Targeting Measurable Residual Disease (MRD) in Acute Myeloid Leukemia (AML): Moving beyond Prognostication
Abstract
1. Introduction
2. Emerging MRD Markers
2.1. AML-Defining Molecular MRD Markers
2.2. Persistent Clonal Hematopoiesis with Oncogenic Potential (CHOP)
2.3. Signaling Pathway Mutations, Focusing on FLT3-ITD
3. Impact of Novel AML Therapies on MRD Endpoints
3.1. VEN and Hypomethylating Agent (HMA)/Low Dose Cytarabine (LDAC)
3.2. FLT3 Inhibitors
3.3. IDH1 and IDH2 Inhibitors
3.4. Gemtuzumab Ozogamicin (GO)
3.5. CC-486
| Therapy | Population | N | MRD Marker | Thresholds (% of N) | Timepoint | Outcomes (neg vs. pos) or (< vs. ≥ Threshold) |
|---|---|---|---|---|---|---|
| Venetoclax (VEN) | ||||||
| VEN-AZA [68] | New AML | 164 | MFC | BM < 0.1% (41) | Any | Median OS NR vs. 19 m 1 y OS 94% vs. 68% |
| VEN-DEC10 [69] | New AML | 83 | MFC | BM < 0.1% (54) | 1–4 m | Median OS 25 m vs. 7 m (2 m timepoint) |
| VEN-AZA [70] | New AML | 63 | MFC | BM < 0.1% (38) | 1–3 m | 18 m CIR 13% vs. 57% 18 m OS 70% vs. 35% Median OS 26.5 m vs. 10 m |
| VEN-HMA/LDAC [71] | New AML | 55 | NPM1 cDNA | BM < 0.005% (49) | ≤6 m | 18 m OS 87% vs. 39% |
| VEN-CLAD-LDAC alternating with VEN-AZA [72] | New AML | 51 | MFC | BM < 0.1% (84) | Any | 2 y OS 80% vs. 45% Median DFS NR vs. 5.9 m |
| VEN-FLAG-IDA [73] | New AML | 40 | MFC | BM < 0.1% (93) | Any | Median OS NR vs. 16 m |
| FLT3 inhibitor | ||||||
| Quizartinib [82] | New AML | 161 | FLT3-ITD | BM < 0.01% (24.6) | PC1 | Median OS NR vs. 29.4 m |
| Gilteritinib [80] | R/R | 49 | FLT3-ITD | BM < 0.01% (16) | Any | Median OS 131.4 weeks vs. 43.3 weeks |
| VEN-Gilteritinib [81] | R/R | 25 | FLT3-ITD | BM < 0.1% (60; 20% < 0.01%) | Any | Median OS 11.6 m vs. 8.2 m |
| IDH1/2 inhibitors | ||||||
| Ivosidenib + intensive chemotherapy [91] | New AML | 41 | IDH1 | BM < 0.02–0.04% (39) | Any | Not reported |
| 20 | MFC | BM < 0.1% (80) | Any | Not reported | ||
| Ivosidenib [83] | R/R | 34 | IDH1 | BM < 0.02–0.04% (21) | Any | Median OS 11.1 m vs. 6.5 m |
| Ivosidenib [86] | New AML | 14 | IDH1 | BM < 0.02–0.04% (64) | Any | Not reported |
| Ivosidenib + VEN ± AZA [90] | New AML or R/R | 31 | IDH1 | BM < 0.1–0.25% (67) | Any | Median OS NR vs. 8 m |
| MFC | BM < 0.1% (63) | |||||
| Enasidenib + intensive chemotherapy [91] | New AML | 64 | IDH2 | BM < 0.02–0.04% (23) | Any | Not reported |
| Enasidenib [85] | R/R | 101 | IDH2 | BM < 0.02–0.04% (12) | Any | Median OS 22.9 m vs. 8.8 m |
| Gemtuzumab ozogamicin | ||||||
| ALFA-0701 [95] | New AML | 61 | NPM1 cDNA | BM < 0.1% (25) | PC1 | 2 y CIR 21% vs. 55% |
| BM < 0.1% (78) | EOT | 2 y CIR 45% vs. 67% | ||||
| AMLSG 09-09 [96] | New AML | 232 | NPM1 cDNA | BM reduction ≥ 3 log10 (87) | PC2 | 4 y CIR 28.5% vs. 60% |
| PB neg (53) | PC2 | 4 y CIR 18% vs. 53% | ||||
| PB neg (78) | EOT | 4 y CIR 28% vs. 70% | ||||
| CC-486 | ||||||
| CC-486 [99] | AML in first remission | 236 | MFC | BM < 0.1% (37) | Any | Median OS 41.3 m vs. 9.0 m Median RFS 20.4 m vs. 2.8 m |
4. MRD-Guided Therapy in AML
4.1. MRD-Directed Therapy Using AZA
4.2. MRD-Directed Therapy Using Intensive Chemotherapy
4.3. MRD-Directed Therapy Using VEN
4.4. MRD-Directed Therapy Using FLT3 Inhibitors
4.5. Future Directions in MRD-Directed Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rollig, C.; Bornhauser, M.; Thiede, C.; Taube, F.; Kramer, M.; Mohr, B.; Aulitzky, W.; Bodenstein, H.; Tischler, H.J.; Stuhlmann, R.; et al. Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: Evaluation of the proposed reporting system. J. Clin. Oncol. 2011, 29, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Freeman, S.D.; Ossenkoppele, G.J.; Buccisano, F.; Hourigan, C.S.; Ngai, L.L.; Tettero, J.M.; Bachas, C.; Baer, C.; Béné, M.C.; et al. 2021 Update on MRD in acute myeloid leukemia: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2021, 138, 2753–2767. [Google Scholar] [CrossRef] [PubMed]
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Bene, M.C.; Buccisano, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S.; et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2018, 131, 1275–1291. [Google Scholar] [CrossRef] [PubMed]
- Dohner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Kronos Bio Speeds Development of Genetically Targeted Leukemia Drug with Unique Trial Design. Available online: https://www.statnews.com/2021/03/04/kronos-bio-speeds-development-of-genetically-targeted-leukemia-drug-with-unique-trial-design (accessed on 6 May 2021).
- Hirsch, P.; Zhang, Y.; Tang, R.; Joulin, V.; Boutroux, H.; Pronier, E.; Moatti, H.; Flandrin, P.; Marzac, C.; Bories, D.; et al. Genetic hierarchy and temporal variegation in the clonal history of acute myeloid leukaemia. Nat. Commun. 2016, 7, 12475. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.A.; Wood, B.L.; Othus, M.; Hourigan, C.S.; Ustun, C.; Linden, M.A.; DeFor, T.E.; Malagola, M.; Anthias, C.; Valkova, V.; et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: A meta-analysis. Haematologica 2017, 102, 865–873. [Google Scholar] [CrossRef]
- Short, N.J.; Fu, C.; Berry, D.A.; Walter, R.B.; Freeman, S.D.; Hourigan, C.S.; Huang, X.; Gonzalez, G.N.; Hwang, H.; Qi, X.; et al. Association of hematologic response and assay sensitivity on the prognostic impact of measurable residual disease in acute myeloid leukemia: A systematic review and meta-analysis. Leukemia 2022, 36, 2817–2826. [Google Scholar] [CrossRef]
- Wei, A.H.; Tiong, I.S. Midostaurin, enasidenib, CPX-351, gemtuzumab ozogamicin, and venetoclax bring new hope to AML. Blood 2017, 130, 2469–2474. [Google Scholar] [CrossRef]
- Kayser, S.; Levis, M.J. Updates on targeted therapies for acute myeloid leukaemia. Br. J. Haematol. 2022, 196, 316–328. [Google Scholar] [CrossRef]
- Tiong, I.S.; Dillon, R.; Ivey, A.; Teh, T.C.; Nguyen, P.; Cummings, N.; Taussig, D.C.; Latif, A.L.; Potter, N.E.; Runglall, M.; et al. Venetoclax induces rapid elimination of NPM1 mutant measurable residual disease in combination with low-intensity chemotherapy in acute myeloid leukaemia. Br. J. Haematol. 2021, 192, 1026–1030. [Google Scholar] [CrossRef]
- Tiong, I.S.; Hiwase, D.; Abro, E.U.; Bajel, A.; Palfreyman, E.; Loo, S.; Fleming, S.; Fong, C.Y.; Teh, T.-C.; Ivey, A.; et al. A Prospective Phase 2 Study of Venetoclax and Low Dose Ara-C (VALDAC) to Target Rising Molecular Measurable Residual Disease and Early Relapse in Acute Myeloid Leukemia. Blood 2022, 140, 1453–1455. [Google Scholar] [CrossRef]
- Platzbecker, U.; Wermke, M.; Radke, J.; Oelschlaegel, U.; Seltmann, F.; Kiani, A.; Klut, I.M.; Knoth, H.; Rollig, C.; Schetelig, J.; et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: Results of the RELAZA trial. Leukemia 2012, 26, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Platzbecker, U.; Middeke, J.M.; Sockel, K.; Herbst, R.; Wolf, D.; Baldus, C.D.; Oelschlagel, U.; Mutherig, A.; Fransecky, L.; Noppeney, R.; et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): An open-label, multicentre, phase 2 trial. Lancet Oncol. 2018, 19, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Grimwade, D.; Jovanovic, J.V.; Hills, R.K.; Nugent, E.A.; Patel, Y.; Flora, R.; Diverio, D.; Jones, K.; Aslett, H.; Batson, E.; et al. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J. Clin. Oncol. 2009, 27, 3650–3658. [Google Scholar] [CrossRef] [PubMed]
- Gokbuget, N.; Dombret, H.; Bonifacio, M.; Reichle, A.; Graux, C.; Faul, C.; Diedrich, H.; Topp, M.S.; Bruggemann, M.; Horst, H.A.; et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 2018, 131, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Grimwade, D.; Hills, R.K.; Moorman, A.V.; Walker, H.; Chatters, S.; Goldstone, A.H.; Wheatley, K.; Harrison, C.J.; Burnett, A.K.; National Cancer Research Institute Adult Leukaemia Working Group. Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010, 116, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Scholl, C.; Schlenk, R.F.; Eiwen, K.; Dohner, H.; Frohling, S.; Dohner, K.; AML Study Group. The prognostic value of MLL-AF9 detection in patients with t(9;11)(p22;q23)-positive acute myeloid leukemia. Haematologica 2005, 90, 1626–1634. [Google Scholar]
- Issa, G.C.; Aldoss, I.; Di Persio, J.F.; Cuglievan, B.; Stone, R.M.; Arellano, M.L.; Thirman, M.J.; Patel, M.R.; Dickens, D.; Shenoy, S.; et al. The menin inhibitor SNDX-5613 (revumenib) leads to durable responses in patients (pts) with KMT2A-rearranged or NPM1 mutant AML: Updated results of a phase (Ph) 1 study. Blood 2022, 140, 150–152. [Google Scholar] [CrossRef]
- Ommen, H.B.; Touzart, A.; MacIntyre, E.; Kern, W.; Haferlach, T.; Haferlach, C.; Tobal, K.; Hokland, P.; Schnittger, S. The kinetics of relapse in DEK-NUP214-positive acute myeloid leukemia patients. Eur. J. Haematol. 2015, 95, 436–441. [Google Scholar] [CrossRef]
- Welch, J.S.; Ley, T.J.; Link, D.C.; Miller, C.A.; Larson, D.E.; Koboldt, D.C.; Wartman, L.D.; Lamprecht, T.L.; Liu, F.; Xia, J.; et al. The origin and evolution of mutations in acute myeloid leukemia. Cell 2012, 150, 264–278. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Jongen-Lavrencic, M.; Grob, T.; Hanekamp, D.; Kavelaars, F.G.; Al Hinai, A.; Zeilemaker, A.; Erpelinck-Verschueren, C.A.J.; Gradowska, P.L.; Meijer, R.; Cloos, J.; et al. Molecular minimal residual disease in acute myeloid leukemia. N. Engl. J. Med. 2018, 378, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Klco, J.M.; Miller, C.A.; Griffith, M.; Petti, A.; Spencer, D.H.; Ketkar-Kulkarni, S.; Wartman, L.D.; Christopher, M.; Lamprecht, T.L.; Helton, N.M. Association between mutation clearance after induction therapy and outcomes in acute myeloid leukemia. JAMA 2015, 314, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Kantarjian, H.M.; Wang, F.; Yan, Y.; Bueso-Ramos, C.; Sasaki, K.; Issa, G.C.; Wang, S.; Jorgensen, J.; Song, X.; et al. Clearance of somatic mutations at remission and the risk of relapse in acute myeloid leukemia. J. Clin. Oncol. 2018, 36, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg-Thurley, M.; Amler, S.; Goerlich, D.; Kohnke, T.; Konstandin, N.P.; Schneider, S.; Sauerland, M.C.; Herold, T.; Hubmann, M.; Ksienzyk, B.; et al. Persistence of pre-leukemic clones during first remission and risk of relapse in acute myeloid leukemia. Leukemia 2018, 32, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Gabdoulline, R.; Liebich, A.; Klement, P.; Schiller, J.; Kandziora, C.; Hambach, L.; Stadler, M.; Koenecke, C.; Flintrop, M.; et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood 2018, 132, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Moon, J.H.; Ahn, J.S.; Kim, Y.K.; Lee, S.S.; Ahn, S.Y.; Jung, S.H.; Yang, D.H.; Lee, J.J.; Choi, S.H.; et al. Next-generation sequencing-based posttransplant monitoring of acute myeloid leukemia identifies patients at high risk of relapse. Blood 2018, 132, 1604–1613. [Google Scholar] [CrossRef]
- Hourigan, C.S.; Dillon, L.W.; Gui, G.; Logan, B.R.; Fei, M.; Ghannam, J.; Li, Y.; Licon, A.; Alyea, E.P.; Bashey, A.; et al. Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease. J. Clin. Oncol. 2020, 38, 1273–1283. [Google Scholar] [CrossRef]
- Heuser, M.; Heida, B.; Buttner, K.; Wienecke, C.P.; Teich, K.; Funke, C.; Brandes, M.; Klement, P.; Liebich, A.; Wichmann, M.; et al. Posttransplantation MRD monitoring in patients with AML by next-generation sequencing using DTA and non-DTA mutations. Blood Adv. 2021, 5, 2294–2304. [Google Scholar] [CrossRef]
- Patkar, N.; Kakirde, C.; Shaikh, A.F.; Salve, R.; Bhanshe, P.; Chatterjee, G.; Rajpal, S.; Joshi, S.; Chaudhary, S.; Kodgule, R.; et al. Clinical impact of panel-based error-corrected next generation sequencing versus flow cytometry to detect measurable residual disease (MRD) in acute myeloid leukemia (AML). Leukemia 2021, 35, 1392–1404. [Google Scholar] [CrossRef]
- Tsai, C.H.; Tang, J.L.; Tien, F.M.; Kuo, Y.Y.; Wu, D.C.; Lin, C.C.; Tseng, M.H.; Peng, Y.L.; Hou, M.F.; Chuang, Y.K.; et al. Clinical implications of sequential MRD monitoring by NGS at 2 time points after chemotherapy in patients with AML. Blood Adv. 2021, 5, 2456–2466. [Google Scholar] [CrossRef] [PubMed]
- Hourigan, C.S.; Dillon, L.W.; Gui, G.; Ravindra, N.; Wong, Z.; Andrew, G.; Mukherjee, D.; El Chaer, F.; Yoon, C.; Spellman, S.; et al. Pre-MEASURE: Multicenter evaluation of the prognostic significance of measurable residual disease testing prior to allogeneic transplantation for adult patients with AML in first remission. J. Clin. Oncol. 2022, 40, 7006. [Google Scholar] [CrossRef]
- Hirsch, P.; Lambert, J.; Bucci, M.; Deswarte, C.; Lambert, J.; Fenwarth, L.; Dombret, H.; Duployez, N.; Preudhomme, C.; Itzykson, R.; et al. Persistence of mutations in complete remission including DNMT3A, TET2 and ASXL1 mutations is associated with worse prognosis in patients with acute myeloid leukemia treated in ALFA 0702 study. Blood 2022, 140, 549–550. [Google Scholar] [CrossRef]
- Metzeler, K.H.; Herold, T.; Rothenberg-Thurley, M.; Amler, S.; Sauerland, M.C.; Gorlich, D.; Schneider, S.; Konstandin, N.P.; Dufour, A.; Braundl, K.; et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood 2016, 128, 686–698. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Kahler, A.K.; Handsaker, R.E.; Lindberg, J.; Rose, S.A.; Bakhoum, S.F.; Chambert, K.; Mick, E.; Neale, B.M.; Fromer, M.; et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 2014, 371, 2477–2487. [Google Scholar] [CrossRef]
- Desai, P.; Mencia-Trinchant, N.; Savenkov, O.; Simon, M.S.; Cheang, G.; Lee, S.; Samuel, M.; Ritchie, E.K.; Guzman, M.L.; Ballman, K.V.; et al. Somatic mutations precede acute myeloid leukemia years before diagnosis. Nat. Med. 2018, 24, 1015–1023. [Google Scholar] [CrossRef]
- Ferret, Y.; Boissel, N.; Helevaut, N.; Madic, J.; Nibourel, O.; Marceau-Renaut, A.; Bucci, M.; Geffroy, S.; Celli-Lebras, K.; Castaigne, S.; et al. Clinical relevance of IDH1/2 mutant allele burden during follow-up in acute myeloid leukemia. A study by the French ALFA group. Haematologica 2018, 103, 822–829. [Google Scholar] [CrossRef]
- Ok, C.Y.; Loghavi, S.; Sui, D.; Wei, P.; Kanagal-Shamanna, R.; Yin, C.C.; Zuo, Z.; Routbort, M.J.; Tang, G.; Tang, Z.; et al. Persistent IDH1/2 mutation in remission can predict relapse in patients with acute myeloid leukemia. Haematologica 2018, 104, 305–311. [Google Scholar] [CrossRef]
- Cappelli, L.V.; Meggendorfer, M.; Baer, C.; Nadarajah, N.; Hutter, S.; Jeromin, S.; Dicker, F.; Kern, W.; Haferlach, T.; Haferlach, C.; et al. Indeterminate and oncogenic potential: CHIP vs CHOP mutations in AML with NPM1 alteration. Leukemia 2021, 36, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, D.H.; Williams, E.L.; Wilks, D.P.; Sun Leong, H.; Somerville, T.D.; Dennis, M.W.; Struys, E.A.; Bakkali, A.; Salomons, G.S.; Somervaille, T.C. Frequent reconstitution of IDH2(R140Q) mutant clonal multilineage hematopoiesis following chemotherapy for acute myeloid leukemia. Leukemia 2016, 30, 1946–1950. [Google Scholar] [CrossRef] [PubMed]
- Bill, M.; Jentzsch, M.; Bischof, L.; Kohlschmidt, J.; Grimm, J.; Schmalbrock, L.K.; Backhaus, D.; Brauer, D.; Goldmann, K.; Franke, G.N.; et al. Impact of IDH1 and IDH2 mutation detection at diagnosis and in remission in patients with AML receiving allogeneic transplantation. Blood Adv. 2023, 7, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Cloos, J.; Goemans, B.F.; Hess, C.J.; van Oostveen, J.W.; Waisfisz, Q.; Corthals, S.; de Lange, D.; Boeckx, N.; Hahlen, K.; Reinhardt, D.; et al. Stability and prognostic influence of FLT3 mutations in paired initial and relapsed AML samples. Leukemia 2006, 20, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Kottaridis, P.D.; Gale, R.E.; Langabeer, S.E.; Frew, M.E.; Bowen, D.T.; Linch, D.C. Studies of FLT3 mutations in paired presentation and relapse samples from patients with acute myeloid leukemia: Implications for the role of FLT3 mutations in leukemogenesis, minimal residual disease detection, and possible therapy with FLT3 inhibitors. Blood 2002, 100, 2393–2398. [Google Scholar] [CrossRef] [PubMed]
- Amatangelo, M.D.; Quek, L.; Shih, A.; Stein, E.M.; Roshal, M.; David, M.D.; Marteyn, B.; Farnoud, N.R.; de Botton, S.; Bernard, O.A.; et al. Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response. Blood 2017, 130, 732–741. [Google Scholar] [CrossRef] [PubMed]
- McMahon, C.M.; Ferng, T.; Canaani, J.; Wang, E.S.; Morrissette, J.J.D.; Eastburn, D.J.; Pellegrino, M.; Durruthy-Durruthy, R.; Watt, C.D.; Asthana, S.; et al. Clonal selection with RAS pathway activation mediates secondary clinical resistance to selective FLT3 inhibition in acute myeloid leukemia. Cancer Discov. 2019, 9, 1050–1063. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Tiong, I.S.; Quaglieri, A.; MacRaild, S.; Loghavi, S.; Brown, F.C.; Thijssen, R.; Pomilio, G.; Ivey, A.; Salmon, J.M.; et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 2020, 135, 791–803. [Google Scholar] [CrossRef]
- Helbig, G.; Koclęga, A.; Wieczorkiewicz-Kabut, A.; Woźniczka, K.; Kopińska, A.; Boral, K.; Grygoruk-Wiśniowska, I.; Stachowicz, M.; Karolczyk, A. Pre-transplant FLT3/ITD status predicts outcome in FLT3-mutated acute myeloid leukemia following allogeneic stem cell transplantation. Ann. Hematol. 2020, 99, 1845–1853. [Google Scholar] [CrossRef]
- Gaballa, S.; Saliba, R.; Oran, B.; Brammer, J.E.; Chen, J.; Rondon, G.; Alousi, A.M.; Kebriaei, P.; Marin, D.; Popat, U.R.; et al. Relapse risk and survival in patients with FLT3 mutated acute myeloid leukemia undergoing stem cell transplantation. Am. J. Hematol. 2017, 92, 331–337. [Google Scholar] [CrossRef]
- Spencer, D.H.; Abel, H.J.; Lockwood, C.M.; Payton, J.E.; Szankasi, P.; Kelley, T.W.; Kulkarni, S.; Pfeifer, J.D.; Duncavage, E.J. Detection of FLT3 internal tandem duplication in targeted, short-read-length, next-generation sequencing data. J. Mol. Diagn. 2013, 15, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Bibault, J.E.; Figeac, M.; Helevaut, N.; Rodriguez, C.; Quief, S.; Sebda, S.; Renneville, A.; Nibourel, O.; Rousselot, P.; Gruson, B.; et al. Next-generation sequencing of FLT3 internal tandem duplications for minimal residual disease monitoring in acute myeloid leukemia. Oncotarget 2015, 6, 22812–22821. [Google Scholar] [CrossRef] [PubMed]
- Levis, M.J.; Perl, A.E.; Altman, J.K.; Gocke, C.D.; Bahceci, E.; Hill, J.; Liu, C.; Xie, Z.; Carson, A.R.; McClain, V.; et al. A next-generation sequencing-based assay for minimal residual disease assessment in AML patients with FLT3-ITD mutations. Blood Adv. 2018, 2, 825–831. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Devine, D.J.; Tu, Z.J.; Mai, M.; Chen, D.; Nguyen, P.L.; Oliveira, J.L.; Hoyer, J.D.; Reichard, K.K.; Ollila, P.L.; et al. Hybridization capture-based next generation sequencing reliably detects FLT3 mutations and classifies FLT3-internal tandem duplication allelic ratio in acute myeloid leukemia: A comparative study to standard fragment analysis. Mod. Pathol. 2020, 33, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Cao, Y.; Jiang, Y.; Cong, X.; Lu, S.; Shen, J.; Liu, Q.; Han, C.; Zhan, Y.; Cao, Y. Detection the frequency and characteristics of FLT3 internal tandem duplication mutations by capillary electrophoresis assay and next-generation sequencing in. Clin. Lab. 2016, 62, 2065–2072. [Google Scholar] [CrossRef]
- Blatte, T.J.; Schmalbrock, L.K.; Skambraks, S.; Lux, S.; Cocciardi, S.; Dolnik, A.; Dohner, H.; Dohner, K.; Bullinger, L. getITD for FLT3-ITD-based MRD monitoring in AML. Leukemia 2019, 33, 2535–2539. [Google Scholar] [CrossRef]
- Loo, S.; Dillon, R.; Ivey, A.; Anstee, N.S.; Othman, J.; Tiong, I.S.; Potter, N.; Jovanovic, J.; Runglall, M.; Chong, C.C.; et al. Pretransplant FLT3-ITD MRD assessed by high-sensitivity PCR-NGS determines posttransplant clinical outcome. Blood 2022, 140, 2407–2411. [Google Scholar] [CrossRef]
- Lee, J.M.; Park, S.; Hwang, I.; Kang, D.; Cho, B.S.; Kim, H.J.; Ahn, A.; Kim, M.; Kim, Y. FLT3-ITD measurable residual disease monitoring in acute myeloid leukemia using next-generation sequencing. Cancers 2022, 14, 6121. [Google Scholar] [CrossRef]
- Dillon, L.W.; Gui, G.; Ravindra, N.; Wong, Z.; Andrew, G.; Mukherjee, D.; Zeger, S.L.; El Chaer, F.; Spellman, S.; Howard, A.; et al. Pre-MEASURE: FLT3-ITD and mutated NPM1 measurable residual disease before allogeneic transplant in adults with AML in first remission. medRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Grob, T.; Sanders, M.A.; Vonk, C.M.; Kavelaars, F.G.; Rijken, M.; Hanekamp, D.W.; Gradowska, P.L.; Cloos, J.; Floisand, Y.; van Marwijk Kooy, M.; et al. Prognostic value of FLT3-internal tandem duplication residual disease in acute myeloid leukemia. J. Clin. Oncol. 2022, 41, 756–765. [Google Scholar] [CrossRef]
- Schranz, K.; Hubmann, M.; Harin, E.; Vosberg, S.; Herold, T.; Metzeler, K.H.; Rothenberg-Thurley, M.; Janke, H.; Braundl, K.; Ksienzyk, B.; et al. Clonal heterogeneity of FLT3-ITD detected by high-throughput amplicon sequencing correlates with adverse prognosis in acute myeloid leukemia. Oncotarget 2018, 9, 30128–30145. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Onozawa, M.; Yoshida, S.; Miyashita, N.; Kimura, H.; Takahashi, S.; Matsukawa, T.; Fujisawa, S.; Miki, K.; Hidaka, D.; et al. Subclinical minute FLT3-ITD clone can be detected in clinically FLT3-ITD-negative acute myeloid leukemia. Blood 2022, 140, 9094–9095. [Google Scholar] [CrossRef]
- Rucker, F.G.; Du, L.; Luck, T.J.; Benner, A.; Krzykalla, J.; Gathmann, I.; Voso, M.T.; Amadori, S.; Prior, T.W.; Brandwein, J.M.; et al. Molecular landscape and prognostic impact of FLT3-ITD insertion site in acute myeloid leukemia: RATIFY study results. Leukemia 2022, 36, 90–99. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Dohner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Montesinos, P.; Ivanov, V.; DiNardo, C.D.; Novak, J.; Laribi, K.; Kim, I.; Stevens, D.A.; Fiedler, W.; Pagoni, M.; et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: A phase 3 randomized placebo-controlled trial. Blood 2020, 135, 2137–2145. [Google Scholar] [CrossRef]
- Pratz, K.; Jonas, B.A.; Pullarkat, V.A.; Thirman, M.J.; Garcia, J.S.; Fiedler, W.; Yamamoto, K.; Wang, J.; Yoon, S.S.; Wolach, O.; et al. Long-term follow-up of the phase 3 Viale-a clinical trial of venetoclax plus azacitidine for patients with untreated acute myeloid leukemia ineligible for intensive chemotherapy. Blood 2022, 135, 2137–2145. [Google Scholar] [CrossRef]
- Pratz, K.W.; Jonas, B.A.; Pullarkat, V.; Recher, C.; Schuh, A.C.; Thirman, M.J.; Garcia, J.S.; DiNardo, C.D.; Vorobyev, V.; Fracchiolla, N.S.; et al. Measurable residual disease response and prognosis in treatment-naive acute myeloid leukemia with venetoclax and azacitidine. J. Clin. Oncol. 2022, 40, 855–865. [Google Scholar] [CrossRef]
- Maiti, A.; DiNardo, C.D.; Wang, S.A.; Jorgensen, J.; Kadia, T.M.; Daver, N.G.; Short, N.J.; Yilmaz, M.; Pemmaraju, N.; Borthakur, G.; et al. Prognostic value of measurable residual disease after venetoclax and decitabine in acute myeloid leukemia. Blood Adv. 2021, 5, 1876–1883. [Google Scholar] [CrossRef]
- Ong, S.Y.; Tan Si Yun, M.; Abdul Halim, N.A.; Christopher, D.; Jen, W.Y.; Gallardo, C.; Tan Hwee Yim, A.; Woon, Y.K.; Ng, H.J.; Ooi, M.; et al. Real-world experience of measurable residual disease response and prognosis in acute myeloid leukemia treated with venetoclax and azacitidine. Cancers 2022, 14, 3576. [Google Scholar] [CrossRef]
- Othman, J.; Tiong, I.S.; Mokretar, K.; Ivey, A.; Austin, M.J.; Latif, A.L.; Crawley, C.; Amer, M.; Crolla, F.; Cross, J.W.; et al. Molecular MRD assessment is strongly prognostic in patients with NPM1 mutated AML receiving venetoclax based non-intensive therapy. Blood 2022, 140, 2033–2035. [Google Scholar] [CrossRef]
- Kadia, T.M.; Reville, P.K.; Wang, X.; Rausch, C.R.; Borthakur, G.; Pemmaraju, N.; Daver, N.G.; DiNardo, C.D.; Sasaki, K.; Issa, G.C.; et al. Phase II study of venetoclax added to cladribine plus low-dose cytarabine alternating with 5-azacitidine in older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2022, 40, 3848–3857. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Lachowiez, C.A.; Takahashi, K.; Loghavi, S.; Kadia, T.; Daver, N.; Xiao, L.; Adeoti, M.; Short, N.J.; Sasaki, K.; et al. Venetoclax combined with FLAG-IDA induction and consolidation in newly diagnosed acute myeloid leukemia. Am. J. Hematol. 2022, 97, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.C.; Hammond, D.; Kent, A.; Tiong, I.S.; Konopleva, M.Y.; Pollyea, D.A.; DiNardo, C.D.; Wei, A.H. Treatment-free remission after ceasing venetoclax-based therapy in patients with acute myeloid leukemia. Blood Adv. 2022, 6, 3879–3883. [Google Scholar] [CrossRef] [PubMed]
- Gutman, J.A.; Winters, A.C.; Kent, A.; Amaya, M.L.; McMahon, C.M.; Smith, C.; Jordan, C.T.; Stevens, B.M.; Minhajuddin, M.; Pei, S.; et al. Higher-dose venetoclax with measurable residual disease-guided azacitidine discontinuation in newly diagnosed patients with acute myeloid leukemia: Phase 2 Hiddav study. Blood 2022, 140, 3275–3276. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Dohner, K.; Marcucci, G.; et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Levis, M.; Shi, W.; Chang, K.; Laing, C.; Pollner, R.; Gocke, C.; Adams, E.; Berisha, F.; Lameh, J.; Lesegretain, A. FLT3 inhibitors added to induction therapy induce deeper remissions. Blood 2020, 135, 75–78. [Google Scholar] [CrossRef]
- Herzig, J.K.; Rücker, F.G.; Schmalbrock, L.K.; Blätte, T.J.; Weber, D.; Skambraks, S.; Kapp-Schwoerer, S.; Corbacioglu, A.; Gaidzik, V.I.; Paschka, P.; et al. Next-Generation Sequencing (NGS)-Based Measurable Residual Disease (MRD) Monitoring in Acute Myeloid Leukemia with FLT3 Internal Tandem Duplication (FLT3-ITD+ AML) Treated with Additional Midostaurin. Blood 2020, 136, 21–22. [Google Scholar] [CrossRef]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef]
- Altman, J.K.; Perl, A.E.; Hill, J.E.; Rosales, M.; Bahceci, E.; Levis, M.J. The impact of FLT3 mutation clearance and treatment response after gilteritinib therapy on overall survival in patients with FLT3 mutation-positive relapsed/refractory acute myeloid leukemia. Cancer Med. 2021, 10, 797–805. [Google Scholar] [CrossRef]
- Daver, N.; Perl, A.E.; Maly, J.; Levis, M.; Ritchie, E.; Litzow, M.; McCloskey, J.; Smith, C.C.; Schiller, G.; Bradley, T.; et al. Venetoclax plus gilteritinib for FLT3-mutated relapsed/refractory acute myeloid leukemia. J. Clin. Oncol. 2022, 40, 4048–4059. [Google Scholar] [CrossRef]
- Levis, M.J.; Erba, H.P.; Montesinos, P.; Vrhovac, R.; Patkowska, E.; Kim, H.J.; Zak, P.; Wang, P.N.; Rohrbach, J.E.C.; Chang, K.C.N.; et al. Quantum-First trial: FLT3-ITD-specific MRD clearance is associated with improved overall survival. Blood 2022, 140, 546–548. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Swords, R.; Collins, R.H.; Mannis, G.N.; Pollyea, D.A.; et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N. Engl. J. Med. 2018, 378, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; DiNardo, C.D.; Pollyea, D.A.; Fathi, A.T.; Roboz, G.J.; Altman, J.K.; Stone, R.M.; DeAngelo, D.J.; Levine, R.L.; Flinn, I.W.; et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017, 130, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; DiNardo, C.D.; Fathi, A.T.; Pollyea, D.A.; Stone, R.M.; Altman, J.K.; Roboz, G.J.; Patel, M.R.; Collins, R.; Flinn, I.W. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood 2019, 133, 676–687. [Google Scholar] [CrossRef]
- Roboz, G.J.; DiNardo, C.D.; Stein, E.M.; de Botton, S.; Mims, A.S.; Prince, G.T.; Altman, J.K.; Arellano, M.L.; Donnellan, W.; Erba, H.P.; et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood 2020, 135, 463–471. [Google Scholar] [CrossRef]
- Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Bertani, G.; Heuser, M.; Calado, R.T.; Schuh, A.C.; Yeh, S.P.; et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N. Engl. J. Med. 2022, 386, 1519–1531. [Google Scholar] [CrossRef]
- Dohner, H.; Marchione, D.M.; Choe, S.; Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Cerchione, C.; Heuser, M.; et al. Molecular characterization of clinical response and relapse in patients with IDH1m ND-AML treated with ivo+AZA in the AGILE study. Blood 2022, 539–542. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Schuh, A.C.; Stein, E.M.; Montesinos, P.; Wei, A.H.; de Botton, S.; Zeidan, A.M.; Fathi, A.T.; Kantarjian, H.M.; Bennett, J.M.; et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): A single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021, 22, 1597–1608. [Google Scholar] [CrossRef]
- Lachowiez, C.A.; Garcia, J.S.; Borthakur, G.; Loghavi, S.; Zeng, Z.; Tippett, G.D.; Kadia, T.; Masarova, L.; Yilmaz, M.; Maiti, A.; et al. A phase Ib/II study of ivosidenib with venetoclax +/− azacitidine in IDH1-mutated hematologic malignancies. J. Clin. Oncol. 2022, 39, 7012. [Google Scholar] [CrossRef]
- Stein, E.M.; DiNardo, C.D.; Fathi, A.T.; Mims, A.S.; Pratz, K.W.; Savona, M.R.; Stein, A.S.; Stone, R.M.; Winer, E.S.; Seet, C.S.; et al. Ivosidenib or enasidenib combined with intensive chemotherapy in patients with newly diagnosed AML: A phase 1 study. Blood 2021, 137, 1792–1803. [Google Scholar] [CrossRef]
- Hills, R.K.; Castaigne, S.; Appelbaum, F.R.; Delaunay, J.; Petersdorf, S.; Othus, M.; Estey, E.H.; Dombret, H.; Chevret, S.; Ifrah, N.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014, 15, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.; Pautas, C.; Terre, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: Final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 2019, 104, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Hills, R.K.; Virgo, P.; Couzens, S.; Clark, N.; Gilkes, A.; Richardson, P.; Knapper, S.; Grimwade, D.; Russell, N.H.; et al. Expression of CD33 is a predictive factor for effect of gemtuzumab ozogamicin at different doses in adult acute myeloid leukaemia. Leukemia 2017, 31, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.; Lambert, J.; Nibourel, O.; Pautas, C.; Hayette, S.; Cayuela, J.M.; Terre, C.; Rousselot, P.; Dombret, H.; Chevret, S.; et al. MRD assessed by WT1 and NPM1 transcript levels identifies distinct outcomes in AML patients and is influenced by gemtuzumab ozogamicin. Oncotarget 2014, 5, 6280–6288. [Google Scholar] [CrossRef]
- Kapp-Schwoerer, S.; Weber, D.; Corbacioglu, A.; Gaidzik, V.I.; Paschka, P.; Kronke, J.; Theis, F.; Rucker, F.G.; Teleanu, M.V.; Panina, E.; et al. Impact of gemtuzumab ozogamicin on MRD and relapse risk in patients with NPM1-mutated AML: Results from the AMLSG 09-09 trial. Blood 2020, 136, 3041–3050. [Google Scholar] [CrossRef]
- Yin, J.A.; O’Brien, M.A.; Hills, R.K.; Daly, S.B.; Wheatley, K.; Burnett, A.K. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: Results of the United Kingdom MRC AML-15 trial. Blood 2012, 120, 2826–2835. [Google Scholar] [CrossRef]
- Wei, A.H.; Dohner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.H.; Porkka, K.; et al. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N. Engl. J. Med. 2020, 383, 2526–2537. [Google Scholar] [CrossRef]
- Roboz, G.J.; Ravandi, F.; Wei, A.H.; Dombret, H.; Thol, F.; Voso, M.T.; Schuh, A.C.; Porkka, K.; La Torre, I.; Skikne, B.; et al. Oral azacitidine prolongs survival of patients with AML in remission independently of measurable residual disease status. Blood 2022, 139, 2145–2155. [Google Scholar] [CrossRef]
- Dohner, H.; Wei, A.H.; Roboz, G.J.; Montesinos, P.; Thol, F.R.; Ravandi, F.; Dombret, H.; Porkka, K.; Sandhu, I.; Skikne, B.; et al. Prognostic impact of NPM1 and FLT3 mutations in patients with AML in first remission treated with oral azacitidine. Blood 2022, 140, 1674–1685. [Google Scholar] [CrossRef]
- Short, N.J.; Zhou, S.; Fu, C.; Berry, D.A.; Walter, R.B.; Freeman, S.D.; Hourigan, C.S.; Huang, X.; Nogueras Gonzalez, G.; Hwang, H.; et al. Association of measurable residual disease with survival outcomes in patients with acute myeloid leukemia: A systematic review and meta-analysis. JAMA Oncol. 2020, 6, 1890–1899. [Google Scholar] [CrossRef]
- Yu, S.; Fan, Z.; Ma, L.; Wang, Y.; Huang, F.; Zhang, Q.; Huang, J.; Wang, S.; Xu, N.; Xuan, L.; et al. Association between measurable residual disease in patients with intermediate-risk acute myeloid leukemia and first remission, treatment, and outcomes. JAMA Netw. Open 2021, 4, e2115991. [Google Scholar] [CrossRef] [PubMed]
- Craddock, C. Transplant in AML with measurable residual disease: Proceed or defer? Hematology 2022, 2022, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Loke, J.; Vyas, H.; Craddock, C. Optimizing transplant approaches and post-transplant strategies for patients with acute myeloid leukemia. Front. Oncol. 2021, 11, 666091. [Google Scholar] [CrossRef] [PubMed]
- Spyridonidis, A. How I treat measurable (minimal) residual disease in acute leukemia after allogeneic hematopoietic cell transplantation. Blood 2020, 135, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Gaut, D.; Mead, M. Measurable residual disease in hematopoietic stem cell transplantation-eligible patients with acute myeloid leukemia: Clinical significance and promising therapeutic strategies. Leuk. Lymphoma 2021, 62, 8–31. [Google Scholar] [CrossRef]
- Araki, D.; Wood, B.L.; Othus, M.; Radich, J.P.; Halpern, A.B.; Zhou, Y.; Mielcarek, M.; Estey, E.H.; Appelbaum, F.R.; Walter, R.B. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: Time to move toward a minimal residual disease-based definition of complete remission? J. Clin. Oncol. 2016, 34, 329–336. [Google Scholar] [CrossRef]
- Gilleece, M.H.; Shimoni, A.; Labopin, M.; Robinson, S.; Beelen, D.; Socie, G.; Unal, A.; Ganser, A.; Vitek, A.; Sengeloev, H.; et al. Measurable residual disease status and outcome of transplant in acute myeloid leukemia in second complete remission: A study by the acute leukemia working party of the EBMT. Blood Cancer J. 2021, 11, 88. [Google Scholar] [CrossRef]
- Sockel, K.; Wermke, M.; Radke, J.; Kiani, A.; Schaich, M.; Bornhauser, M.; Ehninger, G.; Thiede, C.; Platzbecker, U. Minimal residual disease-directed preemptive treatment with azacitidine in patients with NPM1-mutant acute myeloid leukemia and molecular relapse. Haematologica 2011, 96, 1568–1570. [Google Scholar] [CrossRef]
- Bataller, A.; Onate, G.; Diaz-Beya, M.; Guijarro, F.; Garrido, A.; Vives, S.; Tormo, M.; Arnan, M.; Salamero, O.; Sampol, A.; et al. Acute myeloid leukemia with NPM1 mutation and favorable European LeukemiaNet category: Outcome after preemptive intervention based on measurable residual disease. Br. J. Haematol. 2020, 191, 52–61. [Google Scholar] [CrossRef]
- Short, N.J.; Macaron, W.; Kadia, T.; Dinardo, C.; Issa, G.C.; Daver, N.; Wang, S.; Jorgensen, J.; Nguyen, D.; Bidikian, A.; et al. Clinical outcomes and impact of therapeutic intervention in patients with acute myeloid leukemia who experience measurable residual disease (MRD) recurrence following MRD-negative remission. Am. J. Hematol. 2022, 97, E408–E411. [Google Scholar] [CrossRef]
- Dillon, R.; Hills, R.; Freeman, S.; Potter, N.; Jovanovic, J.; Ivey, A.; Kanda, A.S.; Runglall, M.; Foot, N.; Valganon, M.; et al. Molecular MRD status and outcome after transplantation in NPM1-mutated AML. Blood 2020, 135, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Wood, H.; Bourlon, C.; Kulasekararaj, A.; Borg, A.; Pavlu, J.; Elder, P.; Taussig, D.; Veitch, S.; Knapper, S. Venetoclax-based non-intensive combinations successfully salvage molecular relapse of acute myeloid leukemia and are an important bridge to cellular therapy in relapsed/refractory disease—Real-world data from a UK-wide programme. Blood 2022, 140, 9016–9018. [Google Scholar] [CrossRef]
- Othman, J.; Potter, N.; Mokretar, K.; Taussig, D.; Khan, A.; Krishnamurthy, P.; Latif, A.L.; Cahalin, P.; Aries, J.; Amer, M.; et al. High molecular response rate and overall survival with FLT3 inhibitors as MRD-guided salvage treatment for molecular failure in AML. Blood 2022, 140, 2002–2004. [Google Scholar] [CrossRef]
- Tiong, I.S.; Dillon, R.; Ivey, A.; Kuzich, J.A.; Thiagarajah, N.; Sharplin, K.M.; Kok, C.H.; Tedjaseputra, A.; Rowland, J.P.; Grove, C.S.; et al. Clinical impact of NPM1-mutant molecular persistence after chemotherapy for acute myeloid leukemia. Blood Adv. 2021, 5, 5107–5111. [Google Scholar] [CrossRef] [PubMed]
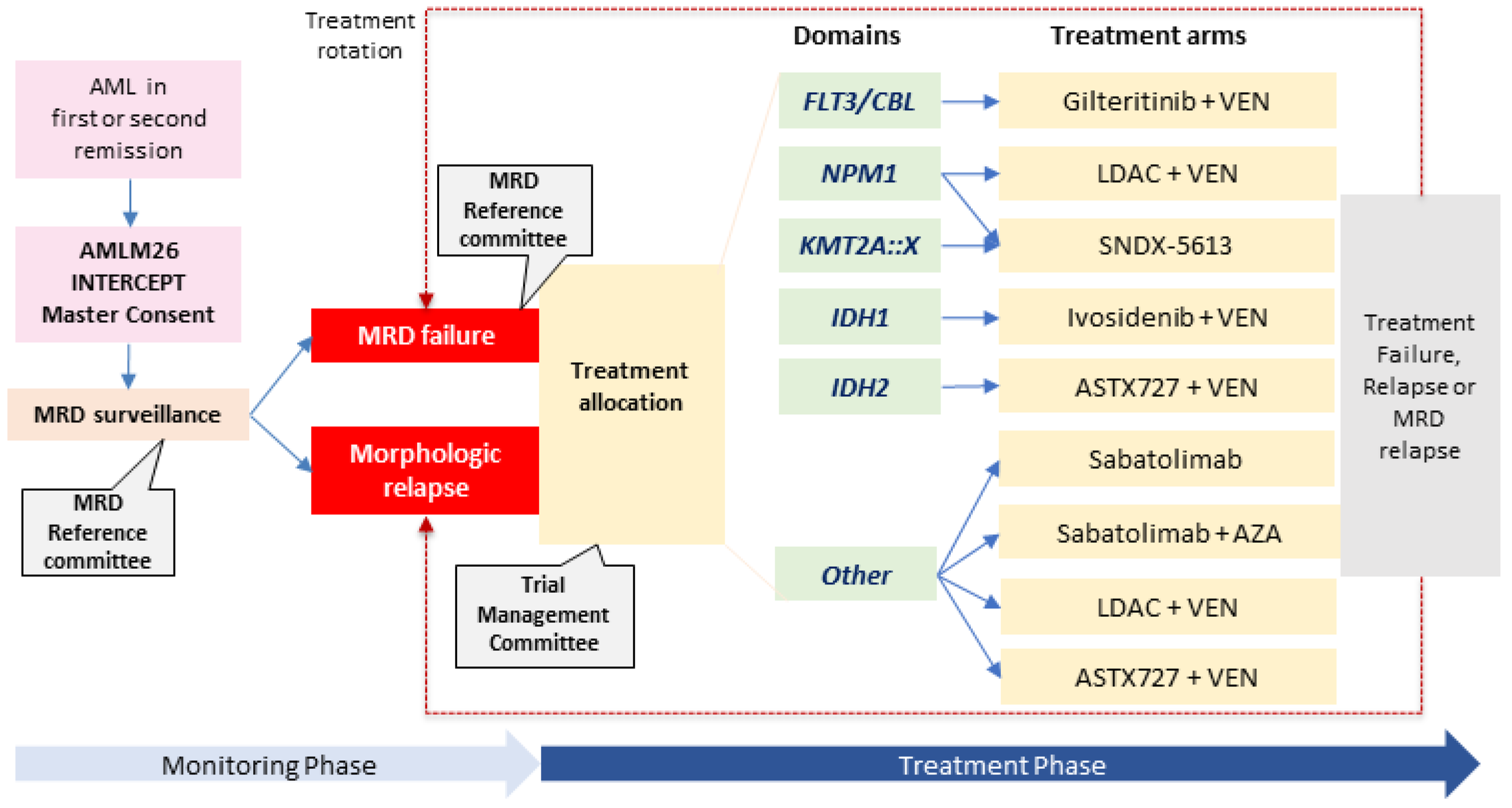
- Wei, A.; Iland, H.; Reynolds, J.; Loo, S.; Chua, C.C.; Westerman, D.; Tiong, I.S.; Ivey, A.; Blombery, P.; Anstee, N.S.; et al. ALLG AMLM26 Phase 1B/2 study investigating novel therapies to target early relapse and clonal evolution as pre-emptive therapy in AML (INTERCEPT): A multi-arm, precision-based, recursive, platform trial. Blood 2022, 3341–3343. [Google Scholar] [CrossRef]
- Hourigan, C.S. Achieving MRD negativity in AML: How important is this and how do we get there? Hematol. Am. Soc. Hematol. Educ. Program 2022, 2022, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Little, R.F.; Othus, M.; Assouline, S.; Ansher, S.; Atallah, E.L.; Lindsley, R.C.; Freidlin, B.; Gore, S.D.; Harris, L.; Hourigan, C.S.; et al. Umbrella trial in myeloid malignancies: The Myelomatch National Clinical Trials Network Precision Medicine Initiative. Blood 2022, 140, 9057–9060. [Google Scholar] [CrossRef]
- Mission Bio. Mission Bio Launches Early Access Program for Single-Cell Measurable Residual Disease Detection in Blood Cancers. Available online: https://missionbio.com/press/single-cell-mrd-early-access-program/ (accessed on 28 July 2022).
- Robinson, T.; Bowman, R.L.; Persaud, S.; Liu, Y.; Gao, Q.; Zhang, J.; Sun, X. Single cell genotypic and phenotypic analysis of measurable residual disease in acute myeloid leukemia. Blood 2022, 140, 2276–2277. [Google Scholar] [CrossRef]
| Mutation Types | Clonal Hematopoiesis | AML-Defining | Signaling Pathway | |
|---|---|---|---|---|
| Characteristics | ||||
| Examples | DNMT3A, TET2, ASXL1, IDH1, IDH2, TP53, SRSF2, and RUNX1, among others | NPM1, CBFB::MYH11, RUNX1::RUNX1T1, PML::RARA, and KMT2A fusions, among others | FLT3 (ITD and TKD), NRAS, KRAS, KIT, CBL, and PTPN11, among others | |
| Clonal hierarchy | Preleukemic, but overlaps with leukemia-initiating clone | Leukemia-initiating clone | Subclone | |
| Presence in clonal hematopoiesis | Yes, but some mutations are deemed higher risk than others (“high risk CCUS”) | Rare | Rare | |
| Clearance post morphological remission | Frequently persists | Yes | Yes | |
| Predictive value | Low value for DTA mutations (see text), and some likely have more oncogenic potential | High if detectable above the threshold or if serially increasing (MRD relapse) | High positive predictive value but low negative predictive value | |
| Stable at relapse | Yes | Yes, but note branched evolution | No | |
| Sample source | gDNA | Usually cDNA | gDNA | |
| Molecular assays | Targeted NGS panel ddPCR (only for hotspots) | RT-qPCR (or RT-dPCR) NGS-MRD panel (gDNA) | Targeted NGS panel FLT3-ITD NGS-MRD panel | |
| Study | N | PB vs. BM | Timepoint | Mutations | Thresholds (% of N) | Risks (neg vs. pos) (or < vs. > Threshold)) |
|---|---|---|---|---|---|---|
| Klco, 2015 [24] | 50 | BM | PC1 | Any | <2.5% (52) | Median OS 42.2 m vs. 10.5 m |
| Morita, 2018 [25] | 122 | BM | PC1 | Any a | Negative (48) | 2 y CIR 24% vs. 46% 2 y OS 77% vs. 60% |
| Jongen-Lavrencic, 2018 [23] | 430 | Either | PC2 | Non-DTA | Negative (72) | 4 y CIR 31.9% vs. 55.4% 4 y RFS 58.1% vs. 36.6% 4 y OS 66.1% vs. 41.9% |
| Rothenberg-Thurley, 2018 [26] | 126 | Either | First remission | Any b | <2% (60) | Median RFS 55.7 m vs. 11.7 m Median OS NR vs. 31.3 m |
| Thol, 2018 [27] | 96 | Either | Pre-HCT | Non-DNMT3A | Negative (55) | 5 y CIR 17% vs. 66% 5 y RFS 74% vs. 31% 5 y OS 78% vs. 41% |
| Kim, 2018 [28] | 104 | BM | Post-HCT D21 | Any | <2% (85) | 3 y CIR 16.0% vs. 56.2% 3 y OS 67.0% vs. 36.5% |
| Hourigan, 2020 [29] | 190 | PB | Pre-HCT | Any c | Negative (35) | Neg: 3 y OS 56% vs. 63% (MAC vs. RIC) Pos: 3 y OS 61% vs. 43% (MAC vs. RIC) Pos: 3 y CIR 19% vs. 67% (MAC vs. RIC) |
| Heuser, 2021 [30] | 131 | Either | Post-HCT D90 ± 180 | Non-DTA | Negative (80) | 5 y CIR 25% vs. 62% 5 y RFS 68% vs. 35% 5 y OS 73% vs. 49% |
| Patkar, 2021 [31] | 201 | BM | PC1 | Any | Negative (29) | 3 y CIR 25.7% vs. 47.5% Median RFS NR vs. 17 m Median OS NR vs. 27 m |
| Tsai, 2021 [32] | 335 | BM | PC2 | Non-DTA | Negative (71) | Median CIR 4.8 y—NR vs. 0.6–1.1 y Median OS NR vs. 3.1–3.6 y |
| Hourigan, 2022 (Pre-MEASURE) [33] | 1075 | PB | Pre-HCT | FLT3, NPM1, IDH1, IDH2, and KIT | <0.01% (70) | 3 y CIR 23% vs. 62% 3 y RFS 59% vs. 25% 3 y OS 66% vs. 36% |
| Study | Population | N | Treatment | MRD Response | Survival |
|---|---|---|---|---|---|
| Sockel, 2011 [109] | NPM1 MRD relapse or persistence > 1% | 10 | AZA | ≥1 log10 decrease (70%) | Not reported |
| Platzbecker, 2018 [14] | RT-qPCR > 1% or donor chimerism loss | 53 (32 NPM1mut) | AZA | 36% MRDneg | 1 y RFS 46% |
| Bataller, 2020 [110] | NPM1 MRD failure (ELN 2017 favorable risk) | 33 (Eight morphologic relapses) | 20 chemo/HMA ± HCT 13 direct HCT | 80% MRDneg (8/10 chemo) | 2 y OS 86% |
| Short, 2022 [111] | MFC-MRD relapse | 16 | Seven HMA-based chemo Nine direct HCT | 43% MRDneg (3/7 chemo) | 5 y RFS 31% 5 y OS 45% |
| Dillon, 2020 [112] | NPM1 MRD relapse | 30 | 27 chemo + HCT Three direct HCT | 59% MRDneg (16/27 chemo) | 2 y OS 63% |
| Tiong, 2021 [11] | NPM1 MRD relapse | Seven | VEN + HMA/LDAC | 86% CR MRDneg | Not reported |
| Wood, 2022 [113] | Molecular MRD failure (marker not specified) | 19 (103 morphologic disease) | VEN ± LDAC or HMA or other | 84% molecular remission | Median OS 18.4 m |
| Tiong, 2022 [12] | MRD relapse | 26 (20 NPM1mut) | VEN-LDAC | ≥1 log10 decrease (69%) 54% MRDneg | 2 y EFS 54% 2 y OS 73% |
| Othman, 2022 [114] | MRD (NPM1 or other gene fusions) failure with baseline FLT3mut | 48 (39 NPM1mut) | 32 gilteritinib Eight quizaritinib Eight sorafenib | 40% MRDneg | 2 y OS 80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiong, I.S.; Loo, S. Targeting Measurable Residual Disease (MRD) in Acute Myeloid Leukemia (AML): Moving beyond Prognostication. Int. J. Mol. Sci. 2023, 24, 4790. https://doi.org/10.3390/ijms24054790
Tiong IS, Loo S. Targeting Measurable Residual Disease (MRD) in Acute Myeloid Leukemia (AML): Moving beyond Prognostication. International Journal of Molecular Sciences. 2023; 24(5):4790. https://doi.org/10.3390/ijms24054790
Chicago/Turabian StyleTiong, Ing S., and Sun Loo. 2023. "Targeting Measurable Residual Disease (MRD) in Acute Myeloid Leukemia (AML): Moving beyond Prognostication" International Journal of Molecular Sciences 24, no. 5: 4790. https://doi.org/10.3390/ijms24054790
APA StyleTiong, I. S., & Loo, S. (2023). Targeting Measurable Residual Disease (MRD) in Acute Myeloid Leukemia (AML): Moving beyond Prognostication. International Journal of Molecular Sciences, 24(5), 4790. https://doi.org/10.3390/ijms24054790





