Structural and Functional Diversity of Two ATP-Driven Plant Proton Pumps
Abstract
1. Introduction
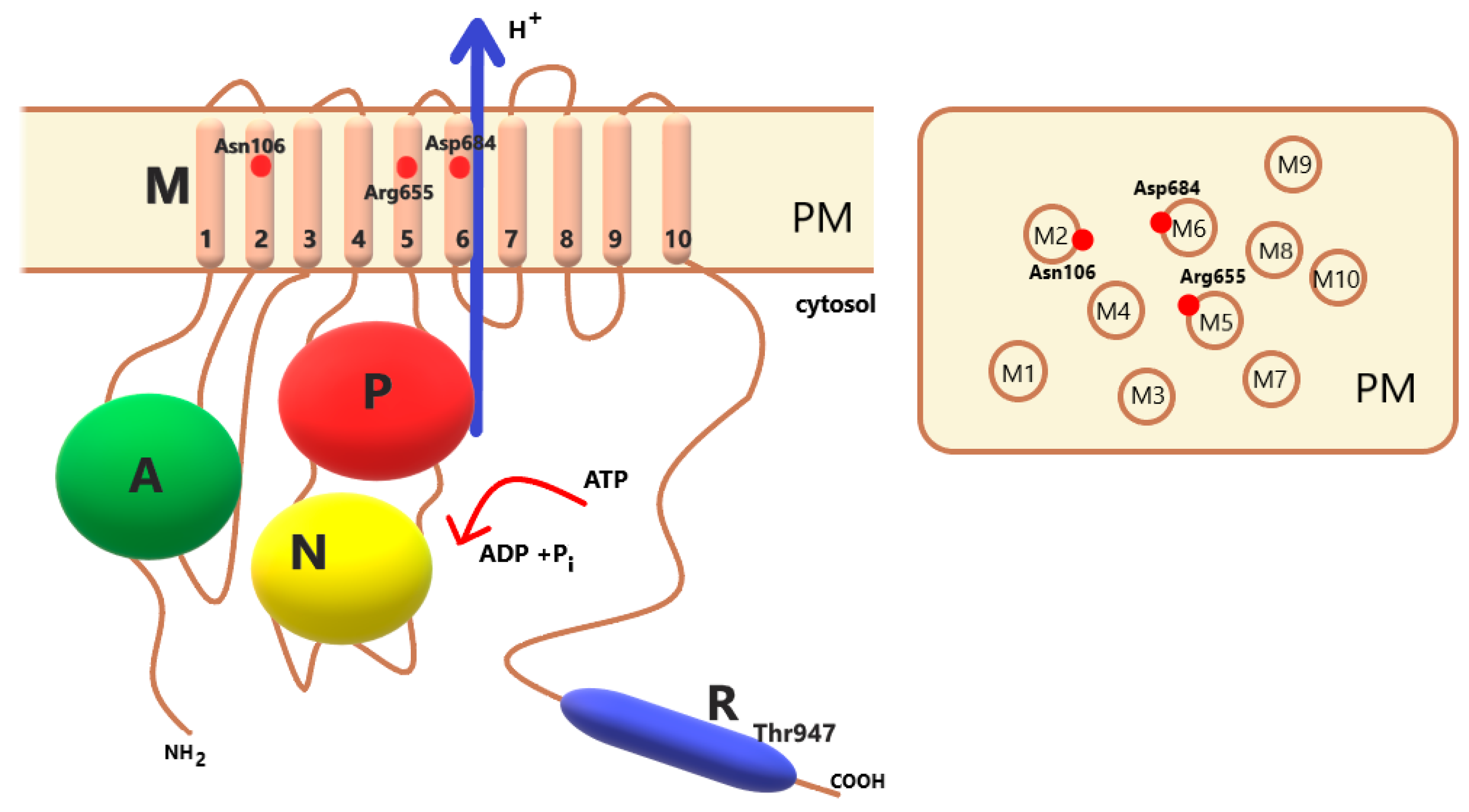
2. PM H+-ATPase
2.1. Multigene Family
2.2. Transport of Protons
2.3. Regulation by Phosphorylation and 14-3-3 Protein Binding
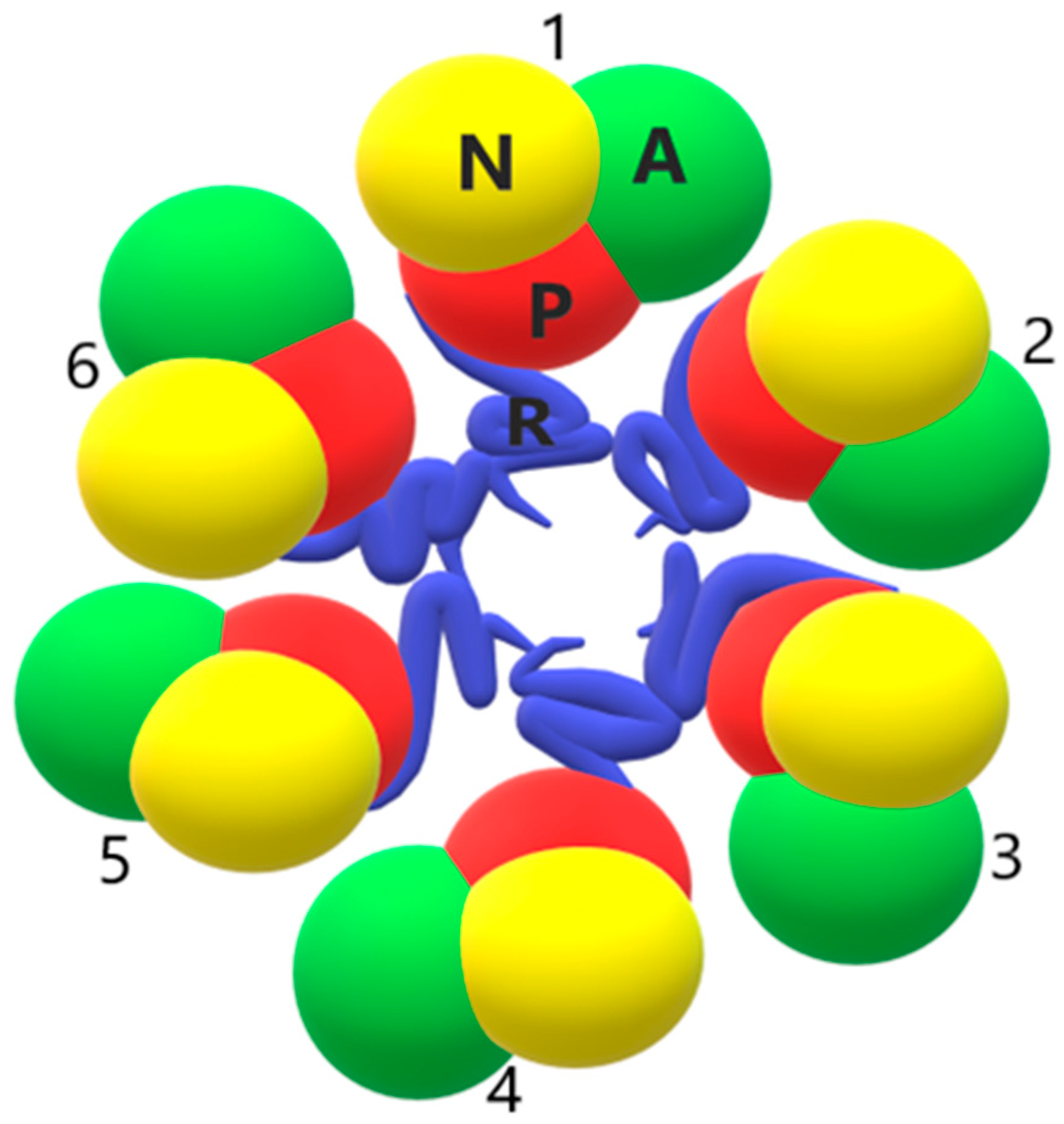
2.4. Oligomerization
3. V-ATPase
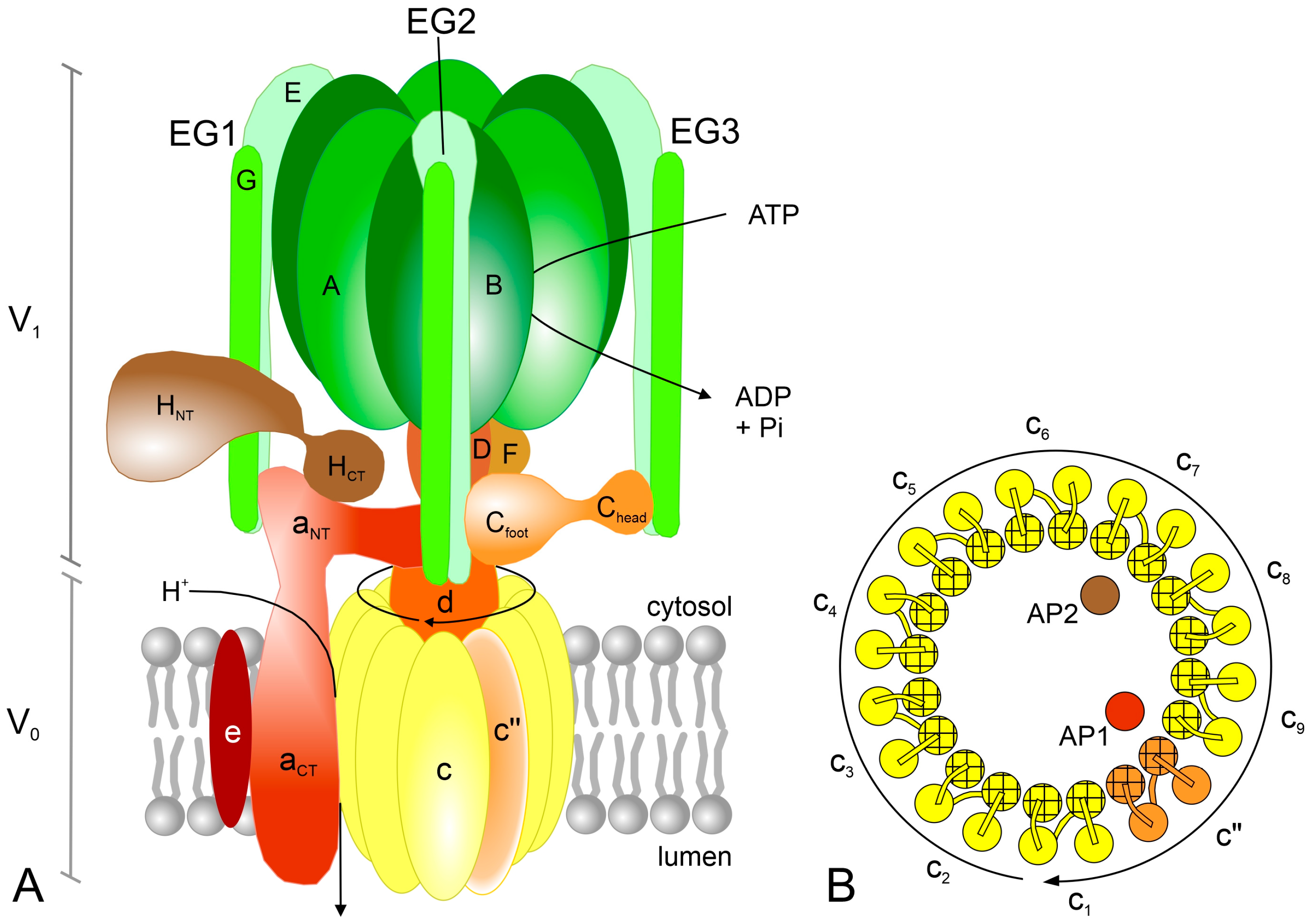
3.1. Overall Structure
3.1.1. V1 Subcomplex
3.1.2. V0 Subcomplex
3.1.3. Interactions with Other Proteins and Regulation at Post-Translational Level
3.2. Rotary Mechanism—Rotor and Stator Functions
3.3. Isoforms of VHA Subunits
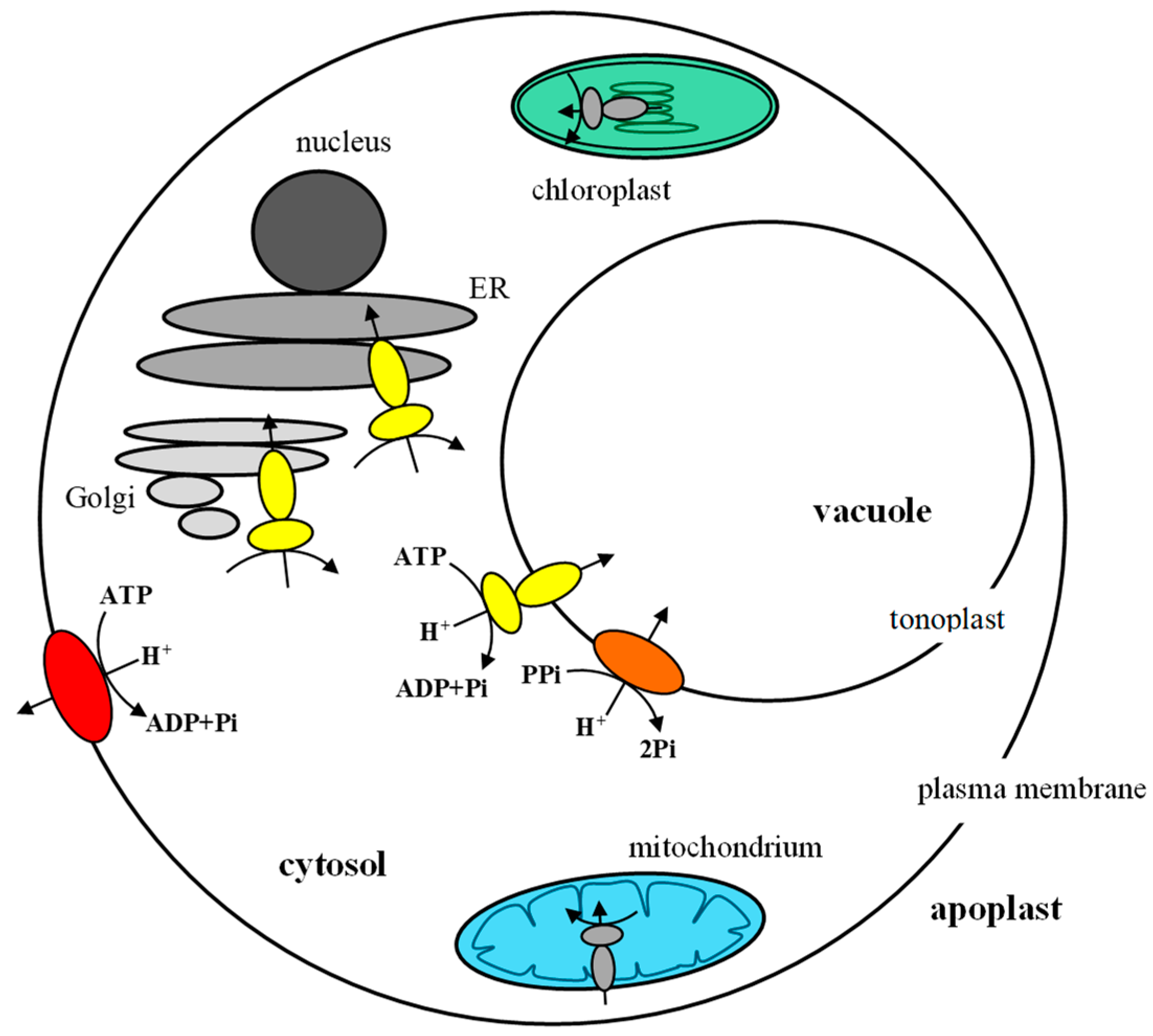
4. Coordination of Plant Proton Pump Functions
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gaxiola, R.A.; Palmgren, M.G.; Schumacher, K. Plant proton pumps. FEBS Lett. 2007, 581, 2204–2214. [Google Scholar] [CrossRef] [PubMed]
- Cosse, M.; Seidel, T. Plant Proton Pumps and Cytosolic pH-Homeostasis. Front. Plant Sci. 2021, 12, 672873. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.J.; Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A.; Wdowikowska, A.; Janicka, M. Plant Plasma Membrane Proton Pump: One Protein with Multiple Functions. Cells 2022, 11, 4052. [Google Scholar] [CrossRef] [PubMed]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma Membrane H+-ATPase Regulation in the Center of Plant Physiology. Mol. Plant. 2016, 9, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Kirpichnikova, A.; Chen, T.; Teplyakova, S.; Shishova, M. Proton pump and plant cell elongation. Biol. Commun. 2018, 63, 32–42. [Google Scholar] [CrossRef]
- Wang, C.; Xiang, Y.; Qian, D. Current progress in plant V-ATPase: From biochemical properties to physiological functions. J. Plant Physiol. 2021, 266, 153525. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, H.; Xu, F.; Yan, F.; Xu, W. H+-ATPases in Plant Growth and Stress Responses. Annu. Rev. Plant Biol. 2022, 73, 495–521. [Google Scholar] [CrossRef]
- Seidel, T. The Plant V-ATPase. Front. Plant Sci. 2022, 13, 931777. [Google Scholar] [CrossRef]
- Kanczewska, J.; Marco, S.; Vandermeeren, C.; Maudoux, O.; Rigaud, J.L.; Boutry, M. Activation of the plant plasma membrane H+-ATPase by phosphorylation and binding of 14-3-3 proteins converts a dimer into a hexamer. Proc. Natl. Acad. Sci. USA 2005, 102, 11675–11680. [Google Scholar] [CrossRef]
- Zhao, J.; Benlekbir, S.; Rubinstein, J. Electron cryomicroscopy observation of rotational states in a eukaryotic V-ATPase. Nature 2015, 521, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Axelsen, K.B.; Palmgren, M.G. Evolution of Substrate Specificities in the P-Type ATPase Superfamily. J. Mol. Evol. 1998, 46, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, M.G.; Nissen, P. P-type ATPases. Annu. Rev. Biophys. 2011, 40, 243–266. [Google Scholar] [CrossRef]
- Palmgren, M.; Morsomme, P. The plasma membrane H+-ATPase, a simple polypeptide with a long history. Yeast 2019, 36, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Cyrklaff, M.; Auer, M.; Kühlbrandt, W.; Scarborough, G.A. 2-D structure of the Neurospora crassa plasma membrane ATPase as determined by electron cryomicroscopy. EMBO J. 1995, 14, 1854–1857. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.P.; Buch-Pedersen, M.J.; Morth, J.P.; Palmgren, M.G.; Nissen, P. Crystal structure of the plasma membrane proton pump. Nature 2007, 450, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Duby, G.; Boutry, M. The plant plasma membrane proton pump ATPase: A highly regulated P-type ATPase with multiple physiological roles. Pflug. Arch. Eur. J. Physiol. 2009, 57, 645–655. [Google Scholar] [CrossRef]
- Ekberg, K.; Palmgren, M.G.; Veierskov, B.; Buch-Pedersen, M.J. A novel mechanism of P-type ATPase autoinhibition involving both termini of the protein. J. Biol. Chem. 2010, 285, 7344–7350. [Google Scholar] [CrossRef]
- Arango, M.; Gevaudant, F.; Oufattole, M.; Boutry, M. The plasma membrane proton pump ATPase: The significance of gene subfamilies. Planta 2003, 216, 355–365. [Google Scholar] [CrossRef]
- Palmgren, M. Plant plasma membrane H+-ATPase: Powerhous for nutrient uptake. Ann. Rev. Plant Physiol. Mol. Biol. 2001, 52, 817–845. [Google Scholar] [CrossRef]
- Wdowikowska, A. Kłobus, The plasma membrane proton pump gene family in cucumber. Acta Physiol. Plant. 2016, 38, 135. [Google Scholar] [CrossRef]
- Kalampanayil, B.; Wimmers, L. Identification and characterization of a salt-stressed-induced plasma membrane H+ATPase in tomato. Plant Cell Environ. 2001, 24, 999–1005. [Google Scholar] [CrossRef]
- Baxter, I.; Tchieu, J.; Sussman, M.; Boutry, M.; Palmgren, M.; Gribskov, M.; Harper, J.; Axelsen, K. Genomic comparison of P-type ATPase ion pumps in Arabidopsis and rice. Plant Physiol. 2003, 132, 618–828. [Google Scholar] [CrossRef] [PubMed]
- Ouffatole, M.; Arango, M.; Boutry, M. Identification and expression of tree new Nicotianaplumbaginifolia genes which encode isoforms of a plasma-membrane H+-ATPase, and one of which is induced by mechanical stress. Planta 2000, 210, 715–722. [Google Scholar] [CrossRef]
- Santi, S.; Locci, G.; Monte, R.; Pinton, R.; Varanini, Z. Induction of nitrate uptake in maize roots: Expression of a putative high-affinity nitrate transporter and plasma membrane H+-ATPase isoforms. J. Exp. Bot. 2003, 54, 1851–1864. [Google Scholar] [CrossRef]
- Moriau, L.; Michelet, B.; Bogaerts, P.; Lambert, L.; Michel, A.; Oufattole, M.; Boutry, M. Expression analysis of two gene subfamilies encoding the plasma membrane H+-ATPase in Nicotiana plumbaginifolia reveals the major transport functions of this enzyme. Plant J. 1999, 19, 31–41. [Google Scholar] [CrossRef]
- Portillo, F. Regulation of plasma membrane H+-ATPase in fungi and plants. Biochim. Biophys. Acta 2000, 1469, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Buch-Pedersen, M.J.; Palmgren, M.G. Mechanism of proton transport by plant plasma membrane proton ATPases. J. Plant Res. 2003, 116, 507–515. [Google Scholar] [CrossRef]
- Buch-Pedersen, M.; Rudashevskaya, E.; Berner, T.; Venema, K.; Palmgren, M. Potassium as an intrinsic uncoupler of the plasma membrane H+-ATPase. J. Biol. Chem. 2006, 281, 38285–38292. [Google Scholar] [CrossRef]
- Buch-Pedersen, M.; Pedersen, P.; Veierskov, B.; Nissen, P.; Palmgren, M. Protons how they are transported by proton pumps. Pflug. Arch. Eur. J. Phyl. 2009, 457, 573–579. [Google Scholar] [CrossRef]
- Focht, D.; Croll, T.I.; Pedersen, B.P.; Nissen, P. Improved Model of Proton Pump Crystal Structure Obtained by Interactive Molecular Dynamics Flexible Fitting Expands the Mechanistic Model for Proton Translocation in P-Type ATPases. Front. Physiol. 2017, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Jahn, T.; Fuglsang, A.T.; Olsson, A.A.; Brüntrup, M.; Collinge, D.B.; Volkmann, D.; Sommarin, M.; Palmgren, M.G.; Larsson, C. The 14-3-3 protein interacts directly with the C-terminal region of the plant plasma membrane H+-ATPase. Plant Cell 1997, 9, 1805–1814. [Google Scholar]
- Fuglsang, A.T.; Palmgren, M. Proton and calcium pumping P-type ATPases and their regulation of plant responses to the environment. Plant Physiol. 2021, 187, 1856–1875. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, M.G.; Sommarin, M.; Serrano, R.; Larsson, C. Identification of an autoinhibitory domain in the C-terminal region of the plant plasma membranę H(+)-ATPase. J. Biol. Chem. 1991, 266, 20470–20475. [Google Scholar] [CrossRef] [PubMed]
- Baunsgaard, L.; Fuglsang, A.T.; Jahn, T.; Korthout, H.A.; de Boer, A.H.; Palmgren, M.G. The 14-3-3 proteins associate with the plant plasma membrane H+-ATPase to generate a fusicoccin binding complex and a fusicoccin responsive system. Plant J. 1998, 13, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Morth, J.; Pedersen, B.; Buch-Pedersen, M.; Andersen, J.P.; Vilsen, P.; Palmgren, M.G.; Nissen, P. A structural overview of the plasma membrane Na+, K+-ATPase and H+-ATPase ion pumps. Nat. Rev. Mol. Cell Biol. 2011, 12, 60–70. [Google Scholar] [CrossRef]
- Zhang, S.; Habets, M.; Holger Breuninger, H.; Dolan, L.; Offringa, R.; van Duijn, B. Evolutionary and Functional Analysis of a Chara Plasma Membrane H+-ATPase. Front. Plant Sci. 2020, 10, 1707. [Google Scholar] [CrossRef]
- Okumura, M.; Inoue, S.; Takahashi, K.; Ishizaki, K.; Kohchi, T.; Kinoshita, T. Characterization of the plasma membrane H + -ATPase in the liverwort Marchantia polymorpha. Plant Physiol. 2012, 159, 826–834. [Google Scholar] [CrossRef]
- Okumura, M.; Takahashi, K.; Inoue, S.; Kinoshita, T. Evolutionary appearance of the plasma membrane H+-ATPase containing a penultimate threonine in the bryophyte. Plant Signal. Behav. 2012, 7, 979–982. [Google Scholar] [CrossRef]
- Stéger, A.; Hayashi, M.; Lauritzen, E.W.; Herburger, K.; Shabala, L.; Wang, C.; Bendtsen, A.K.; Nørrevang, A.F.; Madriz-Ordeñana, K.; Ren, S.; et al. The evolution of plant proton pump regulation via the R domain may have facilitated plant terrestrialization. Commun. Biol. 2022, 5, 1312. [Google Scholar] [CrossRef]
- Olsson, A.; Svennelid, F.; Ek, B.; Sommarin, M.; Larsson, C. A phosphothreonine residue at the C-terminal end of the plasma membrane Hþ-ATPase is protected by fusicoccin-induced 14-3-3 binding. Plant Physiol. 1998, 118, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Duby, G.; Poreba, W.; Piotrowiak, D.; Bobik, K.; Derua, R.; Waelkens, E.; Boutry, M. Activation of plant plasma membrane H+-ATPase by 14-3-3 proteins is negatively controlled by two phosphorylation sites within the H+-ATPase C-terminal region. J. Biol. Chem. 2009, 284, 4213–4221. [Google Scholar] [CrossRef] [PubMed]
- Fuglsang, A.T.; Borch, J.; Bych, K.; Jahn, T.P.; Roepstorff, P.; Palmgren, M.G. The binding site for regulatory 14-3-3 protein in plant plasma membrane Hþ-ATPase: Involvement of a region promoting phosphorylation-independent interaction in addition to the phosphorylation-dependent C-terminal end. J. Biol. Chem. 2003, 278, 42266–42272. [Google Scholar] [CrossRef] [PubMed]
- Fuglsang, A.T.; Guo, Y.; Cuin, T.A.; Qiu, Q.; Song, C.; Kristiansen, K.A.; Bych, K.; Schulz, A.; Shabala, S.; Schumaker, K.S.; et al. Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. Plant Cell 2007, 19, 1617–1634. [Google Scholar] [CrossRef]
- Boer, D. Fusicoccin—A key to multiple 14-3-3 locks? Trends Plant Sci. 1997, 2, 60–66. [Google Scholar] [CrossRef]
- Briskin, D.P.; Basu, S.; Assmann, S.M. Characterization of the red beet plasma membrane H+-ATPase reconstituted in a planar bilayer system. Plant Physiol. 1995, 108, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Jahn, T.; Dietrich, J.; Andersen, B.; Leidvik, B.; Otter, C.; Briving, C.; Kuhlbrandt, W.; Palmgren, M.G. Large scale expression, purification and 2D crystallization of recombinant plant plasma membrane H+-ATPase. J. Mol. Biol. 2001, 309, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Ottmann, C.; Marco, S.; Jaspert, N.; Marcon, C.; Schauer, N.; Weyand, M.; Vandermeeren, C.; Duby, G.; Boutry, M.; Wittinghofer, A.; et al. Structure of a 14-3-3 coordinated hexamer of the plant plasma membrane H-ATPase by combining x-ray crystallography and electron cryomicroscopy. Mol. Cell 2007, 25, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Sabat, G.; Sussman, M.R. In vivo cross-linking supports a head-to-tail mechanism for regulation of the plant plasma membrane P-type H(+)-ATPase. J. Biol. Chem. 2018, 293, 17095–17106. [Google Scholar] [CrossRef]
- Kuhlbrandt, W.; Zeelen, J.; Dietrich, J. Structure, mechanism, and regulation of the Neurospora plasma membrane H+-ATPase. Science 2002, 297, 1692–1696. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, C.; Chen, D.; Yun, C.; Li, H.; Bai, L. Structure and activation mechanism of the hexameric plasma membrane H+-ATPase. Nat. Commun. 2021, 12, 6439. [Google Scholar] [CrossRef] [PubMed]
- Heit, S.; Geurts, M.M.G.; Murphy, B.J.; Corey, R.A.; Mills, D.J.; Kühlbrandt, W.; Bublitz, M. Structure of the hexameric fungal plasma membrane proton pump in its autoinhibited state. Sci. Adv. 2021, 7, eabj5255. [Google Scholar] [CrossRef] [PubMed]
- Lapshin, N.K.; Piotrovskii, M.S.; Trofimowa, M.S. Sterol Extraction from Isolated Plant Plasma Membrane Vesicles Affects H+-ATPase Activity and H+-Transport. Biomolecules 2021, 11, 1891. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, K.; Krebs, M. The V-ATPase: Small cargo, large effects. Curr. Opin. Plant Biol. 2010, 13, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Y.M.; Wu, D.; Bueler, S.A.; Robinson, C.V.; Rubinstein, J.L. Structure of V-ATPase from the mammalian brain. Science 2020, 367, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Holliday, L.S. Vacuolar H+-ATPase: An Essential Multitasking Enzyme in Physiology and Pathophysiology. New J. Sci. 2014, 2014, 675430. [Google Scholar] [CrossRef]
- Vasanthakumar, T.; Rubinstein, J.L. Structure and Roles of V-type ATPases. Trends Biochem. Sci. 2020, 45, 295–307. [Google Scholar] [CrossRef]
- Lupanga, U.; Röhrich, R.; Askani, J.; Hilmer, S.; Kiefer, C.; Krebs, M.; Kanazawa, T.; Ueda, T.; Schumacher, K. The Arabidopsis V-ATPase is localized to the TGN/EE via a seed plant-specific motif. eLife 2020, 9, e60568. [Google Scholar] [CrossRef]
- Sze, H.; Schumacher, K.; Müller, M.L.; Padmanaban, S.; Taiz, L. A simple nomenclature for a complex proton pump: VHA genes encode the vacuolar H+-ATPase. Trends Plant Sci. 2002, 7, 157–161. [Google Scholar] [CrossRef]
- Seidel, T. Structure and Regulation of Plant Vacuolar H+-ATPase. In Progress in Botany; Lüttge, U., Beyschlag, W., Büdel, B., Francis, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 70, pp. 93–126. [Google Scholar]
- Oot, R.A.; Couoh-Cardel, S.; Sharma, S.; Stam, N.J.; Wilkens, S. Breaking up and making up: The secret life of the vacuolar H+-ATPase. Protein Sci. 2017, 26, 896–909. [Google Scholar] [CrossRef]
- Ratajczak, R. Structure, function and regulation of the plant vacuolar H+-translocating ATPase. Biochim. Biophys. Acta 2000, 1465, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Colina-Tenorio, L.; Dautant, A.; Miranda-Astudillo, H.; Giraud, M.-F.; González-Halphen, D. The Peripheral Stalk of Rotary ATPases. Front. Physiol. 2018, 9, 1243. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.P.; Forgac, M. Regulation and function of V-ATPases in physiology and disease. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183341. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Sun, F.; Yan, L.; Li, R.; Bai, J.; Caetano-Anollés, G. Genome-Wide Identification and Characterization of the Vacuolar H+-ATPase Subunit H Gene Family in Crop Plants. Int. J. Mol. Sci. 2019, 20, 5125. [Google Scholar] [CrossRef]
- Sharma, S.; Oot, R.A.; Wilkens, S. MgATP hydrolysis destabilizes the interaction between subunit H and yeast V1-ATPase, highlighting H’s role in V-ATPase regulation by reversible disassembly. J. Biol. Chem. 2018, 293, 10718–10730. [Google Scholar] [CrossRef]
- Oot, R.A.; Kane, P.M.; Berry, E.A.; Wilkens, S. Crystal structure of yeast V1-ATPase in the autoinhibited state. EMBO J. 2016, 35, 1694–1706. [Google Scholar] [CrossRef]
- Wang, R.; Long, T.; Hassan, A.; Wang, J.; Sun, Y.; Xie, X.S.; Li, X. Cryo-EM structures of intact V-ATPase from bovine brain. Nat. Commun. 2020, 11, 3921. [Google Scholar] [CrossRef]
- Tan, Y.Z.; Keon, K.A.; Abdelaziz, R.; Imming, P.; Schulze, W.; Schumacher, K.; Rubinstein, J.L. Structure of V-ATPase from citrus fruit. Structure 2022, 30, 1403–1410. [Google Scholar] [CrossRef]
- Mazhab-Jafari, M.T.; Rohou, A.; Schmidt, C.; Bueler, S.A.; Benlekbir, S.; Robinson, C.V.; Rubinstein, J.L. Atomic model for the membrane-embedded VO motor of a eukaryotic V-ATPase. Nature 2016, 539, 118–122. [Google Scholar] [CrossRef]
- Roh, S.H.; Stam, N.J.; Hryc, C.F.; Couoh-Cardel, S.; Pintilie, G.; Chiu, W.; Wilkens, S. The 3.5-Å CryoEM Structure of Nanodisc-Reconstituted Yeast Vacuolar ATPase Vo Proton Channel. Mol. Cell 2018, 69, 993–1004. [Google Scholar] [CrossRef]
- Seidel, T.; Schnitzer, D.; Golldack, D.; Sauer, M.; Dietz, K.J. Organelle-specific isoenzymes of plant V-ATPase as revealed by in vivo-FRET analysis. BMC Cell Biol. 2008, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Li, Z.; Zhang, X. Cloning and sequencing of V-ATPase subunit d from mung bean and its function in passive proton transport. J. Bioenerg. Biomembr. 2008, 40, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.H.; Shekhar, M.; Pintilie, G.; Chipot, C.; Wilkens, S.; Singharoy, A.; Chiu, W. Cryo-EM and MD infer water-mediated proton transport and autoinhibition mechanisms of Vo complex. Sci. Adv. 2020, 6, eabb9605. [Google Scholar] [CrossRef]
- Toei, M.; Toei, S.; Forgac, M. Definition of membrane topology and identification of residues important for transport in subunit a of the vacuolar ATPase. J. Biol. Chem. 2011, 286, 35176–35186. [Google Scholar] [CrossRef] [PubMed]
- Barkla, B.J.; Vera-Estrella, R.; Hernández-Coronado, M.; Pantoja, O. Quantitative proteomics of the tonoplast reveals a role for glycolytic enzymes in salt tolerance. Plant Cell 2009, 21, 4044–4058. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Qian, D.; Nan, Q.; Tan, C.; An, L.; Xiang, Y. Arabidopsis Vacuolar H+-ATPase (V-ATPase) B Subunits Are Involved in Actin Cytoskeleton Remodeling via Binding to, Bundling, and Stabilizing F-actin. J. Biol. Chem. 2012, 287, 19008–19017. [Google Scholar] [CrossRef]
- Liu, J.; Ji, Y.; Zhou, J.; Xing, D. Phosphatidylinositol 3-Kinase Promotes Activation and Vacuolar Acidification and Delays Methyl Jasmonate-Induced Leaf Senescence. Plant Physiol. 2016, 170, 1714–1731. [Google Scholar] [CrossRef]
- Batelli, G.; Verslues, P.E.; Agius, F.; Qiu, Q.; Fujii, H.; Pan, S.; Schumaker, K.S.; Grillo, S.; Zhu, J.K. SOS2 promotes salt tolerance in part by interacting with the vacuolar H+-ATPase and upregulating its transport activity. Mol Cell Biol. 2007, 27, 7781–7790. [Google Scholar] [CrossRef]
- Cho, Y.H.; Yoo, S.D.; Sheen, J. Regulatory functions of nuclear hexokinase1 complex in glucose signaling. Cell 2006, 127, 579–589. [Google Scholar] [CrossRef]
- Hong-Hermesdorf, A.; Brüx, A.; Grüber, A.; Grüber, G.; Schumacher, K. A WNK kinase binds and phosphorylates V-ATPase subunit C. FEBS Lett. 2006, 580, 932–939. [Google Scholar] [CrossRef]
- Klychnikov, O.I.; Li, K.W.; Lill, H.; de Boer, A.H. The V-ATPase from etiolated barley (Hordeum vulgare L.) shoots is activated by blue light and interacts with 14-3-3 proteins. J. Exp. Bot. 2007, 58, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Yin, X.R.; Xie, X.L.; Allan, A.C.; Ge, H.; Shen, S.L.; Chen, K.S. The Citrus transcription factor, CitERF13, regulates citric acid accumulation via a protein-protein interaction with the vacuolar proton pump, CitVHA-c4. Sci. Rep. 2016, 6, 20151. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Lorenzo, A.; Skinner, M.; El Annan, J.; Futai, M.; Sun-Wada, G.H.; Bourgoin, S.; Casanova, J.; Wildeman, A.; Bechoua, S.; Ausiello, D.A.; et al. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat. Cell Biol. 2006, 8, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Merkulova, M.; Bakulina, A.; Thaker, Y.R.; Grüber, G.; Marshansky, V. Specific motifs of the V-ATPase a2-subunit isoform interact with catalytic and regulatory domains of ARNO. Biochim. Biophys. Acta 2010, 1797, 1398–1409. [Google Scholar] [CrossRef]
- Krah, A.; Marzinek, J.K.; Bond, P.J. Insights into water accessible pathways and the inactivation mechanism of proton translocation by the membrane-embedded domain of V-type ATPases. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1004–1010. [Google Scholar] [CrossRef]
- Chu, A.; Zirngibl, R.A.; Manolson, M.F. The V-ATPase a3 Subunit: Structure, Function and Therapeutic Potential of an Essential Biomolecule in Osteoclastic Bone Resorption. Int. J. Mol. Sci. 2021, 22, 6934. [Google Scholar] [CrossRef]
- Schep, D.G.; Zhao, J.; Rubinstein, J.L. Models for the A subunits of the Thermus thermophilus V/A-ATPase and Saccharomyces cerevisiae V-ATPase enzymes by cryo-EM and evolutionary covariance. Proc. Natl. Acad. Sci. USA 2016, 113, 3245–3250. [Google Scholar] [CrossRef]
- Hohlweg, W.; Wagner, G.E.; Hofbauer, H.F.; Sarkleti, F.; Setz, M.; Gubensäk, N.; Lichtenegger, S.; Falsone, S.F.; Wolinski, H.; Kosol, S.; et al. A cation-π interaction in a transmembrane helix of vacuolar ATPase retains the proton-transporting arginine in a hydrophobic environment. J. Biol. Chem. 2018, 293, 18977–18988. [Google Scholar] [CrossRef]
- Banerjee, S.; Kane, P.M. Regulation of V-ATPase Activity and Organelle pH by Phosphatidylinositol Phosphate Lipids. Front. Cell Dev. Biol. 2020, 8, 510. [Google Scholar] [CrossRef]
- Kabała, K.; Janicka-Russak, M.; Reda, M.; Migocka, M. Transcriptional regulation of the V-ATPase subunit c and V-PPase isoforms in Cucumis sativus under heavy metal stress. Physiol. Plant. 2014, 150, 32–45. [Google Scholar] [CrossRef]
- Zhou, H.; Huang, W.; Luo, S.; Hu, H.; Zhang, Y.; Zhang, L.; Li, P. Genome-Wide Identification of the Vacuolar H+-ATPase Gene Family in Five Rosaceae Species and Expression Analysis in Pear (Pyrus bretschneideri). Plants 2020, 9, 1661. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.Y.; Hussain, S.B.; Guo, L.X.; Yang, H.; Ning, D.Y.; Liu, Y.Z. Genome-wide identification and transcript analysis of vacuolar-ATPase genes in citrus reveal their possible involvement in citrate accumulation. Phytochemistry 2018, 155, 147–154. [Google Scholar] [CrossRef]
- Banerjee, S.; Kane, P.M. Direct interaction of the Golgi V-ATPase a-subunit isoform with PI(4)P drives localization of Golgi V-ATPases in yeast. Mol. Biol. Cell 2017, 28, 2518–2530. [Google Scholar] [CrossRef] [PubMed]
- Vasanthakumar, T.; Bueler, S.A.; Wu, D.I.; Beilsten-Edmands, V.; Robinson, C.V.; Rubinstein, J.L. Structural comparison of the vacuolar and Golgi V-ATPases from Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2019, 116, 7272–7277. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, J.; Hong-Hermesdorf, A.; Stierhof, Y.D.; Schumacher, K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 2006, 18, 715–730. [Google Scholar] [CrossRef]
- Oot, R.A.; Yao, Y.; Manolson, M.F.; Wilkens, S. Purification of active human vacuolar H+-ATPase in native lipid-containing nanodiscs. J. Biol. Chem. 2021, 297, 100964. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, J.; Liu, T.Y.; Schumacher, K. Functional analysis of Arabidopsis V-ATPase subunit VHA-E isoforms. Eur. J. Cell Biol. 2010, 89, 152–156. [Google Scholar] [CrossRef]
- Bageshwar, U.K.; Taneja-Bageshwar, S.; Moharram, H.M.; Binzel, M.L. Two isoforms of the A subunit of the vacuolar H+-ATPase in Lycopersicon esculentum: Highly similar proteins but divergent patterns of tissue localization. Planta 2005, 220, 632–643. [Google Scholar] [CrossRef]
- Jezek, M.; Blatt, M.R. The Membrane Transport System of the Guard Cell and Its Integration for Stomatal Dynamics. Plant Physiol. 2017, 174, 487–519. [Google Scholar] [CrossRef]
- Eisenach, C.; De Angeli, A. Ion Transport at the Vacuole during Stomatal Movements. Plant Physiol. 2017, 174, 520–530. [Google Scholar] [CrossRef]
- Feng, S.; Peng, Y.; Liu, E.; Ma, H.; Qiao, K.; Zhou, A.; Liu, S.; Bu, Y. Arabidopsis V-ATPase d2 Subunit Plays a Role in Plant Responses to Oxidative Stress. Genes 2020, 11, 701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yan, X.; Han, X.; Tang, R.; Chu, M.; Yang, Y.; Yang, Y.H.; Zhao, F.; Fu, A.; Luan, S.; et al. A Defective Vacuolar Proton Pump Enhances Aluminum Tolerance by Reducing Vacuole Sequestration of Organic Acids. Plant Physiol. 2019, 181, 743–761. [Google Scholar] [CrossRef] [PubMed]
- Martinoia, E. Vacuolar Transporters—Companions on a Longtime Journey. Plant Physiol. 2018, 176, 1384–1407. [Google Scholar] [CrossRef]
- Appelhagen, I.; Nordholt, N.; Seidel, T.; Spelt, K.; Koes, R.; Quattrochio, F.; Sagasser, M.; Weisshaar, B. TRANSPARENT TESTA 13 is a tonoplast P3A-ATPase required for vacuolar deposition of proanthocyanidins in Arabidopsis thaliana seeds. Plant J. 2015, 82, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liao, L.; Fang, T.; Peng, Q.; Ogutu, C.; Zhou, H.; Ma, F.; Han, Y.A. Ma10 gene encoding P-type ATPase is involved in fruit organic acid accumulation in apple. Plant Biotechnol. J. 2019, 17, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Paulic, M.; Seidel, T. Interactome of Arabidopsis thaliana. Plants 2022, 11, 350. [Google Scholar] [CrossRef]
| Subunit | Chlamydomonas reinhardtii | Citrus sinensis | Cucumis sativus | Oryza sativa | Arabidopsis thaliana | Malus × domestica | Glycine max | Homo sapiens | Saccharomyces cerevisiae |
|---|---|---|---|---|---|---|---|---|---|
| V1 sector | |||||||||
| A | VHA-A | VHA-A | VHA-A | VHA-A1 | VHA-A | VHA-A1 | VHA-A1 | ATP6V1A | Vma1p |
| VHA-A2 | VHA-A2 | VHA-A2 | |||||||
| B | VHA-B | VHA-B | VHA-B | VHA-B1 | VHA-B1 | VHA-B1 | VHA-B1 | ATP6V1B1 | Vma2p |
| VHA-B2 | VHA-B2 | VHA-B2 | VHA-B2 | ATP6V1B2 | |||||
| VHA-B3 | VHA-B3 | ||||||||
| VHA-B4 | |||||||||
| C | VHA-C | VHA-C | VHA-C | VHA-C | VHA-C | VHA-C1 | VHA-C1 | ATP6V1C1 | Vma5p |
| VHA-C2 | VHA-C2 | ATP6V1C2 | |||||||
| VHA-C3 | VHA-C3 | ||||||||
| VHA-C4 | VHA-C4 | ||||||||
| D | VHA-D | VHA-D | VHA-D | VHA-D | VHA-D | VHA-D1 | VHA-D1 | ATP6V1D | Vma8p |
| VHA-D2 | VHA-D2 | ||||||||
| E | VHA-E | VHA-E1 | VHA-E | VHA-E1 | VHA-E1 | VHA-E1 | VHA-E1 | ATP6V1E1 | Vma4p |
| VHA-E2 | VHA-E2 | VHA-E2 | VHA-E2 | VHA-E2 | ATP6V1E2 | ||||
| VHA-E3 | VHA-E3 | VHA-E3 | VHA-E3 | ||||||
| VHA-E4 | VHA-E4 | ||||||||
| VHA-E5 | |||||||||
| VHA-E6 | |||||||||
| VHA-E7 | |||||||||
| F | VHA-F | VHA-F1 | VHA-F | VHA-F1 | VHA-F | VHA-F1 | VHA-F1 | ATP6V1F | Vma7p |
| VHA-F2 | VHA-F2 | VHA-F2 | VHA-F2 | ||||||
| VHA-F3 | |||||||||
| G | VHA-G | VHA-G | VHA-G1 | VHA-G1 | VHA-G1 | VHA-G1 | VHA-G1 | ATP6V1G1 | Vma10p |
| VHA-G2 | VHA-G2 | VHA-G2 | VHA-G2 | VHA-G2 | ATP6V1G2 | ||||
| VHA-G3 | VHA-G3 | VHA-G3 | ATP6V1G3 | ||||||
| VHA-G4 | VHA-G4 | ||||||||
| VHA-G5 | VHA-G5 | ||||||||
| VHA-G6 | |||||||||
| H | VHA-H | VHA-H1 | VHA-H | VHA-H | VHA-H | VHA-H1 | VHA-H1 | ATP6V1H | Vma13p |
| VHA-H2 | VHA-H2 | VHA-H2 | |||||||
| V0 sector | |||||||||
| a | VHA-a1 | VHA-a1 | VHA-a1 | VHA-a1 | VHA-a1 | VHA-a1 | VHA-a1 | ATP6V0a1 | Vph1p |
| VHA-a2 | VHA-a2 | VHA-a2 | VHA-a2 | VHA-a2 | VHA-a2 | VHA-a2 | ATP6V0a2 | Stv1p | |
| VHA-a3 | VHA-a3 | VHA-a3 | VHA-a3 | VHA-a3 | VHA-a3 | ATP6V0a3 | |||
| VHA-a4 | VHA-a4 | ATP6V0a4 | |||||||
| VHA-a5 | VHA-a5 | ||||||||
| VHA-a6 | VHA-a6 | ||||||||
| VHA-a7 | VHA-a7 | ||||||||
| VHA-a8 | |||||||||
| c | VHA-c | VHA-c1 | VHA-c1 | VHA-c1 | VHA-c1 | VHA-c1 | VHA-c1 | ATP6V0c | Vma3p |
| VHA-c2 | VHA-c2 | VHA-c2 | VHA-c2 | VHA-c2 | VHA-c2 | ||||
| VHA-c3 | VHA-c3 | VHA-c3 | VHA-c3 | VHA-c3 | VHA-c3 | ||||
| VHA-c4 | VHA-c4 | VHA-c4 | VHA-c4 | VHA-c4 | |||||
| VHA-c5 | VHA-c5 | VHA-c5 | |||||||
| VHA-c6 | VHA-c6 | ||||||||
| VHA-c7 | VHA-c7 | ||||||||
| VHA-c8 | VHA-c8 | ||||||||
| VHA-c9 | VHA-c9 | ||||||||
| VHA-c10 | |||||||||
| c′ | - | - | - | - | - | - | Vma11p | ||
| c″ | VHA-c″ | VHA-c″ | VHA-c″1 | VHA-c″ | VHA-c″1 | VHA-c″ | VHA-c″1 | ATP6V0b | Vma16p |
| VHA-c″2 | VHA-c″2 | VHA-c″2 | |||||||
| d | VHA-d | VHA-d | VHA-d | VHA-d | VHA-d1 | VHA-d1 | VHA-d1 | ATP6V0d1 | Vma6p |
| VHA-d2 | VHA-d2 | VHA-d2 | ATP6V0d2 | ||||||
| e | VHA-e | VHA-e | VHA-e1 | VHA-e | VHA-e1 | VHA-e1 | VHA-e1 | ATP6V0e | - |
| VHA-e2 | VHA-e2 | VHA-e2 | VHA-e2 | ||||||
| VHA-e3 | VHA-e3 | ||||||||
| VHA-e4 | VHA-e4 | ||||||||
| f | - | - | - | - | - | - | RNAseK | YPR170W-B | |
| total | 15 | 20 | 20 | 24 | 28 | 48 | 54 | 23 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabała, K.; Janicka, M. Structural and Functional Diversity of Two ATP-Driven Plant Proton Pumps. Int. J. Mol. Sci. 2023, 24, 4512. https://doi.org/10.3390/ijms24054512
Kabała K, Janicka M. Structural and Functional Diversity of Two ATP-Driven Plant Proton Pumps. International Journal of Molecular Sciences. 2023; 24(5):4512. https://doi.org/10.3390/ijms24054512
Chicago/Turabian StyleKabała, Katarzyna, and Małgorzata Janicka. 2023. "Structural and Functional Diversity of Two ATP-Driven Plant Proton Pumps" International Journal of Molecular Sciences 24, no. 5: 4512. https://doi.org/10.3390/ijms24054512
APA StyleKabała, K., & Janicka, M. (2023). Structural and Functional Diversity of Two ATP-Driven Plant Proton Pumps. International Journal of Molecular Sciences, 24(5), 4512. https://doi.org/10.3390/ijms24054512






