Study of Potential Synergistic Effect of Probiotic Formulas on Acrylamide Reduction
Abstract
1. Introduction
2. Results and Discussion
2.1. Acrylamide Reduction by Probiotics in Chemical Solution
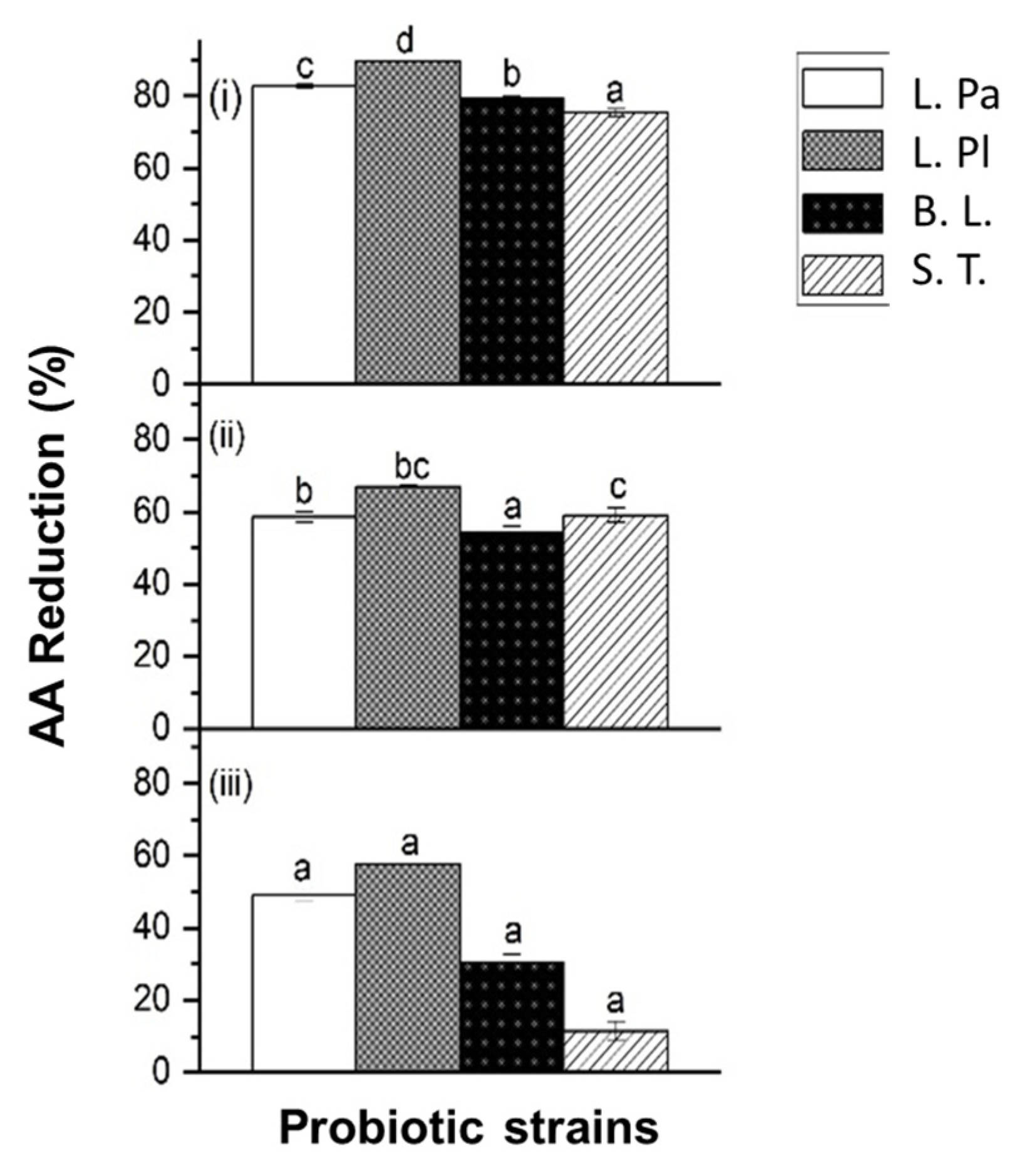
2.1.1. Acrylamide Reduction by Single Strains of Probiotic Bacteria
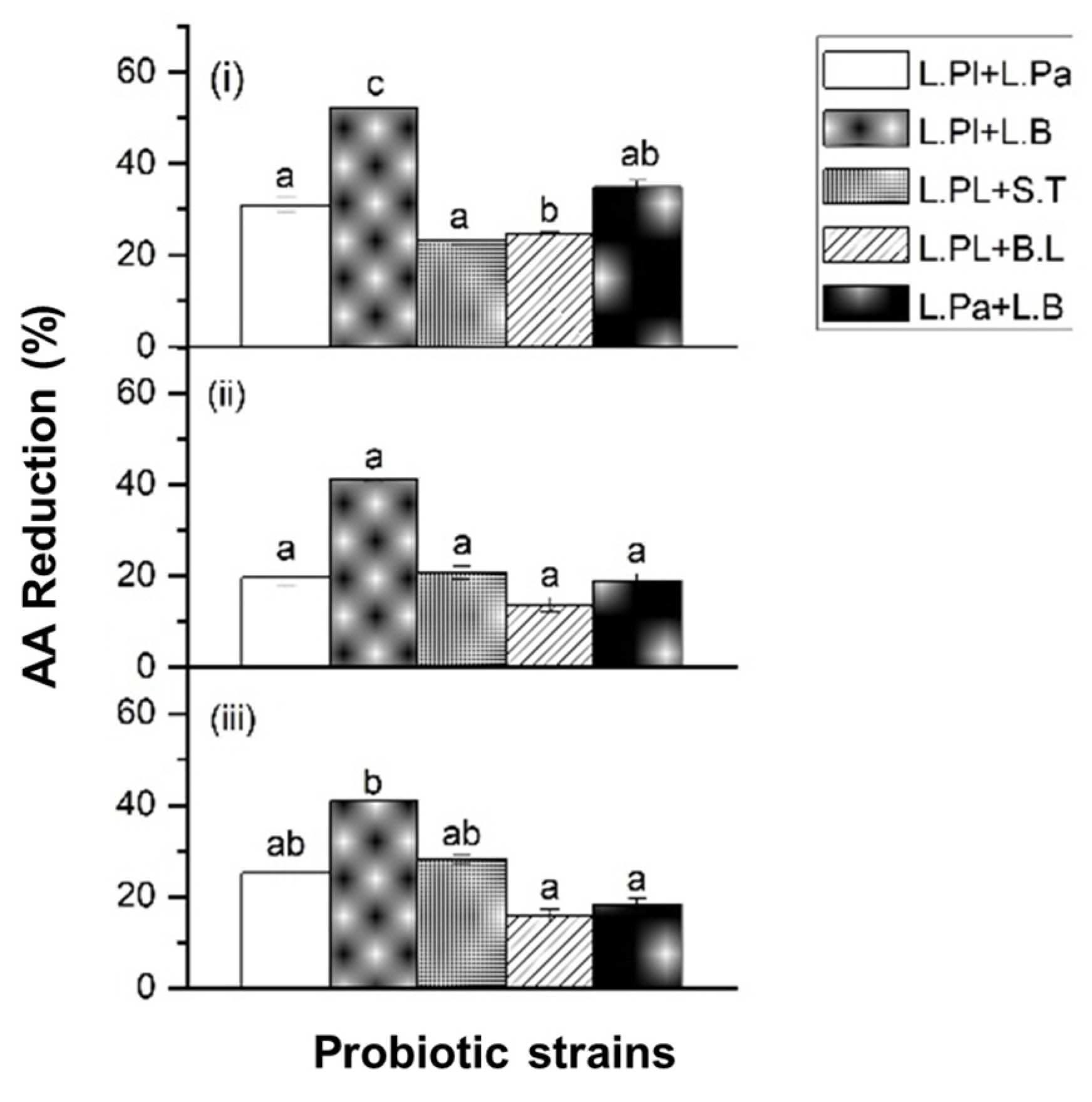
2.1.2. Acrylamide (AA) Reduction by Probiotic Formulas in Chemical Solution
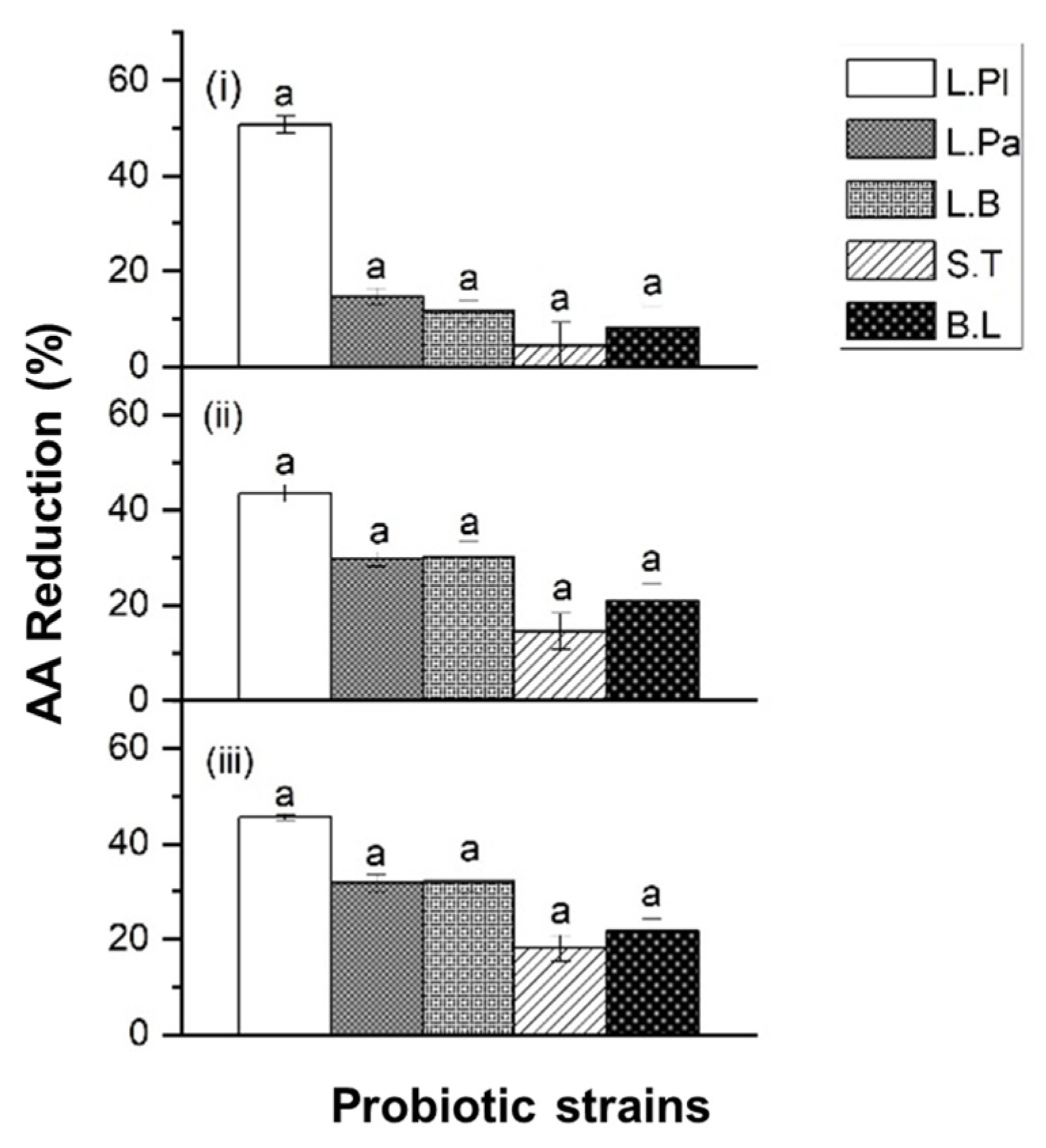
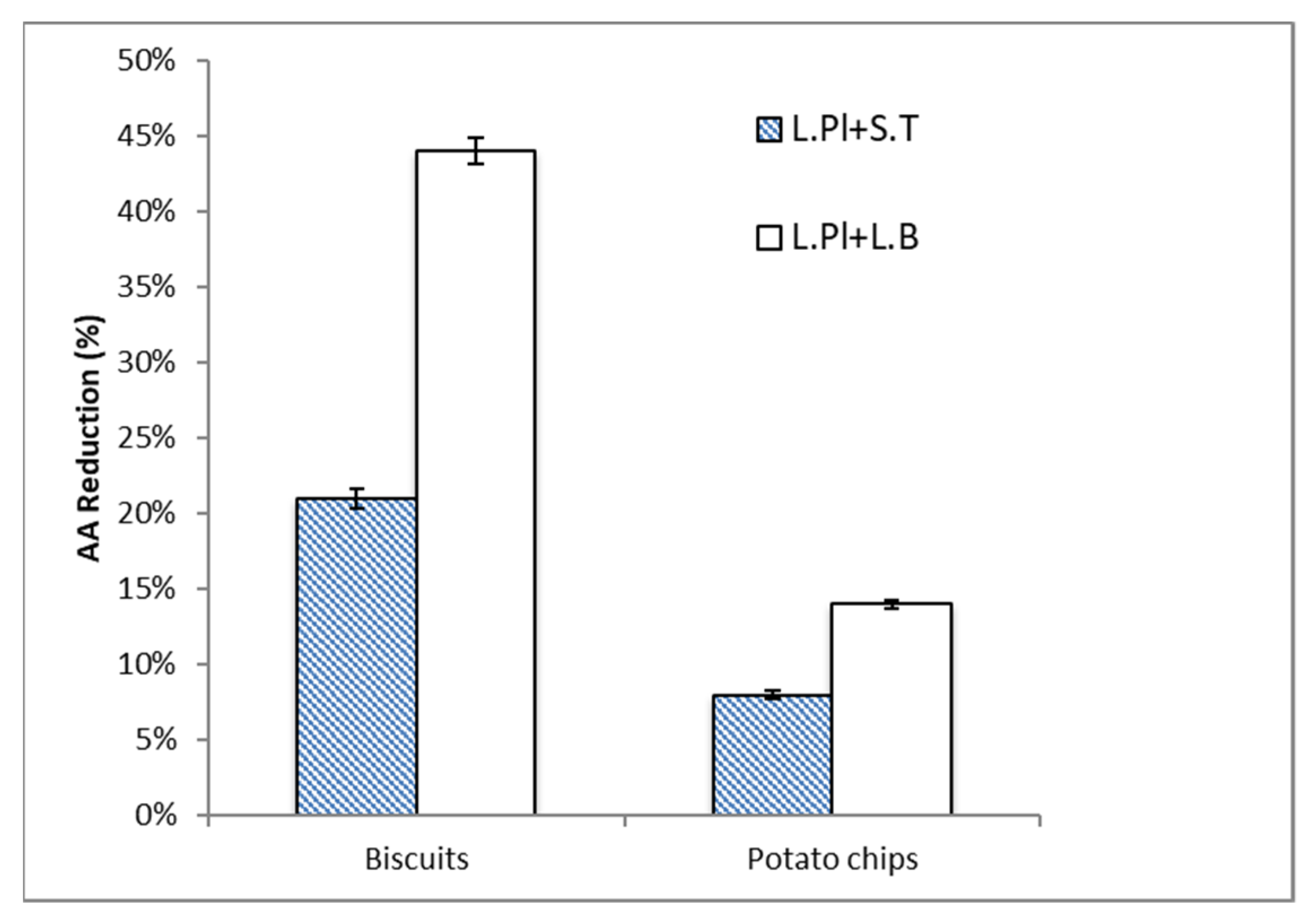
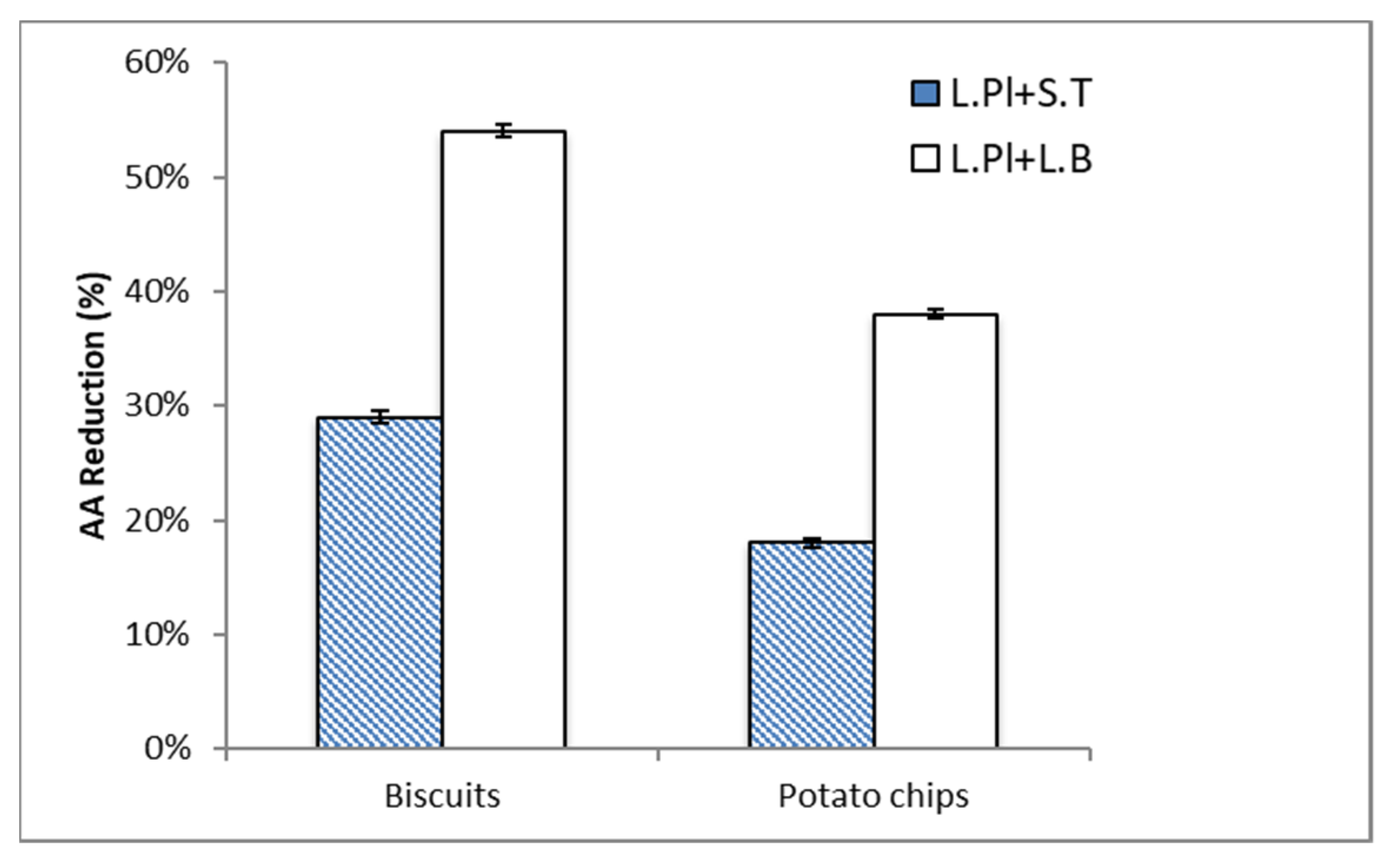
2.2. Acrylamide (AA) Reduction by Probiotic Formulas in Food Matrices
2.3. Acrylamide (AA) Reduction by Probiotic Formulas in Food Matrices under In Vitro Digestion
3. Materials and Methods
3.1. Probiotic Strains and Culture Preparation
3.2. Reagents
3.3. Acrylamide Reduction Ability of Single Probiotic Strains and Probiotic Formulas in Standard Chemical Solutions
- (1)
- Lactiplantibacillus plantarum ATCC14917 (50%) + Lacticaseibacillus paracasei ATCC25302 (50%) (L. Pl. + L. Pa.).
- (2)
- Lactiplantibacillus plantarum ATCC14917 (50%) + Lactobacillus bulgaricus ATCC11842 (50%) (L. Pl. + L. B.).
- (3)
- Lactiplantibacillus plantarum ATCC14917 (50%) + Streptococcus thermophilus ATCC19258 (50%) (L. Pl. + S. T.).
- (4)
- Lactiplantibacillus plantarum ATCC14917 (50%) + Bifidobacterium longum ATCC15707 (50%) (L. Pl. + B. L.).
- (5)
- Lacticaseibacillus paracasei ATCC25302 (50%) + Lactobacillus bulgaricus ATCC11842 (50%) (L. Pa. + L. B.).
3.4. Acrylamide Reduction Ability of Probiotic Formulas in Selected Food Matrices
3.5. Acrylamide Reduction Ability of Probiotic Formulas under In Vitro Digestion Model
3.6. Solid-Phase Extraction
3.7. LC-MS Method for the Analysis of Acrylamide
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kermat, J.; LeBail, A.; Prost, C.; Jafari, M. Acrylamide in Baking Products: A Review Article. Food Bioprocess Technol. 2010, 4, 530–543. [Google Scholar] [CrossRef]
- Alam, S.; Ahmad, R.; Pranaw, K.; Mishra, P.; Knare, S. Asparaginase conjugated magnetic nanoparticles used for reducing acrylamide formation in food model system. Bioresour. Technol. 2018, 269, 121–126. [Google Scholar] [CrossRef]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.C.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef]
- Rosén, J.; Hellenäs, K.E. Analysis of acrylamide in cooked foods by liquid chromate tandem mass spectrometry. Analyst 2002, 127, 880–882. [Google Scholar] [CrossRef]
- Becalski, A.; Lau, B.P.; Lewis, D.; Seaman, S.W. Acrylamide in foods: Occurrence, sources, and mdeling. J. Agric. Food Chem. 2003, 51, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Becalski, A.; Lau, B.P.; Lewis, D.; Seaman, S.W.; Hayward, S.; Sahagian, M.; Ramesh, M.; Leclerc, Y. Acrylamide in French fries: Influence of free amino acids and sugars. J. Agric. Food Chem. 2004, 52, 3801–3806. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives. Safety Evaluation of Certain Contaminants in Food/Prepared by the Seventy-Second Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Food Addit. Ser. 2011, 63, 9–20. [Google Scholar]
- Huang, L.; Liu, Y.; Sun, Y.; Yan, Q.; Jiang, Z. Biochemical characterization of a novel L-Asparaginase with low glutaminase activity from Rhizomucor miehei and its application in food safety and leukemia treatment. Appl. Environ. Microbiol. 2014, 80, 1561–1569. [Google Scholar] [CrossRef]
- Lee, H.W.; Pyo, S. Acrylamide induces adipocyte differentiation and obesity in mice. Chem. Biol. Interact. 2019, 298, 24–34. [Google Scholar] [CrossRef]
- Pedreschi, F.; Kaack, K.; Granby, K. Reduction of acrylamide formation in potato slices during frying. LWT-Food Sci. Technol. 2004, 37, 679–685. [Google Scholar] [CrossRef]
- Pedreschi, F.; Kaack, K.; Granby, K. Acrylamide content and color development in fried potato strips. Food Res. Int. 2006, 39, 40–46. [Google Scholar] [CrossRef]
- Zyzak, D.V.; Sanders, R.A.; Stokanovic, M.; Tallmadge, D.H.; Eberhart, B.L.; Ewald, D.K.; Grubrt, D.C.; Morsh, T.R.; Strothers, M.A.; Rizzi, G.P.; et al. Acrylamide formation mechanism in heated foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [Google Scholar] [CrossRef]
- Pedreschi, F.; Granby, K.; Risum, J. Acrylamide mitigation in potato chips by using NaCl. Food Bioprocess Technol. 2010, 3, 917–921. [Google Scholar] [CrossRef]
- Rydberg, P.; Eriksson, S.; Tareke, E.; Karlsson, P.; Ehrenberg, L.; Törnqvist, M. Investigations of factors that influence the acrylamide content of heated foodstuffs. J. Agric. Food Chem. 2003, 51, 7012–7018. [Google Scholar] [CrossRef] [PubMed]
- Nachi, I.; Fhoula, I.; Smida, I.; Ben, T.I.; Chouaibi, M.; Jaunbergs, J. Assessment of lactic acid bacteria application for the reduction of acrylamide formation in bread. LWT-Food Sci. Technol. 2018, 92, 435–441. [Google Scholar] [CrossRef]
- Long, S.S.; Prober, C.G.; Fischer, M. Principles and Practice of Pediatric Infectious Diseases E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Fuquay, J.W.A.; McSweeney, P.L.B.; Fox, P.F.C. Encyclopedia of Dairy Sciences, 3rd ed.; Academic Press: London, UK, 2021; Volume 4, pp. 144–150. [Google Scholar]
- Robinson, R.K. Encyclopedia of Food Microbiology, 2nd ed.; Academic press: London, UK, 2014; pp. 535–560. [Google Scholar]
- Serrano-Niño, J.C.; Cavazos-Garduño, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A.; García, H.S. In vitro Study of the Potential Protective Role of Lactobacillus Strains by Acrylamide Binding. J. Food Saf. 2014, 34, 62–68. [Google Scholar] [CrossRef]
- Serrano-Niño, J.C.; Cavazos-Garduño, A.; Cantú-Cornelio, F.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A.; García, H.S. In vitro reduced availability of aflatoxin B1 and acrylamide by bonding interactions with teichoic acids from lactobacillus strains. LWT-Food Sci. Technol. 2015, 64, 1334–1341. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, W.; Li, L.; Zhao, H.Y.; Sun, H.Y.; Meng, M.H.; Zhang, S.; Shao, M.L. Key role of peptidoglycan on acrylamide binding by lactic acid bacteria. Food Sci. Biotechnol. 2017, 26, 271–277. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, S.; Zhao, X.; Sun, H.; Shao, M.; Xu, H. In vitro adsorption mechanism of acrylamide by lactic acid bacteria. LWT-Food Sci. Technol. 2019, 100, 119–125. [Google Scholar] [CrossRef]
- Khorshidian, N.; Yousefi, M.; Shadnoush, M.; Siadat, S.D.; Mohammadi, M.; Mortazavian, A.M. Using LABs for mitigation of acrylamide in food products: A mini review. Curr. Opin. Food Sci. 2020, 32, 67–75. [Google Scholar] [CrossRef]
- Shoukat, S. Potential anti-carcinogenic effect of probiotic and lactic acid bacteria in detoxification of benzo [a] pyrene: A review. Trends Food Sci. Technol. 2020, 99, 450–459. [Google Scholar] [CrossRef]
- Sansano, M.; Heredia, A.; Peinado, I.; Andres, A. Dietary acrylamide: What happens during digestion. Food Chem. 2017, 237, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.J. N-Acetylmuramoyl-l- alanine amidase, In Handbook of Proteolytic Enzymes, 2nd ed.; Barrett, A.J., Woessner, J.F., Rawlings, N.D., Eds.; Academic Press: London, UK, 2004; Volume 1, pp. 866–868. [Google Scholar]
- Yousefi, M.; Shariatifar, N.; Tajabadi Ebrahimi, M.; Mortazavian, A.M.; Mohammadi, A.; Khorshidian, N.; Arab, M.; Hosseini, H. In vitro removal of polycyclic aromatic hydrocarbons by lactic acid bacteria. J. Appl. Microbiol. 2019, 126, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K.; Nowak, A.; Chlebicz, A.; Żbikowski, A.; Pawłowski, K.; Szeleszczuk, P. Probiotic microorganisms detoxify ochratoxin A in both a chicken liver cell line and chickens. J. Sci. Food Agric. 2019, 99, 4309–4318. [Google Scholar] [CrossRef]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-culture systems and technologies: Taking synthetic biology to the next level. J. R. Soc. Interface 2014, 11, 20140065. [Google Scholar] [CrossRef]
- Takeuchi, S.; Hashimoto, S.; Ogawa, N. Roles of probiotics and prebiotics in colon cancer prevention: Postulated mechanisms and in-vivo evidence. Int. J. Mol. Sci. 2008, 9, 854–863. [Google Scholar]
- Desrouilleres, K.; Millette, M.; Bagheri, L.; Maherani, B. The synergistic effect of cell wall extracted from probiotic biomass containing Lactobacillus acidophilus CL1285, L. casei LBC80R, and L. rhamnosus CLR2 on the anticancer activity of cranberry juice-HPLC fractions. J. Food Biochem. 2020, 44, e13195. [Google Scholar] [CrossRef]
- Bertkova, I.; Hijova, E.; Chmelarova, A.; Mojzisova, G.; Petrasova, D.; Strojny, L.; Bomba, A.; Zitnan, R. The effect of probiotic microorganisms and bioactive compounds on chemically induced carcinogenesis in rats. Neoplasma 2010, 57, 422–428. [Google Scholar] [CrossRef]
- Lin, H.L.; Shiu, Y.L.; Chiu, C.S.; Huang, S.L. Screening probiotic candidates for a mixture of probiotics to enhance the growth performance, immunity, and disease resistance of Asian seabass, Lates calcarifer (Bloch), against Aeromonas hydrophila. Fish Shellfish. Immunol. 2017, 60, 474–482. [Google Scholar] [CrossRef]
- El-Nezami, H.; Kankaanpaa, P.; Salminen, P.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998, 36, 321–326. [Google Scholar] [CrossRef]
- Heikkila, J.E.; Nybom, S.M.; Salminen, S.J.; Meriluoto, J.A. Removal of cholera toxin from aqueous solution by probiotic bacteria. Pharmaceuticals 2012, 5, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Pizzolitto, R.P.; Dalcero, A.M.; Bueno, D.J.; Cavaglieri, L.; Armando, M.R.; Salvano, M.A. Binding of Aflatoxin B1 to Lactic Acid Bacteria and Saccharomyces Cerevisiae In Vitro: A Useful Model to Determine the Most Efficient Microorganism; IntechOpen Access Publsher: London, UK, 2011; pp. 323–346. [Google Scholar]
- Choi, S.M.; Yang, L.; Chang, Y.X.; Chu, I.K.; Dong, N.P. Study of the efficacy of probiotic bacteria to reduce acrylamide in food and in vitro digestion. Foods 2022, 11, 1263. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.M.; Lin, H.; Xie, W.; Chu, I.K. Study of Potential Synergistic Effect of Probiotic Formulas on Acrylamide Reduction. Int. J. Mol. Sci. 2023, 24, 4693. https://doi.org/10.3390/ijms24054693
Choi SM, Lin H, Xie W, Chu IK. Study of Potential Synergistic Effect of Probiotic Formulas on Acrylamide Reduction. International Journal of Molecular Sciences. 2023; 24(5):4693. https://doi.org/10.3390/ijms24054693
Chicago/Turabian StyleChoi, Siu Mei, Hongyu Lin, Weiying Xie, and Ivan K. Chu. 2023. "Study of Potential Synergistic Effect of Probiotic Formulas on Acrylamide Reduction" International Journal of Molecular Sciences 24, no. 5: 4693. https://doi.org/10.3390/ijms24054693
APA StyleChoi, S. M., Lin, H., Xie, W., & Chu, I. K. (2023). Study of Potential Synergistic Effect of Probiotic Formulas on Acrylamide Reduction. International Journal of Molecular Sciences, 24(5), 4693. https://doi.org/10.3390/ijms24054693





