Chitosan as a Promising Support of a CDH Activity Preservation System for Biomedical and Industrial Applications
Abstract
1. Introduction
2. Results and Discussion
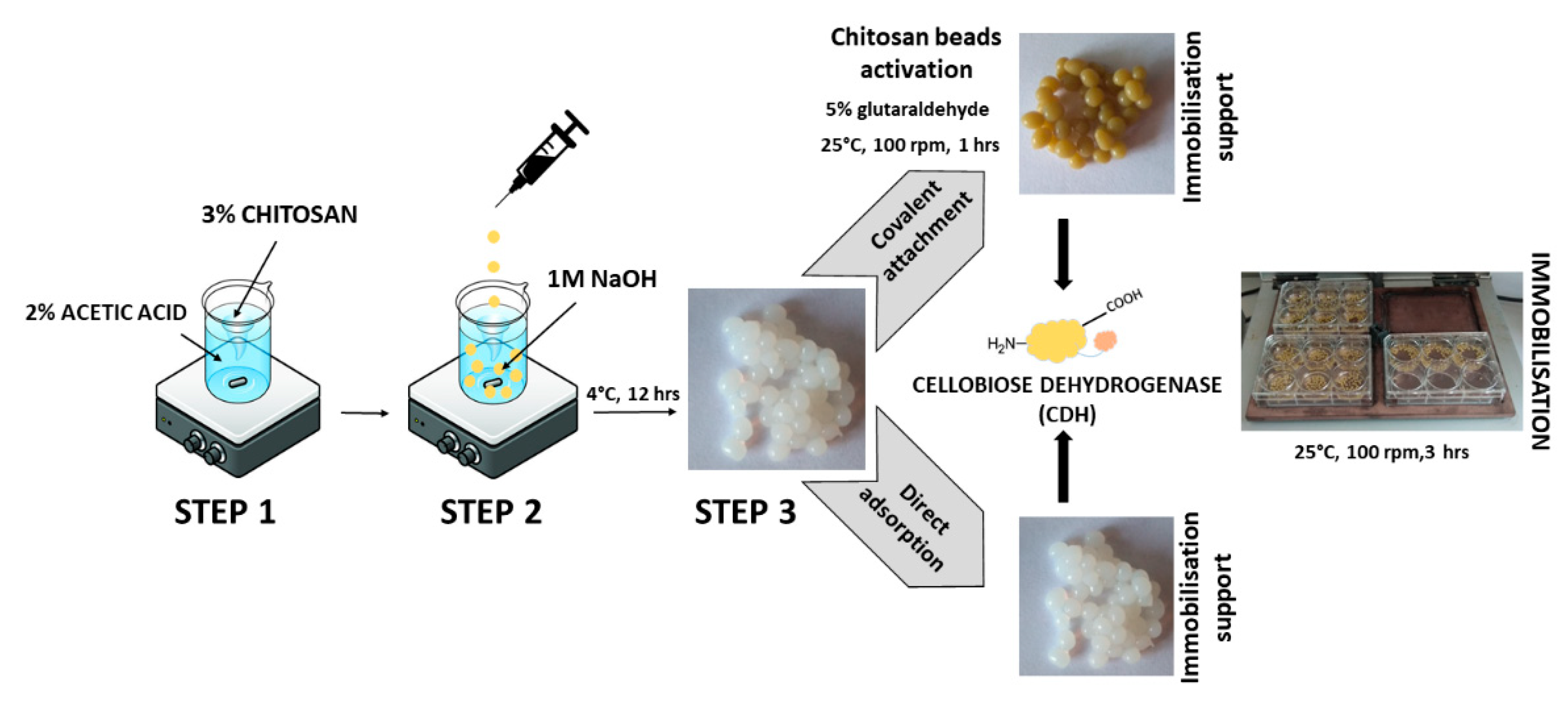
2.1. Immobilization of Cellobiose Dehydrogenase on Chitosan Beads
2.1.1. Direct Adsorption
2.1.2. Covalent Immobilization Using Glutaraldehyde
2.2. Characterization of the Chitosan Beads
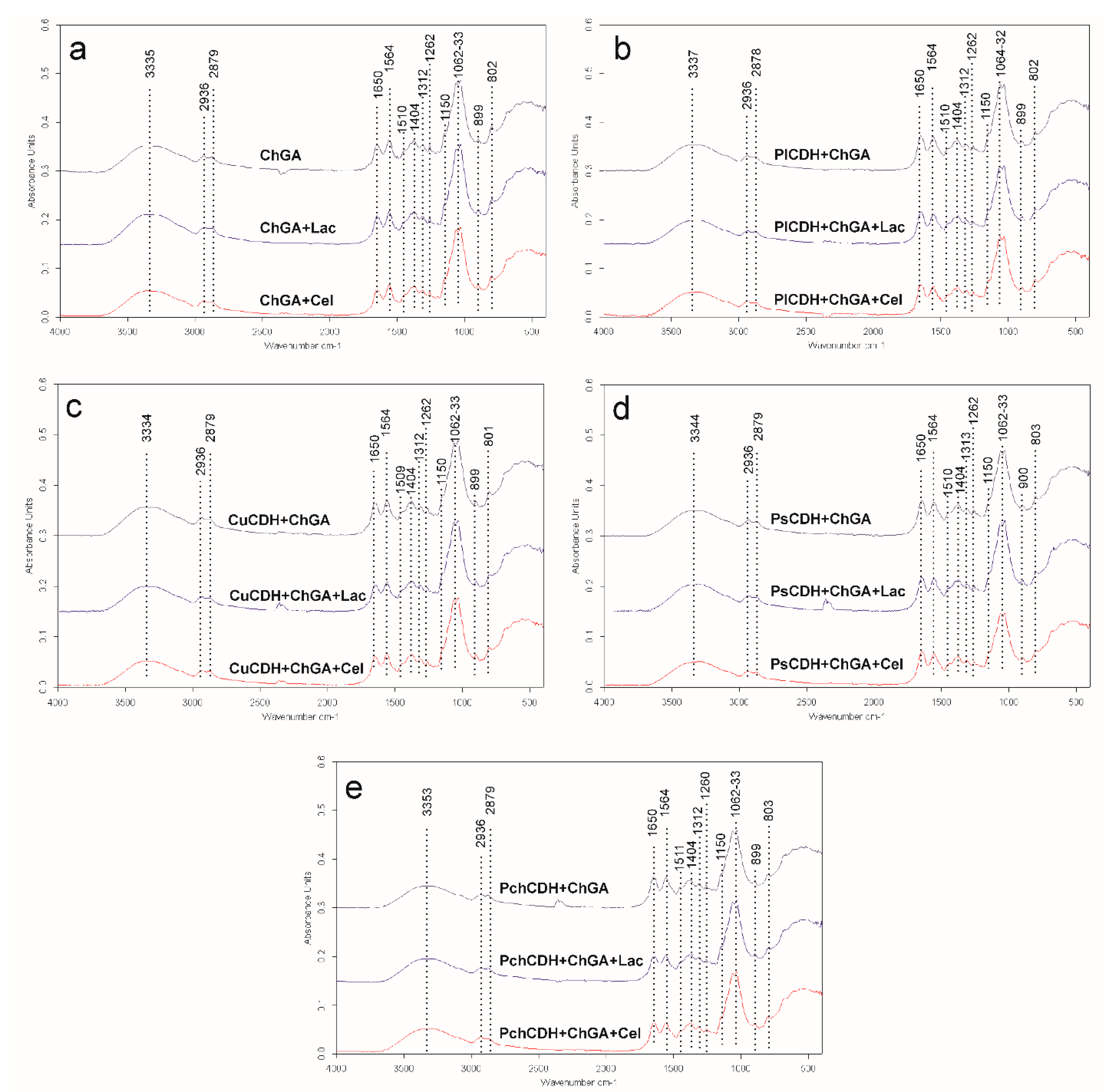
2.2.1. FTIR
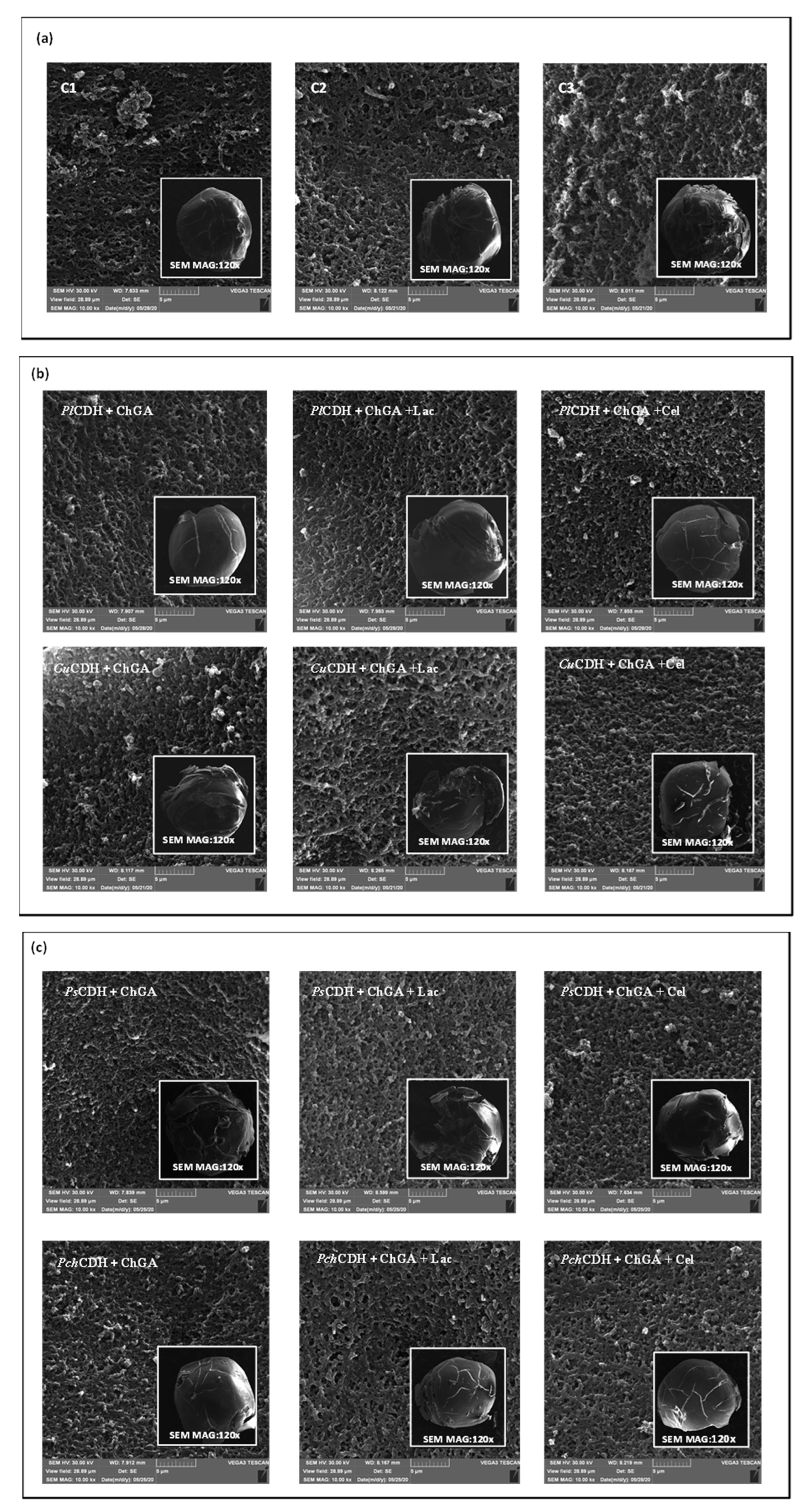
2.2.2. Examination of the Sample Surface Using the SEM Microscopy Technique
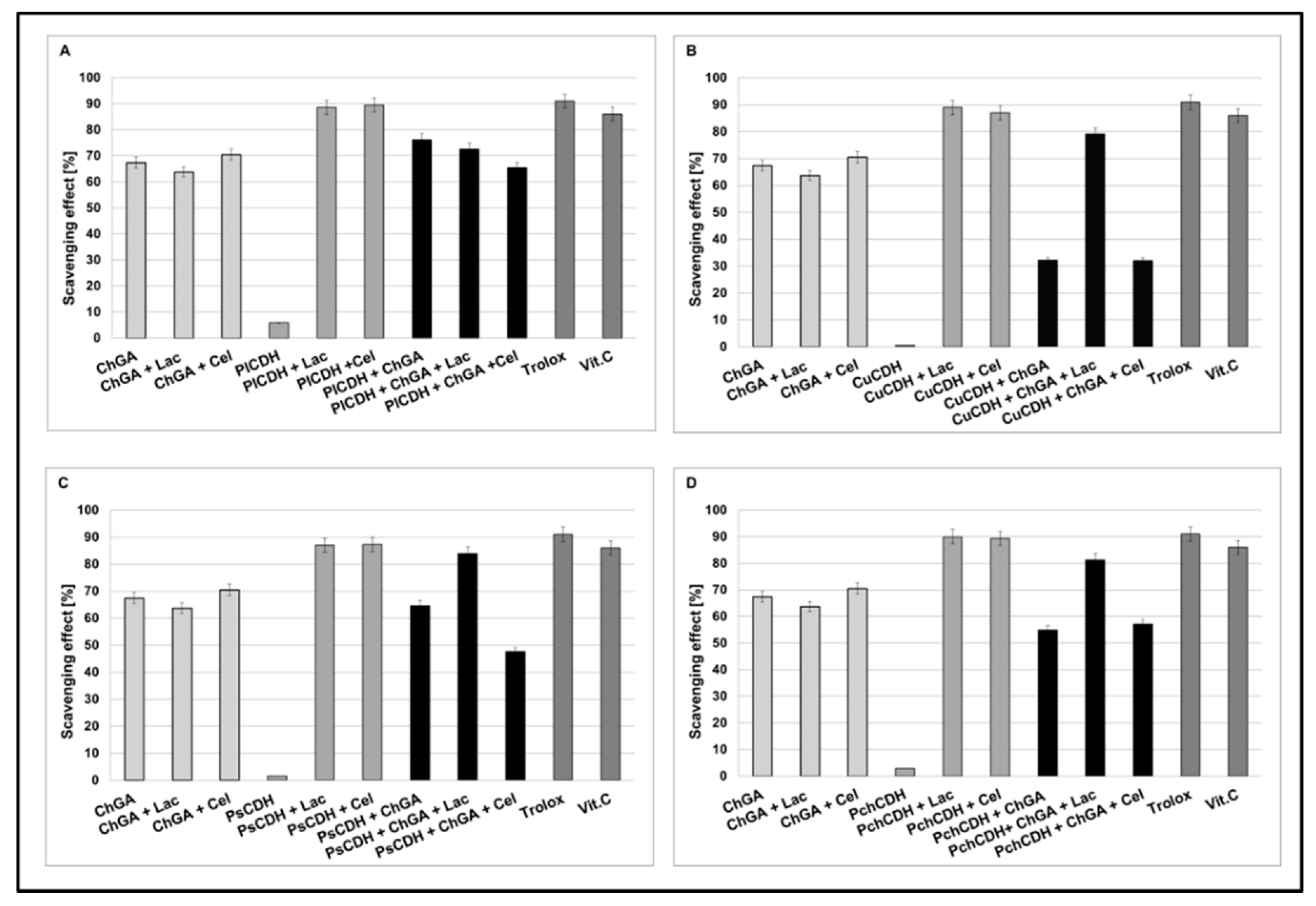
2.2.3. Antioxidant Properties
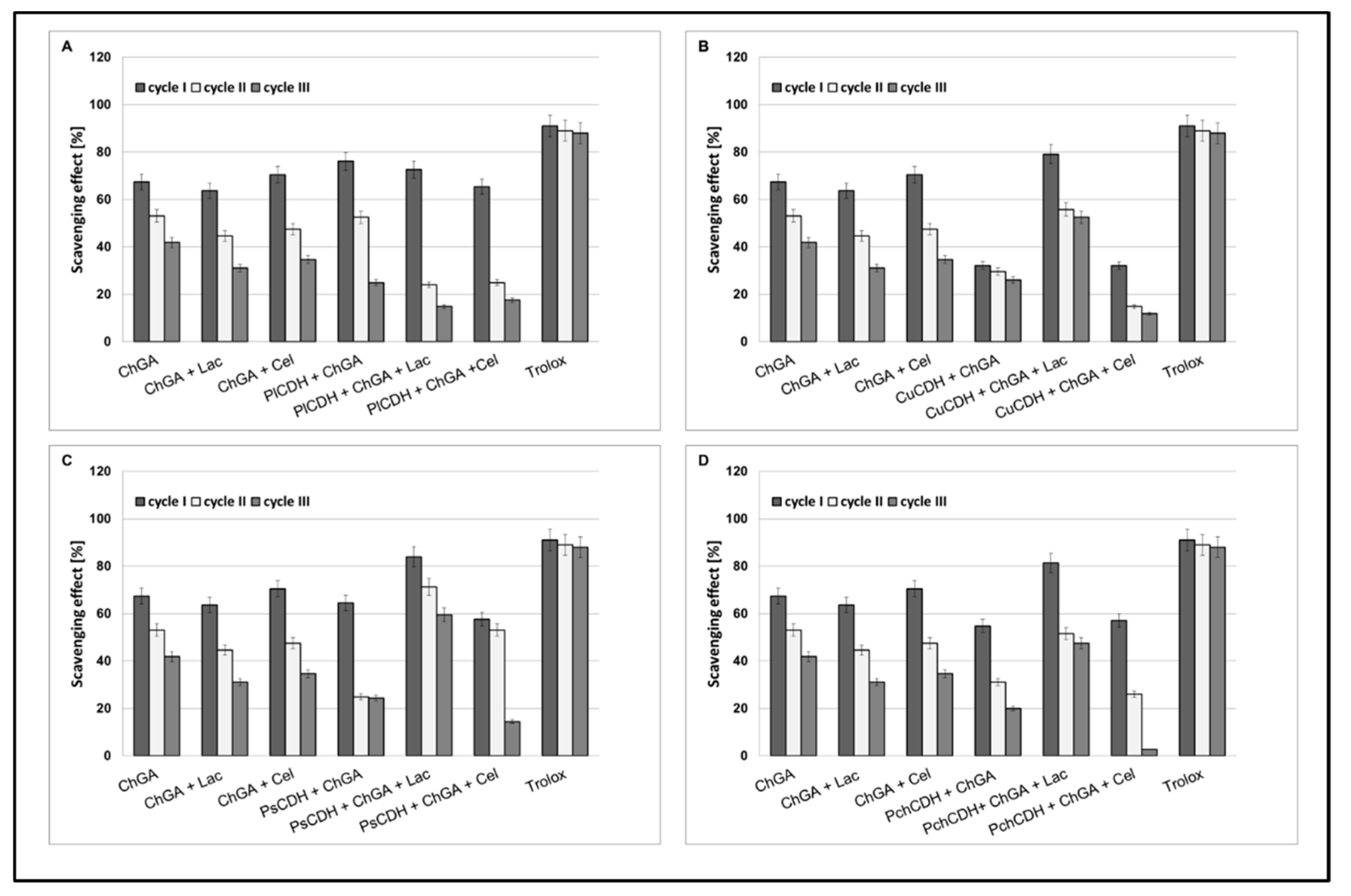
2.2.4. Operational Stability of Chitosan Beads
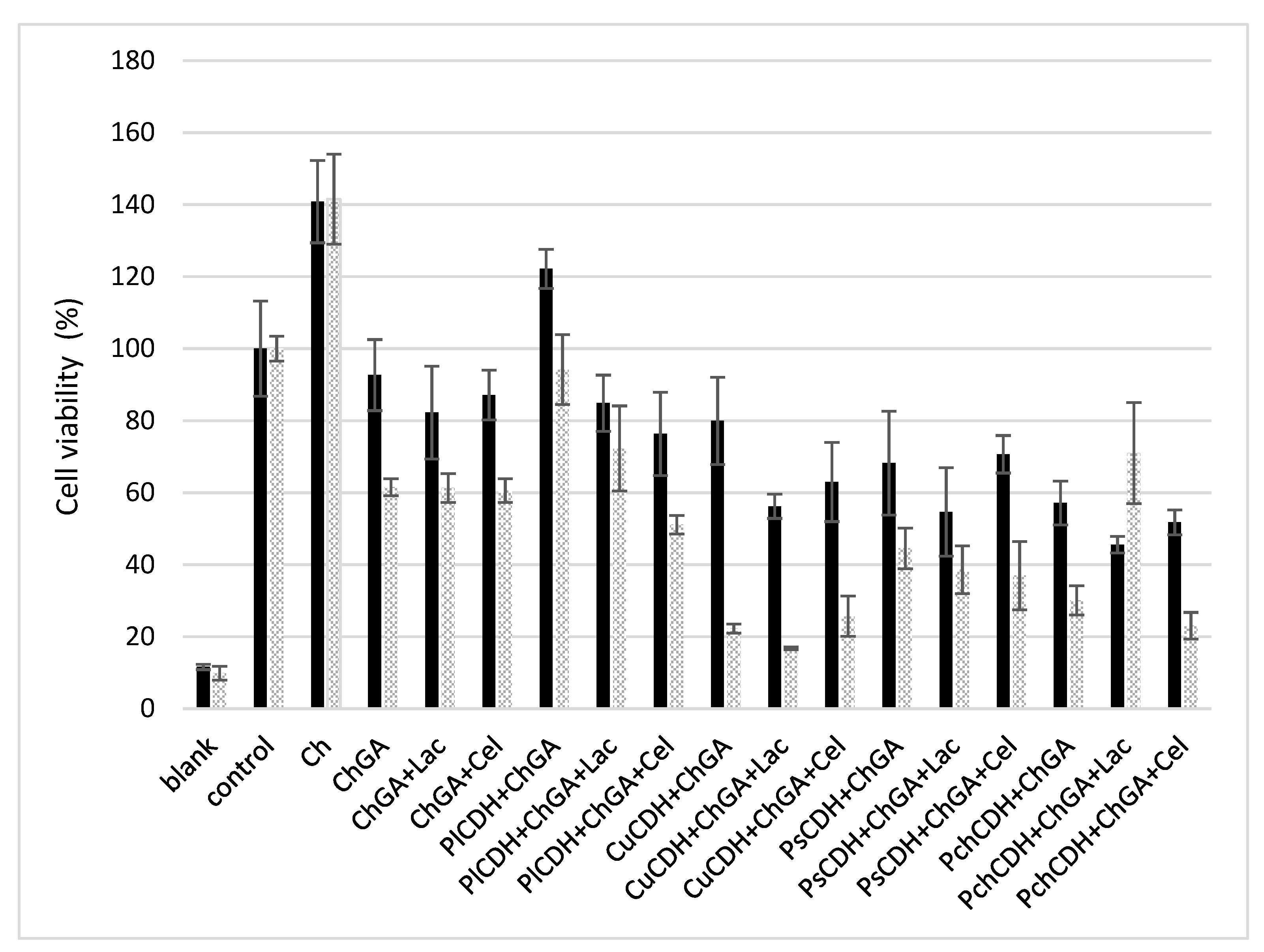
2.2.5. In Vitro Cytotoxicity Assessment
3. Materials and Methods
3.1. Microorganisms
3.2. Materials
3.3. Enzyme Preparation
3.4. Enzyme Activity Assay and Protein Determination
3.5. Immobilization of Cellobiose Dehydrogenase on Chitosan Beads
3.5.1. Preparation of Chitosan Beads
3.5.2. Direct Adsorption
3.5.3. Covalent Immobilization Using Glutaraldehyde/Activation of Chitosan Beads
3.6. Characterization of Chitosan Beads
3.6.1. FTIR
3.6.2. Scanning Microscopy (SEM) of Modified Chitosan Beads
3.6.3. Antioxidant Properties of CDH Immobilized on Chitosan Beads
3.6.4. Operational Stability
3.6.5. Cytotoxicity Evaluation
3.6.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Shah, K.; Kwok, I.; Wang, M.; Rome, L.H.; Mahendra, S. Immobilized fungal enzymes: Innovations and potential applications in biodegradation and biosynthesis. Biotechnol. Adv. 2022, 57, 107936. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef] [PubMed]
- Hartl, L.; Zach, S.; Seidl-Seiboth, V. Fungal chitinases: Diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol. 2012, 93, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Tamayo-Cabezas, J.; Karboune, S. Optimizing immobilization and stabilization of feruloyl esterase from Humicola insolens and its application for the feruloylation of oligosaccharides. Process Biochem. 2020, 98, 11–20. [Google Scholar] [CrossRef]
- Nousiainen, P.; Kontro, J.; Manner, H.; Hatakka, A.; Sipilä, J. Phenolic mediators enhance the manganese peroxidase catalyzed oxidation of recalcitrant lignin model compounds and synthetic lignin. Fungal Genet. Biol. 2014, 72, 137–149. [Google Scholar] [CrossRef]
- Dubey, M.K.; Zehra, A.; Aamir, M.; Meena, M.; Ahirwal, L.; Singh, S.; Shukla, S.; Upadhyay, R.S.; Bueno-Mari, R.; Bajpai, V.K. Improvement strategies, cost effective production, and potential applications of fungal glucose oxidase (GOD): Current updates. Front. Microbiol. 2017, 8, 1032. [Google Scholar] [CrossRef]
- Yang, J.; Xu, P.; Long, L.; Ding, S. Production of lactobionic acid using an immobilized cellobiose dehydrogenase/laccase system on magnetic chitosan spheres. Process Biochem. 2021, 100, 1–9. [Google Scholar] [CrossRef]
- Østergaard, L.H.; Olsen, H.S. Industrial applications of fungal enzymes. In Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2011; pp. 269–290. [Google Scholar]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech. 2016, 6, 174. [Google Scholar] [CrossRef]
- McKelvey, S.M.; Murphy, R.A. Biotechnological use of fungal enzymes. Fungi Biol. Appl. 2017, 201–225. [Google Scholar] [CrossRef]
- Franssen, M.C.; Steunenberg, P.; Scott, E.L.; Zuilhof, H.; Sanders, J.P. Immobilised enzymes in biorenewables production. Chem. Soc. Rev. 2013, 42, 6491–6533. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Kim, M. An overview of techniques in enzyme immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar] [CrossRef]
- Domínguez, A.; Gomez, J.; Lorenzo, M.; Sanromán, Á. Enhanced production of laccase activity by Trametes versicolor immobilized into alginate beads by the addition of different inducers. World J. Microbiol. Biotechnol. 2007, 23, 367–373. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Recent advances in engineered chitosan-based nanogels for biomedical applications. J. Mater. Chem. B 2017, 5, 6986–7007. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.R.; Singh, U.S.; Momin, M.; Bhavsar, C. Chitosan: Application in tissue engineering and skin grafting. J. Polym. Res. 2017, 24, 125. [Google Scholar] [CrossRef]
- Desbrières, J.; Guibal, E. Chitosan for wastewater treatment. Polym. Int. 2018, 67, 7–14. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Recent advances of chitosan applications in plants. Polymers 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, B. Application of chitin-and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Wang, K. Enzyme immobilization on chitosan-based supports. Chitosan-Based Hydrogels 2011, 18, 339–406. [Google Scholar]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Enzyme immobilization on chitin and chitosan-based supports for biotechnological applications. Sustain. Agric. Rev. 35 2019, 147–173. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; de Farias, B.S.; Junior, T.R.S.A.C.; de Almeida Pinto, L.A.; Diaz, P.S. Chitosan–based nanofibers for enzyme immobilization. Int. J. Biol. Macromol. 2021, 183, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Csarman, F.; Wohlschlager, L.; Ludwig, R. Cellobiose dehydrogenase. In The Enzymes; Elsevier: Amsterdam, The Netherlands, 2020; Volume 47, pp. 457–489. [Google Scholar]
- Bao, W.; Renganathan, V. Cellobiose oxidase of Phanerochaete chrysosporium enhances crystalline cellulose degradation by cellulases. FEBS Lett. 1992, 302, 77–80. [Google Scholar] [CrossRef]
- Henriksson, G.; Sild, V.; Szabó, I.J.; Pettersson, G.; Johansson, G. Substrate specificity of cellobiose dehydrogenase from Phanerochaete chrysosporium. Biochim. Et Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1998, 1383, 48–54. [Google Scholar] [CrossRef]
- Sulej, J.; Osińska-Jaroszuk, M.; Jaszek, M.; Grąz, M.; Kutkowska, J.; Pawlik, A.; Chudzik, A.; Bancerz, R. Antimicrobial and antioxidative potential of free and immobilised cellobiose dehydrogenase isolated from wood degrading fungi. Fungal Biol. 2019, 123, 875–886. [Google Scholar] [CrossRef]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme entrapment, biocatalyst immobilization without covalent attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Romero-Fernández, M.; Paradisi, F. General overview on immobilization techniques of enzymes for biocatalysis. Catal. Immobil. Methods Appl. 2020, 409–435. [Google Scholar]
- Betancor, L.; López-Gallego, F.; Alonso-Morales, N.; Dellamora, G.; Mateo, C.; Fernandez-Lafuente, R.; Guisan, J.M. Glutaraldehyde in protein immobilization. In Immobilization of Enzymes and Cells; Springer: Berlin/Heidelberg, Germany, 2006; pp. 57–64. [Google Scholar]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. Rsc Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Tegl, G.; Thallinger, B.; Beer, B.; Sygmund, C.; Ludwig, R.; Rollett, A.; Nyanhongo, G.S.; Guebitz, G.M. Antimicrobial cellobiose dehydrogenase-chitosan particles. ACS Appl. Mater. Interfaces 2016, 8, 967–973. [Google Scholar] [CrossRef]
- Roig, M. Immobilised Cells and Enzymes—A Practical Approach; Woodward, J., Ed.; IRL Press: Oxford, UK, 1986; pp. 91, 177, 1985.£ 13; ISBN 947946-21-7. [Google Scholar]
- Brena, B.M.; Batista-Viera, F. Immobilization of enzymes. In Immobilization of Enzymes and Cells, 2nd ed.; Guisan, J.M., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2006; pp. 123–124. [Google Scholar]
- Labus, K.; Wolanin, K.; Radosiński, Ł. Comparative study on enzyme immobilization using natural hydrogel matrices—Experimental studies supported by molecular models analysis. Catalysts 2020, 10, 489. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q.; Dang, Y.; Shu, G. The effect of glutaraldehyde cross-linking on the enzyme activity of immobilized β-galactosidase on chitosan bead. Adv. J. Food Sci. Technol. 2013, 5, 932–935. [Google Scholar] [CrossRef]
- Gonawan, F.N.; Romli, M.; Zuhan, M.; Jaya, M. Immobilization of Candida Rugosa Lipase on the Glutaraldehyde-Activated Chitosan Beads. J. Chem. Eng. Ind. Biotechnol. 2022, 8, 33–41. [Google Scholar] [CrossRef]
- Bilal, M.; Jing, Z.; Zhao, Y.; Iqbal, H.M. Immobilization of fungal laccase on glutaraldehyde cross-linked chitosan beads and its bio-catalytic potential to degrade bisphenol A. Biocatal. Agric. Biotechnol. 2019, 19, 101174. [Google Scholar] [CrossRef]
- Mehandia, S.; Sharma, S.; Arya, S.K. Immobilization of laccase on chitosan-clay composite beads to improve its catalytic efficiency to degrade industrial dyes. Mater. Today Commun. 2020, 25, 101513. [Google Scholar] [CrossRef]
- Helal, S.E.; Abdelhady, H.M.; Abou-Taleb, K.A.; Hassan, M.G.; Amer, M.M. Lipase from Rhizopus oryzae R1: In-depth characterization, immobilization, and evaluation in biodiesel production. J. Genet. Eng. Biotechnol. 2021, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Islam, N.; Wang, H.; Maqbool, F.; Ferro, V. In vitro enzymatic digestibility of glutaraldehyde-crosslinked chitosan nanoparticles in lysozyme solution and their applicability in pulmonary drug delivery. Molecules 2019, 24, 1271. [Google Scholar] [CrossRef]
- Li, B.; Shan, C.-L.; Zhou, Q.; Fang, Y.; Wang, Y.-L.; Xu, F.; Han, L.-R.; Ibrahim, M.; Guo, L.-B.; Xie, G.-L. Synthesis, characterization, and antibacterial activity of cross-linked chitosan-glutaraldehyde. Mar. Drugs 2013, 11, 1534–1552. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Devi, B.; Sudha, P.; Venkatesan, J.; Anil, S. Synthesis, characterization and applications of nanochitosan/sodium alginate/microcrystalline cellulose film. J. Nanomed. Nanotechnol. 2016, 7, 419. [Google Scholar] [CrossRef]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, M. Infrared spectrum and crystal structure of cellulose. J. Polym. Sci. 1957, 25, 159–171. [Google Scholar] [CrossRef]
- Pearson, F.; Marchessault, R.; Liang, C. Infrared spectra of crystalline polysaccharides. V. Chitin. J. Polym. Sci. 1960, 43, 101–116. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Krimm, S.; Bandekar, J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv. Protein Chem. 1986, 38, 181–364. [Google Scholar] [PubMed]
- Toldra, F.; Lequerica, J. Use of scanning electron microscopy techniques in the biocatalyst immobilization. Food/Nahrung 1991, 35, 739–744. [Google Scholar] [CrossRef]
- Gür, S.D.; İdil, N.; Aksöz, N. Optimization of enzyme co-immobilization with sodium alginate and glutaraldehyde-activated chitosan beads. Appl. Biochem. Biotechnol. 2018, 184, 538–552. [Google Scholar] [CrossRef]
- Singh, A.N.; Singh, S.; Suthar, N.; Dubey, V.K. Glutaraldehyde-activated chitosan matrix for immobilization of a novel cysteine protease, procerain B. J. Agric. Food Chem. 2011, 59, 6256–6262. [Google Scholar] [CrossRef]
- Gilani, S.L.; Najafpour, G.D.; Moghadamnia, A.; Kamaruddin, A.H. Stability of immobilized porcine pancreas lipase on mesoporous chitosan beads: A comparative study. J. Mol. Catal. B Enzym. 2016, 133, 144–153. [Google Scholar] [CrossRef]
- Sulej, J.; Janusz, G.; Osińska-Jaroszuk, M.; Małek, P.; Mazur, A.; Komaniecka, I.; Choma, A.; Rogalski, J. Characterization of cellobiose dehydrogenase and its FAD-domain from the ligninolytic basidiomycete Pycnoporus sanguineus. Enzym. Microb. Technol. 2013, 53, 427–437. [Google Scholar] [CrossRef]
- Sulej, J.; Janusz, G.; Osińska-Jaroszuk, M.; Rachubik, P.; Mazur, A.; Komaniecka, I.; Choma, A.; Rogalski, J. Characterization of cellobiose dehydrogenase from a biotechnologically important Cerrena unicolor strain. Appl. Biochem. Biotechnol. 2015, 176, 1638–1658. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 2009, 75, 15–21. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Panda, S.K.; Navitha, M.; Asha, K.; Anandan, R.; Mathew, S. Vanillic acid and coumaric acid grafted chitosan derivatives: Improved grafting ratio and potential application in functional food. J. Food Sci. Technol. 2015, 52, 7153–7162. [Google Scholar] [CrossRef]
- Podrepšek, G.H.; Primožič, M.; Knez, Ž.; Habulin, M. Immobilization of cellulase for industrial production. Chem. Eng. 2012, 27, 235–240. [Google Scholar]
- Martins, A.F.; Facchi, S.P.; Monteiro, J.P.; Nocchi, S.R.; Silva, C.T.; Nakamura, C.V.; Girotto, E.M.; Rubira, A.F.; Muniz, E.C. Preparation and cytotoxicity of N, N, N-trimethyl chitosan/alginate beads containing gold nanoparticles. Int. J. Biol. Macromol. 2015, 72, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Baminger, U.; Nidetzky, B.; Kulbe, K.D.; Haltrich, D. A simple assay for measuring cellobiose dehydrogenase activity in the presence of laccase. J. Microbiol. Methods 1999, 35, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Paduch, R.; Matysik, G.; Wójciak-Kosior, M.; Kandefer-Szerszen, M.; Skalska-Kaminska, A.; Nowak-Kryska, M.; Niedziela, P. Lamium Album Extracts Express Free Radical Scavenging and Cytotoxic Activities. Pol. J. Environ. Stud. 2008, 17, 569–580. [Google Scholar]
- Huber, D.; Tegl, G.; Mensah, A.; Beer, B.; Baumann, M.; Borth, N.; Sygmund, C.; Ludwig, R.; Guebitz, G.M. A dual-enzyme hydrogen peroxide generation machinery in hydrogels supports antimicrobial wound treatment. ACS Appl. Mater. Interfaces 2017, 9, 15307–15316. [Google Scholar] [CrossRef]
- Augustin, M.; Ali-Vehmas, T.; Atroshi, F. Assessment of enzymatic cleaning agents and disinfectants against bacterial biofilms. J. Pharm. Pharm. Sci. 2004, 7, 55–64. [Google Scholar]
- Ludwig, R.; Harreither, W.; Tasca, F.; Gorton, L. Cellobiose dehydrogenase: A versatile catalyst for electrochemical applications. ChemPhysChem 2010, 11, 2674–2697. [Google Scholar] [CrossRef] [PubMed]
- Alt, E.; Leipold, F.; Milatovic, D.; Lehmann, G.; Heinz, S.; Schömig, A. Hydrogen peroxide for prevention of bacterial growth on polymer biomaterials. Ann. Thorac. Surg. 1999, 68, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Nyanhongo, G.S.; Thallinger, B.; Guebitz, G.M. Cellobiose dehydrogenase-based biomedical applications. Process Biochem. 2017, 59, 37–45. [Google Scholar] [CrossRef]
- Thallinger, B.; Argirova, M.; Lesseva, M.; Ludwig, R.; Sygmund, C.; Schlick, A.; Nyanhongo, G.S.; Guebitz, G.M. Preventing microbial colonisation of catheters: Antimicrobial and antibiofilm activities of cellobiose dehydrogenase. Int. J. Antimicrob. Agents 2014, 44, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Öhlknecht, C.; Tegl, G.; Beer, B.; Sygmund, C.; Ludwig, R.; Guebitz, G.M. Cellobiose dehydrogenase and chitosan-based lysozyme responsive materials for antimicrobial wound treatment. Biotechnol. Bioeng. 2017, 114, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.X.; Campagna, A.N.; Bobek, L.A. Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J. Antimicrob. Chemother. 2006, 57, 1100–1109. [Google Scholar] [CrossRef] [PubMed]

| Sample Type | Applied Protein [mg/g Carrier] | Bound Protein [mg/g Carrier] | Protein Yield [%] | CDH Activity before Immobilization [U/g Carrier] | Activity of CDH Bound with Chitosan [U/g Carrier] | Activity Yield [%] |
|---|---|---|---|---|---|---|
| PlCDH + Ch | 8.25 ± 0.02 | 1.76 ± 0.02 | 21.35 | 14.21 ± 0.03 | 0 | 0 |
| PlCDH + Ch + Lac | 8.23± 0.02 | 2.24 ± 0.02 | 27.21 | 15.27 ± 0.03 | 7.07 ± 0.04 | 46.29 |
| PlCDH + Ch + Cel | 9.61 ± 0.02 | 3.18 ± 0.01 | 33.14 | 15.28 ± 0.03 | 2.24 ± 0.02 | 14.64 |
| CuCDH + Ch | 6.49 ± 0.02 | 2.68 ± 0.02 | 41.27 | 37.53 ± 0.08 | 0.39 ± 0.001 | 1.04 |
| CuCDH + Ch + Lac | 5.76 ± 0.01 | 3.12 ± 0.03 | 54.12 | 40.17 ± 0.06 | 31.62 ± 0.08 | 78.74 |
| CuCDH + Ch + Cel | 6.18 ± 0.02 | 2.97 ± 0.03 | 48.04 | 36.93 ± 0.05 | 16.04 ± 0.05 | 43.44 |
| PsCDH + Ch | 8.19 ± 0.02 | 2.12 ± 0.02 | 25.91 | 17.10 ± 0.03 | 0 | 0 |
| PsCDH + Ch + Lac | 9.23 ± 0.03 | 4.77 ± 0.03 | 51.72 | 17.88 ± 0.03 | 6.60 ± 0.03 | 36.93 |
| PsCDH + Ch + Cel | 8.90 ± 0.02 | 6.12 ± 0.03 | 68.73 | 16.65 ± 0.03 | 2.54 ± 0.02 | 15.28 |
| PchCDH + Ch | 9.70 ± 0.03 | 5.89 ± 0.04 | 60.68 | 12.58 ± 0.02 | 1.56 ± 0.02 | 12.37 |
| PchCDH + Ch + Lac | 10.17 ± 0.04 | 2.59 ± 0.02 | 25.44 | 11.27 ± 0.03 | 2.11 ± 0.01 | 18.74 |
| PchCDH + Ch + Cel | 10.41 ± 0.04 | 3.27 ± 003 | 31.45 | 14.55 ± 0.03 | 2.66 ± 0.02 | 18.30 |
| Sample Type | Applied Protein [mg/g Carrier] | Bound Protein [mg/g Carrier] | Protein Yield [%] | CDH Activity before Immobilization [U/g Carrier] | Activity of CDH Bound with Chitosan [U/g Carrier] | Activity Yield [%] |
|---|---|---|---|---|---|---|
| PlCDH + ChGA | 8.25 ± 0.02 | 3.58 ± 0.02 | 43.37 | 14.21 ± 0.03 | 4.41 ± 0.02 | 31.03 |
| PlCDH + ChGA + Lac | 8.23 ± 0.02 | 5.05 ± 0.03 | 61.36 | 15.27 ± 0.03 | 15.14 ± 0.06 | 99.18 |
| PlCDH + ChGA + Cel | 9.61 ± 0.02 | 6.85 ± 0.02 | 71.32 | 15.28 ± 0.03 | 13.64 ± 0.05 | 89.23 |
| CuCDH + ChGA | 6.49 ± 0.02 | 6.04 ± 0.04 | 93.06 | 37.53 ± 0.08 | 34.63 ± 0.08 | 92.28 |
| CuCDH + ChGA + Lac | 5.76 ± 0.01 | 3.57 ± 0.02 | 61.87 | 40.17 ± 0.06 | 29.60 ± 0.05 | 73.69 |
| CuCDH + ChGA + Cel | 6.18 ± 0.02 | 4.67 ± 0.01 | 75.49 | 36.93 ± 0.05 | 28.55 ± 0.04 | 77.31 |
| PsCDH + ChGA | 8.19 ± 0.02 | 3.95 ± 0.03 | 48.29 | 17.10 ± 0.03 | 4.89 ± 0.02 | 28.59 |
| PsCDH + ChGA + Lac | 9.23 ± 0.03 | 7.65 ± 0.05 | 82.87 | 17.88 ± 0.03 | 16.45 ± 0.03 | 92.02 |
| PsCDH + ChGA + Cel | 8.90 ± 0.02 | 7.07 ± 0.04 | 79.39 | 16.65 ± 0.03 | 12.91 ± 0.02 | 77.55 |
| PchCDH + ChGA | 9.70 ± 0.03 | 3.57 ± 0.02 | 36.76 | 12.58 ± 0.02 | 7.53 ± 0.03 | 59.84 |
| PchCDH + ChGA + Lac | 10.17 ± 0.04 | 7.76 ± 0.03 | 76.34 | 11.27 ± 0.03 | 10.13 ± 0.04 | 89.85 |
| PchCDH + ChGA + Cel | 10.41± 0.04 | 9.29 ± 0.03 | 89.28 | 14.55 ± 0.03 | 12.24 ± 0.04 | 84.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulej, J.; Osińska-Jaroszuk, M.; Jaszek, M.; Olszewska, A.; Belcarz, A.; Piątek-Gołda, W. Chitosan as a Promising Support of a CDH Activity Preservation System for Biomedical and Industrial Applications. Int. J. Mol. Sci. 2023, 24, 4535. https://doi.org/10.3390/ijms24054535
Sulej J, Osińska-Jaroszuk M, Jaszek M, Olszewska A, Belcarz A, Piątek-Gołda W. Chitosan as a Promising Support of a CDH Activity Preservation System for Biomedical and Industrial Applications. International Journal of Molecular Sciences. 2023; 24(5):4535. https://doi.org/10.3390/ijms24054535
Chicago/Turabian StyleSulej, Justyna, Monika Osińska-Jaroszuk, Magdalena Jaszek, Anna Olszewska, Anna Belcarz, and Wiktoria Piątek-Gołda. 2023. "Chitosan as a Promising Support of a CDH Activity Preservation System for Biomedical and Industrial Applications" International Journal of Molecular Sciences 24, no. 5: 4535. https://doi.org/10.3390/ijms24054535
APA StyleSulej, J., Osińska-Jaroszuk, M., Jaszek, M., Olszewska, A., Belcarz, A., & Piątek-Gołda, W. (2023). Chitosan as a Promising Support of a CDH Activity Preservation System for Biomedical and Industrial Applications. International Journal of Molecular Sciences, 24(5), 4535. https://doi.org/10.3390/ijms24054535






