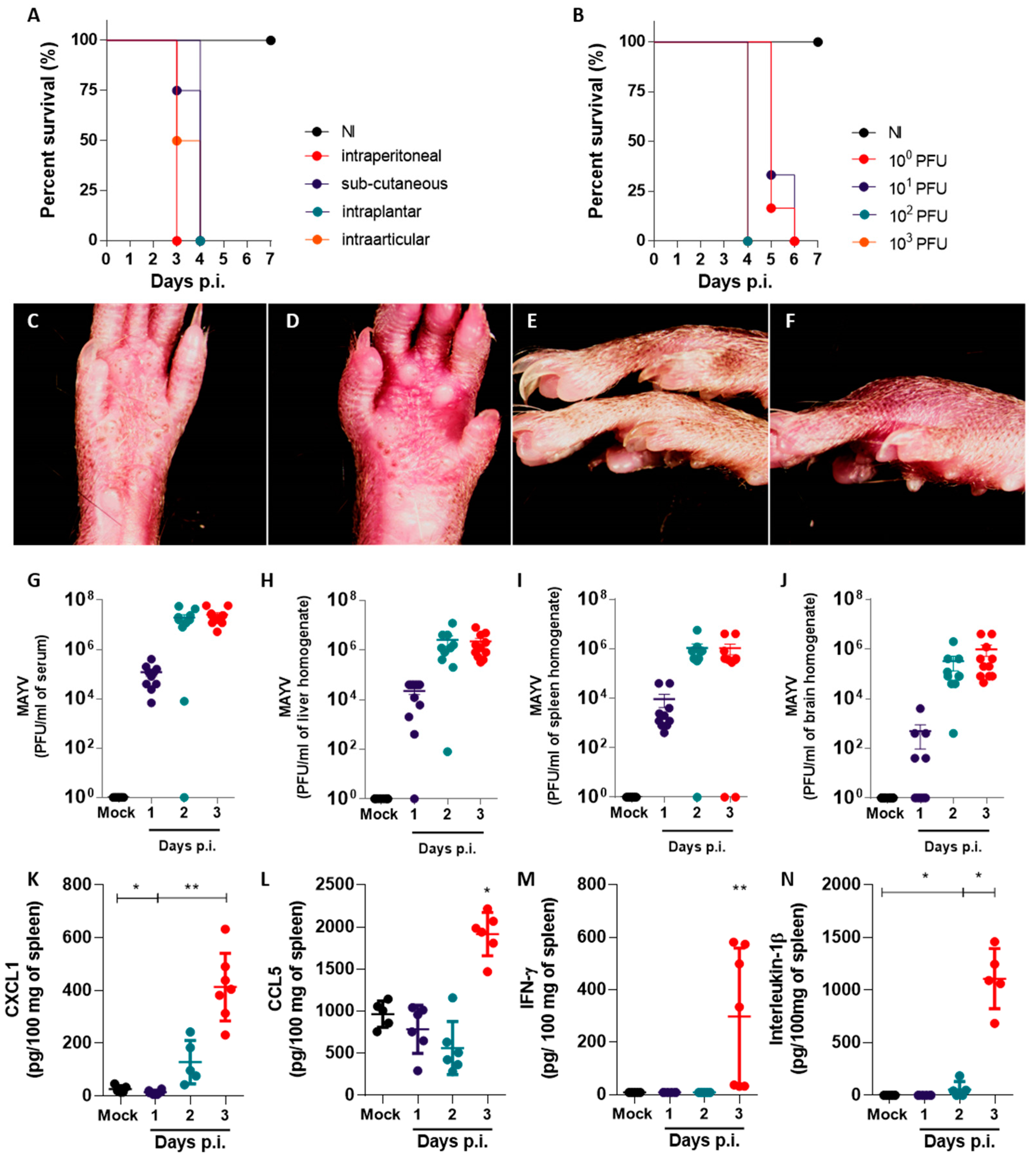
In this study, we established a mouse model of MAYV infection in susceptible type I interferon receptor-deficient (IFNAR−/−) mice to characterize disease development and paw inflammation. We correlated the onset of edema with other aspects of inflammatory responses induced by the MAYV infection, such as the production of proinflammatory cytokines and leukocyte recruitment. Using a semi-automated phase–contrast X-ray microtomography performed at a synchrotron source, we quantified and characterized MAYV-induced paw edema in soft tissues with precision and high reproducibility. Detailed features of MAYV-induced paw inflammation observed using microtomography should be useful in studying the pathogenesis of other viral diseases in which inflammation is a critical feature.
3. Discussion
In vivo models of the alphavirus infection are important tools for investigating the mechanisms of alphavirus-induced disease development and resistance, as well as for assessing the efficacy and safety of candidate treatments [
11,
13]. Adult immunocompetent wild-type (WT) mice are resistant to MAYV inoculation, while young mice or mice deficient in Type-I IFN responses are susceptible and develop disease [
8,
14]. Our objective was to better understand the main features of MAYV-induced disease in vivo and thus establish the susceptible mouse model of MAYV infection using IFNAR−/− mice. MAYV infection promoted an acute and lethal disease, with manifestations that corroborate previous studies [
14,
15]. The footpad injection of IFNAR−/− mice with MAYV inocula as low as 1 PFU led to the development of disease signs and death up to 6 days p.i. Paw inflammation was observed from day 1 p.i. and was characterized by edema, the disruption of tissue architecture and the infiltration of leukocytes. Eye irritation was observed in infected mice in all experiments, corroborating a study by Santos and colleagues [
12], and was predictive of death in this model. Ocular symptoms, although commonly described in human MAYV cases, are overlooked in animal models. Ocular manifestations take place in human infections with other alphaviruses (Chikungunya, Ross River, Eastern, Western and Venezuelan Equine Encephalitis viruses) and might indicate the involvement of the central nervous system (CNS) [
16,
17,
18]. MAYV, Chikungunya virus (CHIKV) and other alphaviruses of the Semliki Forest complex cause diseases with similar clinical manifestations in humans [
1], suggesting that these viruses may share mechanisms of pathogenicity. We expect our findings on MAYV-induced paw inflammation and systemic disease to be translatable to other models of alphaviral disease, including the application of soft-tissue µCT in research.
We observed elevated viremia and the presence of MAYV in the spleen, liver and brain of MAYV-infected mice one day after intraplantar infection (
Figure 1). We interpret the accumulation of MAYV in the brains of IFNAR−/− as a possible limitation of our in vivo infection model, arising from the severe susceptibility of the IFNAR−/− mouse strain to alphavirus infection due to an abrogated type-I interferon response. Such conditions result in the widespread dissemination of the virus to mouse tissues, some of which may not recapitulate disease in humans. Changes in proinflammatory cytokine and chemokine levels, leukocyte populations and histological scores, observed later in infection, were concomitant with disease signs (
Figure 3). Chemokine and cytokine expression is concentrated mostly in the spleen of infected mice. The expression of CXCL1, CCL5, IFN-γ and IL-1β were found to be increased in the spleen, in accordance with previous reports for IFNAR−/− mice MAYV models [
11,
14]. However, cytokines such as IL-6 and TNF-α that are typically associated with inflammatory responses were absent in all the organs and time points evaluated. The expression of CXCL10, a proinflammatory chemokine induced by IFN-γ and important in antiviral responses [
19], was absent in the brain, spleen and liver of infected mice, though IFN-γ was upregulated in the spleen. Collectively, these observations suggest that expression of proinflammatory mediators such as IL-6, TNF-α and CXCL10 may depend on intact type-I interferon responses and that MAYV-induced inflammation and disease are not associated with these mediators in this model of infection.
In addition to the inoculated paw, the spleen seems to be most affected by MAYV infection in this model. Although spleens of MAYV-infected mice are heavier than spleens from mock-infected mice, they contain, on average, less than half of the cells, indicating reduced cellularity and edema in the organ. The total numbers of CD4+ T lymphocytes (T CD4+), T CD8+, macrophages and CD11b- dendritic cells were reduced, while neutrophils and CD11b+ dendritic cells remained unchanged. The reduction in the absolute number of macrophages (more than 20-fold reduction) and T CD4+ lymphocytes (2-fold reduction), the latter being the most abundant leukocyte population in the spleen, are likely to account for the total reduction in spleen cellularity observed in MAYV-infected mice. MAYV replicates in RAW 264.7 and J774 macrophage cell lines and primary cultures of peritoneal and bone marrow-derived macrophages. It leads to the production of reactive oxygen species, secretion of proinflammatory chemokines, apoptosis and cell death [
9,
20]. Thus, we speculate that MAYV infection in splenic macrophages is leading to cell death in vivo, ultimately resulting in the reduction of this population and spleen inflammation in infected mice. Moreover, the loss of functional splenic macrophages may explain the absence of TNF-α expression in the spleen, as macrophages are important sources of this cytokine [
21,
22].
Our results suggest that neutrophils participate in the inflammatory response to MAYV infection in both the paw and the spleen. The neutrophil chemoattractant CXCL1 was upregulated in the MAYV-infected mice in all organs tested, including the inflamed paw. De Castro-Jorge and colleagues [
9] demonstrated that neutrophils are recruited to MAYV-infected paws in WT immunocompetent mice, which corroborates our observation of PMN recruitment to MAYV-infected muscle and ligaments in susceptible IFNAR−/− mice and indicates that this process is independent of intact type I IFN responses (
Figure 3). Interestingly, the neutrophil population in the spleen, although similar in number between infected and non-infected mice, was activated in infected mice and expressed higher levels of CD62L (
Figure 2). CD62L, also known as L-selectin, is an adhesion molecule involved in the recruitment of leukocytes to inflamed tissues, which is typically shed from the surface of neutrophils upon integrin activation and after transmigration from the bloodstream to the tissue [
23]. Thus, activated neutrophils in target tissues would have lower levels of CD62L on the surface, as assessed by conventional flow cytometry. Neutrophils in the spleen of MAYV-infected mice express higher levels of CD62L than uninfected control mice, indicating an abnormal activation status. The implication of higher CD62L expression on neutrophils during alphavirus diseases is unknown, but previous studies from our group with bronchoalveolar lavage samples from severe COVID-19 infected human patients in ICU also presented abnormal activation of neutrophils, pointing towards a possible role for neutrophils contributing to the severity of inflammation upon viral infections [
24].
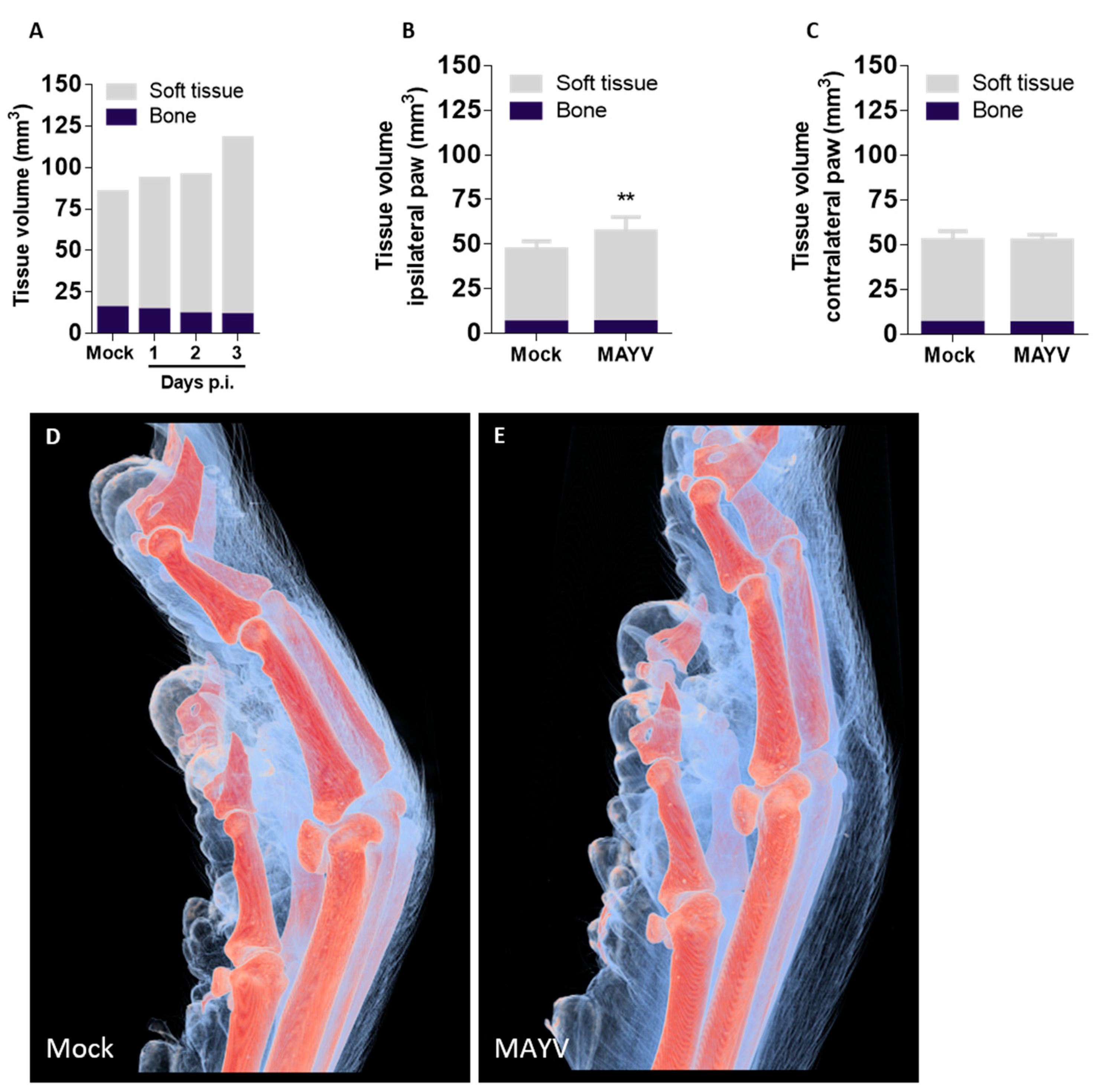
Although three-dimensional visualization of mice anatomy was previously demonstrated, imaging was often restricted to bone or other tissues with high mineral content or required extensive sample preparation with the use of toxic, corrosive reagents for tissue clearance or enhancement prior to measurements [
25,
26]. We established the first µCT technique that also allows imaging of soft tissue to visualize unappreciated features of MAYV infection and disease without contrasting agents. The 2D virtual slices obtained from the reconstructed volumes corroborate results obtained by conventional histopathology assays, with the advantage of visualizing multiple axes or points of view without the need to prepare new samples from the start, a requirement for histological methods [
27,
28]. Using soft-tissue µCT, we were able to confirm previous results on MAYV-induced edema produced using visual observation and histology and added detailed information on edema localization and tissue architecture. A main motivation for the use of soft tissue µCT is the possibility of obtaining quantitative measurements of tissue volume with high precision. Each pixel in 2D virtual slices of mouse paws corresponds to 16.9 μm
2, and each voxel in 3D volumes to 69 μm
3, which represent a significant increase in precision and sensitivity in comparison to instruments such as a paquimeter or plethysmometer, used to assess edema in biological samples. Although the manual segmentation of volumes can be more precise, the process is time-consuming and has low reproducibility and scalability. Thus, to overcome these limitations and allow for comparisons between individuals and experimental groups, we limited manual intervention in the soft tissue µCT analysis process to a minimum: sample positioning according to an anatomical region (the joint between distal and intermediate phalanges in the middle finger). Soft tissue µCT should be useful not only for the study of edema and inflammation but it could be adapted for imaging different target tissues or anatomical structures. Different sample preparation strategies and tissue-specific staining using X-rays would allow the segmentation of multiple soft tissues and structures [
29,
30].
The scalability of segmentation is a reflection not only of the chosen segmentation algorithms but also due to the robustness of the µCT measurement and reconstruction achieved by the rigorous control of all the imaging and reconstruction parameters described above. Moreover, when combined with labeling techniques for the detection of specific molecules, such as immunogold or in situ hybridization, soft tissue µCT becomes a valuable resource for the characterization of cell types and molecules involved in many aspects of the inflammatory process. Such a combination would allow virtual histology analyses and the precise quantification of healthy and damaged tissues.
In conclusion, this in vivo model of MAYV infection in IFNAR−/− mice allowed observation of important features of disease and inflammation induced by MAYV and is corroborated by previous studies [
8,
9,
11,
12,
14,
15]. We are the second group to describe eye irritation in mice, a common manifestation in Mayaro Fever patients that is overlooked in animal studies. The systemic spread of MAYV with high viremia and the upregulated expression of proinflammatory chemokines and cytokines was observed. The absence of CXCL10, IL-1β and TNF-α expression may be explained by the abrogated type-I interferon response and a sharp reduction in splenic macrophages, one of the main sources of TNF-α. Neutrophils also participate in MAYV pathogenesis in this model, and an abnormal activation status was observed in the spleen; however, their participation in disease pathogenesis needs further studies. Finally, we established a μCT technique that allows visualization of both bones and soft tissue in high definition, enabling the exploration of unappreciated features of MAYV-induced disease. As a perspective, the combination of paw μCT with staining techniques based on heavy metal labeling should further increase the scope of the application of μCT in biomedical research.
4. Materials and Methods
4.1. Viral Stock
The MAYV strain IQT 4235 (GenBank accession number MK070491.1), originally isolated from a symptomatic patient in the Peruvian Amazon, was provided by Dr. Scott Weaver (UTMB, Galveston, TX, USA). Viral stocks used in the experiments were generated by passages in mycoplasma-free Vero CCL81 cells (ATCC, Manassas, VA, USA), cultivated in T75 culture flasks with DMEM supplemented with 10% v/v FBS and 1% v/v penicillin–streptomycin (Gibco, Billings, MT, USA) and incubated at 37 °C in a 5% v/v CO2 atmosphere. Culture supernatant was harvested on observation of a 50% cytopathic effect, clarified (3000 rpm, 8 min), aliquoted and stored at −80 °C until use. Viral titers of each stock were determined by a plaque assay.
4.2. Mice
Adult (8–12 weeks old) male and female IFNAR−/− mice in the SV129 background, which are deficient for the type I IFN receptor, were bred in the Animal Facility of CNPEM. Up to five mice were allocated in cages with access to water and food ad libitum. The Animal facility operates in specific pathogen-free conditions with controlled temperature and photoperiod (21 °C, 12 h/12 h dark/light cycle). All experiments were performed under the guidelines and approval of the CNPEM Committee on Ethics and Use of Animals (CEUA) under protocols 30B and 79.
4.3. In Vivo MAYV Infection and Disease Model
IFNAR−/− mice were injected with MAYV IQT 4235 at different inocula (from 105 PFU/animal to 100 PFU/animal) through intra-articular (knee), subcutaneous (back), intraplantar or intraperitoneal routes. Mock-infected controls received the same volume of PBS. In lethality experiments, weight loss and disease signs (changes in behavior and movement, hunched posture, ruffled fur, loss of grip, ocular inflammation) were monitored daily from day 0 post-infection (p.i.) until day 6 p.i. For assessment of viral replication, histopathology, ELISA, flow cytometry and X-ray microtomography experiments, mice were injected with MAYV IQT 4235 at 102 PFU/animal via the intraplantar route, and weight loss and disease signs were monitored daily from day 0 to day 3 p.i. Blood, brain, liver, spleen and hind paws were collected on days 1, 2 and 3 p.i. and prepared according to the subsequent experiment. Mice were euthanized with an overdose of anesthetic (ketamine and xylazine in PBS) with subsequent cervical displacement prior to sample collection.
4.4. Viral Load Quantification
Viral load in all samples and viral stocks were determined through a plaque assay in Vero CCL81 cells. Briefly, organs were weighed and macerated to a 10% w/v solution with PBS. Organ suspensions, or liquid samples such as blood plasma or culture supernatant, were serially diluted 10-fold ranging from 10−1 to 10−6 and incubated with confluent Vero cell monolayers in 24 wells plates, for 1h at 37 °C, 5% CO2. After adsorption, supernatant was removed, and 2mL of viscous complete DMEM supplemented with 2% w/v carboxymethylcellulose was added to each well and incubated for 4 days. Assay plates were fixed in 10% w/v paraformaldehyde and stained with 1% w/v methylene blue. Viral titers in samples were determined by counting viral plaques, and results were expressed as PFU/mL of serum/culture supernatant or PFU/mg of organ.
4.5. Histopathology
Mice were euthanized with 1:2 ketamine–xylazine solution (85%
v/v in PBS) intraperitoneally. Hind paws were collected, fixed in paraformaldehyde 4%
w/v in PBS and kept in 70% ethanol until paraffin embedding. Sections 5 μm thick were obtained from longitudinal cuts parallel to the footpad, mounted on glass slides and stained with hematoxylin–eosin (H&E). Analysis of edema, vascularity/diapedesis and cell infiltration on observed tissues was made through light microscopy. A histological score was created to assess progression of disease signs at tissue level based on previous work by Ierna et al. [
31]. For the skin, muscle and fascia, the following parameters were scored: infiltration of mononuclear cells: none (0); mild (1); moderate (2); severe (3); infiltration of PMN cells: none (0); mild (1); moderate (2); severe (3); Vascularity/Diapedesis: none (0); mild (1); moderate (2); severe (3); Edema: none (0); mild (1); moderate (2); severe (3). For bone: infiltration of mononuclear cells: none (0); mild (1); moderate (2); severe (3); infiltration of PMN cells: none (0); mild (1); moderate (2); severe (3); Erosion: none (0); mild surface (1); moderate (2); extensive full thickness (3); Hyperplasia (synovia): none (0); mild surface (1); moderate (2); extensive full thickness (3).
4.6. Quantification of Cytokines and Chemokines
Enzyme-linked immunosorbent assays (ELISA) were used to quantify the levels of the cytokines and chemokines CXCL1, CCL5, IFN-γ, IL-1β, CXCL10, IL-17, TNF-α and IL-6 in samples of mouse spleen, liver and brain. Samples were weighed and homogenized to a 10% w/v solution in tissue extraction buffer. Assays were performed using R&D’s DuoSet® ELISA Development Systems kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Samples with cytokine levels below the limit of detection of each assay are indicated by N.D. (not detected).
4.7. Hematological Parameters
Peripheral blood from brachial plexus of mice was collected in EDTA-coated tubes on days 1 to 3 p.i. For total leukocyte counting, 10 µL of fresh blood was diluted in 90 µL Turk’s solution for erythrocyte lysis and leukocyte staining. Counting was performed with 10 µL of the Turk-blood dilution into Neubauer chambers under optical microscopy. For differential leukocyte counting, smear slides were made with 10 µL of blood, followed by staining with the Panoptic Fast Stain Kit (Laborclin) according to the manufacturer’s instructions. Stained smear slides were observed in an optical microscope. A minimum of 100 cells were counted per slide and leukocytes were identified and counted as mononuclear or polymorphonuclear cells according to the nuclear morphology. Numbers of mononuclear and polymorphonuclear cells observed were used to calculate the proportion of each population within the total leukocyte counting. Total and differential leukocyte counting results are expressed as 104 cells/mL of blood.
4.8. Flow Cytometry
Mice spleens were collected and macerated in 3.5 mL ice-cold DMEM with 10% v/v FBS and centrifuged at 1200 rpm, 4 °C, for 10 min. Erythrocytes were lysed using ACK Lysing Buffer (Gibco) according to the manufacturer protocols. Cells were counted under optical microscopy and 2 × 106 cells per well were seeded in a 96-well plate in 200 μL per well. Single-cell suspensions from the spleen were incubated with 2.5 g/mL Fc block (clone 93, Biolegend, San Diego, CA, USA) to block non-specific binding for 10 min at room temperature.
Cells were stained with monoclonal antibodies anti-CD3 APC (17A2), CD4 PE (GK1.5), CD8 FITC (53-6.7), CD11b PE-Cy7 (M1/70), CD11c APC-Cy7 (N418), CD25 APC-Cy7 (PC61), CD62L FITC (MEL-14), CD69 PE-Cy7 (H1.2F3), F4/80 PE (BM8), Ly6G APC (1A8), IA/IE Percp-Cy5.5 (M5/114.15.2), all from BioLegend (San Diego, CA, USA), for 30 min in FACS Buffer at 4 °C, washed and resuspended in paraformaldehyde 2% before acquisition on the flow cytometer (FACS Canto II; BD Biosciences, San Diego, CA, USA). Data were analyzed using FlowJo software (BD Bioscience).
4.9. Microtomography
The mouse paws were placed inside a pipette tip in an upright position and mounted at the rotation stage. By revolving the sample around a fixed rotation axis by 180 degrees in uniformly spaced angular steps to compose a sinogram that was later computationally transformed into the 3D map of the sample, 1024 X-ray transmission images were acquired. The transmission images were obtained using radiation from the 1.67 T bending magnet of the 1.37 GeV UVX storage ring, filtered by 0.55 mm Si filters. These setups produced a polychromatic beam with peak energy at approximately 13 keV and 50% bandwidth. The radiographs were recorded by an indirect detector system (PCO.2000 14-bit CCD cooled camera) and 2X objective, with a pixel size of 4.11 × 4.11 μm2. Considering the detector pixel array size of 2048 × 2048, the field of view (FOV) of the radiographs was 8.4 mm × 8.4 mm. This FOV was sufficient to guarantee compact support for the tomography (horizontal axis) but not for imaging the entire paw in the vertical direction. Thus, for each paw 3 tomograms on 3 different heights were obtained. Data were reconstructed using the standard filtered back projection with parzen window, as implemented at the ASTRA toolbox. Finally, the 3D reconstructions of each height were digitally combined.
4.10. Segmentation and Volume Analysis
The 3D reconstructed data obtained, as mentioned previously, were combined using the Avizo software. Each sample consisted of 3 independent tomograms that were aligned and merged into a single 3D volume. Following, all post-processing was performed using the free Fiji software. Those would consist of data cropping and slice denoising by non-local means. After denoising, volume quantification involved two distinct strategies for both soft-tissue and bone segmentation.
For the soft-tissue segmentation, the Level Sets algorithm (Level Sets and Fast Marching,
https://imagej.net/plugins/level-sets, accessed on 23 August 2022) allowed the segmentation of paw boundary on a 2D reconstructed slice. Briefly, this algorithm iterates a contour line until it hits the object boundary. Some parameters like curvature and convergence will prevent the contour from leaking if there are gaps in the object boundary and were properly adjusted for proper segmentation avoiding most of the paws’ hair without leaking into the paw slice. Finally, as the Level Set algorithm is a 2D segmentation tool, a slice-by-slice strategy with each slice using a dilated initial guess based on the previous slice was implemented. In this sense, this was a semiautomatic segmentation strategy, as the initial slice had to be segmented under supervision for parameter optimization.
For the bone segmentation, a threshold using the minimum criteria on the entire volume histogram generated a segmentation of almost all the bones. Still, a morphological filter based on a minimum volume and minimum perimeter-to-area ratio excluded any structure that was not the bone tissue. Finally, the morphologically filtered image was used as a seed for the Morphological Reconstruction 3D from MorphlibJ, resulting in the final bone binary mask.
Afterward, the obtained binary masks were inspected, and, if necessary, the segmented regions were manually refined. After segmentation of both soft tissue and bone, the entire 3D model was digitally sectioned at the joint connecting the distal and intermediate phalange in the middle finger. A box of approximately 6 × 6 × 6 mm was used to delimit the paw so that all the samples would account for the same anatomic features. The quantitative analysis of each tissue and paw was performed using the software MorpholibJ 3D analyze.
4.11. Statistical Analysis
Viral load, ELISA cytokine levels, flow cytometry and total leukocyte counting data were analyzed using Kruskal–Wallis nonparametric test and Dunn’s multiple comparisons’ post-test. Differential leukocyte counts and segmented volume data were analyzed with ordinary 2-way Analysis of Variance (ANOVA), followed by Tukey’s multiple comparison’s test. Weight loss was analyzed by multiple t-tests corrected for multiple comparisons using the Holm-Sidak method. Survival curves were compared by Mantel–Cox log-rank test. Differences between groups were considered significant if p < 0.05. All analyses were performed using the GraphPad Prism 8 software.