Abstract
Cilia and flagella are evolutionarily conserved organelles that form protrusions on the surface of many growth-arrested or differentiated eukaryotic cells. Due to the structural and functional differences, cilia can be roughly classified as motile and non-motile (primary). Genetically determined dysfunction of motile cilia is the basis of primary ciliary dyskinesia (PCD), a heterogeneous ciliopathy affecting respiratory airways, fertility, and laterality. In the face of the still incomplete knowledge of PCD genetics and phenotype-genotype relations in PCD and the spectrum of PCD-like diseases, a continuous search for new causative genes is required. The use of model organisms has been a great part of the advances in understanding molecular mechanisms and the genetic basis of human diseases; the PCD spectrum is not different in this respect. The planarian model (Schmidtea mediterranea) has been intensely used to study regeneration processes, and—in the context of cilia—their evolution, assembly, and role in cell signaling. However, relatively little attention has been paid to the use of this simple and accessible model for studying the genetics of PCD and related diseases. The recent rapid development of the available planarian databases with detailed genomic and functional annotations prompted us to review the potential of the S. mediterranea model for studying human motile ciliopathies.
1. Introduction–Cilia and Ciliopathies
Cilia and flagella are evolutionarily conserved organelles that form protrusions on the surface of many growth-arrested or differentiated eukaryotic cells, from simple unicellular organisms such as Chlamydomonas to specialized cells in higher animals—fish and mammals [1,2,3]. The cilium is anchored at the cell membrane via the basal body, which extends into the axoneme, the main part of the cilium protruding outside the cell; the transition zone between the basal body and axoneme has a function of ciliary gating [4]. Due to structural and functional differences, cilia can be roughly classified as motile and non-motile (also called primary or sensory), although their detailed classification is much more complicated [5,6].
Motile cilia are ancient organelles that were already present in the Last Eukaryotic Common Ancestor, LECA [2]. Their main function is associated with their ability to move; they also perform some sensory functions [5,7,8]. In unicellular organisms, such as Chlamydomonas, Paramecium, or Tetrahymena, cilia or flagella (structurally related to motile cilia, but much longer) are responsible for the movement of cells. In higher organisms, motile cilia (5–10 um long) form a dense carpet (hundreds of organelles per cell) on the apical side of multiciliated cells (MCCs), and through their coordinated planar beating are responsible for the flow of fluids covering epithelium [9]. Single flagella are responsible for sperm motility [10], and single motile cilia in the mammalian embryonal node regulate the directional flow of signals required for the establishment of left-right patterning [11]. Primary cilia found as singular entities on the surface of almost every differentiated metazoan cell type [1], are not in focus in this review, but it should be mentioned that they evolved from motile cilia. Due to the difference in their structure, they are immotile [12], but they play an important role in the reception and transmission of signals involved in the regulation of cellular processes essential for development and the maintenance of tissue homeostasis. The main signaling pathways coordinated by primary cilia include those regulated by Hedgehog (HH), G-protein-coupled receptors (GPCR), wingless (WNT), receptor-tyrosine kinases (RTKs), and TGFβ/BMP receptors [12,13,14,15].
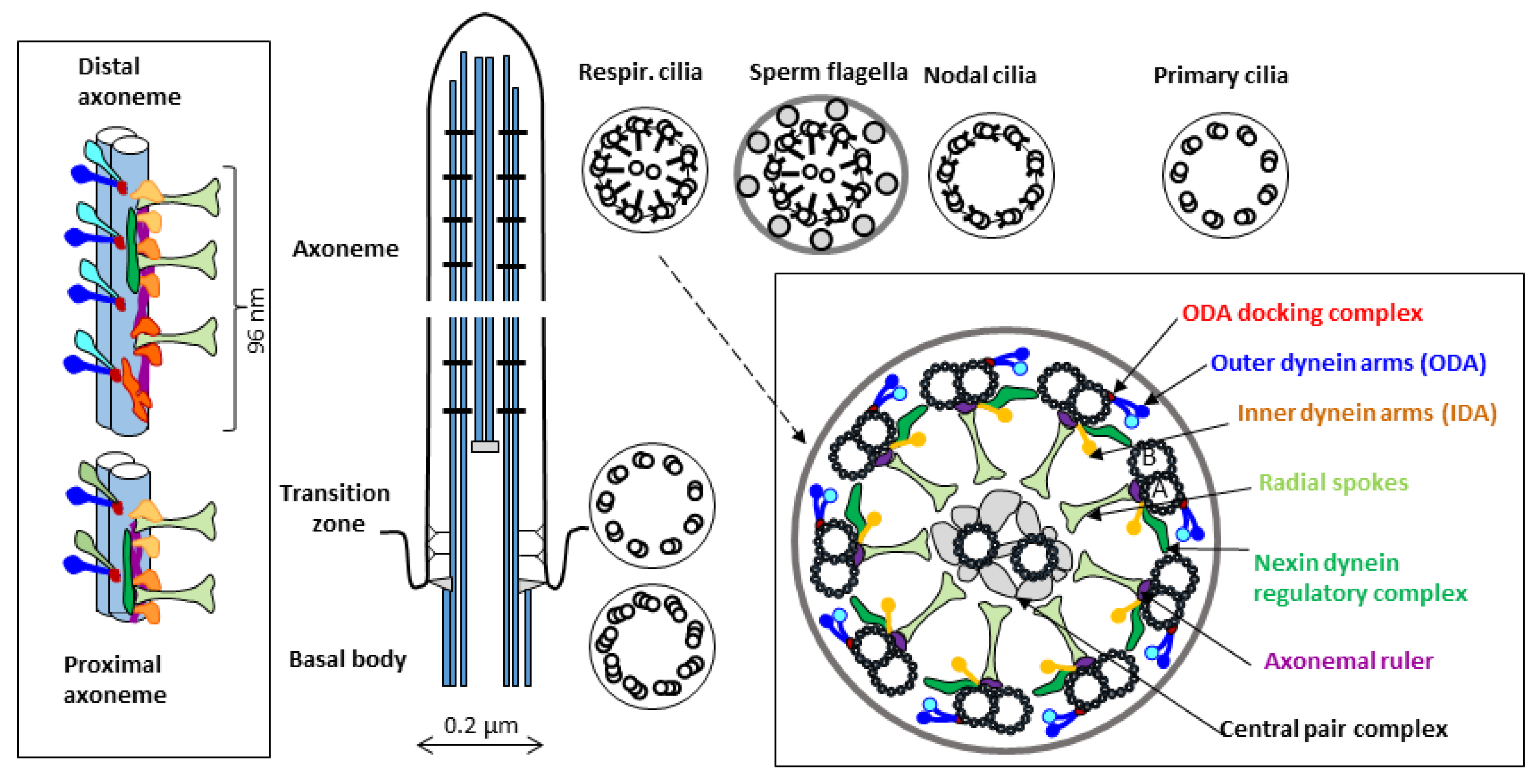
In the axoneme of a typical motile cilium or flagellum (Figure 1), nine peripheral doublets of microtubules (A and B) surround two single central microtubules (C1 and C2). In the cross-section of the axoneme observed in the transmission electron microscope (TEM), this arrangement is known as the 9 × 2 + 2 pattern. Additional protein elements attached to the microtubules are distributed periodically along the axoneme length. Multiprotein complexes associated with the peripheral A microtubules form characteristic auxiliary structures: outer and inner dynein arms (ODA and IDA), ODA docking complexes, radial spokes (RS), and nexin-dynein regulatory complexes (N-DRC). Several detailed reviews describing the axonemal structure components have been published in recent years, e.g., [16,17,18].
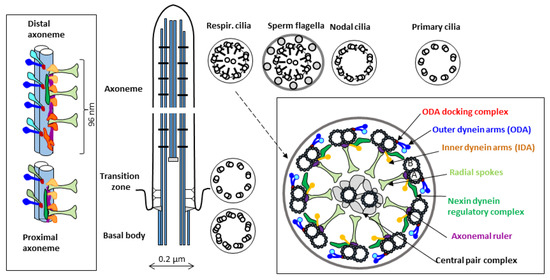
Figure 1.
Simplified schematic representation of the ultrastructure of a motile cilium (longitudinal and cross sections detailing microtubule-associated elements). Cross-sections of the axoneme of different types of motile cilia (respiratory and nodal cilia, sperm flagellum) are compared with that of a primary cilium. The periodic longitudinal unit (organized in 96 nm repeats) consists of four ODAs (attached to A microtubules of the peripheral dublets through the ODA-docking complexes), three radial spokes, and a set of IDAs (consisting of different complexes identified using a cryoelectron tomography [16,19]). The length of this unit is determined by the molecular ruler proteins (in humans, CCDC39 and CCDC40, homologs of Chlamydomonas proteins FAP59 and FAP172 [20]). In mammalian motile cilia, ODAs differ in the proximal and distal parts of the axoneme (by the presence of DNAH11 and DNAH9 proteins, respectively); in sperm flagella, they are replaced by a single protein DNAH17 [21]. Several small elements attached to the A microtubule, not visible in classical TEM, have been identified in model organisms using cryoelectron tomography [22]; additional small proteins are docked at the luminal side of both A- and B-microtubules [23,24]. The central microtubules, connected by a multiprotein bridge, and surrounded by a set of projections, form the central pair complex [25].
Cilia movement is powered by ODAs and IDAs, which act as ATP-dependent molecular motors. They move in synchrony along the B microtubule of the adjacent doublet. The resulting mutual sliding of the peripheral doublets is restricted by the N-DRCs, which connect neighboring doublets; the net result is a bend of the cilium [26]. Radial spokes, attached to the peripheral A microtubules and transiently contacting the projections of the central microtubules, stabilize the structure and functionally connect the central apparatus to the dynein arms [27]. The cooperation of all these ultrastructural elements results in a coordinated planar beating of motile cilia, with a fast power stroke and a slower recovery stroke occurring in the same plane; for the review see, e.g., [18].
The ciliogenesis of motile cilia is a multi-step process, starting from the cell cycle exit and involving many signaling factors; it has been the subject of many excellent reviews, e.g., [28]. The inhibition of the NOTCH1 signal and activation of the MCIDAS-dependent pathway initiates the biogenesis process specific to MCCs [29]. In MCCs, the MCIDAS pathway orchestrates massive centriole amplification and their docking in the apical cell membrane (through the activation of cyclin O) [30]. The expression of the FOXJ1 transcription factor switches on the synthesis of proteins directly involved in the formation of motile cilia ultrastructure [31]. Cilia elongation and maintenance are possible thanks to the presence of a dedicated protein shuttle system, named intraflagellar transport (IFT), which involves the transport of molecules from the cell body through the basal body, transition zone to the tip of cilia and back [32,33]. Some of the multiprotein ciliary elements of motile cilia (e.g., dynein arms, ODA docking complexes) are preassembled in the cytoplasm in a process that requires the presence of proteins, which are physically not a part of the axonemal ultrastructure [34,35].
Genetically determined dysfunction of cilia is the cause of a large group of diseases, collectively referred to as ciliopathies. With the expanding knowledge of cilia biology and genetics, their number is now estimated as at least 35 [36]. The majority of ciliopathies are caused by the dysfunction of primary/sensory cilia [37]. Consistent with the presence of primary cilia on almost all cell types and their function in basic cellular pathways, their dysfunction affects multiple systems, including kidneys, brain, heart, skeleton, and eyes (e.g., polycystic kidney disease, PKD; nephronophthisis, NPHP; Bardet-Biedl syndrome, BBS; Joubert syndrome, JBTS; Meckel syndrome, MKS; Senior-Locken syndrome, SLSN; retinitis pigmentosa, RP); for the reviews see [36,37,38]. In this review, however, we focus on the diseases caused by the genetically determined defects of the structure and/or function of motile cilia.
1.1. Primary Ciliary Dyskinesia
Primary ciliary dyskinesia, PCD (OMIM244400; population frequency of 1:10,000 to 1:20,000), is the flagship ciliopathy resulting from the dysfuntion of motile cilia [34,35,39,40,41,42,43]. It has to be emphasized that the word “primary” in the name of the disease refers to the fact that PCD is a genetically-based condition, as opposed to a “secondary” ciliary dyskinesia, where the dysfunction of motile cilia is caused by environmental factors.
Typical clinical symptoms of PCD reflect the role of motile cilia in different parts of the human body. Defects of cilia on the apical surface of the epithelial cells lining the respiratory tract impair mucociliary clearance; as a result, PCD patients suffer from recurrent respiratory airway infections leading to chronic bronchopulmonary disease, recurrent sinusitis, rhinitis, otitis media, and bronchiectasis. The immotility of sperm flagella is a cause of male infertility, and the immotility of cilia on the epithelial cells lining fallopian tubes reduces fertility in females. Dysfunction of cilia present on the ependymal cells in the brain, which are responsible for cerebrospinal fluid flow, can lead to hydrocephalus. Finally, the defects of single motile cilia in the embryonic node impair the flow of morphogens and body patterning (situs), resulting in the randomization of body organ symmetry (situs inversus totalis in about 50% of PCD patients).
Due to the largely nonspecific clinical symptoms of PCD and insufficient diagnostic methods, PCD patients are often diagnosed late. The range and severity of PCD symptoms depend on the mutated gene, reflecting different impacts of the dysfunctional protein on the ciliary structure and/or function [34,35,42,43,44,45].
Theoretically, the diagnostic problems can be overcome by applying genetic tests with high efficiency of mutation detection. However, the genetic basis of PCD is highly heterogeneous, reflecting a large number of proteins involved in the structure and function of motile cilia [46,47]. The inheritance of PCD in most families is autosomal recessive; the X-linked or autosomal dominant inheritance is rare. To date, ~50 genes have been reported to be involved in PCD pathogenesis (Table 1), and the involvement of more is still under investigation [34,35,39,40,42,44] (Table 2). The role of these genes in the axonemal structure, ciliary function, or in the biogenesis of motile cilia has been confirmed in animal models using a range of approaches, including analysis of mutant organisms, knockout of the gene in question by targeted mutation or gene knockdown using double-stranded RNA (dsRNA) or morpholinos.

Table 1.
Genes involved in PCD pathogenesis.

Table 2.
Examples of genes with an uncertain role in PCD or involved in PCD-like diseases.
Pathogenic variants in many of the PCD genes result in obvious ultrastructural and/or functional defects of cilia, which can be readily visualized using transmission electron microscopy (TEM) or analysis of the ciliary beat [45,169,170]. The most frequent ultrastructural defects include the absence or shortening of ODA or both ODA and IDA, disorganization of microtubule arrangement, scarcity, or a complete lack of cilia. The defects of the ciliary beat can manifest as immotility, flickering, slow beating, or disturbed pattern of beating [169,170]. On the other hand, mutations identified in some of the genes, e.g., encoding central pair complex proteins, N-DRC proteins, or some of the dynein arm elements, have no clear effect on the axonemal structure or on the cilia beat frequency [102,119,123,139,147,171].
Importantly, even the use of high-throughput genome sequencing fails to detect mutations in known PCD genes in ~1/3 of the patients [34,35]. This may be due to the presence of unknown pathogenic variants, lying outside the most commonly studied coding sequences or impossible to detect with copy number insensitive techniques, as well as the presence of pathogenic mutations in yet-unidentified PCD genes. Moreover, the association of some candidate genes with PCD pathogenesis remains a matter of debate, especially when mutations have been described in single families. In addition, there is an expanding list of genes from the so-called PCD spectrum [172], in which mutations in cilia-related genes are associated with an atypical clinical picture of the disease, with a syndromic presentation [51,52,54,55,173] or without (or very mild) respiratory symptoms [10,102,174,175,176]. Classification and curation of gene-disease relationships involving PCD and related motile cilia disorders are currently the focus of the Motile Ciliopathy Gene Curation Expert Panel, a part of the ClinicalGenome consortium [https://www.clinicalgenome.org/ (accessed on 20 February 2023)] [177].
The overall result of the heterogeneity of heritable motile ciliopathies is that—to better characterize the molecular/genetic basis of the unsolved cases of PCD and to differentiate them from the PCD-like spectrum diseases—the search for new candidate genes potentially involved in the pathogenesis is still needed.
1.2. The Validation of New Genes Underlying PCD and PCD-like Ciliopathies—The Role of Model Organisms
The easy access to NGS-based genetic screening of PCD patients increases the chances to reveal new candidate genes. Validation of their impact on motile cilia structure and function requires functional studies. The use of primary cell cultures in such studies requires obtaining respiratory epithelial biopsies from patients with pathogenic variants in candidate genes; the amount of the biological material obtained this way is limited, and often insufficient for detailed biochemical and molecular analyses. Another approach, silencing candidate genes in the in vitro culture of healthy human respiratory epithelium (HRE), is not in a routine laboratory method (reviewed in [178]). Primary HRE cells have limited ability to proliferate in culture. While the recent development of conditionally reprogrammed HRE cell cultures increased the proliferative lifespan of these cells and their ability to differentiate, this model is very demanding and still not sufficiently robust and replicable [178]. This is especially important in functional studies, where genome modification is required to overexpress or silence specific candidate genes.
Thanks to the high level of evolutionary conservation of motile cilia [3], a variety of model organisms have been successfully used for studying cilia biology. The same approach, which has allowed explaining the molecular basis of cilia assembly, structure, and function across Eukaryotic species, is widely used as a tool in the functional analysis of candidate genes underlying the pathogenesis of PCD and other cilia-related diseases.
For almost half of the causative genes identified during over twenty years of PCD research, their involvement in motile cilia function has been revealed by earlier (non-PCD) forward genetics studies in model organisms, performed to study cilia biology–the identity of proteins, their ultrastructural localization, interactions, and function. The loss-of-function variants in these genes, when found among patients, have been directly associated with PCD pathogenesis. For another half of PCD genes, with deleterious variants identified during the genetic screening of patients, their involvement in motile cilia dysfunction has been confirmed by follow-up reverse genetics studies, involving candidate gene silencing in model organisms.
The majority of all these studies had been based on a model of double-flagella unicellular alga, Chlamydomonas reinhardtii. DNAI1, the first gene identified as involved in the pathogenesis of PCD, has been earlier associated with the flagellar dysfunction caused by the mutation of IC78, DNAI1 homolog in a double-flagella unicellular alga, Ch. reinhardtii [88]. Other models, which had supported the role of then-candidate PCD genes in motile cilia dysfunction, include unicellular organisms (P. reinhardtii, Trypanosoma brucei, Tetrahymena thermophila), invertebrates (Drosophila melanogaster, Schmidtea mediterranea) or vertebrates (frog Xenopus leavis, fish Danio rerio/zebrafish, mouse, dog); reviewed in [179,180]. Among these model organisms considered in the context of PCD, relatively little attention has been put to the use of S. mediterranea.
2. Schmidtea mediterranea
S. mediterranea is a representative of freshwater planarians, free-living invertebrates from the phylum Platyhelminthes (flatworms). These animals belong to the group of organisms that have three germ layers (endoderm, mesoderm, and ectoderm), bilateral symmetry, and tissues with separate organs. The manner of reproduction of the freshwater planarians varies across the species and can be exclusively asexual (by transverse fission), seasonally sexual, and exclusively sexual (by the cross-fertilization of hermaphrodites) [181]. Planarians achieved popularity due to their great ability to regenerate after amputation or injury. In some cases, a full organism can be rebuilt after several days from 1/279 piece of a single worm, although the regenerative abilities of planarians are different across the species [182,183,184,185]. This regenerative potential makes planarians practically immortal and enables researchers to use them as efficient model organisms in a variety of studies.
There are several hundred species of planarians, but their use as animal models in molecular and genetic studies has been mostly limited to the Dugesiidae family (e.g., genera Dugesia, Girardia, Schmidtea), with the majority of research conducted using two species, S. mediterranea and Dugesia japonica [186]. D. japonica is favored for behavioral studies and toxicology screening, while S. mediterranea is popular and attractive for molecular experiments [187]. Two distinct strains of S. mediterranea exist in nature: a sexual strain (2 cm long) and an asexual strain (slightly shorter). Both are diploid, with four pairs of chromosomes (2n = 8); the asexual form results from a chromosome translocation between the sexual strain chromosomes 1 and 3 [181,188].
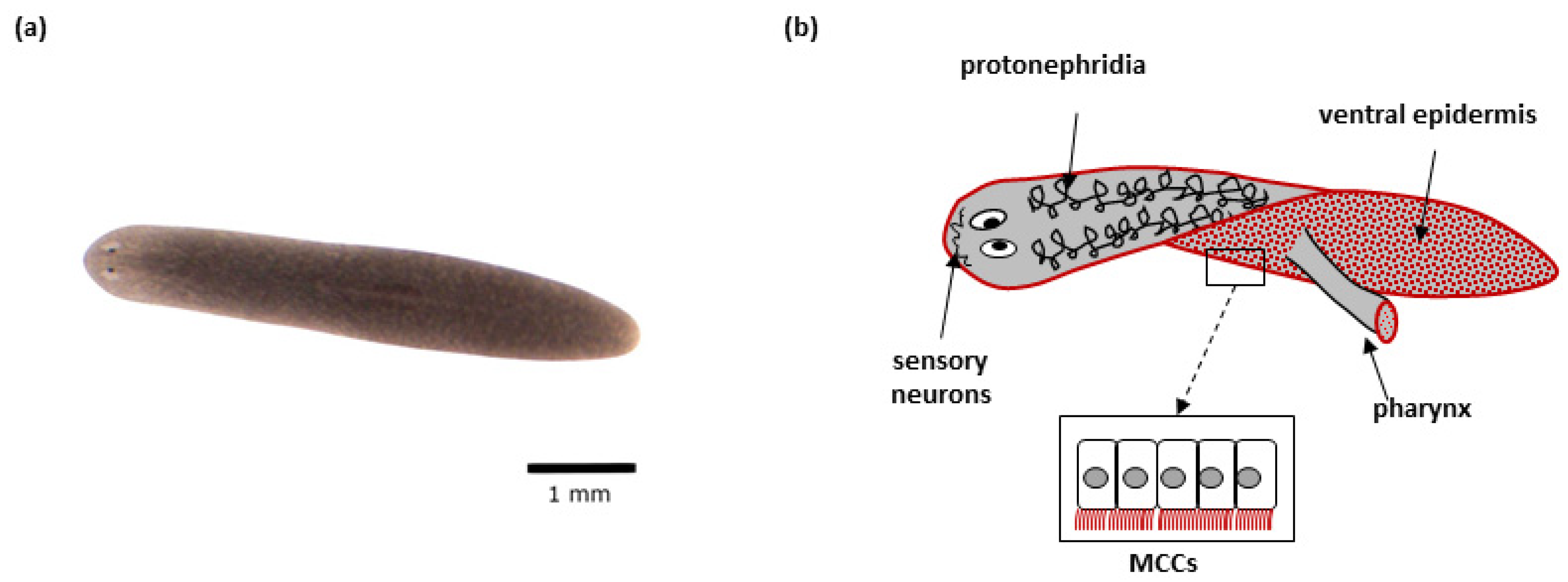
A planarian organism has a complex anatomy. The nervous system of flatworms is comprised of a bilobed ‘brain’ with different types of neurons and glia, and two longitudinal nerve cords connected by many transverse nerves [181,185]. Photo-, chemo- and rheoreceptors located at the front of the planarian’s body send signals to the brain, where they are processed, leading to behavioral responses [181,184]. Paired ‘eyes’ located on the planarian head allow the detection of light and shadow, and consist of two types of cells: pigmented optic cup cells, and photoreceptor neurons [189,190]. Due to the lack of respiratory and circulatory systems in planarians, oxygen is obtained and transported by diffusion. A centrally located pharynx is in charge of food intake and removal and is connected to a highly branched intestine, which circulates nutrients within the body. The excretory system (protonephridia) is responsible for the removal of waste products and osmoregulation [181]. Internal organs are surrounded by a mesenchymal tissue, parenchyma, consisting of adult pluripotent stem cells (neoblasts), which are essential for worms’ regeneration ability and comprise ~30% of the cells in the adult animal [185,191]. Planarians possess a set of muscle fibers, organized in longitudinal, diagonal, and circular orientations. The planarians body is covered with an epidermis; the ventral epidermis consists of a single layer of multiciliated cells (MCCs), and gland cells involved in the production and secretion of mucus, which is used by flatworms for protection, locomotion, catching food, and adhesion to substrates [181].
2.1. Advantages of the S. mediterranea as a Model Organism
Although planarians do not fully reflect the complexity of the human organism, many of the annotated S. mediterranea’s genes have known orthologs (or at least homologs) in the human genome, and researchers increasingly use S. mediterranea in studies aiming to better understand aspects related to human development and function involving certain cell types or tissues.
The maintenance of planarians is relatively easy and cheap, and does not require specialized equipment; only habitat conditions, such as temperature, darkness, feeding, and water culture, have to be provided, and many methodological guidelines have been published [e.g., [188,192,193]]. An important feature of using planarians as a model organism is the easy way to perform simple modifications of their gene expression. This can be achieved by knockdown/silencing genes of interest through RNA interference (RNAi) using double-stranded RNA (dsRNA). DsRNA can be administered to the worms by microinjection, by feeding them with dsRNA-containing bacteria, or with food mixed with free dsRNA [194,195,196]. The efficacy of gene silencing on the mRNA level can be evaluated using reverse transcription polymerase chain reaction (RT-PCR) or quantitative reverse transcription PCR (RT-qPCR), while the gene expression pattern of a silenced gene can be determined using whole-mount fluorescent in situ hybridization (FISH) and whole-mount in situ hybridization (WMISH). The phenotypic effect/s of gene silencing is typically observed within a week or two after implementing the RNAi procedure [188].
The genome of S. mediterranea has been well annotated, which makes this species more attractive than other planarians. The advances in single-cell RNA sequencing increased molecular knowledge about planarian stem cell differentiation, and have allowed for determining the transcriptomes for each cell type in S. mediterranea, and tracking the transcriptomic changes during the regeneration process [197,198,199,200]. The genomic and transcriptomic data are deposited in specialized databases and freely available to the research community [197,201,202,203,204].
2.2. S. mediterranea Model in the Context of Studying Cilia Biology
The potential of using S. mediterranea as a model organism to study evolutionarily conserved motile cilia was first described in 2009, and its value has been confirmed in later publications [188,192,205,206].
Motile cilia are present in many planarian cell types (Figure 2). Multiple motile cilia (9 × 2 + 2) covering the apical side of MCCs (~80 per cell) in the planarian body epidermis beat in a synchronized way and are responsible for worms’ locomotion [188,207]. MCCs are also present in the epithelium that covers the feeding organ (pharynx) [188,207,208]. Specialized ciliated cells at the proximal end of protonephridia (so-called flame cells) play role in fluid ultrafiltration and circulation. Cilia with the same ultrastructure as motile ones are also found in the sensory neurons in planarians, although their ability to move is not clear [188,207]. Finally, sperm cells with flagella are present in the sexual strains of planarians [209,210]. In planarians, basal bodies are assembled de novo during terminal differentiation of ciliated cells from neoblast progenies, and never have the function of a centrosome [1,58,207]. While it is currently unclear how the flatworms generate multiple centrioles in cells that are initially centriole-free, RNAi experiments show that the known key factors of centriole duplication are crucial for their amplification [208].
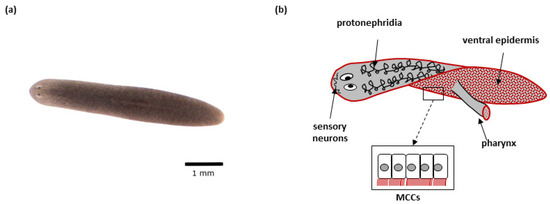
Figure 2.
Schmidtea mediterranea: (a) Representative S. mediterranea individual–top view; (b) Schematic representation of a planarian. Tissues with cells featuring motile cilia are indicated. The red color indicates tissues/organs with multiciliated cells (MCCs).
Both ventral and pharyngeal epidermis are easily accessible and form cilia at high density and in known orientation [188]. The great advantage of using S. mediterranea as a model in motile cilia studies is that the effect of gene silencing on cilia function can be readily analyzed by recording the change in the speed of planarian locomotion. Importantly, defects that compromise the function and structure of the cilia are not detrimental to planarians, making them an ideal system for loss-of-function studies concerning cilia-related genes [192]. Under normal conditions, planarians move by the use of ciliated epithelium covering the ventral side of worms’ body (so-called gliding movement), while cilia-related gene silencing manifests in a so-called “inch-worming” movement that engages the muscles (waves of whole-body contraction and extension) [188,207]. This phenotype (inch-worming) is easily visible to even unaided human eyes; a stereoscope (with camera) makes it more precise and allows to record movies showing the movement of planarians, which can then be used to measure the distance traveled by worms (using ImageJ software) [192]. The motility impairment may be associated with edema, which results from the dysfunction of cilia in protonephridia [211]. The beating of cilia covering the lateral part of worms can be recorded using high-speed video camera microscopy (HSVM), and the cilia beating frequency, pattern, and synchrony can be analyzed in slow motion under a microscope. The number and length of cilia can be inspected using a fluorescence microscope after immunofluorescence staining (IF) with cilia-specific antibodies (acetylated alpha-tubulin, a marker of the axoneme). After RNAi, changes in the gene expression pattern of epidermal markers can be tracked using WMISH. In addition, the possibility to stimulate cell differentiation through worms’ fragmentation (cutting) that triggers the regeneration process allows for tracing changes in the gene expression during the differentiation of the neoblasts into ciliated cells. The effect of gene silencing on the ultrastructure of planarian cilia can be also examined using a transmission electron microscope (TEM). In addition, the flatworms bloat due to the inhibition of ciliary function in flame cells, which leads to defective osmoregulation and edema formation [207].
Planarians have been widely used as a model for studying signaling networks implicated in the maintenance of tissue homeostasis, regeneration, and polarity. A large number of studies were devoted to essential cellular pathways, including Wnt and Hedgehog signaling in establishing polarity [212], Akt signaling in tissue maintenance and regeneration [213], EGFR signaling in the regulation of excretory system [211]. Like in most bilaterally symmetric animals, canonical Wnt signal is transduced through frizzled receptor and with the help of disheveled stabilizes beta-catenin, which activates expression cascade controlling anterior/posterior axis during regeneration [212,214]. Wnt signals transduced through frizzled receptors to various non-canonical pathways (disheveled-dependent or Ca2+-dependent) control cell movement and planar cell polarity (apical positioning of the basal bodies of epithelial cells). Hedgehog signaling modulates Wnt/beta-catenin’s role in establishing the anterior/posterior axis; when Wnt signaling is low, heads develop, and when it is high, tails are formed [145,215]. The majority of these signaling networks have the ciliary context, linking various aspects of Hedgehog signaling, regeneration, and the biogenesis of cilia, e.g., [145,213,216,217].
2.3. S. mediterranea Model in the Context of Studying PCD and PCD-like Ciliopathies
RNAi-mediated silencing of a variety of genes in S. mediterranea has been used to explain/confirm the connection between the homologous genes, defects of the ciliary ultrastructure, and cilia dysfunction in other organisms. The explicit use of S. mediterranea as an animal model to elucidate the pathogenesis of motile ciliopathies (and in particular, the role of candidate PCD genes) includes relatively few studies, where the effect of gene silencing on motile cilia function has been examined using dsRNA-mediated knockdown of the planarian homologs of human candidate genes not previously linked to PCD. In many more S. mediterranea studies, the demonstrated phenotypic effects of motile cilia-related genes’ silencing strongly resemble those seen when PCD genes are mutated, but their involvement in the pathogenesis remains to be confirmed by finding deleterious variants in PCD patients.
Deleterious variants of CFAP298 (C21orf59) [81], CCDC151 [109], and CFAP300 (C11orf70) [63] have been found in PCD patients. Knockdown of the planarian homologs of these genes has revealed the impaired locomotion phenotype in worms. The details of this phenotype have been explained using further assays. HSVM analysis of planarians with silenced CFAP300 (C11orf70) demonstrated changes in cilia motility pattern and lowered beat frequency, while TEM analysis of cilia in planarians with CFAP298 (C21orf59) and CCDC151 knockdown revealed ODA assembly defects of dynein arms and loss of ODA, respectively. The effects of these three genes’ silencing are consistent with the observations in other animal models. Ch. reinhardtii and zebrafish mutants lacking CCDC151 orthologues featured a loss of ODAs [111,218]; silencing of CCDC151 in zebrafish and mice was shown to alter ODA assembly [109]. Knockdown of CCDC298 in zebrafish and Ch. reinhardtii, and of CFAP300 in P. tetraurelia and Ch. reinhardtii resulted in a complete lack of ODA and IDA [61,81]. All three genes are presently considered PCD genes, involved in the assembly of dynein arms (CFAP298, CFAP300), and proper functioning of the ODA docking complex (CCD151).
The role of FOXJ1 as the key transcription factor controlling motile cilia biogenesis has been reported in various FOXJ1-deficient model organisms, including mice [49], X. laevis, and zebrafish [50]. The S. mediterranea model has been used to demonstrate the conserved role of vertebrate FOXJ1. Among four FOXJ1 homologs found in planarians, silencing of FOXJ1-4 caused the absence of motile cilia, resulting in a characteristic inch-worming locomotion and edema formation [48]. The FOXJ1 involvement in PCD pathogenesis in humans has been demonstrated several years later, when dominant pathogenic variants in FOXJ1 were found in PCD patients with mild respiratory symptoms and hydrocephalus, caused by the severely reduced number of cilia per MCC due to defect in the apical docking of basal bodies [31].
Proteins essential to basal body assembly in S. mediterranea include orthologs of many conserved genes required for centriole assembly or function in humans. In planarians, depleting the ortholog of OFD1 (among other proteins) results in the decreased locomotion of knocked-down animals, apparently due to the inhibition of basal body docking [58]. A similar ciliary phenotype has been recently demonstrated in PCD patients with the disease caused by nonsense mutations in the few last exons of the OFD1 gene [55]. In humans, the truncation of the C-terminus of the protein causes PCD without severe neurological, skeletal, or renal symptoms characteristic for other OFD1-related syndromes associated with the loss of a larger part of the OFD1 protein cause syndromic diseases (e.g., oral-facial-digital syndrome type 1 or Joubert syndrome type 10). While the effect of the gene knockdown in planarians does not explain truncation size-dependent differences in human clinical phenotype, it corroborates the proposed mechanism for the ciliary phenotype in PCD patients, showing that apical docking of basal bodies in planarians and in humans employ, at least in part, the same molecular components.
DAW1 (WDR69/ODA16) encodes a WD repeat protein, whose role as a dynein assembly factor has been shown in many model organisms. Depletion of DAW1 protein homologs results in ultrastructural defect characterized by the reduced number of ODAs in Ch. reinhardtii [219], zebrafish [220], and mouse [221]; Ch. reinhardtii studies have shown that DAW1 is involved in ODA transport through interaction with IFT46 protein [86,222,223]. The knockdown of DAW1 homolog in S. mediterranea results in shortened epidermal cilia and decreased abundance of ciliated protonephridia [85]. The recent finding of deleterious DAW1 variants in patients with disturbed laterality and respiratory symptoms has confirmed the predicted involvement of this gene in PCD pathogenesis, although only in patients whose cilia are characterized by subtle beating abnormalities [84].
Deleterious variants in two other genes, CFAP45/CCDC19/NESG1, and CFAP52/WDR16, have been found in human individuals whose clinical presentation, with situs inversus and asthenozoospermia, but only mild respiratory symptoms, did not allow for classifying them as classical PCD cases [151]. Earlier studies in Ch. reinhardtii have localized these two proteins in the lumen of the B microtubule of the peripheral doublet [24]. The knockdown of the planarian homologs resulted in significant impairment of planarian locomotion in viscous but not in a normal medium; TEM of the silenced worms has shown normal ciliary ultrastructure, consistent with TEM cross-sections of CFAP45- and CFAP52-deficient respiratory cilia from CRISPR-Cas9 generated mice or from humans with mutated genes [151]. Therefore, planarian results confirm the uncertain status of CFAP45 and CFAP52 as PCD genes.
IC2 and LC1 are S. mediterranea homologs of human DNAI2 and DNAL1 genes, respectively, encoding integral components of ODA. Mutations in DNAI2(IC2) cause defects in ODA resulting in the reduction in ciliary beat frequency in Ch. reinhardtii, and are known to cause PCD in humans [90]. Mutations in DNAL1(LC1) disturb the proper function of ODA in Ch. reinhardtii [224], but the data supporting this gene’s role in human PCD are scarce [97,225]. The knockdown of either of these two genes in S. mediterranea severely decreases worms’ motility, due to the reduction in the ciliary beat frequency and coordination (metachronal synchrony). However, while TEM and IF reveal the loss of ODA in IC2-silenced planarians, no ODA defects are visible in LC1-silenced worms [91]. This is consistent with the still uncertain role of DNAL1 in PCD pathogenesis in humans.
The ODA-docking complex is a microtubule-associated structure that targets ODA to its binding site on the axonemal microtubule [226]. In Ch. reinhardtii it contains three proteins, referred to as DC1, DC2, and DC3, of which DC1 and DC2 can assemble ODA in the absence of DC3 [227]. Ch. reinhardtii mutants with the loss of DC2 (a major subunit of the ODA-docking complex) have two flagella of normal length but show slow jerky swimming [228]. Two DC2 homologs, CCDC63 and CCDC114, function in ODA docking in vertebrates. Respiratory cilia in PCD patients with deleterious variants in CCDC114 have normal length, but lack ODAs due to the defects in ODA docking to microtubules [229]. In mice, in which CCDC63 (the testis-specific DC2 homolog) is knocked out, spermatozoa flagella are shortened, but ODAs remain unaffected, probably due to the compensation by overexpression of CCDC114 [230]. The knockdown of DC2 orthologue in S. mediterranea impairs worms’ locomotion due to the low-frequency, uncoordinated ciliary beating caused by the inefficient ODA docking; in addition, cilia density and length are decreased [231]. The importance of these findings for PCD pathogenesis remains to be explored.
WDR92 is a highly conserved WD-repeat protein. Silencing of the planarian homolog of WDR92 results in a phenotype similar to those observed when acknowledged PCD genes are knocked down. Peristaltic contractions instead of smooth gliding of the worms reduced and uncoordinated the ciliary beat; in TEM analysis, partial loss of dynein arms, incomplete closure of the B-microtubule, and lack of normal central pair complex are observed [232]. WDR92 is required for the assembly of ODAs and IDAs in D. melanogaster and Ch. reinhardtii [233,234,235]. Based on these observations, WDR92 has been proposed to act as a part of a cytoplasmic chaperone required for the proper folding and stability of key axonemal components. So far, no pathogenic variants have been found in human PCD patients.
IFT88 (Tg737) encodes a component of the IFT complex; its mutations in Ch. reinhardtii results in a lack of flagella, while in mice they cause shortening of primary cilia, as well as kidney and liver defects [236]. The importance of IFT88 in motile cilia biogenesis has been confirmed in the S. mediterranea model, where silencing of IFT88 significantly reduced planarians motility and caused the complete absence of cilia on the ventral surface of knocked-down animals [91]. Defects in IFT are likely to affect motile cilia in humans. Defects in the Tg737 gene in mice are very similar to those seen in humans with autosomal recessive polycystic kidney disease [237], but so far no pathogenic IFT88 variants have been reported in PCD patients.
Ch. reinhardtii protein FAP163 is an intermediate dynein chain closely related to the FAP133 intermediate dynein chain that powers retrograde IFT required for the assembly of cilia. The functional role of FAP163 has been examined by the knockdown of the orthologous gene WD60 (FAP163) in S. mediterranea [238]. The silenced animals exhibited severely impaired movement (reduced velocity and inch-worming), resulting from a dramatic reduction in both the number and length of cilia. Cilia and ciliary stubs examined by TEM contained doublet microtubules and associated structures but had an enlarged diameter due to the presence of large quantities of amorphous electron-dense material located between the axonemal doublet microtubules and the ciliary membrane. These observations suggest that WD60(FAP163) is required for ciliary assembly. So far, no pathogenic variants have been found in human PCD patients.
An interesting application of RNAi-mediated gene silencing in S. mediterranea concerns the analysis of planarian protonephridia as a model of pathological features of human cystic kidney diseases (CKDs), in which fluid-filled cysts develop from nephric tubules due to defective flow sensing, cell proliferation, and differentiation. In contrast to mammalian kidneys that contain only immotile sensory cilia, the excretory system of planarians is equipped with motile cilia that drive fluid flow into and through the tubules [239,240]. Interestingly, structure and function comparisons revealed that the combination of ultrafiltration and flow-associated filtrate modification is remarkably conserved between the planarian excretory system (flame cells) and the vertebrate nephrons (podocytes) [67]. The genome of S. mediterranea contains many genes that cause cysts when their equivalents are mutated in humans. Silencing of planarian homologs of human DNAH1 and LRRC50 genes resulted in abnormal worms locomotion due to the loss of cilia beating; animals also developed edema and formed protonephridial cysts [67]. These results suggest that cilia-driven fluid flow is crucial for maintaining cell homeostasis in planarian protonephridia and establish planarians as a novel and experimentally accessible invertebrate model for the study of human kidney pathologies.
In the majority of the aforementioned studies, S. mediterranea was not the only organism used in the functional assessment of cilia-related genes, which are or can be considered candidate PCD genes. While the application of the planarian model in these studies may seem redundant, it can be seen that abundant work using S. mediterranea genes silencing has been performed to analyze the role of various proteins in the assembly, maintenance, and function of motile cilia, without immediate referring to PCD pathogenesis. These results are often used later, whenever deleterious variants in candidate PCD genes are found in human patients (see, e.g., the cases of FOXJ1 or DAW1). On the other hand, when a new candidate PCD gene comes into focus based on genetic screening in humans, using the planarian model is perhaps the most efficient way to perform preliminary functional studies. When compared to the most popular single-cell organisms (Ch. reinhardtii, P. tetraurelia, T. brucei, T. thermophila), S. mediterranea offers an advantage of studying the epidermis that closely resembles human epithelium with MCCs, and compared to fish or mammals allows a much faster, easier and more affordable alternative modeling.
3. Perspectives
For many years, the use of the planarian model in the analysis of human candidate genes has been hampered by the lack of information on human-planarian orthologues. Recently, the rapid and extensive growth of the genomic, transcriptomic, phenotypic, and phylogenetic data generated by the planarian research community has alleviated this problem.
In this review, the human gene names or aliases are used to facilitate comparison with the part of the text describing human cilia and ciliopathies. However, it should be emphasized that to establish a uniform method for naming genes and proteins and for describing RNAi experiments in S. mediterranea, nomenclature guidelines have been developed [241], where the main rule is that the name of the gene is preceded by a ‘Smed’ prefix. In addition, to enhance cross-platform and cross-species searchability, the Planarian Anatomy Ontology (PLANA), an extendable relational framework of defined S. mediterranea anatomical terms has been recently developed [242].
The rapid growth of the amount of genomic and functional data on S. mediterranea prompted the development of integration tools that would enable the collection and use of these data by the scientific community. In response to these needs, the SmedGD database has been developed by the Sánchez Alvarado team [201,202]. The data deposited in SmedGD refer to the S. mediterranea genome and include predicted and annotated genes, protein homologies, gene expression patterns, and RNAi phenotypes, among others. SmedGD has been a stand-alone web resource for 15 years. Recently, the S. mediterranea genome assembly from SmedGD has been transferred to SIMRbase at the Stowers Institute for Medical Research (https://simrbase.stowers.org/ (accessed on 20 February 2023)).
Another high-quality S. mediterranea genome assembly can be found in the Planmine database (https://planmine.mpinat.mpg.de/planmine/begin.do (accessed on 20 February 2023)), developed in 2016 by the Rink team [199,203,243]. Originally, Planmine was based on the independently assembled transcriptomes from the Rink team and contributors from the planarian community. The updated database provides also genomic information, including a gene prediction set that assigns existing transcripts to defined genomic coordinates. In addition, Planmine uses recent datasets from the single-cell RNA-seq (e.g., from Digiworm resource and Planaria Single Cell Atlas), allowing for the expansion of the available gene expression information [197,198]. Both Digiworm and Planaria Single Cell Atlas refer to transcriptomes published by the Rink team, which makes these resources compatible with data in the Planmine database. Moreover, in contrast to other planarian databases, Planmine is also a resource of transcriptomes from other flatworm species. Planmine can be used to search for the planarian homologs of interesting transcripts and corresponding predicted genes. Planmine provides information about functional annotations (gene ontology), the best BLAST hits, expression patterns of homologs in planarian cell types, as well as phenotypes after RNAi of specified genes, among others.
Planosphere (https://planosphere.stowers.org/ (accessed on 20 February 2023)) is a new website dedicated to S. mediterranea, which contains a collection of data and tools from the Sánchez Alvarado laboratory [242]. One of the Planosphere tools is “gene search”, which can be used to search homologs in S. mediterranea, and to define experimentally determined cell/tissue-specific gene expression patterns. In addition to transcriptomic and genomic data, Planosphere provides information about predicted protein sequences. This website is linked to the data deposited in the Planmine database and refers to data (e.g., from RNAseq) published by other teams.
For researchers studying cilia or cilia-related diseases, it is important that RNAseq, together with other techniques, has allowed to characterize and reconstruct epidermal cell lineages, including the stages between neoblasts and fully differentiated epidermal cells [197,198,244,245,246]. Using Digiworm, researchers can check at which stage of epidermal lineage the gene of interest is expressed (it is possible for all cell/tissue types, e.g., protonephridia).
The available web resources, especially Planmine, Planosphere, and Digiworm, provide researchers with a powerful tool to design experiments using S. mediterranea as a model organism. They can be used to simply explore their contents to better understand planarian biology. Importantly, they allow the cross-searching of databases devoted to other organisms, using gene names, sequences, annotation terms, etc. (the details of such searches differ among planarian websites). This facilitates using the growing planarian knowledge in applications related to studies of human ciliopathies.
Author Contributions
The authors (A.R. and E.Z.) wrote the original draft; reviewing and editing, E.Z.; drawings and tables, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grants funded by the National Science Centre, Poland: 2018/29/N/NZ5/00810 to A.R. and 2018/31/B/NZ2/03248 to E.Z.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carvalho-Santos, Z.; Azimzadeh, J.; Pereira-Leal, J.B.; Bettencourt-Dias, M. Evolution: Tracing the origins of centrioles, cilia, and flagella. J. Cell Biol. 2011, 194, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.R. Evolution of cilia. Cold Spring Harb. Perspect. Biol. 2017, 9, a028290. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Scholey, J.M. Assembly, functions and evolution of archaella, flagella and cilia. Curr. Biol. 2018, 28, R278–R292. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalo, F.R.; Reiter, J.F. Open sesame: How transition fibers and the transition zone control ciliary composition. Cold Spring Harb. Perspect. Biol. 2017, 9, a028134. [Google Scholar] [CrossRef]
- Takeda, S.; Narita, K. Structure and function of vertebrate cilia, towards a new taxonomy. Differentiation 2012, 83, S4–S11. [Google Scholar] [CrossRef]
- Kempeneers, C.; Chilvers, M.A. To beat, or not to beat, that is question! The spectrum of ciliopathies. Pediatr. Pulmonol. 2018, 53, 1122–1129. [Google Scholar] [CrossRef]
- Shah, A.S.; Ben-Shahar, Y.; Moninger, T.O.; Kline, J.N.; Welsh, M.J. Motile cilia of human airway epithelia are chemosensory. Science 2009, 325, 1131–1134. [Google Scholar] [CrossRef]
- Jain, R.; Javidan-Nejad, C.; Alexander-Brett, J.; Horani, A.; Cabellon, M.C.; Walter, M.J.; Brody, S.L. Sensory functions of motile cilia and implication for bronchiectasis. Front. Biosci. 2012, 4, 1088–1098. [Google Scholar] [CrossRef][Green Version]
- Brooks, E.R.; Wallingford, J.B. Multiciliated cells. Curr. Biol. 2014, 24, R973–R982. [Google Scholar] [CrossRef]
- Sironen, A.; Shoemark, A.; Patel, M.; Loebinger, M.R.; Mitchison, H.M. Sperm defects in primary ciliary dyskinesia and related causes of male infertility. Cell. Mol. Life Sci. 2020, 77, 2029–2048. [Google Scholar] [CrossRef]
- Shinohara, K.; Hamada, H. Cilia in left-right symmetry breaking. Cold Spring Harb. Perspect. Biol. 2017, 9, a028282. [Google Scholar] [CrossRef] [PubMed]
- Anvarian, Z.; Mykytyn, K.; Mukhopadhyay, S.; Pedersen, L.B.; Christensen, S.T. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 2019, 15, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Goetz, S.C.; Anderson, K.V. The primary cilium: A signalling centre during vertebrate development. Nat. Rev. Genet. 2010, 11, 331–344. [Google Scholar] [CrossRef]
- Nishimura, Y.; Kasahara, K.; Shiromizu, T.; Watanabe, M.; Inagaki, M. Primary cilia as signaling hubs in health and disease. Adv. Sci. 2019, 6, 1801138. [Google Scholar] [CrossRef] [PubMed]
- Nachury, M.V.; Mick, D.U. Establishing and regulating the composition of cilia for signal transduction. Nat. Rev. Mol. Cell Biol. 2019, 20, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Samsel, Z.; Sekretarska, J.; Osinka, A.; Wloga, D.; Joachimiak, E. Central apparatus, the molecular kickstarter of ciliary and flagellar nanomachines. Int. J. Mol. Sci. 2021, 22, 3013. [Google Scholar] [CrossRef]
- Kurkowiak, M.; Ziętkiewicz, E.; Witt, M. Recent advances in primary ciliary dyskinesia genetics. J. Med. Genet. 2015, 52, 1–9. [Google Scholar] [CrossRef]
- Osinka, A.; Poprzeczko, M.; Zielinska, M.M.; Fabczak, H.; Joachimiak, E.; Wloga, D. Ciliary proteins: Filling the gaps. Recent advances in deciphering the protein composition of motile ciliary complexes. Cells 2019, 8, 730. [Google Scholar] [CrossRef]
- Nicastro, D.; Schwartz, C.; Pierson, J.; Gaudette, R.; Porter, M.E.; McIntosh, J.R. The molecular architecture of axonemes revealed by cryoelectron tomography. Science 2006, 313, 944–948. [Google Scholar] [CrossRef]
- Oda, T.; Yanagisawa, H.; Kamiya, R.; Kikkawa, M. A molecular ruler determines the repeat length in eukaryotic cilia and flagella. Science 2014, 346, 857–860. [Google Scholar] [CrossRef]
- Whitfield, M.; Thomas, L.; Bequignon, E.; Schmitt, A.; Stouvenel, L.; Montantin, G.; Tissier, S.; Duquesnoy, P.; Copin, B.; Chantot, S.; et al. Mutations in DNAH17, encoding a sperm-specific axonemal outer dynein arm heavy chain, cause isolated male infertility due to asthenozoospermia. Am. J. Hum. Genet. 2019, 105, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Bazan, R.; Schröfel, A.; Joachimiak, E.; Poprzeczko, M.; Pigino, G.; Wloga, D. Ccdc113/Ccdc96 complex, a novel regulator of ciliary beating that connects radial spoke 3 to dynein g and the nexin link. PLoS Genet. 2021, 17, e1009388. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Stoyanova, M.; Rademacher, G.; Dutcher, S.K.; Brown, A.; Zhang, R. Structure of the decorated ciliary doublet microtubule. Cell 2019, 179, 909–922.e12. [Google Scholar] [CrossRef]
- Owa, M.; Uchihashi, T.; Yanagisawa, H.-A.; Yamano, T.; Iguchi, H.; Fukuzawa, H.; Wakabayashi, K.-I.; Ando, T.; Kikkawa, M. Inner lumen proteins stabilize doublet microtubules in cilia and flagella. Nat. Commun. 2019, 10, 1143. [Google Scholar] [CrossRef] [PubMed]
- Joachimiak, E.; Osinka, A.; Farahat, H.; Świderska, B.; Sitkiewicz, E.; Poprzeczko, M.; Fabczak, H.; Wloga, D. Composition and function of the C1b/C1f region in the ciliary central apparatus. Sci. Rep. 2021, 11, 11760. [Google Scholar] [CrossRef]
- Satir, P.; Heuser, T.; Sale, W.S. A structural basis for how motile cilia beat. Bioscience 2014, 64, 1073–1083. [Google Scholar] [CrossRef]
- Pigino, G.; Ishikawa, T. Axonemal radial spokes: 3D structure, function and assembly. Bioarchitecture 2012, 2, 50–58. [Google Scholar] [CrossRef]
- Choksi, S.P.; Lauter, G.; Swoboda, P.; Roy, S. Switching on cilia: Transcriptional networks regulating ciliogenesis. Development 2014, 141, 1427–1441. [Google Scholar] [CrossRef]
- Boon, M.; Wallmeier, J.; Ma, L.; Loges, N.T.; Jaspers, M.; Olbrich, H.; Dougherty, G.W.; Raidt, J.; Werner, C.; Amirav, I.; et al. MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat. Commun. 2014, 5, 4418. [Google Scholar] [CrossRef]
- Wallmeier, J.; Al-Mutairi, D.A.; Chen, C.-T.; Loges, N.T.; Pennekamp, P.; Menchen, T.; Ma, L.; Shamseldin, H.E.; Olbrich, H.; Dougherty, G.W.; et al. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat. Genet. 2014, 46, 646–651. [Google Scholar] [CrossRef]
- Wallmeier, J.; Frank, D.; Shoemark, A.; Nöthe-Menchen, T.; Cindric, S.; Olbrich, H.; Loges, N.T.; Aprea, I.; Dougherty, G.W.; Pennekamp, P.; et al. De novo mutations in FOXJ1 result in a motile ciliopathy with hydrocephalus and randomization of left/right body asymmetry. Am. J. Hum. Genet. 2019, 105, 1030–1039. [Google Scholar] [CrossRef]
- Lechtreck, K.F. IFT-Cargo interactions and protein transport in cilia. Trends Biochem. Sci. 2015, 40, 765–778. [Google Scholar] [CrossRef]
- Ishikawa, H.; Marshall, W.F. Intraflagellar transport and ciliary dynamics. Cold Spring Harb. Perspect. Biol. 2017, 9, a021998. [Google Scholar] [CrossRef] [PubMed]
- Wallmeier, J.; Nielsen, K.G.; Kuehni, C.E.; Lucas, J.S.; Leigh, M.W.; Zariwala, M.A.; Omran, H. Motile ciliopathies. Nat. Rev. Dis. Prim. 2020, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Legendre, M.; Zaragosi, L.-E.; Mitchison, H.M. Motile cilia and airway disease. Semin. Cell Dev. Biol. 2021, 110, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.F.; Leroux, M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Focșa, I.O.; Budișteanu, M.; Bălgrădean, M. Clinical and genetic heterogeneity of primary ciliopathies (review). Int. J. Mol. Med. 2021, 48, 176. [Google Scholar] [CrossRef] [PubMed]
- Tobin, J.L.; Beales, P.L. The nonmotile ciliopathies. Genet. Med. 2009, 11, 386–402. [Google Scholar] [CrossRef]
- Horani, A.; Ferkol, T.W.; Dutcher, S.K.; Brody, S.L. Genetics and biology of primary ciliary dyskinesia. Paediatr. Respir. Rev. 2016, 18, 18–24. [Google Scholar] [CrossRef]
- Mirra, V.; Werner, C.; Santamaria, F. Primary ciliary dyskinesia: An update on clinical aspects, genetics, diagnosis, and future treatment strategies. Front. Pediatr. 2017, 5, 135. [Google Scholar] [CrossRef]
- Damseh, N.; Quercia, N.; Rumman, N.; Dell, S.D.; Kim, R.H. Primary ciliary dyskinesia: Mechanisms and management. Appl. Clin. Genet. 2017, 10, 67–74. [Google Scholar] [CrossRef]
- Lucas, J.S.; Davis, S.D.; Omran, H.; Shoemark, A. Primary ciliary dyskinesia in the genomics age. Lancet Respir. Med. 2020, 8, 202–216. [Google Scholar] [CrossRef]
- Horani, A.; Ferkol, T.W. Understanding primary ciliary dyskinesia and other ciliopathies. J. Pediatr. 2021, 230, 15–22. [Google Scholar] [CrossRef]
- Wheway, G.; Thomas, N.S.; Carroll, M.; Coles, J.; Doherty, R.; Genomics England Research Consortium; Goggin, P.; Green, B.; Harris, A.; Hunt, D.; et al. Whole genome sequencing in the diagnosis of primary ciliary dyskinesia. BMC Med. Genom. 2021, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.K.; Ferkol, T.W.; Davis, S.D. Emerging genotype-phenotype relationships in primary ciliary dyskinesia. Int. J. Mol. Sci. 2021, 22, 8272. [Google Scholar] [CrossRef] [PubMed]
- van Dam, T.J.; Wheway, G.; Slaats, G.G.; SYSCILIA Study Group; Huynen, M.A.; Giles, R.H. The SYSCILIA gold standard (SCGSv1) of known ciliary components and its applications within a systems biology consortium. Cilia 2013, 2, 7. [Google Scholar] [CrossRef]
- Vasquez, S.S.V.; van Dam, J.; Wheway, G. An updated SYSCILIA gold standard (SCGSv2) of known ciliary genes, revealing the vast progress that has been made in the cilia research field. Mol. Biol. Cell 2021, 32, br13. [Google Scholar] [CrossRef]
- Vij, S.; Rink, J.C.; Ho, H.K.; Babu, D.; Eitel, M.; Narasimhan, V.; Tiku, V.; Westbrook, J.; Schierwater, B.; Roy, S. Evolutionarily ancient association of the FoxJ1 transcription factor with the motile ciliogenic program. PLoS Genet. 2012, 8, e1003019. [Google Scholar] [CrossRef] [PubMed]
- Brody, S.L.; Yan, X.H.; Wuerffel, M.K.; Song, S.K.; Shapiro, S.D. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-Null mice. Am. J. Respir. Cell. Mol. Biol. 2000, 23, 45–51. [Google Scholar] [CrossRef]
- Stubbs, J.L.; Oishi, I.; Izpisúa Belmonte, J.C.; Kintner, C. The forkhead protein Foxj1 specifies node-like cilia in xenopus and zebrafish embryos. Nat. Genet. 2008, 40, 1454–1460. [Google Scholar] [CrossRef]
- Moore, A.; Escudier, E.; Roger, G.; Tamalet, A.; Pelosse, B.; Marlin, S.; Clément, A.; Geremek, M.; Delaisi, B.; Bridoux, A.-M.; et al. RPGR Is mutated in patients with a complex X linked phenotype combining primary ciliary dyskinesia and retinitis pigmentosa. J. Med. Genet. 2006, 43, 326–333. [Google Scholar] [CrossRef]
- Bukowy-Bieryłło, Z.; Ziętkiewicz, E.; Loges, N.T.; Wittmer, M.; Geremek, M.; Olbrich, H.; Fliegauf, M.; Voelkel, K.; Rutkiewicz, E.; Rutland, J.; et al. RPGR mutations might cause reduced orientation of respiratory cilia. Pediatr. Pulmonol. 2013, 48, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Murga-Zamalloa, C.A.; Chan, L.; Hitchcock, P.F.; Swaroop, A.; Khanna, H. Human retinopathy-associated ciliary protein retinitis pigmentosa GTPase regulator mediates cilia-dependent vertebrate development. Hum. Mol. Genet. 2010, 19, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Hannah, W.B.; DeBrosse, S.; Kinghorn, B.; Strausbaugh, S.; Aitken, M.L.; Rosenfeld, M.; Wolf, W.E.; Knowles, M.R.; Zariwala, M.A. The expanding phenotype of OFD1-related disorders: Hemizygous loss-of-function variants in three patients with primary ciliary dyskinesia. Mol. Genet. Genom. Med. 2019, 7, e911. [Google Scholar] [CrossRef] [PubMed]
- Bukowy-Bieryllo, Z.; Rabiasz, A.; Dabrowski, M.; Pogorzelski, A.; Wojda, A.; Dmenska, H.; Grzela, K.; Sroczynski, J.; Witt, M.; Zietkiewicz, E. Truncating mutations in exons 20 and 21 of OFD1 can cause primary ciliary dyskinesia without associated syndromic symptoms. J. Med. Genet. 2019, 56, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Bengueddach, H.; Lemullois, M.; Aubusson-Fleury, A.; Koll, F. Basal body positioning and anchoring in the multiciliated cell paramecium tetraurelia: Roles of OFD1 and VFL3. Cilia 2017, 6, 6. [Google Scholar] [CrossRef]
- Ferrante, M.I.; Zullo, A.; Barra, A.; Bimonte, S.; Messaddeq, N.; Studer, M.; Dollé, P.; Franco, B. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat. Genet. 2006, 38, 112–117. [Google Scholar] [CrossRef]
- Azimzadeh, J.; Wong, M.L.; Downhour, D.M.; Sánchez Alvarado, A.; Marshall, W.F. Centrosome loss in the evolution of planarians. Science 2012, 335, 461–463. [Google Scholar] [CrossRef]
- Bustamante-Marin, X.M.; Yin, W.-N.; Sears, P.R.; Werner, M.E.; Brotslaw, E.J.; Mitchell, B.J.; Jania, C.M.; Zeman, K.L.; Rogers, T.D.; Herring, L.E.; et al. Lack of GAS2L2 causes PCD by impairing cilia orientation and mucociliary clearance. Am. J. Hum. Genet. 2019, 104, 229–245. [Google Scholar] [CrossRef]
- Mitchison, H.M.; Schmidts, M.; Loges, N.T.; Freshour, J.; Dritsoula, A.; Hirst, R.A.; O’Callaghan, C.; Blau, H.; Al Dabbagh, M.; Olbrich, H.; et al. Mutations in axonemal dynein assembly factor DNAAF3 cause primary ciliary dyskinesia. Nat. Genet. 2012, 44, 381–389, S1-2. [Google Scholar] [CrossRef]
- Fassad, M.R.; Shoemark, A.; le Borgne, P.; Koll, F.; Patel, M.; Dixon, M.; Hayward, J.; Richardson, C.; Frost, E.; Jenkins, L.; et al. C11orf70 mutations disrupting the intraflagellar transport-dependent assembly of multiple axonemal dyneins cause primary ciliary dyskinesia. Am. J. Hum. Genet. 2018, 102, 956–972. [Google Scholar] [CrossRef]
- Höben, I.M.; Hjeij, R.; Olbrich, H.; Dougherty, G.W.; Nöthe-Menchen, T.; Aprea, I.; Frank, D.; Pennekamp, P.; Dworniczak, B.; Wallmeier, J.; et al. Mutations in C11orf70 cause primary ciliary dyskinesia with randomization of left/right body asymmetry due to defects of outer and inner dynein arms. Am. J. Hum. Genet. 2018, 102, 973–984. [Google Scholar] [CrossRef]
- Zietkiewicz, E.; Bukowy-Bieryllo, Z.; Rabiasz, A.; Daca-Roszak, P.; Wojda, A.; Voelkel, K.; Rutkiewicz, E.; Pogorzelski, A.; Rasteiro, M.; Witt, M. CFAP300: Mutations in slavic patients with primary ciliary dyskinesia and a role in ciliary dynein arms trafficking. Am. J. Respir. Cell Mol. Biol. 2019, 61, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Ostrowski, L.E.; Loges, N.T.; Hurd, T.; Leigh, M.W.; Huang, L.; Wolf, W.E.; Carson, J.L.; Hazucha, M.J.; Yin, W.; et al. Mutations in SPAG1 cause primary ciliary dyskinesia associated with defective outer and inner dynein arms. Am. J. Hum. Genet. 2013, 93, 711–720. [Google Scholar] [CrossRef]
- Loges, N.T.; Olbrich, H.; Becker-Heck, A.; Häffner, K.; Heer, A.; Reinhard, C.; Schmidts, M.; Kispert, A.; Zariwala, M.A.; Leigh, M.W.; et al. Deletions and point mutations of LRRC50 cause primary ciliary dyskinesia due to dynein arm defects. Am. J. Hum. Genet. 2009, 85, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Duquesnoy, P.; Escudier, E.; Vincensini, L.; Freshour, J.; Bridoux, A.-M.; Coste, A.; Deschildre, A.; de Blic, J.; Legendre, M.; Montantin, G.; et al. Loss-of-function mutations in the human ortholog of Chlamydomonas reinhardtii ODA7 disrupt dynein arm assembly and cause primary ciliary dyskinesia. Am. J. Hum. Genet. 2009, 85, 890–896. [Google Scholar] [CrossRef]
- Thi-Kim Vu, H.; Rink, J.C.; McKinney, S.A.; McClain, M.; Lakshmanaperumal, N.; Alexander, R.; Sánchez Alvarado, A. Stem cells and fluid flow drive cyst formation in an invertebrate excretory organ. eLife 2015, 4, e07405. [Google Scholar] [CrossRef]
- Van Rooijen, E.; Giles, R.H.; Voest, E.E.; van Rooijen, C.; Schulte-Merker, S.; van Eeden, F.J. LRRC50, a conserved ciliary protein implicated in polycystic kidney disease. J. Am. Soc. Nephrol. 2008, 19, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Horani, A.; Druley, T.E.; Zariwala, M.A.; Patel, A.C.; Levinson, B.T.; Van Arendonk, L.G.; Thornton, K.C.; Giacalone, J.C.; Albee, A.J.; Wilson, K.S.; et al. Whole-exome capture and sequencing identifies HEATR2 mutation as a cause of primary ciliary dyskinesia. Am. J. Hum. Genet. 2012, 91, 685–693. [Google Scholar] [CrossRef]
- Diggle, C.P.; Moore, D.J.; Mali, G.; zur Lage, P.; Ait-Lounis, A.; Schmidts, M.; Shoemark, A.; Munoz, A.G.; Halachev, M.R.; Gautier, P.; et al. HEATR2 plays a conserved role in assembly of the ciliary motile apparatus. PLoS Genet. 2014, 10, e1004577. [Google Scholar] [CrossRef]
- Paff, T.; Daniels, J.M.A.; Weersink, E.J.; Lutter, R.; Vonk Noordegraaf, A.; Haarman, E.G. A randomised controlled trial on the effect of inhaled hypertonic saline on quality of life in primary ciliary dyskinesia. Eur. Respir. J. 2017, 49, 1601770. [Google Scholar] [CrossRef] [PubMed]
- Olcese, C.; Patel, M.P.; Shoemark, A.; Kiviluoto, S.; Legendre, M.; Williams, H.J.; Vaughan, C.K.; Hayward, J.; Goldenberg, A.; Emes, R.D.; et al. X-linked primary ciliary dyskinesia due to mutations in the cytoplasmic axonemal dynein assembly factor PIH1D3. Nat. Commun. 2017, 8, 14279. [Google Scholar] [CrossRef]
- Lennon, J.; zur Lage, P.; von Kriegsheim, A.; Jarman, A.P. Strongly truncated Dnaaf4 plays a conserved role in drosophila ciliary dynein assembly as part of an R2TP-Like co-chaperone complex with Dnaaf6. Front. Genet. 2022, 13, 943197. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Shinohara, K.; Botilde, Y.; Nabeshima, R.; Asai, Y.; Fukumoto, A.; Hasegawa, T.; Matsuo, M.; Takeda, H.; Shiratori, H.; et al. Pih1d3 is required for cytoplasmic preassembly of axonemal dynein in mouse sperm. J. Cell Biol. 2014, 204, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Zariwala, M.A.; Gee, H.Y.; Kurkowiak, M.; Al-Mutairi, D.A.; Leigh, M.W.; Hurd, T.W.; Hjeij, R.; Dell, S.D.; Chaki, M.; Dougherty, G.W.; et al. ZMYND10 is mutated in primary ciliary dyskinesia and interacts with LRRC6. Am. J. Hum. Genet. 2013, 93, 336–345. [Google Scholar] [CrossRef]
- Moore, D.J.; Onoufriadis, A.; Shoemark, A.; Simpson, M.A.; zur Lage, P.I.; de Castro, S.C.; Bartoloni, L.; Gallone, G.; Petridi, S.; Woollard, W.J.; et al. Mutations in ZMYND10, a gene essential for proper axonemal assembly of inner and outer dynein arms in humans and flies, cause primary ciliary dyskinesia. Am. J. Hum. Genet. 2013, 93, 346–356. [Google Scholar] [CrossRef]
- Kott, E.; Duquesnoy, P.; Copin, B.; Legendre, M.; Dastot-Le Moal, F.; Montantin, G.; Jeanson, L.; Tamalet, A.; Papon, J.-F.; Siffroi, J.-P.; et al. Loss-of-function mutations in LRRC6, a gene essential for proper axonemal assembly of inner and outer dynein arms, cause primary ciliary dyskinesia. Am. J. Hum. Genet. 2012, 91, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Kavlie, R.G.; Kernan, M.J.; Eberl, D.F. Hearing in drosophila requires TilB, a conserved protein associated with ciliary motility. Genetics 2010, 185, 177–188. [Google Scholar] [CrossRef][Green Version]
- Kishimoto, N.; Cao, Y.; Park, A.; Sun, Z. Cystic kidney gene seahorse regulates cilia-mediated processes and wnt pathways. Dev. Cell 2008, 14, 954–961. [Google Scholar] [CrossRef]
- Xue, J.C.; Goldberg, E. Identification of a novel testis-specific leucine-rich protein in humans and mice. Biol. Reprod. 2000, 62, 1278–1284. [Google Scholar] [CrossRef][Green Version]
- Austin-Tse, C.; Halbritter, J.; Zariwala, M.A.; Gilberti, R.M.; Gee, H.Y.; Hellman, N.; Pathak, N.; Liu, Y.; Panizzi, J.R.; Patel-King, R.S.; et al. Zebrafish ciliopathy screen plus human mutational analysis identifies C21orf59 and CCDC65 defects as causing primary ciliary dyskinesia. Am. J. Hum. Genet. 2013, 93, 672–686. [Google Scholar] [CrossRef]
- Omran, H.; Kobayashi, D.; Olbrich, H.; Tsukahara, T.; Loges, N.T.; Hagiwara, H.; Zhang, Q.; Leblond, G.; O’Toole, E.; Hara, C.; et al. Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature 2008, 456, 611–616. [Google Scholar] [CrossRef]
- Tarkar, A.; Loges, N.T.; Slagle, C.E.; Francis, R.; Dougherty, G.W.; Tamayo, J.V.; Shook, B.; Cantino, M.; Schwartz, D.; Jahnke, C.; et al. DYX1C1 is required for axonemal dynein assembly and ciliary motility. Nat. Genet. 2013, 45, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.S.; Hjeij, R.; Vivante, A.; Bearce, E.A.; Dyer, L.; Wang, J.; Rawlins, L.; Kennedy, J.; Ubeyratna, N.; Fasham, J.; et al. Biallelic DAW1 variants cause a motile ciliopathy characterized by laterality defects and subtle ciliary beating abnormalities. Genet. Med. 2022, 24, 2249–2261. [Google Scholar] [CrossRef]
- Lesko, S.L.; Rouhana, L. Dynein assembly factor with WD repeat domains 1 (DAW1) is required for the function of motile cilia in the planarian schmidtea mediterranea. Dev. Growth Differ. 2020, 62, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.T.; Gao, C.; Lucker, B.F.; Cole, D.G.; Mitchell, D.R. ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflagellar transport machinery. J. Cell Biol. 2008, 183, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Bouhouche, K.; Whitfield, M.; Thouvenin, G.; Coste, A.; Louis, B.; Szymanski, C.; Bequignon, E.; Papon, J.-F.; Castelli, M.; et al. TTC12 loss-of-function mutations cause primary ciliary dyskinesia and unveil distinct dynein assembly mechanisms in motile cilia versus flagella. Am. J. Hum. Genet. 2020, 106, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Pennarun, G.; Escudier, E.; Chapelin, C.; Bridoux, A.M.; Cacheux, V.; Roger, G.; Clément, A.; Goossens, M.; Amselem, S.; Duriez, B. Loss-of-function mutations in a human gene related to Chlamydomonas reinhardtii dynein IC78 result in primary ciliary dyskinesia. Am. J. Hum. Genet. 1999, 65, 1508–1519. [Google Scholar] [CrossRef]
- Guichard, C.; Harricane, M.C.; Lafitte, J.J.; Godard, P.; Zaegel, M.; Tack, V.; Lalau, G.; Bouvagnet, P. Axonemal dynein intermediate-chain gene (DNAI1) mutations result in situs inversus and primary ciliary dyskinesia (kartagener syndrome). Am. J. Hum. Genet. 2001, 68, 1030–1035. [Google Scholar] [CrossRef]
- Loges, N.T.; Olbrich, H.; Fenske, L.; Mussaffi, H.; Horvath, J.; Fliegauf, M.; Kuhl, H.; Baktai, G.; Peterffy, E.; Chodhari, R.; et al. DNAI2 mutations cause primary ciliary dyskinesia with defects in the outer dynein arm. Am. J. Hum. Genet. 2008, 83, 547–558. [Google Scholar] [CrossRef]
- Rompolas, P.; Patel-King, R.S.; King, S.M. An outer arm dynein conformational switch is required for metachronal synchrony of motile cilia in planaria. Mol. Biol. Cell 2010, 21, 3669–3679. [Google Scholar] [CrossRef]
- Pennarun, G.; Chapelin, C.; Escudier, E.; Bridoux, A.M.; Dastot, F.; Cacheux, V.; Goossens, M.; Amselem, S.; Duriez, B. The human dynein intermediate chain 2 gene (DNAI2): Cloning, mapping, expression pattern, and evaluation as a candidate for primary ciliary dyskinesia. Hum. Genet. 2000, 107, 642–649. [Google Scholar] [CrossRef]
- Olbrich, H.; Häffner, K.; Kispert, A.; Völkel, A.; Volz, A.; Sasmaz, G.; Reinhardt, R.; Hennig, S.; Lehrach, H.; Konietzko, N.; et al. Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nat. Genet. 2002, 30, 143–144. [Google Scholar] [CrossRef]
- DiBella, L.M.; King, S.M. Dynein motors of the Chlamydomonas flagellum. Int. Rev. Cytol. 2001, 210, 227–268. [Google Scholar] [CrossRef]
- Duriez, B.; Duquesnoy, P.; Escudier, E.; Bridoux, A.-M.; Escalier, D.; Rayet, I.; Marcos, E.; Vojtek, A.-M.; Bercher, J.-F.; Amselem, S. A common variant in combination with a nonsense mutation in a member of the thioredoxin family causes primary ciliary dyskinesia. Proc. Natl. Acad. Sci. USA 2007, 104, 3336–3341. [Google Scholar] [CrossRef] [PubMed]
- Padma, P.; Hozumi, A.; Ogawa, K.; Inaba, K. Molecular cloning and characterization of a thioredoxin/nucleoside diphosphate kinase related dynein intermediate chain from the ascidian, ciona intestinalis. Gene 2001, 275, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Mazor, M.; Alkrinawi, S.; Chalifa-Caspi, V.; Manor, E.; Sheffield, V.C.; Aviram, M.; Parvari, R. Primary ciliary dyskinesia caused by homozygous mutation in DNAL1, encoding dynein light chain 1. Am. J. Hum. Genet. 2011, 88, 599–607. [Google Scholar] [CrossRef][Green Version]
- Piperno, G.; Huang, B.; Luck, D.J. Two-dimensional analysis of flagellar proteins from wild-type and paralyzed mutants of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1977, 74, 1600–1604. [Google Scholar] [CrossRef]
- Baron, D.M.; Kabututu, Z.P.; Hill, K.L. Stuck in reverse: Loss of LC1 in trypanosoma brucei disrupts outer dynein arms and leads to reverse flagellar beat and backward movement. J. Cell Sci. 2007, 120, 1513–1520. [Google Scholar] [CrossRef]
- Schwabe, G.C.; Hoffmann, K.; Loges, N.T.; Birker, D.; Rossier, C.; de Santi, M.M.; Olbrich, H.; Fliegauf, M.; Failly, M.; Liebers, U.; et al. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum. Mutat. 2008, 29, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, G.W.; Loges, N.T.; Klinkenbusch, J.A.; Olbrich, H.; Pennekamp, P.; Menchen, T.; Raidt, J.; Wallmeier, J.; Werner, C.; Westermann, C.; et al. DNAH11 localization in the proximal region of respiratory cilia defines distinct outer dynein arm complexes. Am. J. Respir. Cell. Mol. Biol. 2016, 55, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Loges, N.T.; Antony, D.; Maver, A.; Deardorff, M.A.; Güleç, E.Y.; Gezdirici, A.; Nöthe-Menchen, T.; Höben, I.M.; Jelten, L.; Frank, D.; et al. Recessive DNAH9 loss-of-function mutations cause laterality defects and subtle respiratory ciliary-beating defects. Am. J. Hum. Genet. 2018, 103, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Fassad, M.R.; Shoemark, A.; Legendre, M.; Hirst, R.A.; Koll, F.; le Borgne, P.; Louis, B.; Daudvohra, F.; Patel, M.P.; Thomas, L.; et al. Mutations in outer dynein arm heavy chain DNAH9 cause motile cilia defects and situs inversus. Am. J. Hum. Genet. 2018, 103, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Leigh, M.W.; Ostrowski, L.E.; Huang, L.; Carson, J.L.; Hazucha, M.J.; Yin, W.; Berg, J.S.; Davis, S.D.; Dell, S.D.; et al. Exome sequencing identifies mutations in CCDC114 as a cause of primary ciliary dyskinesia. Am. J. Hum. Genet. 2013, 92, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Onoufriadis, A.; Paff, T.; Antony, D.; Shoemark, A.; Micha, D.; Kuyt, B.; Schmidts, M.; Petridi, S.; Dankert-Roelse, J.E.; Haarman, E.G.; et al. Splice-site mutations in the axonemal outer dynein arm docking complex gene CCDC114 cause primary ciliary dyskinesia. Am. J. Hum. Genet. 2013, 92, 88–98. [Google Scholar] [CrossRef]
- Hjeij, R.; Lindstrand, A.; Francis, R.; Zariwala, M.A.; Liu, X.; Li, Y.; Damerla, R.; Dougherty, G.W.; Abouhamed, M.; Olbrich, H.; et al. ARMC4 mutations cause primary ciliary dyskinesia with randomization of left/right body asymmetry. Am. J. Hum. Genet. 2013, 93, 357–367. [Google Scholar] [CrossRef]
- Wallmeier, J.; Shiratori, H.; Dougherty, G.W.; Edelbusch, C.; Hjeij, R.; Loges, N.T.; Menchen, T.; Olbrich, H.; Pennekamp, P.; Raidt, J.; et al. TTC25 Deficiency results in defects of the outer dynein arm docking machinery and primary ciliary dyskinesia with left-right body asymmetry randomization. Am. J. Hum. Genet. 2016, 99, 460–469. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, J.; Huang, S.; Feng, D.; Zhang, W.; Zhu, X.; Yan, X. Characterization of tetratricopeptide repeat-containing proteins critical for cilia formation and function. PLoS ONE 2015, 10, e0124378. [Google Scholar] [CrossRef]
- Hjeij, R.; Onoufriadis, A.; Watson, C.M.; Slagle, C.E.; Klena, N.T.; Dougherty, G.W.; Kurkowiak, M.; Loges, N.T.; Diggle, C.P.; Morante, N.F.C.; et al. CCDC151 mutations cause primary ciliary dyskinesia by disruption of the outer dynein arm docking complex formation. Am. J. Hum. Genet. 2014, 95, 257–274. [Google Scholar] [CrossRef]
- Alsaadi, M.M.; Erzurumluoglu, A.M.; Rodriguez, S.; Guthrie, P.A.I.; Gaunt, T.R.; Omar, H.Z.; Mubarak, M.; Alharbi, K.K.; Al-Rikabi, A.C.; Day, I.N.M. Nonsense mutation in coiled-coil domain containing 151 gene (CCDC151) causes primary ciliary dyskinesia. Hum. Mutat. 2014, 35, 1446–1448. [Google Scholar] [CrossRef]
- Dean, A.B.; Mitchell, D.R. Chlamydomonas ODA10 is a conserved axonemal protein that plays a unique role in outer dynein arm assembly. Mol. Biol. Cell 2013, 24, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, J.R.; Becker-Heck, A.; Castleman, V.H.; Al-Mutairi, D.A.; Liu, Y.; Loges, N.T.; Pathak, N.; Austin-Tse, C.; Sheridan, E.; Schmidts, M.; et al. CCDC103 mutations cause primary ciliary dyskinesia by disrupting assembly of ciliary dynein arms. Nat. Genet. 2012, 44, 714–719. [Google Scholar] [CrossRef] [PubMed]
- King, S.M.; Patel-King, R.S. The oligomeric outer dynein arm assembly factor CCDC103 is tightly integrated within the ciliary axoneme and exhibits periodic binding to microtubules. J. Biol. Chem. 2015, 290, 7388–7401. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoy, S.; Watson, C.M.; Kernohan, K.D.; Lemos, M.; Hutchinson, S.; Poulter, J.A.; Crinnion, L.A.; Berry, I.; Simmonds, J.; Vasudevan, P.; et al. Biallelic mutations in LRRC56, encoding a protein associated with intraflagellar transport, cause mucociliary clearance and laterality defects. Am. J. Hum. Genet. 2018, 103, 727–739. [Google Scholar] [CrossRef]
- Bustamante-Marin, X.M.; Horani, A.; Stoyanova, M.; Charng, W.-L.; Bottier, M.; Sears, P.R.; Yin, W.-N.; Daniels, L.A.; Bowen, H.; Conrad, D.F.; et al. Mutation of CFAP57, a protein required for the asymmetric targeting of a subset of inner dynein arms in Chlamydomonas, causes primary ciliary dyskinesia. PLoS Genet. 2020, 16, e1008691. [Google Scholar] [CrossRef]
- Lin, J.; Le, T.V.; Augspurger, K.; Tritschler, D.; Bower, R.; Fu, G.; Perrone, C.; O’Toole, E.T.; Mills, K.V.; Dymek, E.; et al. FAP57/WDR65 targets assembly of a subset of inner arm dyneins and connects to regulatory hubs in cilia. Mol. Biol. Cell 2019, 30, 2659–2680. [Google Scholar] [CrossRef]
- Merveille, A.-C.; Davis, E.E.; Becker-Heck, A.; Legendre, M.; Amirav, I.; Bataille, G.; Belmont, J.; Beydon, N.; Billen, F.; Clément, A.; et al. CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat. Genet. 2011, 43, 72–78. [Google Scholar] [CrossRef]
- Becker-Heck, A.; Zohn, I.E.; Okabe, N.; Pollock, A.; Lenhart, K.B.; Sullivan-Brown, J.; McSheene, J.; Loges, N.T.; Olbrich, H.; Haeffner, K.; et al. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat. Genet. 2011, 43, 79–84. [Google Scholar] [CrossRef]
- Olbrich, H.; Cremers, C.; Loges, N.T.; Werner, C.; Nielsen, K.G.; Marthin, J.K.; Philipsen, M.; Wallmeier, J.; Pennekamp, P.; Menchen, T.; et al. Loss-of-function GAS8 mutations cause primary ciliary dyskinesia and disrupt the nexin-dynein regulatory complex. Am. J. Hum. Genet. 2015, 97, 546–554. [Google Scholar] [CrossRef]
- Rupp, G.; Porter, M.E. A subunit of the dynein regulatory complex in chlamydomonas is a homologue of a growth arrest-specific gene product. J. Cell Biol. 2003, 162, 47–57. [Google Scholar] [CrossRef]
- Hutchings, N.R.; Donelson, J.E.; Hill, K.L. Trypanin is a cytoskeletal linker protein and is required for cell motility in African trypanosomes. J. Cell Biol. 2002, 156, 867–877. [Google Scholar] [CrossRef]
- Colantonio, J.R.; Vermot, J.; Wu, D.; Langenbacher, A.D.; Fraser, S.; Chen, J.-N.; Hill, K.L. The dynein regulatory complex is required for ciliary motility and otolith biogenesis in the inner ear. Nature 2009, 457, 10. [Google Scholar] [CrossRef] [PubMed]
- Wirschell, M.; Olbrich, H.; Werner, C.; Tritschler, D.; Bower, R.; Sale, W.S.; Loges, N.T.; Pennekamp, P.; Lindberg, S.; Stenram, U.; et al. The nexin-dynein regulatory complex subunit DRC1 is essential for motile cilia function in algae and humans. Nat. Genet. 2013, 45, 262–268. [Google Scholar] [CrossRef]
- Horani, A.; Brody, S.L.; Ferkol, T.W.; Shoseyov, D.; Wasserman, M.G.; Ta-shma, A.; Wilson, K.S.; Bayly, P.V.; Amirav, I.; Cohen-Cymberknoh, M.; et al. CCDC65 mutation causes primary ciliary dyskinesia with normal ultrastructure and hyperkinetic cilia. PLoS ONE 2013, 8, e72299. [Google Scholar] [CrossRef] [PubMed]
- Bower, R.; Tritschler, D.; VanderWaal, K.; Perrone, C.A.; Mueller, J.; Fox, L.; Sale, W.S.; Porter, M.E. The N-DRC forms a conserved biochemical complex that maintains outer doublet alignment and limits microtubule sliding in motile axonemes. Mol. Biol. Cell 2013, 24, 1134–1152. [Google Scholar] [CrossRef]
- Kott, E.; Legendre, M.; Copin, B.; Papon, J.-F.; Dastot-Le Moal, F.; Montantin, G.; Duquesnoy, P.; Piterboth, W.; Amram, D.; Bassinet, L.; et al. Loss-of-function mutations in RSPH1 cause primary ciliary dyskinesia with central-complex and radial-spoke defects. Am. J. Hum. Genet. 2013, 93, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Ostrowski, L.E.; Leigh, M.W.; Sears, P.R.; Davis, S.D.; Wolf, W.E.; Hazucha, M.J.; Carson, J.L.; Olivier, K.N.; Sagel, S.D.; et al. Mutations in RSPH1 cause primary ciliary dyskinesia with a unique clinical and ciliary phenotype. Am. J. Respir. Crit. Care Med. 2014, 189, 707–717. [Google Scholar] [CrossRef]
- Yin, W.; Livraghi-Butrico, A.; Sears, P.R.; Rogers, T.D.; Burns, K.A.; Grubb, B.R.; Ostrowski, L.E. Mice with a deletion of Rsph1 exhibit a low level of mucociliary clearance and develop a primary ciliary dyskinesia phenotype. Am. J. Respir. Cell Mol. Biol. 2019, 61, 312–321. [Google Scholar] [CrossRef]
- Castleman, V.H.; Romio, L.; Chodhari, R.; Hirst, R.A.; de Castro, S.C.P.; Parker, K.A.; Ybot-Gonzalez, P.; Emes, R.D.; Wilson, S.W.; Wallis, C.; et al. Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. Am. J. Hum. Genet. 2009, 84, 197–209. [Google Scholar] [CrossRef]
- Yoke, H.; Ueno, H.; Narita, A.; Sakai, T.; Horiuchi, K.; Shingyoji, C.; Hamada, H.; Shinohara, K. Rsph4a is essential for the triplet radial spoke head assembly of the mouse motile cilia. PLoS Genet. 2020, 16, e1008664. [Google Scholar] [CrossRef]
- Jeanson, L.; Copin, B.; Papon, J.-F.; Dastot-Le Moal, F.; Duquesnoy, P.; Montantin, G.; Cadranel, J.; Corvol, H.; Coste, A.; Désir, J.; et al. RSPH3 mutations cause primary ciliary dyskinesia with central-complex defects and a near absence of radial spokes. Am. J. Hum. Genet. 2015, 97, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, A.R.; Diener, D.R.; Rosenbaum, J.L.; Sale, W.S. Flagellar radial spoke protein 3 is an a-kinase anchoring protein (Akap). J. Cell Biol. 2001, 153, 443–448. [Google Scholar] [CrossRef] [PubMed]
- El Khouri, E.; Thomas, L.; Jeanson, L.; Bequignon, E.; Vallette, B.; Duquesnoy, P.; Montantin, G.; Copin, B.; Dastot-Le Moal, F.; Blanchon, S.; et al. Mutations in DNAJB13, encoding an HSP40 family member, cause primary ciliary dyskinesia and male infertility. Am. J. Hum. Genet. 2016, 99, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Yuan, L. A heat-shock protein 40, DNAJB13, is an axoneme-associated component in mouse spermatozoa. Mol. Reprod. Dev. 2008, 75, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Owen, H.A.; Yang, P. Dimeric heat shock protein 40 binds radial spokes for generating coupled power strokes and recovery strokes of 9 + 2 flagella. J. Cell Biol. 2008, 180, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.H.; Huh, H.J.; Jeong, I.; Lee, N.Y.; Koh, W.-J.; Park, H.-C.; Ki, C.-S. A nonsense variant in NME5 causes human primary ciliary dyskinesia with radial spoke defects. Clin. Genet. 2020, 98, 64–68. [Google Scholar] [CrossRef]
- Anderegg, L.; Im Hof Gut, M.; Hetzel, U.; Howerth, E.W.; Leuthard, F.; Kyöstilä, K.; Lohi, H.; Pettitt, L.; Mellersh, C.; Minor, K.M.; et al. NME5 frameshift variant in alaskan malamutes with primary ciliary dyskinesia. PLoS Genet. 2019, 15, e1008378. [Google Scholar] [CrossRef]
- Olbrich, H.; Schmidts, M.; Werner, C.; Onoufriadis, A.; Loges, N.T.; Raidt, J.; Banki, N.F.; Shoemark, A.; Burgoyne, T.; Al Turki, S.; et al. Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am. J. Hum. Genet. 2012, 91, 672–684. [Google Scholar] [CrossRef]
- Cindrić, S.; Dougherty, G.W.; Olbrich, H.; Hjeij, R.; Loges, N.T.; Amirav, I.; Philipsen, M.C.; Marthin, J.K.; Nielsen, K.G.; Sutharsan, S.; et al. SPEF2- and HYDIN-mutant cilia lack the central pair-associated protein SPEF2, aiding primary ciliary dyskinesia diagnostics. Am. J. Respir. Cell Mol. Biol. 2020, 62, 382–396. [Google Scholar] [CrossRef]
- Broadhead, R.; Dawe, H.R.; Farr, H.; Griffiths, S.; Hart, S.R.; Portman, N.; Shaw, M.K.; Ginger, M.L.; Gaskell, S.J.; McKean, P.G.; et al. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature 2006, 440, 224–227. [Google Scholar] [CrossRef]
- Lechtreck, K.-F.; Delmotte, P.; Robinson, M.L.; Sanderson, M.J.; Witman, G.B. Mutations in hydin impair ciliary motility in mice. J. Cell Biol. 2008, 180, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Lechtreck, K.-F.; Witman, G.B. Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility. J. Cell Biol. 2007, 176, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Edelbusch, C.; Cindrić, S.; Dougherty, G.W.; Loges, N.T.; Olbrich, H.; Rivlin, J.; Wallmeier, J.; Pennekamp, P.; Amirav, I.; Omran, H. Mutation of serine/threonine protein kinase 36 (STK36) causes primary ciliary dyskinesia with a central pair defect. Hum. Mutat. 2017, 38, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.W.; Nguyen, C.T.; Chen, M.-H.; Yang, J.-H.; Gacayan, R.; Huang, J.; Chen, J.-N.; Chuang, P.-T. Fused has evolved divergent roles in vertebrate hedgehog signaling and motile ciliogenesis. Nature 2009, 459, 98–102. [Google Scholar] [CrossRef]
- Rink, J.C.; Gurley, K.A.; Elliott, S.A.; Sánchez Alvarado, A. Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science 2009, 326, 1406–1410. [Google Scholar] [CrossRef]
- Sironen, A.; Kotaja, N.; Mulhern, H.; Wyatt, T.A.; Sisson, J.H.; Pavlik, J.A.; Miiluniemi, M.; Fleming, M.D.; Lee, L. Loss of SPEF2 function in mice results in spermatogenesis defects and primary ciliary dyskinesia. Biol. Reprod. 2011, 85, 690–701. [Google Scholar] [CrossRef]
- Biebach, L.; Cindrić, S.; Koenig, J.; Aprea, I.; Dougherty, G.W.; Raidt, J.; Bracht, D.; Ruppel, R.; Schreiber, J.; Hjeij, R.; et al. Recessive mutations in CFAP74 cause primary ciliary dyskinesia with normal ciliary ultrastructure. Am. J. Respir. Cell Mol. Biol. 2022, 67, 409–413. [Google Scholar] [CrossRef]
- DiPetrillo, C.G.; Smith, E.F. Pcdp1 is a central apparatus protein that binds Ca2+-calmodulin and regulates ciliary motility. J. Cell Biol. 2010, 189, 601–612. [Google Scholar] [CrossRef]
- Bustamante-Marin, X.M.; Shapiro, A.; Sears, P.R.; Charng, W.-L.; Conrad, D.F.; Leigh, M.W.; Knowles, M.R.; Ostrowski, L.E.; Zariwala, M.A. Identification of genetic variants in CFAP221 as a cause of primary ciliary dyskinesia. J. Hum. Genet. 2020, 65, 175–180. [Google Scholar] [CrossRef]
- Lee, L.; Campagna, D.R.; Pinkus, J.L.; Mulhern, H.; Wyatt, T.A.; Sisson, J.H.; Pavlik, J.A.; Pinkus, G.S.; Fleming, M.D. Primary ciliary dyskinesia in mice lacking the novel ciliary protein Pcdp1. Mol. Cell Biol. 2008, 28, 949–957. [Google Scholar] [CrossRef]
- Dougherty, G.W.; Mizuno, K.; Nöthe-Menchen, T.; Ikawa, Y.; Boldt, K.; Ta-Shma, A.; Aprea, I.; Minegishi, K.; Pang, Y.-P.; Pennekamp, P.; et al. CFAP45 deficiency causes situs abnormalities and asthenospermia by disrupting an axonemal adenine nucleotide homeostasis module. Nat. Commun. 2020, 11, 5520. [Google Scholar] [CrossRef] [PubMed]
- Ta-Shma, A.; Perles, Z.; Yaacov, B.; Werner, M.; Frumkin, A.; Rein, A.J.J.T.; Elpeleg, O. A human laterality disorder associated with a homozygous WDR16 deletion. Eur. J. Hum. Genet. 2015, 23, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Wallmeier, J.; Bracht, D.; Alsaif, H.S.; Dougherty, G.W.; Olbrich, H.; Cindric, S.; Dzietko, M.; Heyer, C.; Teig, N.; Thiels, C.; et al. Mutations in TP73 cause impaired mucociliary clearance and lissencephaly. Am. J. Hum. Genet. 2021, 108, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Nemajerova, A.; Kramer, D.; Siller, S.S.; Herr, C.; Shomroni, O.; Pena, T.; Gallinas Suazo, C.; Glaser, K.; Wildung, M.; Steffen, H.; et al. TAp73 is a central transcriptional regulator of airway multiciliogenesis. Genes. Dev. 2016, 30, 1300–1312. [Google Scholar] [CrossRef]
- Al Mutairi, F.; Alkhalaf, R.; Alkhorayyef, A.; Alroqi, F.; Yusra, A.; Umair, M.; Nouf, F.; Khan, A.; Meshael, A.; Hamad, A.; et al. Homozygous truncating NEK10 mutation, associated with primary ciliary dyskinesia: A case report. BMC Pulm. Med. 2020, 20, 141. [Google Scholar] [CrossRef]
- Porpora, M.; Sauchella, S.; Rinaldi, L.; Delle Donne, R.; Sepe, M.; Torres-Quesada, O.; Intartaglia, D.; Garbi, C.; Insabato, L.; Santoriello, M.; et al. Counterregulation of CAMP-directed kinase activities controls ciliogenesis. Nat. Commun. 2018, 9, 1224. [Google Scholar] [CrossRef]
- Ryan, R.; Failler, M.; Reilly, M.L.; Garfa-Traore, M.; Delous, M.; Filhol, E.; Reboul, T.; Bole-Feysot, C.; Nitschké, P.; Baudouin, V.; et al. Functional characterization of tektin-1 in motile cilia and evidence for TEKT1 as a new candidate gene for motile ciliopathies. Hum. Mol. Genet. 2018, 27, 266–282. [Google Scholar] [CrossRef]
- Devlin, L.A.; Coles, J.; Jackson, C.L.; Barroso-Gil, M.; Green, B.; Walker, W.T.; Thomas, N.S.; Thompson, J.; Rock, S.A.; Neatu, R.; et al. Biallelic variants in CEP164 cause a motile ciliopathy-like syndrome. Clin. Genet. 2022, 103, 330–334. [Google Scholar] [CrossRef]
- Reed, M.; Takemaru, K.-I.; Ying, G.; Frederick, J.M.; Baehr, W. Deletion of CEP164 in mouse photoreceptors post-ciliogenesis interrupts ciliary intraflagellar transport (IFT). PLoS Genet. 2022, 18, e1010154. [Google Scholar] [CrossRef]
- Beckers, A.; Adis, C.; Schuster-Gossler, K.; Tveriakhina, L.; Ott, T.; Fuhl, F.; Hegermann, J.; Boldt, K.; Serth, K.; Rachev, E.; et al. The FOXJ1 target Cfap206 is required for sperm motility, mucociliary clearance of the airways and brain development. Development 2020, 147, dev188052. [Google Scholar] [CrossRef]
- Vasudevan, K.K.; Song, K.; Alford, L.M.; Sale, W.S.; Dymek, E.E.; Smith, E.F.; Hennessey, T.; Joachimiak, E.; Urbanska, P.; Wloga, D.; et al. FAP206 is a microtubule-docking adapter for ciliary radial spoke 2 and dynein c. Mol. Biol. Cell 2015, 26, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Tritschler, D.; Song, K.; Barber, C.F.; Cobb, J.S.; Porter, M.E.; Nicastro, D. Building blocks of the nexin-dynein regulatory complex in Chlamydomonas flagella. J. Biol. Chem. 2011, 286, 29175–29191. [Google Scholar] [CrossRef] [PubMed]
- Ta-Shma, A.; Hjeij, R.; Perles, Z.; Dougherty, G.W.; Abu Zahira, I.; Letteboer, S.J.F.; Antony, D.; Darwish, A.; Mans, D.A.; Spittler, S.; et al. Homozygous loss-of-function mutations in MNS1 cause laterality defects and likely male infertility. PLoS Genet. 2018, 14, e1007602. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, F.; Leu, N.A.; Wang, P.J. MNS1 is essential for spermiogenesis and motile ciliary functions in mice. PLoS Genet. 2012, 8, e1002516. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Tu, C.-F.; Yang, D.-H.; Ding, S.-Z.; Lei, C.; Wang, R.-C.; Liu, L.; Kang, X.; Shen, X.-Q.; Yang, Y.-F.; et al. Bi-Allelic BRWD1 variants cause male infertility with asthenoteratozoospermia and likely primary ciliary dyskinesia. Hum. Genet. 2021, 140, 761–773. [Google Scholar] [CrossRef]
- Rachev, E.; Schuster-Gossler, K.; Fuhl, F.; Ott, T.; Tveriakhina, L.; Beckers, A.; Hegermann, J.; Boldt, K.; Mai, M.; Kremmer, E.; et al. CFAP43 modulates ciliary beating in mouse and xenopus. Dev. Biol. 2020, 459, 109–125. [Google Scholar] [CrossRef]
- Narasimhan, V.; Hjeij, R.; Vij, S.; Loges, N.T.; Wallmeier, J.; Koerner-Rettberg, C.; Werner, C.; Thamilselvam, S.K.; Boey, A.; Choksi, S.P.; et al. Mutations in CCDC11, which encodes a coiled-coil containing ciliary protein, causes situs inversus due to dysmotility of monocilia in the left-right organizer. Hum. Mutat. 2015, 36, 307–318. [Google Scholar] [CrossRef]
- Silva, E.; Betleja, E.; John, E.; Spear, P.; Moresco, J.J.; Zhang, S.; Yates, J.R.; Mitchell, B.J.; Mahjoub, M.R. Ccdc11 is a novel centriolar satellite protein essential for ciliogenesis and establishment of left–right asymmetry. Mol. Biol. Cell 2016, 27, 48–63. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Leigh, M.W. Value of transmission electron microscopy for primary ciliary dyskinesia diagnosis in the era of molecular medicine: Genetic defects with normal and non-diagnostic ciliary ultrastructure. Ultrastruct. Pathol. 2017, 41, 373–385. [Google Scholar] [CrossRef]
- Amirav, I.; Mussaffi, H.; Roth, Y.; Schmidts, M.; Omran, H.; Werner, C. Israeli PCD consortium investigators a reach-out system for video microscopy analysis of ciliary motions aiding PCD diagnosis. BMC Res. Notes 2015, 8, 71. [Google Scholar] [CrossRef]
- Knowles, M.R.; Leigh, M.W.; Carson, J.L.; Davis, S.D.; Dell, S.D.; Ferkol, T.W.; Olivier, K.N.; Sagel, S.D.; Rosenfeld, M.; Burns, K.A.; et al. Mutations of DNAH11 in patients with primary ciliary dyskinesia with normal ciliary ultrastructure. Thorax 2012, 67, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.; Hogg, C. Primary ciliary dyskinesia: A major player in a bigger game. Breathe 2020, 16, 200047. [Google Scholar] [CrossRef] [PubMed]
- Budny, B.; Chen, W.; Omran, H.; Fliegauf, M.; Tzschach, A.; Wisniewska, M.; Jensen, L.R.; Raynaud, M.; Shoichet, S.A.; Badura, M.; et al. A novel x-linked recessive mental retardation syndrome comprising macrocephaly and ciliary dysfunction is allelic to oral-facial-digital type I syndrome. Hum. Genet. 2006, 120, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Yiallouros, P.K.; Kouis, P.; Pirpa, P.; Michailidou, K.; Loizidou, M.A.; Potamiti, L.; Kalyva, M.; Koutras, G.; Kyriacou, K.; Hadjisavvas, A. Wide phenotypic variability in RSPH9-associated primary ciliary dyskinesia: Review of a case-series from cyprus. J. Thorac. Dis. 2019, 11, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sha, Y.; Li, Y.; Mei, L.; Lin, S.; Huang, X.; Lu, J.; Ding, L.; Kong, S.; Lu, Z. Loss-of-function mutations in SPEF2 cause multiple morphological abnormalities of the sperm flagella (MMAF). J. Med. Genet. 2019, 56, 678–684. [Google Scholar] [CrossRef]
- Ostrowski, L.E.; Yin, W.; Smith, A.J.; Sears, P.R.; Bustamante-Marin, X.M.; Dang, H.; Hildebrandt, F.; Daniels, L.A.; Capps, N.A.; Sullivan, K.M.; et al. Expression of a truncated form of ODAD1 associated with an unusually mild primary ciliary dyskinesia phenotype. Int. J. Mol. Sci. 2022, 23, 1753. [Google Scholar] [CrossRef]
- Preston, C.G.; Wright, M.W.; Madhavrao, R.; Harrison, S.M.; Goldstein, J.L.; Luo, X.; Wand, H.; Wulf, B.; Cheung, G.; Mandell, M.E.; et al. ClinGen variant curation interface: A variant classification platform for the application of evidence criteria from ACMG/AMP guidelines. Genome Med. 2022, 14, 6. [Google Scholar] [CrossRef]
- Bukowy-Bieryłło, Z. Long-term differentiating primary human airway epithelial cell cultures: How far are we? Cell Commun. Signal 2021, 19, 63. [Google Scholar] [CrossRef]
- Poprzeczko, M.; Bicka, M.; Farahat, H.; Bazan, R.; Osinka, A.; Fabczak, H.; Joachimiak, E.; Wloga, D. Rare human diseases: Model organisms in deciphering the molecular basis of primary ciliary dyskinesia. Cells 2019, 8, 1614. [Google Scholar] [CrossRef]
- Niziolek, M.; Bicka, M.; Osinka, A.; Samsel, Z.; Sekretarska, J.; Poprzeczko, M.; Bazan, R.; Fabczak, H.; Joachimiak, E.; Wloga, D. PCD genes-from patients to model organisms and back to humans. Int. J. Mol. Sci. 2022, 23, 1749. [Google Scholar] [CrossRef]
- Newmark, P.A.; Sánchez Alvarado, A. Not your father’s planarian: A classic model enters the era of functional genomics. Nat. Rev. Genet. 2002, 3, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.H. Experimental studies of the regeneration of planaria maculata. Arch. Entwickelungsmechanik Org. 1898, 7, 364–397. [Google Scholar] [CrossRef]
- Vila-Farré, M.; C Rink, J. The ecology of freshwater planarians. Methods Mol. Biol. 2018, 1774, 173–205. [Google Scholar] [CrossRef]
- Reddien, P.W.; Sánchez Alvarado, A. Fundamentals of planarian regeneration. Annu. Rev. Cell Dev. Biol. 2004, 20, 725–757. [Google Scholar] [CrossRef] [PubMed]
- Reddien, P.W. The cellular and molecular basis for planarian regeneration. Cell 2018, 175, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, N.J.; Nicolas, C.L.; Adams, D.S.; Levin, M. Planarians: A versatile and powerful model system for molecular studies of regeneration, adult stem cell regulation, aging, and behavior. CSH Protoc. 2008, 2008, pdb.emo101. [Google Scholar] [CrossRef]
- Deochand, N.; Costello, M.S.; Deochand, M.E. Behavioral research with planaria. Perspect. Behav. Sci. 2018, 41, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Basquin, C.; Orfila, A.-M.; Azimzadeh, J. The planarian Schmidtea mediterranea as a model for studying motile cilia and multiciliated cells. Methods Cell Biol. 2015, 127, 243–262. [Google Scholar] [CrossRef]
- Saló, E.; Pineda, D.; Marsal, M.; Gonzalez, J.; Gremigni, V.; Batistoni, R. Genetic network of the eye in Platyhelminthes: Expression and functional analysis of some players during planarian regeneration. Gene 2002, 287, 67–74. [Google Scholar] [CrossRef]
- LoCascio, S.A.; Lapan, S.W.; Reddien, P.W. Eye absence does not regulate planarian stem cells during eye regeneration. Dev. Cell 2017, 40, 381–391.e3. [Google Scholar] [CrossRef]
- Önal, P.; Grün, D.; Adamidi, C.; Rybak, A.; Solana, J.; Mastrobuoni, G.; Wang, Y.; Rahn, H.-P.; Chen, W.; Kempa, S.; et al. Gene expression of pluripotency determinants is conserved between mammalian and planarian stem cells. EMBO J. 2012, 31, 2755–2769. [Google Scholar] [CrossRef] [PubMed]
- Rompolas, P.; Azimzadeh, J.; Marshall, W.F.; King, S.M. Analysis of ciliary assembly and function in planaria. Methods Enzym. 2013, 525, 245–264. [Google Scholar] [CrossRef]
- Merryman, M.S.; Alvarado, A.S.; Jenkin, J.C. Culturing planarians in the laboratory. Methods Mol. Biol. 2018, 1774, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Alvarado, A.; Newmark, P.A. Double-stranded RNA specifically disrupts gene expression during planarian regeneration. Proc. Natl. Acad. Sci. USA 1999, 96, 5049–5054. [Google Scholar] [CrossRef]
- Reddien, P.W.; Bermange, A.L.; Murfitt, K.J.; Jennings, J.R.; Sánchez Alvarado, A. Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev. Cell 2005, 8, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Rouhana, L.; Weiss, J.A.; Forsthoefel, D.J.; Lee, H.; King, R.S.; Inoue, T.; Shibata, N.; Agata, K.; Newmark, P.A. RNA interference by feeding in vitro-synthesized double-stranded rna to planarians: Methodology and dynamics. Dev. Dyn. 2013, 242, 718–730. [Google Scholar] [CrossRef]
- Fincher, C.T.; Wurtzel, O.; de Hoog, T.; Kravarik, K.M.; Reddien, P.W. Cell Type transcriptome atlas for the planarian Schmidtea mediterranea. Science 2018, 360, eaaq1736. [Google Scholar] [CrossRef]
- Plass, M.; Solana, J.; Wolf, F.A.; Ayoub, S.; Misios, A.; Glažar, P.; Obermayer, B.; Theis, F.J.; Kocks, C.; Rajewsky, N. Cell type atlas and lineage tree of a whole complex animal by single-cell transcriptomics. Science 2018, 360, eaaq1723. [Google Scholar] [CrossRef]
- Grohme, M.A.; Schloissnig, S.; Rozanski, A.; Pippel, M.; Young, G.R.; Winkler, S.; Brandl, H.; Henry, I.; Dahl, A.; Powell, S.; et al. The genome of Schmidtea mediterranea and the evolution of core cellular mechanisms. Nature 2018, 554, 56–61. [Google Scholar] [CrossRef]
- Benham-Pyle, B.W.; Brewster, C.E.; Kent, A.M.; Mann, F.G.; Chen, S.; Scott, A.R.; Box, A.C.; Sánchez Alvarado, A. Identification of rare post-mitotic cell states induced by injury and required for whole-body regeneration in Schmidtea mediterranea. Nat. Cell Biol. 2021, 23, 939–952. [Google Scholar] [CrossRef]
- Robb, S.M.C.; Ross, E.; Sánchez Alvarado, A. SmedGD: The Schmidtea mediterranea genome database. Nucleic Acids Res. 2008, 36, D599–D606. [Google Scholar] [CrossRef]
- Robb, S.M.C.; Gotting, K.; Ross, E.; Sánchez Alvarado, A. SmedGD 2.0: The Schmidtea mediterranea genome database. Genesis 2015, 53, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Rozanski, A.; Moon, H.; Brandl, H.; Martín-Durán, J.M.; Grohme, M.A.; Hüttner, K.; Bartscherer, K.; Henry, I.; Rink, J.C. PlanMine 3.0-improvements to a mineable resource of flatworm biology and biodiversity. Nucleic Acids Res. 2019, 47, D812–D820. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Lara, S.; Abril, J.F. PlanNET: Homology-based predicted interactome for multiple planarian transcriptomes. Bioinformatics 2018, 34, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Rompolas, P.; Patel-King, R.S.; King, S.M. Schmidtea mediterranea: A model system for analysis of motile cilia. Methods Cell Biol. 2009, 93, 81–98. [Google Scholar] [CrossRef] [PubMed]
- King, S.M.; Patel-King, R.S. Planaria as a model system for the analysis of ciliary assembly and motility. Methods Mol. Biol. 2016, 1454, 245–254. [Google Scholar] [CrossRef]
- Azimzadeh, J.; Basquin, C. Basal bodies across eukaryotes series: Basal bodies in the freshwater planarian Schmidtea mediterranea. Cilia 2016, 5, 15. [Google Scholar] [CrossRef]
- Li, Y.; Guo, F.; Jing, Q.; Zhu, X.; Yan, X. Characterisation of centriole biogenesis during multiciliation in planarians. Biol. Cell 2020, 112, 398–408. [Google Scholar] [CrossRef]
- Harrath, A.H.; Gammoudi, M.; Mansour, L.; Ahmed, M.; Sirotkin, A.V.; Al Omar, S.Y.; Ibrahim, K.E.; Alwasel, S.H. Investigation of the ultrastructure of dendrocoelum constrictum (Platyhelminthes, Tricladida) spermatogenesis and mature spermatozoa. C. R. Biol. 2014, 337, 513–520. [Google Scholar] [CrossRef]
- Christman, D.A.; Curry, H.N.; Rouhana, L. Heterotrimeric kinesin II is required for flagellar assembly and elongation of nuclear morphology during spermiogenesis in Schmidtea mediterranea. Dev. Biol. 2021, 477, 191–204. [Google Scholar] [CrossRef]
- Rink, J.C.; Vu, H.T.-K.; Sánchez Alvarado, A. The maintenance and regeneration of the planarian excretory system are regulated by EGFR signaling. Development 2011, 138, 3769–3780. [Google Scholar] [CrossRef]
- Katoh, M.; Katoh, M. WNT signaling pathway and stem cell signaling network. Clin. Cancer Res. 2007, 13, 4042–4045. [Google Scholar] [CrossRef]
- Peiris, T.H.; Ramirez, D.; Barghouth, P.G.; Oviedo, N.J. The akt signaling pathway is required for tissue maintenance and regeneration in planarians. BMC Dev. Biol. 2016, 16, 7. [Google Scholar] [CrossRef]
- Petersen, C.P.; Reddien, P.W. Polarized notum activation at wounds inhibits wnt function to promote planarian head regeneration. Science 2011, 332, 852–855. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, E.M. Wnt signaling in axial patterning and regeneration: Lessons from planaria. Sci. Signal. 2010, 3, pe21. [Google Scholar] [CrossRef] [PubMed]
- Glazer, A.M.; Wilkinson, A.W.; Backer, C.B.; Lapan, S.W.; Gutzman, J.H.; Cheeseman, I.M.; Reddien, P.W. The Zn finger protein iguana impacts hedgehog signaling by promoting ciliogenesis. Dev. Biol. 2010, 337, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Currie, K.W.; Pearson, B.J. Transcription factors Lhx1/5-1 and Pitx are required for the maintenance and regeneration of serotonergic neurons in planarians. Development 2013, 140, 3577–3588. [Google Scholar] [CrossRef]
- Jerber, J.; Baas, D.; Soulavie, F.; Chhin, B.; Cortier, E.; Vesque, C.; Thomas, J.; Durand, B. The coiled-coil domain containing protein CCDC151 is required for the function of IFT-dependent motile cilia in animals. Hum. Mol. Genet. 2014, 23, 563–577. [Google Scholar] [CrossRef]
- Ahmed, N.T.; Mitchell, D.R. ODA16p, a Chlamydomonas flagellar protein needed for dynein assembly. Mol. Biol. Cell 2005, 16, 5004–5012. [Google Scholar] [CrossRef]
- Gao, C.; Wang, G.; Amack, J.D.; Mitchell, D.R. Oda16/Wdr69 is essential for axonemal dynein assembly and ciliary motility during zebrafish embryogenesis. Dev. Dyn. 2010, 239, 2190–2197. [Google Scholar] [CrossRef]
- Solomon, G.M.; Francis, R.; Chu, K.K.; Birket, S.E.; Gabriel, G.; Trombley, J.E.; Lemke, K.L.; Klena, N.; Turner, B.; Tearney, G.J.; et al. Assessment of ciliary phenotype in primary ciliary dyskinesia by micro-optical coherence tomography. JCI Insight 2017, 2, e91702. [Google Scholar] [CrossRef] [PubMed]
- Taschner, M.; Mourão, A.; Awasthi, M.; Basquin, J.; Lorentzen, E. Structural basis of outer dynein arm intraflagellar transport by the transport adaptor protein ODA16 and the intraflagellar transport protein IFT46. J. Biol. Chem. 2017, 292, 7462–7473. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Witman, G.B. The N-terminus of IFT46 mediates intraflagellar transport of outer arm dynein and its cargo-adaptor ODA16. Mol. Biol. Cell 2017, 28, 2420–2433. [Google Scholar] [CrossRef] [PubMed]
- Patel-King, R.S.; King, S.M. An outer arm dynein light chain acts in a conformational switch for flagellar motility. J. Cell Biol. 2009, 186, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Horváth, J.; Fliegauf, M.; Olbrich, H.; Kispert, A.; King, S.M.; Mitchison, H.; Zariwala, M.A.; Knowles, M.R.; Sudbrak, R.; Fekete, G.; et al. Identification and analysis of axonemal dynein light chain 1 in primary ciliary dyskinesia patients. Am. J. Respir. Cell Mol. Biol. 2005, 33, 41–47. [Google Scholar] [CrossRef]
- Takada, S.; Wilkerson, C.G.; Wakabayashi, K.; Kamiya, R.; Witman, G.B. The outer dynein arm-docking complex: Composition and characterization of a subunit (Oda1) necessary for outer arm assembly. Mol. Biol. Cell 2002, 13, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.M.; Inaba, K.; Pazour, G.J.; Takada, S.; Wakabayashi, K.; Wilkerson, C.G.; Kamiya, R.; Witman, G.B. DC3, the 21-KDa subunit of the outer dynein arm-docking complex (ODA-DC), is a novel EF-hand protein important for assembly of both the outer arm and the ODA-DC. Mol. Biol. Cell 2003, 14, 3650–3663. [Google Scholar] [CrossRef]
- Takada, S.; Kamiya, R. Functional reconstitution of chlamydomonas outer dynein arms from alpha-beta and gamma subunits: Requirement of a third factor. J. Cell Biol. 1994, 126, 737–745. [Google Scholar] [CrossRef]
- Onoufriadis, A.; Shoemark, A.; Munye, M.M.; James, C.T.; Schmidts, M.; Patel, M.; Rosser, E.M.; Bacchelli, C.; Beales, P.L.; Scambler, P.J.; et al. Combined exome and whole-genome sequencing identifies mutations in ARMC4 as a cause of primary ciliary dyskinesia with defects in the outer dynein arm. J. Med. Genet. 2014, 51, 61–67. [Google Scholar] [CrossRef]
- Young, S.A.M.; Miyata, H.; Satouh, Y.; Kato, H.; Nozawa, K.; Isotani, A.; Aitken, R.J.; Baker, M.A.; Ikawa, M. CRISPR/Cas9-mediated rapid generation of multiple mouse lines identified Ccdc63 as essential for spermiogenesis. Int. J. Mol. Sci. 2015, 16, 24732–24750. [Google Scholar] [CrossRef]
- Kyuji, A.; Patel-King, R.S.; Hisabori, T.; King, S.M.; Wakabayashi, K.-I. Cilia loss and dynein assembly defects in planaria lacking an outer dynein arm-docking complex subunit. Zoolog. Sci. 2020, 37, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Patel-King, R.S.; King, S.M. A prefoldin-associated WD-repeat protein (WDR92) is required for the correct architectural assembly of motile cilia. Mol. Biol. Cell 2016, 27, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Zur Lage, P.; Stefanopoulou, P.; Styczynska-Soczka, K.; Quinn, N.; Mali, G.; von Kriegsheim, A.; Mill, P.; Jarman, A.P. Ciliary dynein motor preassembly is regulated by Wdr92 in association with HSP90 co-chaperone, R2TP. J. Cell Biol. 2018, 217, 2583–2598. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, L.; Pan, J. Chlamydomonas WDR92 in association with R2TP-like complex and multiple DNAAFs to regulate ciliary dynein preassembly. J. Mol. Cell Biol. 2019, 11, 770–780. [Google Scholar] [CrossRef]
- Patel-King, R.S.; Sakato-Antoku, M.; Yankova, M.; King, S.M. WDR92 Is required for axonemal dynein heavy chain stability in cytoplasm. Mol. Biol. Cell 2019, 30, 1834–1845. [Google Scholar] [CrossRef]
- Pazour, G.J.; Dickert, B.L.; Vucica, Y.; Seeley, E.S.; Rosenbaum, J.L.; Witman, G.B.; Cole, D.G. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene Tg737, are required for assembly of cilia and flagella. J. Cell Biol. 2000, 151, 709–718. [Google Scholar] [CrossRef]
- Moyer, J.H.; Lee-Tischler, M.J.; Kwon, H.Y.; Schrick, J.J.; Avner, E.D.; Sweeney, W.E.; Godfrey, V.L.; Cacheiro, N.L.; Wilkinson, J.E.; Woychik, R.P. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science 1994, 264, 1329–1333. [Google Scholar] [CrossRef]
- Patel-King, R.S.; Gilberti, R.M.; Hom, E.F.Y.; King, S.M. WD60/FAP163 Is a dynein intermediate chain required for retrograde intraflagellar transport in cilia. Mol. Biol. Cell 2013, 24, 2668–2677. [Google Scholar] [CrossRef]
- Scimone, M.L.; Srivastava, M.; Bell, G.W.; Reddien, P.W. A regulatory program for excretory system regeneration in planarians. Development 2011, 138, 4387–4398. [Google Scholar] [CrossRef]
- Marra, A.N.; Li, Y.; Wingert, R.A. Antennas of organ morphogenesis: The roles of cilia in vertebrate kidney development. Genesis 2016, 54, 457–469. [Google Scholar] [CrossRef]
- Reddien, P.W.; Newmark, P.A.; Sánchez Alvarado, A. Gene nomenclature guidelines for the planarian Schmidtea mediterranea. Dev. Dyn. 2008, 237, 3099–3101. [Google Scholar] [CrossRef] [PubMed]
- Nowotarski, S.H.; Davies, E.L.; Robb, S.M.C.; Ross, E.J.; Matentzoglu, N.; Doddihal, V.; Mir, M.; McClain, M.; Sánchez Alvarado, A. Planarian anatomy ontology: A resource to connect data within and across experimental platforms. Development 2021, 148, dev196097. [Google Scholar] [CrossRef] [PubMed]
- Brandl, H.; Moon, H.; Vila-Farré, M.; Liu, S.-Y.; Henry, I.; Rink, J.C. PlanMine--a mineable resource of planarian biology and biodiversity. Nucleic Acids Res. 2016, 44, D764–D773. [Google Scholar] [CrossRef] [PubMed]
- van Wolfswinkel, J.C.; Wagner, D.E.; Reddien, P.W. Single-cell analysis reveals functionally distinct classes within the planarian stem cell compartment. Cell Stem Cell 2014, 15, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.C.; Cheng, L.-C.; TK Vu, H.; Lange, J.J.; McKinney, S.A.; Seidel, C.W.; Sánchez Alvarado, A. Egr-5 is a post-mitotic regulator of planarian epidermal differentiation. eLife 2015, 4, e10501. [Google Scholar] [CrossRef]
- Wurtzel, O.; Oderberg, I.M.; Reddien, P.W. Planarian epidermal stem cells respond to positional cues to promote cell type diversity. Dev. Cell 2017, 40, 491–504.e5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).