Aluminum and Fluoride Stresses Altered Organic Acid and Secondary Metabolism in Tea (Camellia sinensis) Plants: Influences on Plant Tolerance, Tea Quality and Safety
Abstract
1. Introduction
2. Results
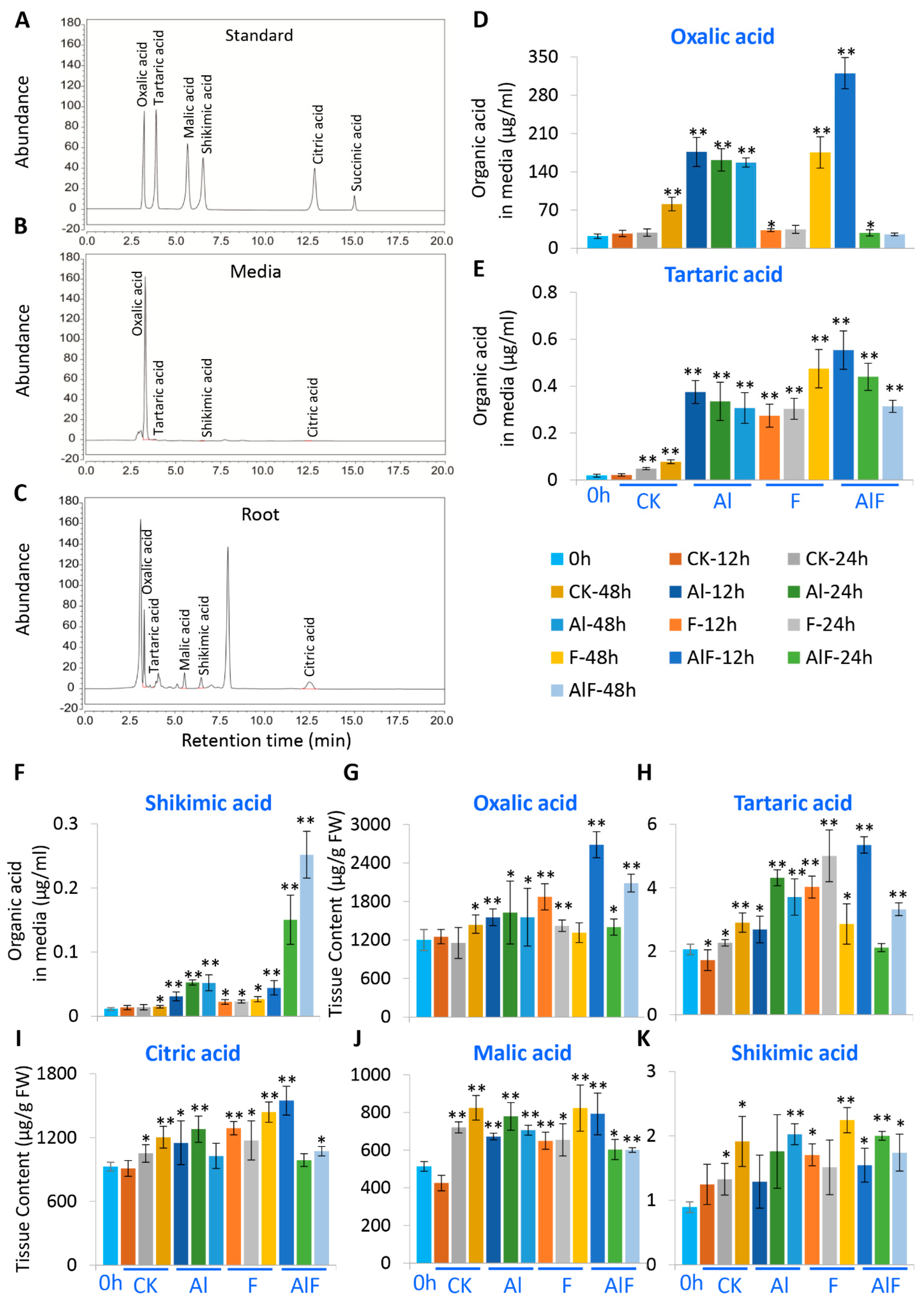
2.1. Analysis of Organic Acid Synthesis and Secretion in Tea Roots Treated with Al and F
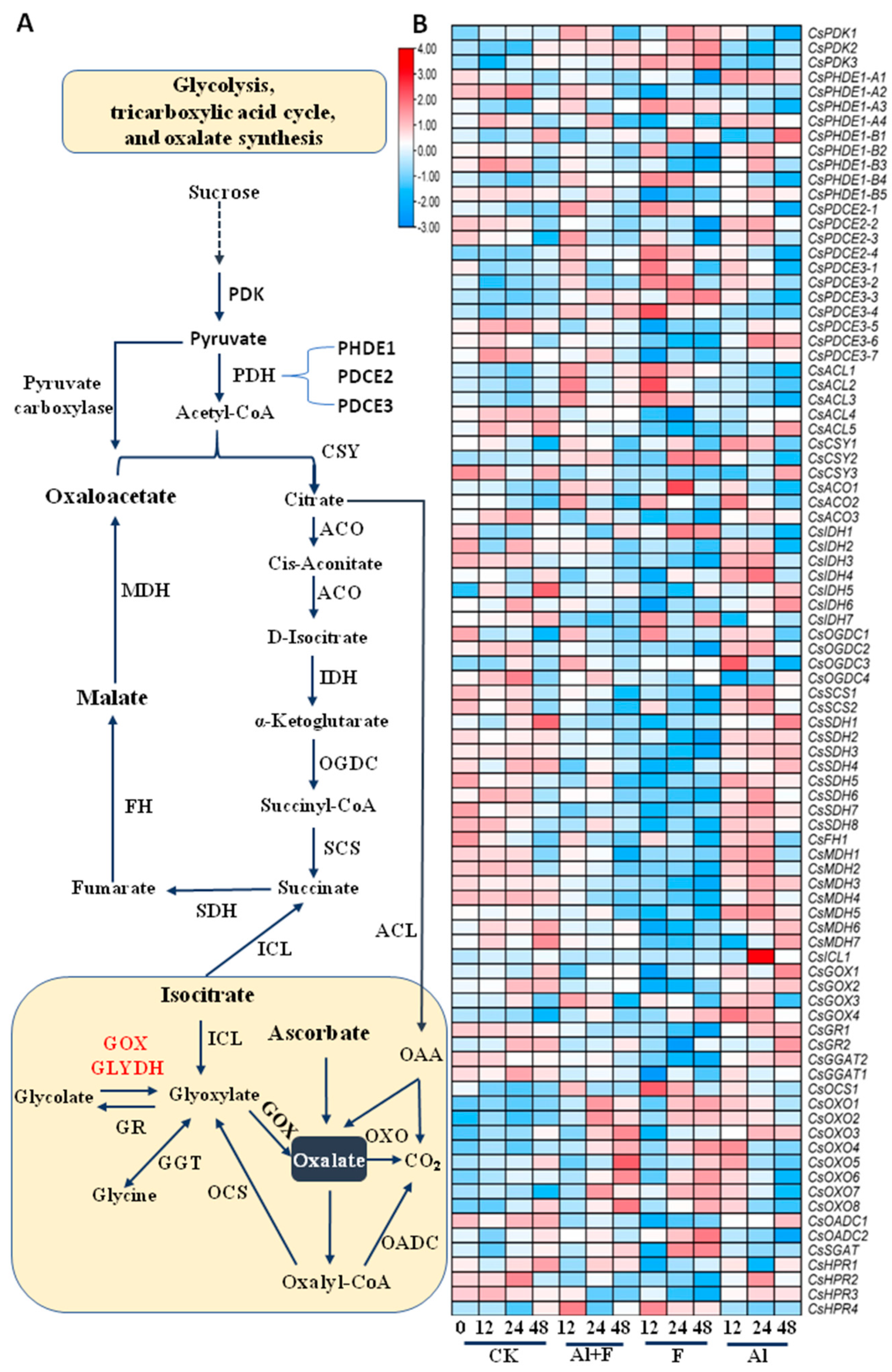
2.2. Altered Expression of TAC Cycle Genes in Tea Plant Roots under Al and F Stresses
2.3. Oxalic Acid Biosynthesis in Tea Plant Roots under Al and F Stresses
2.4. Tartaric Acid Biosynthesis in Tea Plant Roots under Al and F Stresses
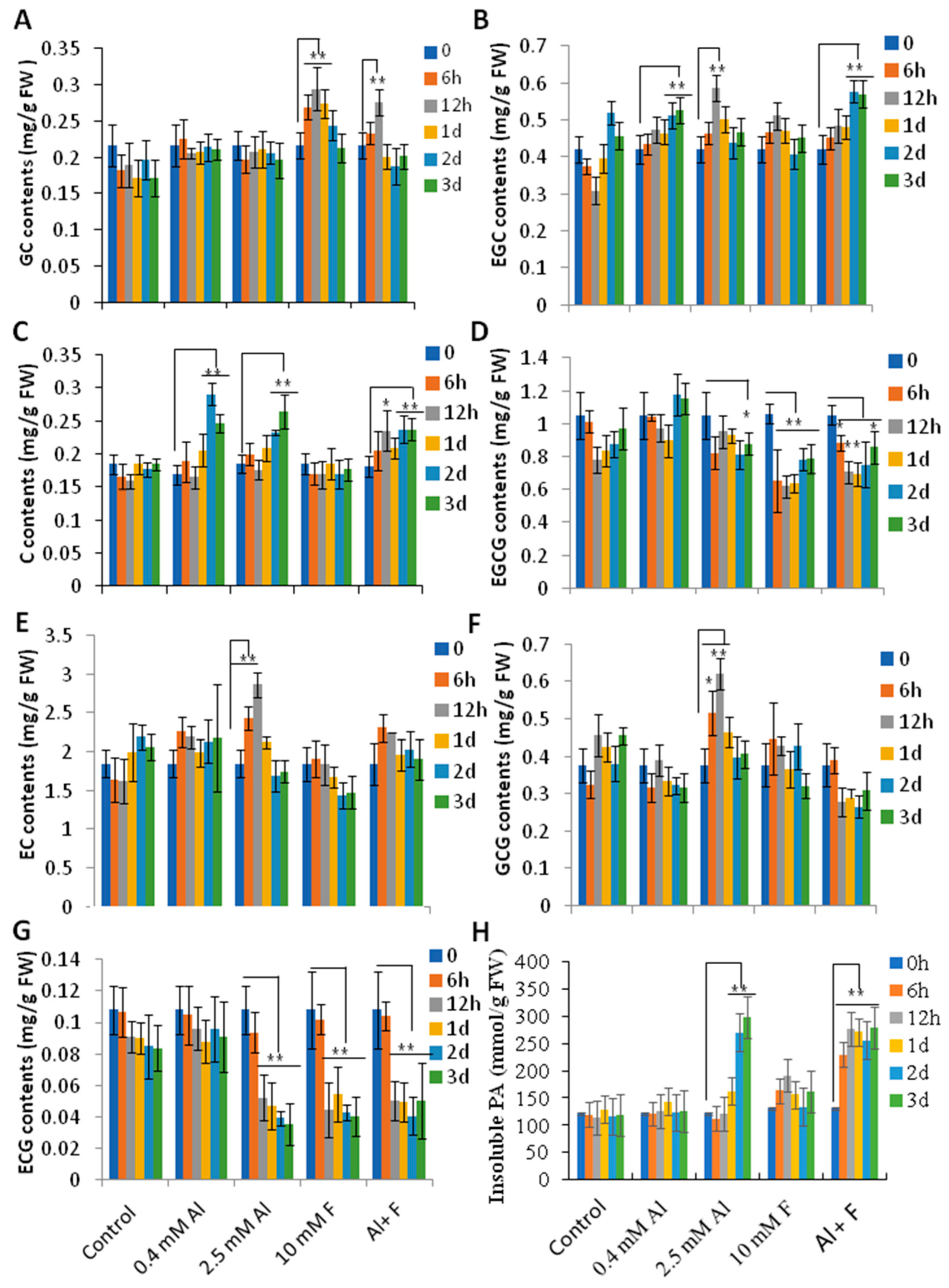
2.5. Catechins Biosynthesis Genes Were Differentially Regulated by Al and F Treatments
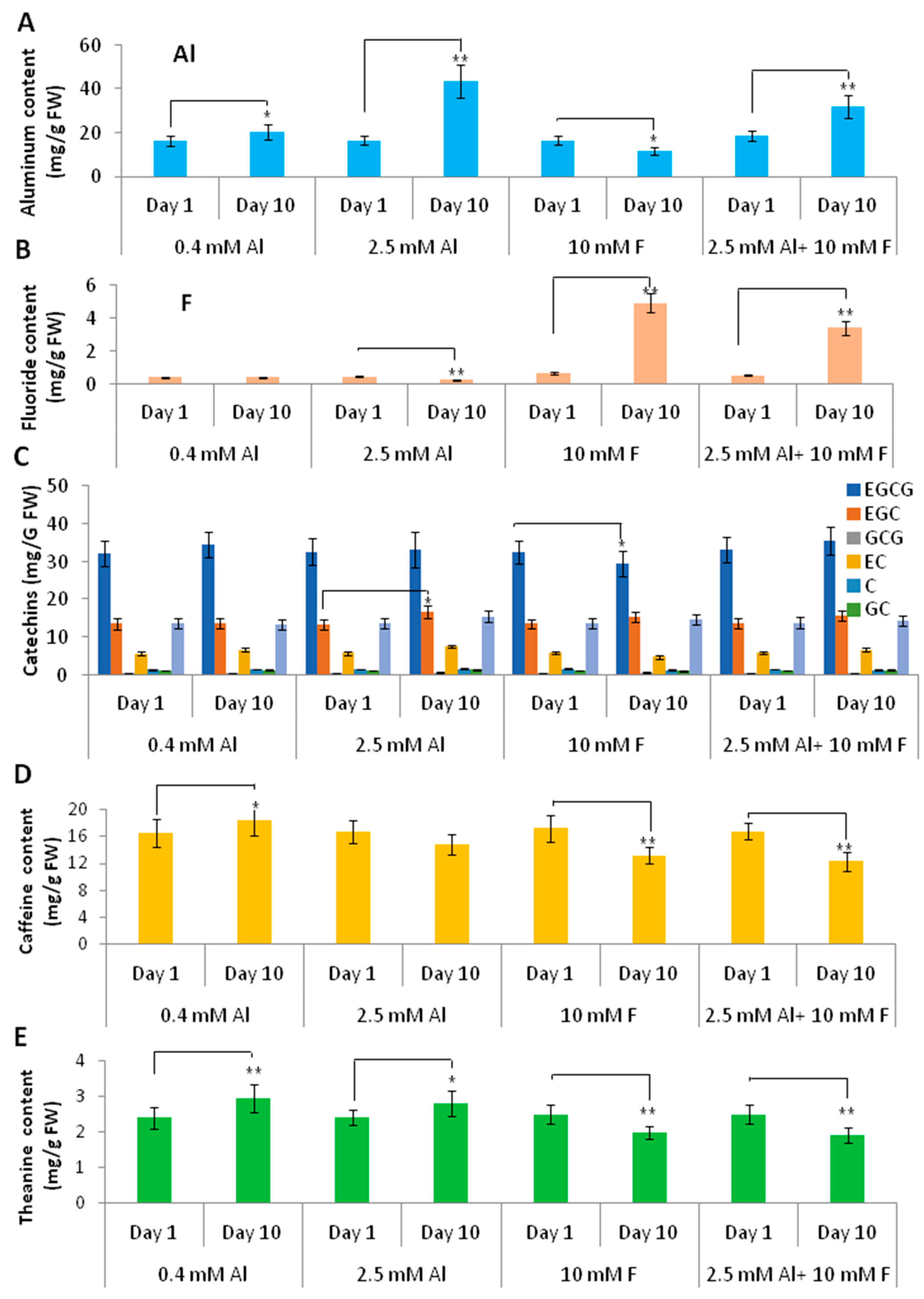
2.6. Al and F Stresses Altered the Accumulation of Catechins and Proanthocyanidins in Roots
2.7. Theanine and Caffeine Synthesis Genes Were Differentially Regulated by Al and F Stresses
2.8. Al and F Stresses Affected Tea Secondary Metabolites in Young Tea Leaves
2.9. qRT-PCR Confirmation of Gene Expression Patterns
3. Discussion
3.1. Tea Roots Had Active TAC Metabolism in Response to Al and F Stresses
3.2. Tea Roots Actively Secreted Organic Acids to Affect Al and F Accumulation
3.3. Al and F Impacted TAC Metabolism and Synthesis of Organic and Amino Acids Differently
3.4. Al and F Stresses Modulated Catechins, Theanine, and Caffeine Synthesis
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Determination of OAs Content in High Performance Liquid Phase
4.3. RNA Isolation and Transcriptome Analysis
4.4. RNA Isolation and qRT-PCR Analysis
4.5. Determination of Catechins, Caffeine, and Theanine in Roots and Young Leaves of Tea Plant Seedlings
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Li, P.; Xia, T.; Wan, X. Exploring plant metabolic genomics: Chemical diversity, metabolic complexity in the biosynthesis and transport of specialized metabolites with the tea plant as a model. Crit. Rev. Biotechnol. 2020, 40, 667–688. [Google Scholar] [CrossRef]
- Yang, C.S.; Hong, J. Prevention of Chronic Diseases by Tea: Possible Mechanisms and Human Relevance. Annu. Rev. Nutr. 2013, 33, 161–181. [Google Scholar] [CrossRef]
- Morita, A.; Horie, H.; Fujii, Y.; Takatsu, S.; Watanabe, N.; Yagi, A.; Yokota, H. Chemical forms of aluminum in xylem sap of tea plants (Camellia sinensis L.). Phytochemistry 2004, 65, 2775–2780. [Google Scholar] [CrossRef]
- Morita, A.; Yanagisawa, O.; Takatsu, S.; Maeda, S.; Hiradate, S. Mechanism for the detoxification of aluminum in roots of tea plant (Camellia sinensis (L.) Kuntze). Phytochemistry 2008, 69, 147–153. [Google Scholar] [CrossRef]
- Gao, H.-J.; Zhao, Q.; Zhang, X.-C.; Wan, X.-C.; Mao, J.-D. Localization of Fluoride and Aluminum in Subcellular Fractions of Tea Leaves and Roots. J. Agric. Food Chem. 2014, 62, 2313–2319. [Google Scholar] [CrossRef]
- Yamashita, H.; Fukuda, Y.; Yonezawa, S.; Morita, A.; Ikka, T. Tissue ionome response to rhizosphere pH and aluminum in tea plants (Camellia sinensis L.), a species adapted to acidic soils. Plant-Environ. Interact. 2020, 1, 152–164. [Google Scholar] [CrossRef]
- Yang, J.; Gou, M. The Research Status of Fluorine Contamination in Soils of China. Ecol. Evol. Sci. 2017, 26, 506–513. [Google Scholar]
- Wang, L.; Fu, Q.; Hu, H.; Min, Y.; Ye, F. Fluorine content in tea leaf and fluorine fractionation in soils of tea gardens in Hubei province. Environ. Chem. 2011, 30, 662–667. [Google Scholar]
- Mou, M.; Shi, Y.; Zhang, X.; Qian, Y.; Zhao, L. Contents and Risk Assessment of Heavy Metal and Fluorine in Selenium Enriched Tea Garden Soils in Enshi Area. J. Hennan Agric. Sci. 2016, 45, 61–65. [Google Scholar]
- Zhang, C.; Li, Z.; Gu, M.; Deng, C.; Liu, M.; Li, L. Spatial and vertical distribution and pollution assessment of soil fluorine in a lead-zinc mining area in the Karst region of Guangxi, China. Plant Soil Environ. 2010, 56, 282–287. [Google Scholar] [CrossRef]
- Ding, Z.J.; Shi, Y.Z.; Li, G.X.; Harberd, N.P.; Zheng, S.J. Tease out the future: How tea research might enable crop breeding for acid soil tolerance. Plant Commun. 2021, 2, 100182. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, W.F.; Yang, X.Q. Fluoride content in tea and its relationship with tea quality. J. Agric. Food Chem. 2004, 52, 4472–4476. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, Y.; Liu, J.; Xirao, R.; Danzeng, S.; Daji, D.; Yan, Y. Brick tea fluoride as a main source of adult fluorosis. Food Chem. Toxicol. 2003, 41, 535–542. [Google Scholar] [CrossRef]
- Karak, T.; Bhagat, R.M. Trace elements in tea leaves, made tea and tea infusion: A review. Food Res. Int. 2010, 43, 2234–2252. [Google Scholar] [CrossRef]
- Hao, J.; Peng, A.; Li, Y.; Zuo, H.; Li, P.; Wang, J.; Yu, K.; Liu, C.; Zhao, S.; Wan, X.; et al. Tea plant roots respond to aluminum-induced mineral nutrient imbalances by transcriptional regulation of multiple cation and anion transporters. BMC Plant Biol. 2022, 22, 203. [Google Scholar] [CrossRef]
- Ruan, J.; Ma, L.; Shi, Y.; Zhang, F. Effects of litter incorporation and nitrogen fertilization on the contents of extractable aluminium in the rhizosphere soil of tea plant (Camallia sinensis (L.) O. Kuntze). Plant Soil 2004, 263, 283–296. [Google Scholar] [CrossRef]
- Wan, Q.; Xu, R.K.; Li, X.H. Proton release by tea plant (Camellia sinensis L.) roots as affected by nutrient solution concentration and pH. Plant Soil Environ. 2012, 58, 429–434. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.-K.; Wang, N.; Li, X.-H. Soil Acidification of Alfisols as Influenced by Tea Cultivation in Eastern China. Pedosphere 2010, 20, 799–806. [Google Scholar] [CrossRef]
- Mukhopadyay, M.; Bantawa, P.; Das, A.; Sarkar, B.; Bera, B.; Ghosh, P.; Mondal, T.K. Changes of growth, photosynthesis and alteration of leaf antioxidative defence system of tea [Camellia sinensis (L.) O. Kuntze] seedlings under aluminum stress. Biometals 2012, 25, 1141–1154. [Google Scholar] [CrossRef]
- Baunthiyal, M.; Ranghar, S. Physiological and Biochemical Responses of Plants under Fluoride Stress: An Overview. Fluoride 2014, 47, 287–293. [Google Scholar]
- Yang, P.; Liu, Z.; Zhao, Y.; Cheng, Y.; Li, J.; Ning, J.; Yang, Y.; Huang, J. Comparative study of vegetative and reproductive growth of different tea varieties response to different fluoride concentrations stress. Plant Physiol. Biochem. 2020, 154, 419–428. [Google Scholar] [CrossRef]
- Makete, N.; Rizzu, M.; Seddaiu, G.; Gohole, L.; Otinga, A. Fluoride toxicity in cropping systems: Mitigation, adaptation strategies and related mechanisms. A review. Sci. Total Environ. 2022, 833, 155129. [Google Scholar] [CrossRef]
- Peng, C.Y.; Xu, X.F.; Ren, Y.F.; Niu, H.L.; Yang, Y.Q.; Hou, R.Y.; Wan, X.C.; Cai, H.M. Fluoride absorption, transportation and tolerance mechanism in Camellia sinensis, and its bioavailability and health risk assessment: A systematic review. J. Sci. Food Agric. 2021, 101, 379–387. [Google Scholar] [CrossRef]
- Huang, D.; Gong, Z.; Chen, X.; Wang, H.; Tan, R.; Mao, Y. Transcriptomic responses to aluminum stress in tea plant leaves. Sci. Rep. 2021, 11, 5800. [Google Scholar] [CrossRef]
- Morita, A.; Yanagisawa, O.; Maeda, S.; Takatsu, S.; Ikka, T. Tea plant (Camellia sinensis L.) roots secrete oxalic acid and caffeine into medium containing aluminum. Soil Sci. Plant Nutr. 2011, 57, 796–802. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Huang, C.-F.; de Silva, J.; Zhao, F.-J. Aluminium alleviates fluoride toxicity in tea (Camellia sinensis). Plant Soil 2016, 402, 179–190. [Google Scholar] [CrossRef]
- Ruan, J.; Ma, L.; Shi, Y.; Han, W. Uptake of fluoride by tea plant (Camellia sinensis L.) and the impact of aluminium. J. Sci. Food Agric. 2003, 83, 1342–1348. [Google Scholar] [CrossRef]
- Tolrà, R.; Vogel-Mikuš, K.; Hajiboland, R.; Kump, P.; Pongrac, P.; Kaulich, B.; Gianoncelli, A.; Babin, V.; Barceló, J.; Regvar, M.; et al. Localization of aluminium in tea (Camellia sinensis) leaves using low energy X-ray fluorescence spectro-microscopy. J. Plant Res. 2010, 124, 165–172. [Google Scholar] [CrossRef]
- Hajiboland, R.; Bahrami Rad, S.; Barceló, J.; Poschenrieder, C. Mechanisms of aluminum-induced growth stimulation in tea (Camellia sinensis). J. Plant Nutr. Soil Sci. 2013, 176, 616–625. [Google Scholar] [CrossRef]
- Chen, Y.M.; Tsao, T.M.; Liu, C.C.; Lin, K.C.; Wang, M.K. Aluminium and nutrients induce changes in the profiles of phenolic substances in tea plants (Camellia sinensis CV TTES, No. 12 (TTE)). J. Sci. Food Agric. 2011, 91, 1111–1117. [Google Scholar] [CrossRef]
- Tolrà, R.; Martos, S.; Hajiboland, R.; Poschenrieder, C. Aluminium alters mineral composition and polyphenol metabolism in leaves of tea plants (Camellia sinensis). J. Inorg. Biochem. 2020, 204, 110956. [Google Scholar] [CrossRef] [PubMed]
- Osawa, H.; Endo, I.; Hara, Y.; Matsushima, Y.; Tange, T. Transient proliferation of proanthocyanidin-accumulating cells on the epidermal apex contributes to highly aluminum-resistant root elongation in camphor tree. Plant Physiol. 2011, 155, 433–446. [Google Scholar] [CrossRef]
- She, G.; Yu, S.; Li, Z.; Peng, A.; Li, P.; Li, Y.; Chang, M.; Liu, L.; Chen, Q.; Shi, C.; et al. Characterization of CsTSI in the Biosynthesis of Theanine in Tea Plants (Camellia sinensis). J. Agric. Food Chem. 2022, 70, 826–836. [Google Scholar] [CrossRef]
- Yang, L.T.; Qi, Y.P.; Jiang, H.X.; Chen, L.S. Roles of organic acid anion secretion in aluminium tolerance of higher plants. BioMed. Res. Int. 2013, 2013, 173682. [Google Scholar] [CrossRef]
- Pan, J.; Li, D.; Zhu, J.; Shu, Z.; Ye, X.; Xing, A.; Wen, B.; Ma, Y.; Zhu, X.; Fang, W.; et al. Aluminum relieves fluoride stress through stimulation of organic acid production in Camellia sinensis. Physiol. Mol. Biol. Plants 2020, 26, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.; Fung, K.F.; Carr, H.P. Aluminium and fluoride contents of tea, with emphasis on brick tea and their health implications. Toxicol. Lett. 2003, 137, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Beard, K.F.M.; Nunes-Nesi, A.; Fernie, A.R.; Ratcliffe, R.G. Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 2010, 15, 462–470. [Google Scholar] [CrossRef]
- Patel, M.S.; Nemeria, N.S.; Furey, W.; Jordan, F. The pyruvate dehydrogenase complexes: Structure-based function and regulation. J. Biol. Chem. 2014, 289, 16615–16623. [Google Scholar] [CrossRef]
- Lin, M.; Oliver, D.J. The role of acetyl-coenzyme a synthetase in Arabidopsis. Plant Physiol. 2008, 147, 1822–1829. [Google Scholar] [CrossRef]
- Foster, J.; Cheng, N.; Paris, V.; Wang, L.; Wang, J.; Wang, X.; Nakata, P.A. An Arabidopsis Oxalyl-CoA Decarboxylase, AtOXC, Is Important for Oxalate Catabolism in Plants. Int. J. Mol. Sci. 2021, 22, 3266. [Google Scholar] [CrossRef]
- Cholet, C.; Claverol, S.; Claisse, O.; Rabot, A.; Osowsky, A.; Dumot, V.; Ferrari, G.; Geny, L. Tartaric acid pathways in Vitis vinifera L. (cv. Ugni blanc): A comparative study of two vintages with contrasted climatic conditions. BMC Plant Biol. 2016, 16, 144. [Google Scholar] [CrossRef]
- Narnoliya, L.K.; Sangwan, R.S.; Singh, S.P. Transcriptome mining and in silico structural and functional analysis of ascorbic acid and tartaric acid biosynthesis pathway enzymes in rose-scanted geranium. Mol. Biol. Rep. 2018, 45, 315–326. [Google Scholar] [CrossRef]
- Burbidge, C.A.; Ford, C.M.; Melino, V.J.; Wong, D.C.J.; Jia, Y.; Jenkins, C.L.D.; Soole, K.L.; Castellarin, S.D.; Darriet, P.; Rienth, M.; et al. Biosynthesis and Cellular Functions of Tartaric Acid in Grapevines. Front. Plant Sci. 2021, 12, 643024. [Google Scholar] [CrossRef]
- Narnoliya, L.K.; Kaushal, G.; Singh, S.P.; Sangwan, R.S. De novo transcriptome analysis of rose-scented geranium provides insights into the metabolic specificity of terpene and tartaric acid biosynthesis. BMC Genom. 2017, 18, 74. [Google Scholar] [CrossRef]
- Jia, Y.; Burbidge, C.A.; Sweetman, C.; Schutz, E.; Soole, K.; Jenkins, C.; Hancock, R.D.; Bruning, J.B.; Ford, C.M. An aldo-keto reductase with 2-keto-l-gulonate reductase activity functions in l-tartaric acid biosynthesis from vitamin C in Vitis vinifera. J. Biol. Chem. 2019, 294, 15932–15946. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino Acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araujo, W.L.; Braun, H.P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Y.; Zhu, L.; Hao, Z.; Hu, L.; Hu, X.; Wang, G.; Cheng, T.; Shi, J.; Chen, J. The role of gamma-aminobutyric acid in aluminum stress tolerance in a woody plant, Liriodendron chinense x tulipifera. Hortic. Res. 2021, 8, 80. [Google Scholar] [CrossRef]
- Akram, M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Ding, Z.; Fan, K.; Ma, D.; Zhang, Y.; Yin, Q. Aluminum induced physiological and proteomic responses in tea (Camellia sinensis) roots and leaves. Plant Physiol. Biochem. 2017, 115, 141–151. [Google Scholar] [CrossRef]
- Kochian, L.V.; Pineros, M.A.; Liu, J.; Magalhaes, J.V. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Chen, Z.C.; Liao, H. Organic acid anions: An effective defensive weapon for plants against aluminum toxicity and phosphorus deficiency in acidic soils. J. Genet. Genom. 2016, 43, 631–638. [Google Scholar] [CrossRef]
- Delhaize, E.; Taylor, P.; Hocking, P.J.; Simpson, R.J.; Ryan, P.R.; Richardson, A.E. Transgenic barley (Hordeum vulgare L.) expressing the wheat aluminium resistance gene (TaALMT1) shows enhanced phosphorus nutrition and grain production when grown on an acid soil. Plant Biotechnol. J. 2009, 7, 391–400. [Google Scholar] [CrossRef]
- Liu, J.; Magalhaes, J.V.; Shaff, J.; Kochian, L.V. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009, 57, 389–399. [Google Scholar] [CrossRef]
- Wang, J.; Hou, Q.; Li, P.; Yang, L.; Sun, X.; Benedito, V.A.; Wen, J.; Chen, B.; Mysore, K.S.; Zhao, J. Diverse functions of multidrug and toxin extrusion (MATE) transporters in citric acid efflux and metal homeostasis in Medicago truncatula. Plant J. 2017, 90, 79–95. [Google Scholar] [CrossRef]
- Delhaize, E.; Ryan, P.R.; Hebb, D.M.; Yamamoto, Y.; Sasaki, T.; Matsumoto, H. Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proc. Natl. Acad. Sci. USA 2004, 101, 15249–15254. [Google Scholar] [CrossRef]
- Furukawa, J.; Yamaji, N.; Wang, H.; Mitani, N.; Murata, Y.; Sato, K.; Katsuhara, M.; Takeda, K.; Ma, J.F. An aluminum-activated citrate transporter in barley. Plant Cell Physiol. 2007, 48, 1081–1091. [Google Scholar] [CrossRef]
- Tesfaye, M.; Temple, S.J.; Allan, D.L.; Vance, C.P.; Samac, D.A. Overexpression of Malate Dehydrogenase in Transgenic Alfalfa Enhances Organic Acid Synthesis and Confers Tolerance to Aluminum. Plant Physiol. 2001, 127, 1836–1844. [Google Scholar] [CrossRef]
- De Angeli, A.; Baetz, U.; Francisco, R.; Zhang, J.; Chaves, M.M.; Regalado, A. The vacuolar channel VvALMT9 mediates malate and tartrate accumulation in berries of Vitis vinifera. Planta 2013, 238, 283–291. [Google Scholar] [CrossRef]
- Lv, A.; Wen, W.; Fan, N.; Su, L.; Zhou, P.; An, Y. Dehydrin MsDHN1 improves aluminum tolerance of alfalfa (Medicago sativa L.) by affecting oxalate exudation from root tips. Plant J. 2021, 108, 441–458. [Google Scholar] [CrossRef]
- Li, D.; Shu, Z.; Ye, X.; Zhu, J.; Pan, J.; Wang, W.; Chang, P.; Cui, C.; Shen, J.; Fang, W.; et al. Cell wall pectin methyl-esterification and organic acids of root tips involve in aluminum tolerance in Camellia sinensis. Plant Physiol. Biochem. 2017, 119, 265–274. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Song, X.; Zhang, Z.; Jiang, Y.; Zhu, Y.; Zhao, H.; Ni, D. An RNA-Seq transcriptome analysis revealing novel insights into aluminum tolerance and accumulation in tea plant. Planta 2017, 246, 91–103. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, M.; Liu, X.; Mao, Q.; Shi, C.; Kochian, L.V.; Liao, H. Aluminium is essential for root growth and development of tea plants (Camellia sinensis). J. Integr. Plant Biol. 2020, 62, 984–997. [Google Scholar] [CrossRef]
- Li, L.; Du, X.; He, C. Absorption and accumulation characteristics of fluorine in nutrient liquid cultured tea plant. J. Sichuan Agric. Univ. 2008, 26, 59–63. [Google Scholar]
- Zhang, G.; Ahmad, M.Z.; Chen, B.; Manan, S.; Zhang, Y.; Jin, H.; Wang, X.; Zhao, J. Lipidomic and transcriptomic profiling of developing nodules reveals the essential roles of active glycolysis and fatty acid and membrane lipid biosynthesis in soybean nodulation. Plant J. 2020, 103, 1351–1371. [Google Scholar] [CrossRef]
- Zuo, H.; Si, X.; Li, P.; Li, J.; Chen, Z.; Li, P.; Chen, C.; Liu, Z.; Zhao, J. Dynamic change of tea (Camellia sinensis) leaf cuticular wax in white tea processing for contribution to tea flavor formation. Food Res. Int. 2023, 163, 112182. [Google Scholar] [CrossRef]
- Chai, Q.; Wang, X.; Gao, M.; Zhao, X.; Chen, Y.; Zhang, C.; Jiang, H.; Wang, J.; Wang, Y.; Zheng, M.; et al. A glutathione S-transferase GhTT19 determines flower petal pigmentation via regulating anthocyanin accumulation in cotton. Plant Biotechnol. J. 2023, 21, 433–448. [Google Scholar] [CrossRef]
- Li, P.; Xu, Y.; Zhang, Y.; Fu, J.; Yu, S.; Guo, H.; Chen, Z.; Chen, C.; Yang, X.; Wang, S.; et al. Metabolite Profiling and Transcriptome Analysis Revealed the Chemical Contributions of Tea Trichomes to Tea Flavors and Tea Plant Defenses. J. Agric. Food Chem. 2020, 68, 11389–11401. [Google Scholar] [CrossRef]
- Li, P.; Fu, J.; Xu, Y.; Shen, Y.; Zhang, Y.; Ye, Z.; Tong, W.; Zeng, X.; Yang, J.; Tang, D.; et al. CsMYB1 integrates the regulation of trichome development and catechins biosynthesis in tea plant domestication. New Phytol. 2022, 234, 902–917. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, A.; Yu, K.; Yu, S.; Li, Y.; Zuo, H.; Li, P.; Li, J.; Huang, J.; Liu, Z.; Zhao, J. Aluminum and Fluoride Stresses Altered Organic Acid and Secondary Metabolism in Tea (Camellia sinensis) Plants: Influences on Plant Tolerance, Tea Quality and Safety. Int. J. Mol. Sci. 2023, 24, 4640. https://doi.org/10.3390/ijms24054640
Peng A, Yu K, Yu S, Li Y, Zuo H, Li P, Li J, Huang J, Liu Z, Zhao J. Aluminum and Fluoride Stresses Altered Organic Acid and Secondary Metabolism in Tea (Camellia sinensis) Plants: Influences on Plant Tolerance, Tea Quality and Safety. International Journal of Molecular Sciences. 2023; 24(5):4640. https://doi.org/10.3390/ijms24054640
Chicago/Turabian StylePeng, Anqi, Keke Yu, Shuwei Yu, Yingying Li, Hao Zuo, Ping Li, Juan Li, Jianan Huang, Zhonghua Liu, and Jian Zhao. 2023. "Aluminum and Fluoride Stresses Altered Organic Acid and Secondary Metabolism in Tea (Camellia sinensis) Plants: Influences on Plant Tolerance, Tea Quality and Safety" International Journal of Molecular Sciences 24, no. 5: 4640. https://doi.org/10.3390/ijms24054640
APA StylePeng, A., Yu, K., Yu, S., Li, Y., Zuo, H., Li, P., Li, J., Huang, J., Liu, Z., & Zhao, J. (2023). Aluminum and Fluoride Stresses Altered Organic Acid and Secondary Metabolism in Tea (Camellia sinensis) Plants: Influences on Plant Tolerance, Tea Quality and Safety. International Journal of Molecular Sciences, 24(5), 4640. https://doi.org/10.3390/ijms24054640







