The Effect of the Stress-Signalling Mediator Triacontanol on Biochemical and Physiological Modifications in Dracocephalum forrestii Culture
Abstract
:1. Introduction
2. Results and Discussion
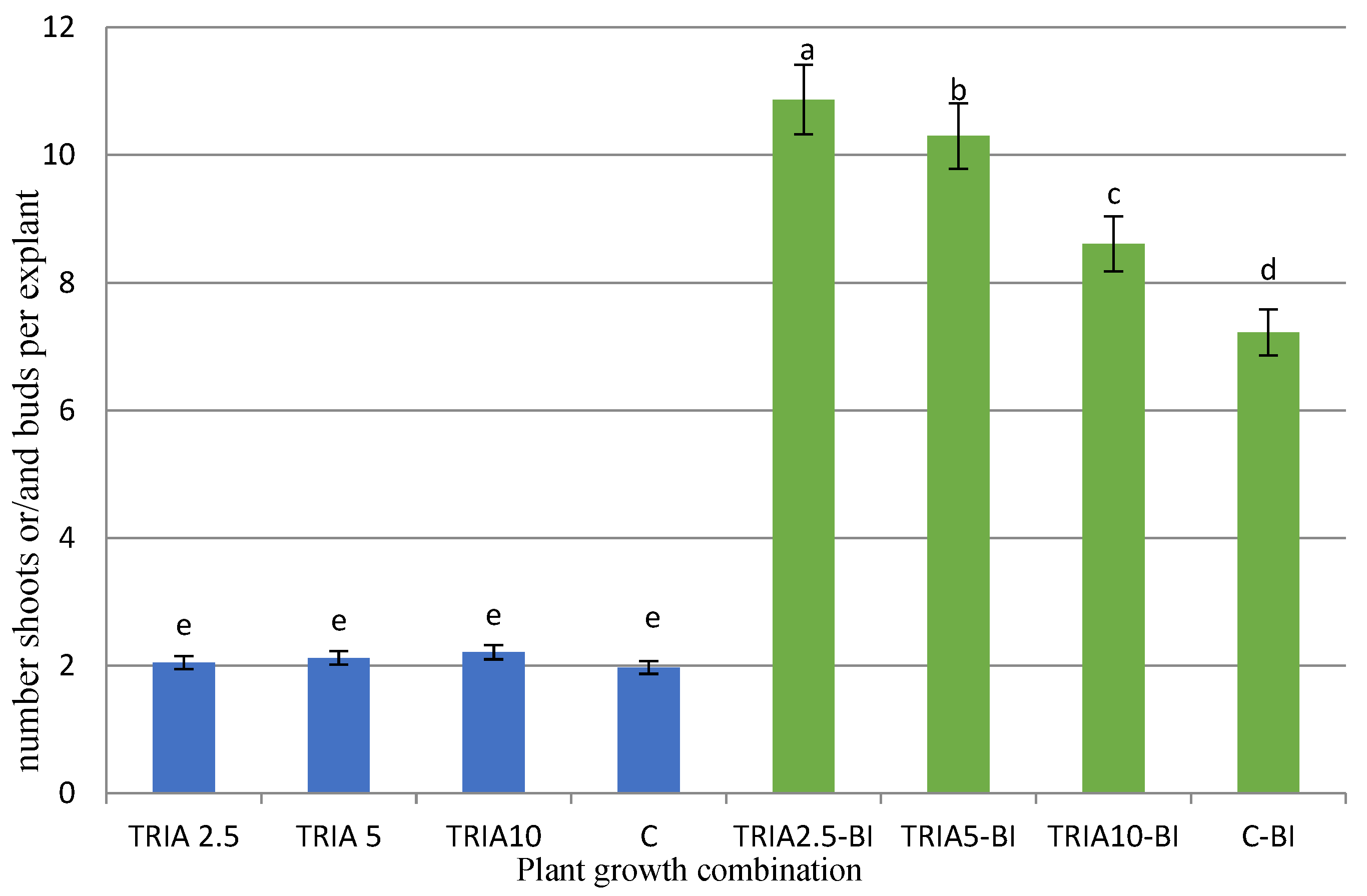
2.1. Effects of TRIA on Culture Growth
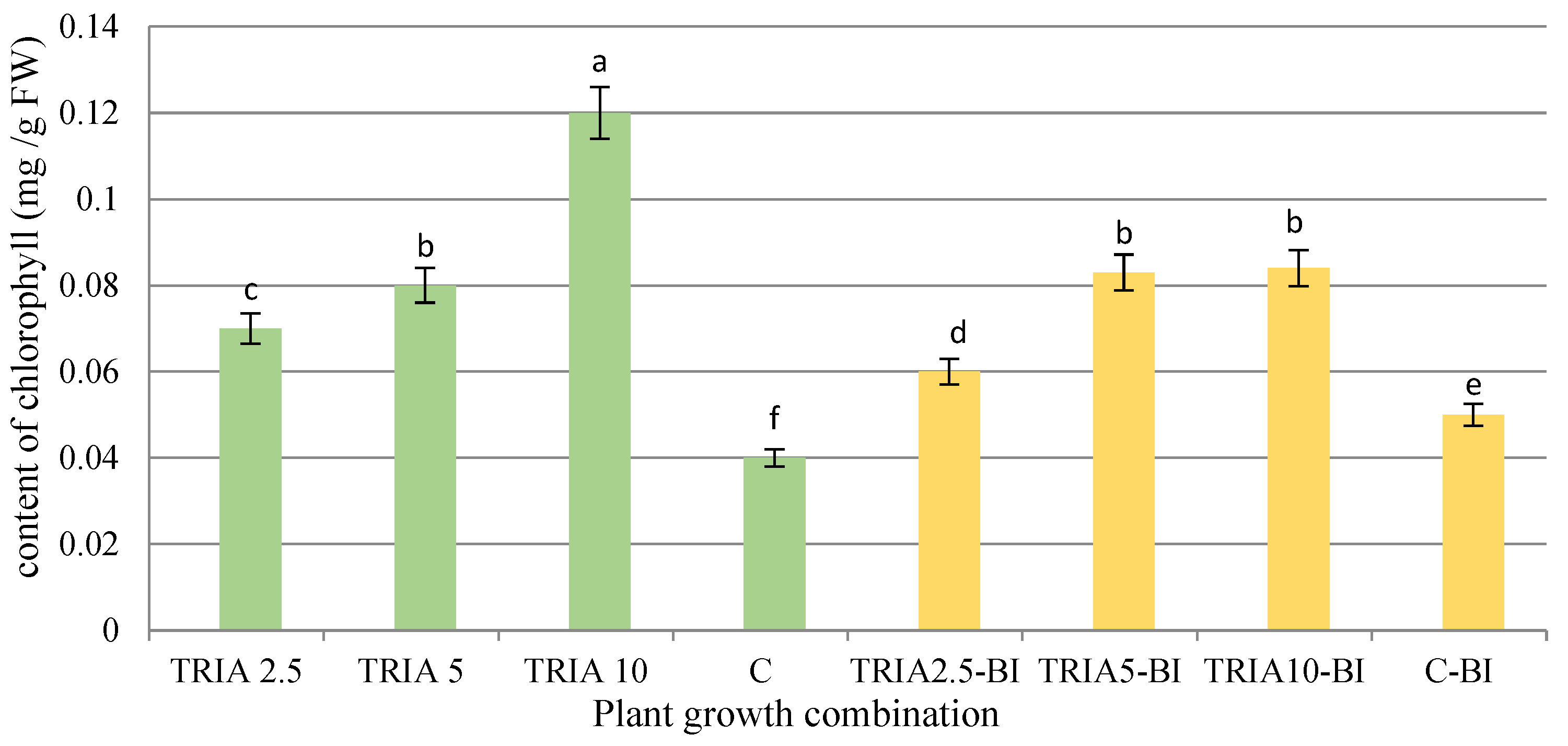
2.2. Determination of Chlorophyll Content
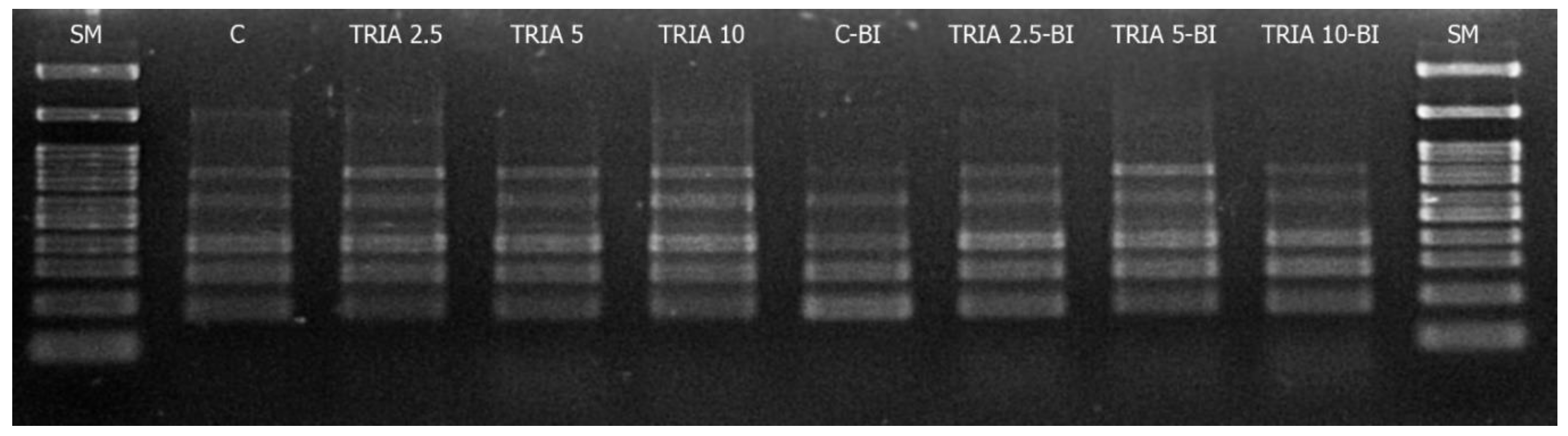
2.3. Genetic Stability
2.4. Effects of Triacontanol on Activities of Antioxidant Enzymes
2.5. Effects of Triacontanol Phenolic Compounds Content
3. Material and Methods
3.1. Plant Material and Experimental Setup
3.2. Measurement of Growth and Chlorophyll Content
3.3. Genetic Stability
3.4. Determination of Phenolic Compound Content
3.5. Estimation of Activities of Antioxidant Enzymes
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanstraelen, M.; Benková, E. Hormonal interactions in the regulation of plant development. Annu. Rev. Cell Dev. Biol. 2012, 28, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, K.; Bhattacharya, S.; Puri, S. Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. J. Appl. Res. Med. Aromat. Plants 2018, 9, 26–38. [Google Scholar] [CrossRef]
- Ogunsanya, H.Y.; Motti, P.; Li, J.; Irinh, H.K.; Xu, L.; Bernaret, N.; Van Droogenbroeck, B.; Murvanidze, N.; Werbrouck, S.P.O.; Mangelinckx, S.; et al. Belgian endive-derived biostimulants promote shoot and root growth in vitro. Sci. Rep. 2022, 12, 8792. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Ferrasse, I.; Shrestha, S.S.; Kumar, G.; Salviero, A.; Sello, S.; Atissimo, A.; Pagni, L.; Gattesco, F.; Dall’Acqua, S. Comparison of biostimulant treatments in Acmella oleracea cultivation for alkylamides production. Plants 2020, 9, 818. [Google Scholar] [CrossRef]
- Naeem, M.; Khan, M.M.A.; Moinuddin. Triacontanol: A potent plant growth regulator in agriculture. J. Plant Interact. 2012, 7, 129–142. [Google Scholar] [CrossRef]
- Ries, S.; Houtz, R. Triacontanol as a plant growth regulator. HortScience 1983, 18, 654–662. [Google Scholar] [CrossRef]
- Freeman, B.; Albrigo, L.G.; Biggs, R.H. Cuticular waxes of developing leaves and fruit of blueberry, Vaccinium ashei Reade cv. Bluegem. J. Am. Soc. Hortic. Sci. 1979, 104, 398–403. [Google Scholar] [CrossRef]
- Uchiyama, T.; Ogasawara, N. Constituents of plant leaf wax contained in rice callus tissues. Agric. Biol. Chem. 1981, 45, 1261–1263. [Google Scholar]
- Aftab, T.; Khan, M.M.A.; Idrees, M.; Naeem, M.; Singh, M.; Ram, M. Stimulation of crop productivity, photosynthesis and artemisinin production in Artemisia annua L. by triacontanol and gibberellic acid application. J. Plant Interact. 2010, 5, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Idrees, M.; Khan, M.M.A.; Aftab, T.; Naeem, M. Synergistic effects of gibberellic acid and triacontanol on growth, physiology, enzyme activities and essential oil content of Coriandrum sativum L. Asian Australas. J. Plant Sci. Biotechnol. 2010, 4, 24–29. [Google Scholar]
- Chen, X.; Yuan, H.; Chen, R.; Zhu, L.; Du, B.; Weng, Q.; He, G. Isolation and characterization of triacontanol-regulated genes in rice (Oryza sativa L.): Possible role of triacontanol as a plant growth stimulator. Plant Cell Physiol. 2002, 43, 869–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S.; Mohammad, F. Triacontanol a dynamic growth regulator for plants under diverse environmental conditions. Physiol. Mol. Biol. Plants 2020, 26, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Khan, M.M.A.; Singh, M.; Nasir, S.; Naeem, M.; Siddiqui, M.H.; Mohammad, F. Gibberellic acid and triacontanol can ameliorate the optimum yield and morphine production in opium poppy (Papaver somniferum L.). Acta Agric. Scand. B Soil Plant Sci. 2007, 57, 307–312. [Google Scholar]
- Grzegorczyk, I.; Bilichowski, I.; Mikiciuk-Olasik, E.; Wysokińska, H. The effect of triacontanol on shoot multiplication and production of antioxidant compounds in shoot of Salvia officinalis. Acta Soc. Biol. Pol. 2006, 1, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Fratelmale, D.; Giomper, L.; Ricci, D.; Rocchi, M.B.L.; Guidi, L.; Epifano, F. The effects of triacontanol on micropropagation and secretory system of Thymus mastichiana. Plant Cell Tissue Organ Cult. 2003, 74, 87–97. [Google Scholar] [CrossRef]
- Wu, Z.X.; Li, X.W. Flora of China; Since Press: Beijing, China, 1979; Volume 2. [Google Scholar]
- Li, S.M.; Yang, X.W.; Li, Y.L.; Shen, Y.H.; Feng, L.; Wang, Y.H.; Zeng, H.W.; Liu, X.H.; Zhang, C.S.; Long, C.L.; et al. Chemical constituents of Dracocephalum forrestii. Planta Med. 2009, 75, 1591–1596. [Google Scholar] [CrossRef] [Green Version]
- Weremczuk-Jeżyna, I.; Hnatuszko-Konka, K.; Lebet, L.; Grzegorczyk-Karolak, I. The protective function and modification of secondary metabolite accumulation in response to light stress in Dracocephalum forrestii shoots. Int. J. Mol. Sci. 2021, 22, 7965. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Kochan, E.; Szymczyk, P.; Lisiecki, P.; Kuźma, Ł.; Grzegorczyk-Karolak, I. The antioxidant and antimicrobial properties of phenol-rich extracts of Dracocephalum forrestii W.W. Smith shoot cultures grown in the nutrient sprinkle bioreactor. Phytochem. Lett. 2019, 30, 254–260. [Google Scholar] [CrossRef]
- Hangarter, R.; Riess, K. Effect of triacontanol on plant cell cultures in vitro. Plant Physiol. 1978, 61, 855–857. [Google Scholar] [CrossRef] [Green Version]
- Abhirami, D.; Annapurna, A.S.; Umesh, T.G. In vitro regeneration, antioxidant potential, and genetic fidelity analysis of Asystasia gangetica (L.) T. Anderson. In Vitro Cell. Dev. Biol. Plant 2021, 57, 447–459. [Google Scholar]
- Tantos, A.; Meszaros, A.; Kissimon, J.; Horvath, G.; Forkas, T. The effect of triacontanol on micropropagation of balm, Melisa officinalis L. Plant Cell Rep. 1999, 19, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.O.; Giridhar, P.; Ravishankar, G.A. The effect of triacontanol on micropropagation of Capsicum frutescens and Decalepsis hamiltoni W.A. Plant Cell Tissue Organ Cult. 2002, 71, 253–258. [Google Scholar] [CrossRef]
- Ahmad, J.; Ali, A.A.; Al-Hugail, A.A.; Qersi, M.J. Triacontanol attenuates drought-induced oxidative stress in Brassica juncea L. regulation lignification, genes calcium metabolism and the antioxidant system. Plant Physiol. Biochem. 2021, 166, 985–998. [Google Scholar] [CrossRef]
- Jaccard, P. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 1908, 44, 223–270. [Google Scholar]
- Perveen, S.; Shahbaz, M.; Ashraf, A. Modulation in activities of antioxidant enzymes in salt stressed and non-stressed wheat (Triticum aestivum L.) plants raised from seed treated with triacontanol. Pak. J. Bot. 2011, 43, 2463–2468. [Google Scholar]
- Verma, A.; Malik, C.P.; Gupta, V.K.; Bajaj, B.K. Effects of in vitro triacontanol on growth antioxidant enzymes and photosynthetic characteristic in Arachis hypogea L. Braz. J. Plant Physiol. 2012, 23, 271–277. [Google Scholar] [CrossRef]
- Karam, E.A.; Keramat, B.; Asrar, Z.; Mozafari, H. Study of interaction effect between triacontanol and nitric oxide on alleviating stress arsenic toxicity in coriander seedlings. J. Plant Interact. 2017, 12, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Shaki, F.; Rezayian, M.; Ebrahimzadeh, H.; Niknam, V. Role of triazolic compounds in underlying mechanisms of plant stress tolerance: A review. Iran. J. Plant Physiol. 2022, 12, 3943–3954. [Google Scholar]
- Perveen, S.; Shahbaz, M.; Ashraf, M. Regulation in gas exchange and quantum yield of photosystem II (PSII) in salt-stressed and non-stressed wheat plants raised from seed treated with triacontanol. Pak. J. Bot. 2012, 42, 3081. [Google Scholar]
- Muthuchelian, K.; Murugan, C.; Harigovindan, R. Ameliorating effect of triacontanol on salt stressed Erythrina variegata seedlings. Changes in growth, biomass, pigments and solute accumulation. Biol. Plant. 1996, 38, 133–136. [Google Scholar]
- Villalva, M.; Jaime, L.; Aguado, E.; Nieto, J.A.; Reglero, G.; Santoyo, S. Anti-Inflammatory and antioxidant activities from the basolateral fraction of Caco-2 Cells exposed to a rosmarinic acid enriched extract. J. Agric. Food Chem. 2018, 66, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Li, G.S.; Jiang, W.L.; Tian, J.W.; Qu, G.W.; Zhu, H.B.; Fu, F.H. In vitro and in vivo antifibrotic effects of rosmarinic acid on experimental liver fibrosis. Phytomedicine 2010, 17, 282–288. [Google Scholar] [CrossRef]
- Ma, Z.J.; Lu, Y.B.; Yang, F.G.; Li, S.P.; He, X.G.; Gao, Y.C.; Zhang, G.Z.; Ren, E.H.; Wang, Y.G.; Kang, X.W. Rosmarinic acid exerts a neuroprotective effect on spinal cord injury by suppressing oxidative stress and inflammation via modulating the Nrf2/HO-1 and TLR4/NF-κ B pathways. Toxicol. Appl. Pharmacol. 2020, 397, 115014. [Google Scholar] [CrossRef] [PubMed]
- Khamse, S.; Sadr, S.S.; Roghani, M.; Hasanzadeh, G.; Mohammadian, M. Rosmarinic acid exerts a neuroprotective effect in the kainate rat model of temporal lobe epilepsy: Underlying mechanisms. Pharm. Biol. 2015, 53, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.F.; Chen, H.H.; Huang, E.; Zhuang, H.L.; Li, D.; Ni, F. Rosmarinic acid inhibits stem-like breast cancer through Hedgehog and Bcl-2/Bax signaling pathways. Pharmacogn. Mag. 2019, 15, 600–606. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Gunasekaran, S.; Jesudoss, V.A.S.; Namasivayam, N. The effect of rosmarinic acid on 1,2-dimethylhydrazine induced colon carcinogenesis. Exp. Toxicol. Pathol. 2013, 65, 409–418. [Google Scholar] [CrossRef]
- Naeem, M.; Ansari, A.A.; Aftab, T.; Shabbir, A.; Alam, M.M.; Khan, M.M.A.; Uddin, M. Application of triacontanol modulates plant growth and physiological activities of Catharanthus roseus (L.). Int. J. Botany Stud. 2019, 4, 131–135. [Google Scholar]
- Perveen, S.; Iqbal, M.; Parveen, A.; Akram, M.S.; Shahbaz, M.; Akber, S.; Mehboob, A. Exogenous triacontanol-mediated increase in phenolics, proline, activity of nitrate reductase, and shoot k+ confers salt tolerance in maize (Zea mays L.). Braz. J. Bot. 2017, 40, 1–11. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Monopoulu, E.; Varzakas, T.; Petsalaki, A. Chlorophyll determination in green pepper using two different extraction methods. Curr. Res. Nutr. Food Sci. 2016, 4, 2–60. [Google Scholar]
- Wellburn, A.R. The spectra determination of chlorophylls a and b as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Grzegorczyk-Karolak, I.; Hnatuszko-Konka, K.; Krzemińska, M.; Olszewska, M.A.; Owczarek, A. Cytokinin-based tissue cultures for stable medicinal plant production: Regeneration and phytochemical profiling of Salvia bulleyana shoots. Biomolecules 2021, 14, 1513. [Google Scholar] [CrossRef] [PubMed]
- Sirivarasai, J.; Kaojarern, S.; Chanprasertyothin, S.; Panpunuan, P.; Petchpoung, K.; Tatsaneeyapant, A.; Yoovathaworn, K.; Sura, T.; Kaojarern, S.; Sritara, P. Environmental lead exposure, catalase gene, and markers of antioxidant and oxidative stress relation to hypertension: An analysis based on the EGAT study. Biomed. Res. Int. 2015, 2015, 856319. [Google Scholar] [CrossRef] [Green Version]
- Ghaderi, N.; Hatami, M.; Aliakbar Mozafari, A.; Siosehmardeh, A. Change in antioxidant enzymes activity and some morpho-physiological characteristics of strawberry under long-term salt stress. Physiol. Mol. Biol. Plants 2018, 24, 833–843. [Google Scholar] [CrossRef]
- Lima, C.C.; Cajueiro Gurgel, E.S.; Borges, E.E.L. Antioxidant enzyme activity in germination of Dalbergia spruceana seeds under different temperatures. J. Seed Sci. 2021, 43, e202143006. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford assay for determining protein concentration. Cold Spring Harb. Protoc. 2020, 4, 102269. [Google Scholar] [CrossRef]


| Activity of Antioxidant Enzymes (U/mg of Protein) | |||
|---|---|---|---|
| Medium with | POD | SOD | CAT |
| TRIA 2.5 | 7.81 ± 0.64 d | 25.11 ± 0.15 b | 35.17 ± 0.15 d |
| TRIA 5 | 15.81 ± 0.31 a | 29.62 ± 0.21 a | 84.98 ± 0.27 a |
| TRIA 10 | 7.39 ± 0.61 d | 23.48 ± 0.09 c | 71.72 ± 0.28 c |
| C | 3.21 ± 0.02 f | 12.10 ± 0.08 g | 11.75 ± 0.09 g |
| TRIA 2.5-BI | 9.54 ± 0.70 c | 20.05 ± 0.11 d | 37.15 ± 0.19 e |
| TRIA 5-BI | 11.14 ± 0.11 b | 16.23 ± 0.11 e | 78.07 ± 0.49 b |
| TRIA 10-BI | 5.07 ± 0.40 e | 15.78 ± 0.18 f | 78.49 ± 0.23 b |
| C-BI | 5.90 ± 0.09 e | 15.03 ± 0.12 f | 22.48 ± 0.18 f |
| Compound | TRIA 2.5 | TRIA 5 | TRIA 10 | C | TRIA 2.5-BI | TRIA 5-BI | TRIA 10-BI | C-BI |
|---|---|---|---|---|---|---|---|---|
| chlorogenic acid | 0.53 ± 0.01 c | 0.31 ± 0.01 d | 0.11 ± 0.03 f | 1.64 ± 0.05 a | 0.15 ± 0.003 e | 0.18 ± 0.001 e | 0.19 ± 0.003 e | 0.60 ± 0.013 b |
| caffeic acid | 0.03 ± 0.002 c | 0.03 ± 0.001 c | 0.04 ± 0.01 bc | 0.04 ± 0.002 bc | 0.10 ± 0.002 a | 0.09 ± 0.001 a | 0.09 ± 0.002 a | 0.05 ± 0.001 b |
| salvianolic acid I/H and salvianolic acid E | 0.45 ± 0.01 b | 0.28 ± 0.01 d | 0.28 ± 0.01 d | 0.67 ± 0.02 a | 0.36 ± 0.003 c | 0.41 ± 0.01 b | 0.44 ± 0.01 b | 0.45 ± 0.04 b |
| rosmarinic acid | 9.51 ± 0.22 d | 9.19 ± 0.23 d | 11.30 ± 0.02 b | 5.02 ± 0.01 f | 10.67 ± 0.21 c | 10.67 ± 0.19 c | 14.79 ± 0.31 a | 6.47 ± 0.01 e |
| salvianolic acid B | 0.19 ± 0.001 e | 0.19 ± 0.01 e | 0.28 ± 0.002 c | 0.23 ± 0.01 d | 0.47 ± 0.01 a | 0.27 ± 0.001 c | 0.21 ± 0.00 d | 0.37 ± 0.01 b |
| methyl rosmarinate | 0.28 ± 0.01 f | 0.39 ± 0.003 e | 0.48 ± 0.03 d | 0.54 ± 0.003 cd | 0.97 ± 0.04 a | 0.72 ± 0.03 b | 0.72 ± 0.01 b | 0.59 ± 0.1 c |
| Total phenol content | 10.96 ± 0.1 c | 10.39 ± 0.3 c | 12.52 ± 0.2 b | 8.14 ± 0.2 d | 12.72 ± 0.1 b | 12.35 ± 0.2 b | 19.44 ± 0.34 a | 8.53 ± 0.1 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weremczuk-Jeżyna, I.; Hnatuszko-Konka, K.; Lebelt, L.; Piotrowska, D.G.; Grzegorczyk-Karolak, I. The Effect of the Stress-Signalling Mediator Triacontanol on Biochemical and Physiological Modifications in Dracocephalum forrestii Culture. Int. J. Mol. Sci. 2022, 23, 15147. https://doi.org/10.3390/ijms232315147
Weremczuk-Jeżyna I, Hnatuszko-Konka K, Lebelt L, Piotrowska DG, Grzegorczyk-Karolak I. The Effect of the Stress-Signalling Mediator Triacontanol on Biochemical and Physiological Modifications in Dracocephalum forrestii Culture. International Journal of Molecular Sciences. 2022; 23(23):15147. https://doi.org/10.3390/ijms232315147
Chicago/Turabian StyleWeremczuk-Jeżyna, Izabela, Katarzyna Hnatuszko-Konka, Liwia Lebelt, Dorota G. Piotrowska, and Izabela Grzegorczyk-Karolak. 2022. "The Effect of the Stress-Signalling Mediator Triacontanol on Biochemical and Physiological Modifications in Dracocephalum forrestii Culture" International Journal of Molecular Sciences 23, no. 23: 15147. https://doi.org/10.3390/ijms232315147
APA StyleWeremczuk-Jeżyna, I., Hnatuszko-Konka, K., Lebelt, L., Piotrowska, D. G., & Grzegorczyk-Karolak, I. (2022). The Effect of the Stress-Signalling Mediator Triacontanol on Biochemical and Physiological Modifications in Dracocephalum forrestii Culture. International Journal of Molecular Sciences, 23(23), 15147. https://doi.org/10.3390/ijms232315147







