The Pivotal Role of Galectin-3 in Viral Infection: A Multifaceted Player in Host–Pathogen Interactions
Abstract
1. Introduction
2. The Comprehensive Impact of Galectin-3 on Viral Infections: From Entry to Immunity, Life Cycle, and Disease Progression
2.1. Galectin-3 and Viral Entry
2.1.1. Galectin-3 as a Facilitator of Viral Attachment
2.1.2. Galectin-3 and Viral Internalization
2.2. Galectin-3 and Immune Responses
2.2.1. Galectin-3 in Immune Cell Activation and Recruitment
2.2.2. Galectin-3 in the Regulation of Immune Signaling Pathways
2.2.3. Galectin-3 in Modulating Apoptosis and Autophagy
2.3. Galectin-3 and Viral Life Cycle
2.3.1. Galectin-3 in Viral Replication
2.3.2. Galectin-3 in Viral Assembly and Release
2.4. Galectin-3 and Viral Pathogenesis
2.4.1. Galectin-3 in Tissue Damage and Inflammation
2.4.2. Galectin-3 in Viral Persistence and Latency
2.4.3. Galectin-3 and Host Protection
2.5. Galectin-3 Interactions with Sulfated Glycosaminoglycans and Implications for Viral Pathogenesis
2.6. Signaling Pathways Triggered by Galectin-3 in Viral Infections
2.7. Galectin-3: Receptor Interactions and Their Implications for Viral Infections
- EGFR: Interaction with Epidermal Growth Factor Receptor (EGFR) could implicate Gal-3 in the regulation of cell growth and survival, thus influencing oncogenesis [61].
- Integrins: Through binding to various integrins, Gal-3 participates in modulating cell adhesion and migration [62].
- CD45: Interactions with CD45 could allow Gal-3 to adjust immune responses [63].
- CD71: Through interaction with CD71, Gal-3 plays a role in cellular iron uptake [64].
- RAGE: Through interacting with Receptor for Advanced Glycation End Products (RAGE), Gal-3 can influence inflammation and oxidative stress responses [65].
3. Galectin-3 in Specific Viral Diseases
3.1. SARS-CoV-2 Virus Infection and Galectin-3
3.1.1. The Role of Galectin-3 in Modulating Immune Response and Inflammation in COVID-19
3.1.2. Potential Role of Human Galectin-3 in SARS-CoV-2 Viral Adherence and Entry
3.1.3. Galectin-3 as a Potential Biomarker for COVID-19 Severity and Prognosis
3.1.4. Comparative Roles and Interactions of Galectin-3 and Galectin-3-Binding Protein in SARS-CoV-2 Infection
3.2. Human Immunodeficiency Virus (HIV) Infection and Galectin-3
3.2.1. Galectin-3 in HIV-1 Entry and Infection
3.2.2. Galectin-3 Expression and HIV-1 Tat Protein
3.2.3. Galectin-3 and HIV-1 Dissemination
3.2.4. Galectin-3 and HIV-1-Infected Macrophage Cell Death
3.2.5. Galectin-3 and HIV-Associated Pathogenesis
3.3. Influenza A Virus Infection and Galectin-3
3.3.1. Galectin-3 and Influenza A Virus Infection
3.3.2. Promoting Viral RNA Synthesis
3.3.3. Modulating Host Immune Response against IAV
3.4. Galectin-3 and Hepatitis Virus Infections
3.4.1. Galectin-3 and Hepatitis B Virus Infections
3.4.2. Galectin-3 and Hepatitis C Virus Infections
3.5. Galectin-3 and Herpes Simplex Virus
3.5.1. Galectin-3 in HSV Attachment and Entry
3.5.2. Galectin-3 and Herpetic Allodynia
3.6. Galectin-3 and Cytomegalovirus
3.7. The Role of Galectin-3 in Other Viral Infections
3.7.1. Kaposi’s Sarcoma-Associated Herpesvirus
3.7.2. Adenovirus
3.7.3. Enterovirus Type 71
3.7.4. Minute Virus of Mice
4. Therapeutic Potential of Targeting Galectin-3
4.1. Small Molecule Inhibitors of Galectin-3
4.2. Monoclonal Antibodies Targeting Galectin-3
4.3. RNA Interference Techniques for Galectin-3 Silencing
5. Challenges and Future Directions
5.1. Delineating the Molecular Mechanisms of Galectin-3 in Viral Infection
5.2. Identifying Galectin-3’s Role in Various Viral Infections
5.3. Evaluating the Therapeutic Potential of Galectin-3 Targeting
5.4. Assessing the Potential Side Effects of Galectin-3 Inhibition
5.5. Investigating the Role of Galectin-3 in the Context of Host Immunity
5.6. Enhancing Understanding of Galectin-3 and Viral Interactions: A Critical Perspective
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sudhan, S.S.; Sharma, P. Human Viruses: Emergence and Evolution. Emerg. Reemerging Viral Pathog. 2020, 1, 53–68. [Google Scholar]
- Louten, J. Virus Structure and Classification. Essent. Hum. Virol. 2016, 19–29. [Google Scholar] [CrossRef]
- Woolhouse, M.; Scott, F.; Hudson, Z.; Howey, R.; Chase-Topping, M. Human viruses: Discovery and emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2864–2871. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; Macgregor-Skinner, G. Dangerous Viral Pathogens of Animal Origin: Risk and Biosecurity: Zoonotic Select Agents. Zoonoses-Infect. Affect. Hum. Anim. 2014, 1015–1062. [Google Scholar] [CrossRef]
- Machala, E.A.; McSharry, B.P.; Rouse, B.T.; Abendroth, A.; Slobedman, B. Gal power: The diverse roles of galectins in regulating viral infections. J. Gen. Virol. 2019, 100, 333–349. [Google Scholar] [CrossRef]
- Mishra, A.; Behura, A.; Mawatwal, S.; Kumar, A.; Naik, L.; Mohanty, S.S.; Manna, D.; Dokania, P.; Mishra, A.; Patra, S.K.; et al. Structure-function and application of plant lectins in disease biology and immunity. Food Chem. Toxicol. 2019, 134, 110827. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.R. Roles of galectins in infection. Nat. Rev. Microbiol. 2009, 7, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Laderach, D.J.; Compagno, D. Unraveling How Tumor-Derived Galectins Contribute to Anti-Cancer Immunity Failure. Cancers 2021, 13, 4529. [Google Scholar] [CrossRef]
- Vasta, G.R. Galectins as pattern recognition receptors: Structure, function, and evolution. Adv. Exp. Med. Biol. 2012, 946, 21–36. [Google Scholar] [CrossRef]
- Díaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediat. Inflamm. 2017, 2017, 9247574. [Google Scholar] [CrossRef]
- Farhad, M.; Rolig, A.S.; Redmond, W.L. The role of Galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. Oncoimmunology 2018, 7, e1434467. [Google Scholar] [CrossRef]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef]
- Caniglia, J.L.; Asuthkar, S.; Tsung, A.J.; Guda, M.R.; Velpula, K.K. Immunopathology of galectin-3: An increasingly promising target in COVID-19. F1000Research 2020, 9, 1078. [Google Scholar] [CrossRef]
- Gallo, V.; Arienzo, A.; Iacobelli, S.; Iacobelli, V.; Antonini, G. Gal-3BP in Viral Infections: An Emerging Role in Severe Acute Respiratory Syndrome Coronavirus 2. Int. J. Mol. Sci. 2022, 23, 7314. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Wang, W.-H.; Huang, S.-W.; Yeh, C.-S.; Yuan, R.-Y.; Yang, Z.-S.; Urbina, A.N.; Tseng, S.-P.; Lu, P.-L.; Chen, Y.-H.; et al. The Examination of Viral Characteristics of HIV-1 CRF07_BC and Its Potential Interaction with Extracellular Galectin-3. Pathogens 2020, 9, 425. [Google Scholar] [CrossRef] [PubMed]
- Odun-Ayo, F.; Reddy, L. Potential Roles of Modified Pectin Targeting Galectin-3 against Severe Acute Respiratory Syndrome Coronavirus-2. J 2021, 4, 824–837. [Google Scholar] [CrossRef]
- Woodward, A.M.; Mauris, J.; Argüeso, P. Binding of transmembrane mucins to galectin-3 limits herpesvirus 1 infection of human corneal keratinocytes. J. Virol. 2013, 87, 5841–5847. [Google Scholar] [CrossRef]
- Nita-Lazar, M.; Banerjee, A.; Feng, C.; Amin, M.N.; Frieman, M.B.; Chen, W.H.; Cross, A.S.; Wang, L.X.; Vasta, G.R. Desialylation of airway epithelial cells during influenza virus infection enhances pneumococcal adhesion via galectin binding. Mol. Immunol. 2015, 65, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-L.; Chen, Y.-C.; Wang, C.-T.; Chong, H.-E.; Chung, N.-H.; Leu, C.-H.; Liu, F.-T.; Lai, M.M.C.; Ling, P.; Wu, C.-L.; et al. Upregulation of galectin-3 in influenza A virus infection promotes viral RNA synthesis through its association with viral PA protein. J. Biomed. Sci. 2023, 30, 14. [Google Scholar] [CrossRef]
- Huang, W.C.; Chen, H.L.; Chen, H.Y.; Peng, K.P.; Lee, Y.; Huang, L.M.; Chang, L.Y.; Liu, F.T. Galectin-3 and Its Genetic Variation rs4644 Modulate Enterovirus 71 Infection. PLoS ONE 2016, 11, e0168627. [Google Scholar] [CrossRef]
- Nita-Lazar, M.; Banerjee, A.; Feng, C.; Vasta, G.R. Galectins regulate the inflammatory response in airway epithelial cells exposed to microbial neuraminidase by modulating the expression of SOCS1 and RIG1. Mol. Immunol. 2015, 68, 194–202. [Google Scholar] [CrossRef]
- Gajovic, N.; Markovic, S.S.; Jurisevic, M.; Jovanovic, M.; Arsenijevic, N.; Mijailovic, Z.; Jovanovic, M.; Jovanovic, I. Galectin-3 as an important prognostic marker for COVID-19 severity. Sci. Rep. 2023, 13, 1460. [Google Scholar] [CrossRef] [PubMed]
- Uluca, Ü.; Şen, V.; Ece, A.; Tan, İ.; Karabel, D.; Aktar, F.; Karabel, M.; Balık, H.; Güneş, A. Serum galectin-3 levels in children with chronic hepatitis B infection and inactive hepatitis B carriers. Med. Sci. Monit. 2015, 21, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Sigamani, A.; Mayo, K.H.; Miller, M.C.; Chen-Walden, H.; Reddy, S.; Platt, D. An Oral Galectin Inhibitor in COVID-19—A Phase II Randomized Controlled Trial. Vaccines 2023, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.S. Virus entry: Molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2004, 2, 109–122. [Google Scholar] [CrossRef]
- Miller, N.L.; Clark, T.; Raman, R.; Sasisekharan, R. Glycans in Virus-Host Interactions: A Structural Perspective. Front. Mol. Biosci. 2021, 8, 666756. [Google Scholar] [CrossRef]
- Rotshenker, S. Galectin-3 (MAC-2) controls phagocytosis and macropinocytosis through intracellular and extracellular mechanisms. Front. Cell. Neurosci. 2022, 16, 949079. [Google Scholar] [CrossRef]
- Liu, F.T.; Stowell, S.R. The role of galectins in immunity and infection. Nat. Rev. Immunol. 2023, 1–16. [Google Scholar] [CrossRef]
- Snarr, B.D.; St-Pierre, G.; Ralph, B.; Lehoux, M.; Sato, Y.; Rancourt, A.; Takazono, T.; Baistrocchi, S.R.; Corsini, R.; Cheng, M.P.; et al. Galectin-3 enhances neutrophil motility and extravasation into the airways during Aspergillus fumigatus infection. PLoS Pathog. 2020, 16, e1008741. [Google Scholar] [CrossRef]
- Lee, M.S.; Tseng, Y.H.; Chen, Y.C.; Kuo, C.H.; Wang, S.L.; Lin, M.H.; Huang, Y.F.; Wang, Y.W.; Lin, Y.C.; Hung, C.H. M2 macrophage subset decrement is an indicator of bleeding tendency in pediatric dengue disease. J. Microbiol. Immunol. Infect. 2018, 51, 829–838. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, X.; Hu, Q.; Chen, X.; Chen, Y.; Huang, L. Galectin-3 activates TLR4/NF-κB signaling to promote lung adenocarcinoma cell proliferation through activating lncRNA-NEAT1 expression. BMC Cancer 2018, 18, 580. [Google Scholar] [CrossRef] [PubMed]
- Darif, D.; Hammi, I.; Kihel, A.; El Idrissi Saik, I.; Guessous, F.; Akarid, K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb. Pathog. 2021, 153, 104799. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Revilla, J.; Deierborg, T.; Venero, J.L.; Boza-Serrano, A. Hyperinflammation and Fibrosis in Severe COVID-19 Patients: Galectin-3, a Target Molecule to Consider. Front. Immunol. 2020, 11, 2069. [Google Scholar] [CrossRef]
- Lin, C.Y.; Yang, Z.S.; Wang, W.H.; Urbina, A.N.; Lin, Y.T.; Huang, J.C.; Liu, F.T.; Wang, S.F. The Antiviral Role of Galectins toward Influenza A Virus Infection-An Alternative Strategy for Influenza Therapy. Pharmaceuticals 2021, 14, 490. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, M.D.; Stojanovic, B.; Arsenijevic, N.; Stojanovic, B. The Role of TLR-4 and Galectin-3 Interaction in Acute Pancreatitis. Exp. Appl. Biomed. Res. (EABR) 2020. [Google Scholar] [CrossRef]
- Arsenijevic, A.; Stojanovic, B.; Milovanovic, J.; Arsenijevic, D.; Arsenijevic, N.; Milovanovic, M. Galectin-3 in Inflammasome Activation and Primary Biliary Cholangitis Development. Int. J. Mol. Sci. 2020, 21, 5097. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhu, H.; Li, C. Galectins and galectin-mediated autophagy regulation: New insights into targeted cancer therapy. Biomark. Res. 2023, 11, 22. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Nakahara, S.; Hogan, V.; Raz, A. Galectin-3 in apoptosis, a novel therapeutic target. J. Bioenerg. Biomembr. 2007, 39, 79–84. [Google Scholar] [CrossRef]
- Li, F.-Y.; Wang, S.-F.; Bernardes, E.S.; Liu, F.-T. Galectins in Host Defense Against Microbial Infections. In Lectin in Host Defense Against Microbial Infections; Hsieh, S.-L., Ed.; Springer: Singapore, 2020; pp. 141–167. [Google Scholar]
- Kulkarni, R.; Prasad, A. Exosomes Derived from HIV-1 Infected DCs Mediate Viral trans-Infection via Fibronectin and Galectin-3. Sci. Rep. 2017, 7, 14787. [Google Scholar] [CrossRef]
- Fogel, S.; Guittaut, M.; Legrand, A.; Monsigny, M.; Hébert, E. The Tat protein of HIV-1 induces galectin-3 expression. Glycobiology 1999, 9, 383–387. [Google Scholar] [CrossRef]
- Wang, S.F.; Tsao, C.H.; Lin, Y.T.; Hsu, D.K.; Chiang, M.L.; Lo, C.H.; Chien, F.C.; Chen, P.; Arthur Chen, Y.M.; Chen, H.Y.; et al. Galectin-3 promotes HIV-1 budding via association with Alix and Gag p6. Glycobiology 2014, 24, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Hung, Y.H.; Tsao, C.H.; Chiang, C.Y.; Teoh, P.G.; Chiang, M.L.; Lin, W.H.; Hsu, D.K.; Jan, H.M.; Lin, H.C.; et al. Galectin-3 facilitates cell-to-cell HIV-1 transmission by altering the composition of membrane lipid rafts in CD4 T cells. Glycobiology 2022, 32, 760–777. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Wang, S.-F.; Weng, I.C.; Hong, M.-H.; Lo, T.-H.; Jan, J.-T.; Hsu, L.-C.; Chen, H.-Y.; Liu, F.-T. Galectin-3 Enhances Avian H5N1 Influenza A Virus–Induced Pulmonary Inflammation by Promoting NLRP3 Inflammasome Activation. Am. J. Pathol. 2018, 188, 1031–1042. [Google Scholar] [CrossRef]
- Oatis, D.; Simon-Repolski, E.; Balta, C.; Mihu, A.; Pieretti, G.; Alfano, R.; Peluso, L.; Trotta, M.C.; D’Amico, M.; Hermenean, A. Cellular and Molecular Mechanism of Pulmonary Fibrosis Post-COVID-19: Focus on Galectin-1, -3, -8, -9. Int. J. Mol. Sci. 2022, 23, 8210. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kumar, D.; Butty, V.; Singh, S.; Esteban, A.; Fink, G.R.; Ploegh, H.L.; Sehrawat, S. Galectin-3 Regulates γ-Herpesvirus Specific CD8 T Cell Immunity. iScience 2018, 9, 101–119. [Google Scholar] [CrossRef]
- Okamoto, M.; Hidaka, A.; Toyama, M.; Baba, M. Galectin-3 is involved in HIV-1 expression through NF-κB activation and associated with Tat in latently infected cells. Virus Res. 2019, 260, 86–93. [Google Scholar] [CrossRef]
- Lukic, R.; Gajovic, N.; Jovanovic, I.; Jurisevic, M.; Mijailovic, Z.; Maric, V.; Popovska Jovicic, B.; Arsenijevic, N. Potential Hepatoprotective Role of Galectin-3 during HCV Infection in End-Stage Renal Disease Patients. Dis. Mrk. 2017, 2017, 6275987. [Google Scholar] [CrossRef]
- Stojanovic, B.; Milovanovic, J.; Arsenijevic, A.; Stojanovic, B.; Strazic Geljic, I.; Arsenijevic, N.; Jonjic, S.; Lukic, M.L.; Milovanovic, M. Galectin-3 Deficiency Facilitates TNF-α-Dependent Hepatocyte Death and Liver Inflammation in MCMV Infection. Front. Microbiol. 2019, 10, 185. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D. Chapter 26 Discovery and Classification of Glycan-Binding Proteins. In Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2017. [Google Scholar]
- Lindahl, U.; Couchman, J.; Kimata, K.; Esko, J.D. Proteoglycans and Sulfated Glycosaminoglycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015; pp. 207–221. [Google Scholar]
- Talaga, M.L.; Fan, N.; Fueri, A.L.; Brown, R.K.; Bandyopadhyay, P.; Dam, T.K. Multitasking Human Lectin Galectin-3 Interacts with Sulfated Glycosaminoglycans and Chondroitin Sulfate Proteoglycans. Biochemistry 2016, 55, 4541–4551. [Google Scholar] [CrossRef]
- Iwaki, J.; Minamisawa, T.; Tateno, H.; Kominami, J.; Suzuki, K.; Nishi, N.; Nakamura, T.; Hirabayashi, J. Desulfated galactosaminoglycans are potential ligands for galectins: Evidence from frontal affinity chromatography. Biochem. Biophys. Res. Commun. 2008, 373, 206–212. [Google Scholar] [CrossRef]
- Tobacman, J.K.; Bhattacharyya, S. Profound Impact of Decline in N-Acetylgalactosamine-4-Sulfatase (Arylsulfatase B) on Molecular Pathophysiology and Human Diseases. Int. J. Mol. Sci. 2022, 23, 13146. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-B.; Yoon, H.J.; Chang, C.Y.; Koh, H.S.; Jeon, S.-H.; Park, E.J. Galectin-3 Exerts Cytokine-Like Regulatory Actions through the JAK–STAT Pathway. J. Immunol. 2010, 185, 7037–7046. [Google Scholar] [CrossRef] [PubMed]
- Ezeonwumelu, I.J.; Garcia-Vidal, E.; Ballana, E. JAK-STAT Pathway: A Novel Target to Tackle Viral Infections. Viruses 2021, 13, 2379. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.M.; Bum-Erdene, K.; Yu, X.; Blanchard, H. Galectin-3 Interactions with Glycosphingolipids. J. Mol. Biol. 2014, 426, 1439–1451. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.; Zhou, Z.; Xiao, Y. Emerging roles of Galectin-3 in diabetes and diabetes complications: A snapshot. Rev. Endocr. Metab. Disord. 2022, 23, 569–577. [Google Scholar] [CrossRef]
- Li, P.; Liu, S.; Lu, M.; Bandyopadhyay, G.; Oh, D.; Imamura, T.; Johnson, A.M.F.; Sears, D.; Shen, Z.; Cui, B.; et al. Hematopoietic-Derived Galectin-3 Causes Cellular and Systemic Insulin Resistance. Cell 2016, 167, 973–984.e12. [Google Scholar] [CrossRef]
- Kuo, H.Y.; Hsu, H.T.; Chen, Y.C.; Chang, Y.W.; Liu, F.T.; Wu, C.W. Galectin-3 modulates the EGFR signalling-mediated regulation of Sox2 expression via c-Myc in lung cancer. Glycobiology 2016, 26, 155–165. [Google Scholar] [CrossRef]
- Sedlář, A.; Trávníčková, M.; Bojarová, P.; Vlachová, M.; Slámová, K.; Křen, V.; Bačáková, L. Interaction between Galectin-3 and Integrins Mediates Cell-Matrix Adhesion in Endothelial Cells and Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 5144. [Google Scholar] [CrossRef]
- Giovannone, N.; Smith, L.K.; Treanor, B.; Dimitroff, C.J. Galectin-Glycan Interactions as Regulators of B Cell Immunity. Front. Immunol. 2018, 9, 2839. [Google Scholar] [CrossRef]
- Carlsson, M.C.; Bengtson, P.; Cucak, H.; Leffler, H. Galectin-3 guides intracellular trafficking of some human serotransferrin glycoforms. J. Biol. Chem. 2013, 288, 28398–28408. [Google Scholar] [CrossRef] [PubMed]
- Daffu, G.; del Pozo, C.H.; O’Shea, K.M.; Ananthakrishnan, R.; Ramasamy, R.; Schmidt, A.M. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int. J. Mol. Sci. 2013, 14, 19891–19910. [Google Scholar] [CrossRef] [PubMed]
- Monserrat, J.; Gómez-Lahoz, A.; Ortega, M.A.; Sanz, J.; Muñoz, B.; Arévalo-Serrano, J.; Rodríguez, J.M.; Gasalla, J.M.; Gasulla, Ó.; Arranz, A.; et al. Role of Innate and Adaptive Cytokines in the Survival of COVID-19 Patients. Int. J. Mol. Sci. 2022, 23, 10344. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef]
- Biji, A.; Khatun, O.; Swaraj, S.; Narayan, R.; Rajmani, R.S.; Sardar, R.; Satish, D.; Mehta, S.; Bindhu, H.; Jeevan, M.; et al. Identification of COVID-19 prognostic markers and therapeutic targets through meta-analysis and validation of Omics data from nasopharyngeal samples. EBioMedicine 2021, 70, 103525. [Google Scholar] [CrossRef]
- Puccini, M.; Jakobs, K.; Reinshagen, L.; Friebel, J.; Schencke, P.-A.; Ghanbari, E.; Landmesser, U.; Haghikia, A.; Kränkel, N.; Rauch, U. Galectin-3 as a Marker for Increased Thrombogenicity in COVID-19. Int. J. Mol. Sci. 2023, 24, 7683. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, N.; Liu, Y.; Yang, X.; He, Y.; Li, Q.; Shen, X.; Zhu, Y.; Yang, Y. The Interaction Between Pulmonary Fibrosis and COVID-19 and the Application of Related Anti-Fibrotic Drugs. Front. Pharm. 2021, 12, 805535. [Google Scholar] [CrossRef]
- Cervantes-Alvarez, E.; la Rosa, N.L.-d.; la Mora, M.S.-d.; Valdez-Sandoval, P.; Palacios-Jimenez, M.; Rodriguez-Alvarez, F.; Vera-Maldonado, B.I.; Aguirre-Aguilar, E.; Escobar-Valderrama, J.M.; Alanis-Mendizabal, J.; et al. Galectin-3 as a potential prognostic biomarker of severe COVID-19 in SARS-CoV-2 infected patients. Sci. Rep. 2022, 12, 1856. [Google Scholar] [CrossRef]
- Baykiz, D.; Emet, S.; Ayduk-Govdeli, E.; Kaytaz, M.; Yavuz, M.L.; Karaca-Ozer, P.; Karaayvaz, E.B.; Medetalibeyoglu, A.; Elitok, A.; Genc, S.; et al. Galectin-3 as a Novel Biomarker for Predicting Clinical Outcomes in Hospitalized COVID-19 Patients. Clin. Lab. 2022, 68, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Özcan, S.; Dönmez, E.; Yavuz, S.T.; Ziyrek, M.; İnce, O.; Küçük, H.S.; Taşdemir, Z.A.; Yılmaz, İ.; Varol, S.; Şahin, İ.; et al. Prognostic significance of serum galectin-3 in hospitalized patients with COVID-19. Cytokine 2022, 158, 155970. [Google Scholar] [CrossRef]
- Reddy, K.; Nichol, A.; McAuley, D.F. Galectin-3 Inhibition in COVID-19. Am. J. Respir. Crit. Care Med. 2023, 207, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Sures, I.; Pompetti, F.; Natoli, G.; Palka, G.; Iacobelli, S. The gene (LGALS3BP) encoding the serum protein 90K, associated with cancer and infection by the human immunodeficiency virus, maps at 17q25. Cytogenet. Cell Genet. 1995, 69, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Xia, Z.; Deng, F.; Liu, L.; Wang, Q.; Yu, Y.; Wang, F.; Zhu, C.; Liu, W.; Cheng, Z.; et al. Inducible LGALS3BP/90K activates antiviral innate immune responses by targeting TRAF6 and TRAF3 complex. PLoS Pathog. 2019, 15, e1008002. [Google Scholar] [CrossRef] [PubMed]
- Kuśnierz-Cabala, B.; Maziarz, B.; Dumnicka, P.; Dembiński, M.; Kapusta, M.; Bociąga-Jasik, M.; Winiarski, M.; Garlicki, A.; Grodzicki, T.; Kukla, M. Diagnostic Significance of Serum Galectin-3 in Hospitalized Patients with COVID-19-A Preliminary Study. Biomolecules 2021, 11, 1136. [Google Scholar] [CrossRef]
- Gallo, V.; Gentile, R.; Antonini, G.; Iacobelli, S. Increased Gal-3BP plasma levels in hospitalized patients infected with SARS-CoV-2. Clin. Exp. Med. 2023, 23, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Boasso, A.; Shearer, G.M.; Chougnet, C. Immune dysregulation in human immunodeficiency virus infection: Know it, fix it, prevent it? J. Intern. Med. 2009, 265, 78–96. [Google Scholar] [CrossRef]
- Wang, W.H.; Yeh, C.S.; Lin, C.Y.; Yuan, R.Y.; Urbina, A.N.; Lu, P.L.; Chen, Y.H.; Chen, Y.A.; Liu, F.T.; Wang, S.F. Amino Acid Deletions in p6(Gag) Domain of HIV-1 CRF07_BC Ameliorate Galectin-3 Mediated Enhancement in Viral Budding. Int. J. Mol. Sci. 2020, 21, 2910. [Google Scholar] [CrossRef]
- Das, A.T.; Harwig, A.; Berkhout, B. The HIV-1 Tat protein has a versatile role in activating viral transcription. J. Virol. 2011, 85, 9506–9516. [Google Scholar] [CrossRef]
- Clark, E.; Nava, B.; Caputi, M. Tat is a multifunctional viral protein that modulates cellular gene expression and functions. Oncotarget 2017, 8, 27569–27581. [Google Scholar] [CrossRef]
- Loregian, A.; Bortolozzo, K.; Boso, S.; Caputo, A.; Palù, G. Interaction of Sp1 transcription factor with HIV-1 Tat protein: Looking for cellular partners. FEBS Lett. 2003, 543, 61–65. [Google Scholar] [CrossRef]
- Wang, W.-H.; Lin, C.-Y.; Chang, M.R.; Urbina, A.N.; Assavalapsakul, W.; Thitithanyanont, A.; Chen, Y.-H.; Liu, F.-T.; Wang, S.-F. The role of galectins in virus infection—A systemic literature review. J. Microbiol. Immunol. Infect. 2020, 53, 925–935. [Google Scholar] [CrossRef]
- Feldmann, J.; Schwartz, O. HIV-1 Virological Synapse: Live Imaging of Transmission. Viruses 2010, 2, 1666–1680. [Google Scholar] [CrossRef]
- Xue, J.; Fu, C.; Cong, Z.; Peng, L.; Peng, Z.; Chen, T.; Wang, W.; Jiang, H.; Wei, Q.; Qin, C. Galectin-3 promotes caspase-independent cell death of HIV-1-infected macrophages. FEBS J. 2017, 284, 97–113. [Google Scholar] [CrossRef]
- Alcendor, D.J.; Knobel, S.M.; Desai, P.; Zhu, W.Q.; Vigil, H.E.; Hayward, G.S. KSHV downregulation of galectin-3 in Kaposi’s sarcoma. Glycobiology 2010, 20, 521–532. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Rezaei, N. Influenza Viruses. In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Oxford, UK, 2022; pp. 67–78. [Google Scholar]
- Li, X.; Gu, M.; Zheng, Q.; Gao, R.; Liu, X. Packaging signal of influenza A virus. Virol. J. 2021, 18, 36. [Google Scholar] [CrossRef]
- Kosik, I.; Yewdell, J.W. Influenza Hemagglutinin and Neuraminidase: Yin-Yang Proteins Coevolving to Thwart Immunity. Viruses 2019, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Zhou, B.; Yuan, J.; Yang, G. Human Infected H5N1 Avian Influenza. Radiol. Influenza 2016, 67–76. [Google Scholar] [CrossRef]
- Yang, Z.-S.; Lin, C.-Y.; Huang, S.-W.; Wang, W.-H.; Urbina, A.N.; Tseng, S.-P.; Lu, P.-L.; Chen, Y.-H.; Wang, S.-F. Regulatory roles of galectins on influenza A virus and their potential as a therapeutic strategy. Biomed. Pharmacother. 2021, 139, 111713. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Yang, T.C.; Lai, C.C.; Huang, S.H.; Liao, J.M.; Wan, L.; Lin, Y.J.; Lin, C.W. Antiviral activity of aloe-emodin against influenza A virus via galectin-3 up-regulation. Eur. J. Pharm. 2014, 738, 125–132. [Google Scholar] [CrossRef]
- Mikušová, M.; Tomčíková, K.; Briestenská, K.; Kostolanský, F.; Varečková, E. The Contribution of Viral Proteins to the Synergy of Influenza and Bacterial Co-Infection. Viruses 2022, 14, 1064. [Google Scholar] [CrossRef]
- Saeed, U.; Waheed, Y.; Ashraf, M. Hepatitis B and hepatitis C viruses: A review of viral genomes, viral induced host immune responses, genotypic distributions and worldwide epidemiology. Asian Pac. J. Trop. Dis. 2014, 4, 88–96. [Google Scholar] [CrossRef]
- Weigand, K.; Peschel, G.; Grimm, J.; Müller, M.; Buechler, C. Serum Galectin-3 in Hepatitis C Virus Infection Declines after Successful Virus Eradication by Direct-Acting Antiviral Therapy. J. Gastrointestin. Liver Dis. 2022, 31, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, I.; Taniguchi, K.; Komatsu, F.; Sasaki, A.; Andoh, T.; Nojima, H.; Shiraki, K.; Hsu, D.K.; Liu, F.-T.; Kato, I.; et al. Contribution of spinal galectin-3 to acute herpetic allodynia in mice. PAIN 2012, 153, 585–592. [Google Scholar] [CrossRef]
- Wang, T.; Fei, Y.; Yao, M.; Tao, J.; Deng, J.; Huang, B. Correlation between Galectin-3 and Early Herpes Zoster Neuralgia and Postherpetic Neuralgia: A Retrospective Clinical Observation. Pain Res. Manag. 2020, 2020, 8730918. [Google Scholar] [CrossRef] [PubMed]
- Schleiss, M.R. Cytomegalovirus. In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Oxford, UK, 2022; pp. 53–58. [Google Scholar]
- Gupta, M.; Shorman, M. Cytomegalovirus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Krmpotic, A.; Bubic, I.; Polic, B.; Lucin, P.; Jonjic, S. Pathogenesis of murine cytomegalovirus infection. Microbes Infect. 2003, 5, 1263–1277. [Google Scholar] [CrossRef]
- Goncalves, P.H.; Ziegelbauer, J.; Uldrick, T.S.; Yarchoan, R. Kaposi sarcoma herpesvirus-associated cancers and related diseases. Curr. Opin HIV AIDS 2017, 12, 47–56. [Google Scholar] [CrossRef]
- Lynch, J.P., 3rd; Kajon, A.E. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [CrossRef]
- Mackinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J.; et al. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537–546. [Google Scholar] [CrossRef]
- Nayak, G.; Bhuyan, S.K.; Bhuyan, R.; Sahu, A.; Kar, D.; Kuanar, A. Global emergence of Enterovirus 71: A systematic review. Beni. Suef. Univ. J. Basic Appl. Sci. 2022, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Garcin, P.O.; Panté, N. Cell migration is another player of the minute virus of mice infection. Virology 2014, 468–470, 150–159. [Google Scholar] [CrossRef]
- Garcin, P.O.; Nabi, I.R.; Panté, N. Galectin-3 plays a role in minute virus of mice infection. Virology 2015, 481, 63–72. [Google Scholar] [CrossRef]
- Caniglia, J.L.; Guda, M.R.; Asuthkar, S.; Tsung, A.J.; Velpula, K.K. A potential role for Galectin-3 inhibitors in the treatment of COVID-19. PeerJ 2020, 8, e9392. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Anam, K.; Ahmed, H. Development of Galectin-3 Targeting Drugs for Therapeutic Applications in Various Diseases. Int. J. Mol. Sci. 2023, 24, 8116. [Google Scholar] [CrossRef] [PubMed]
- Gaughan, E.E.; Quinn, T.M.; Mills, A.; Bruce, A.M.; Antonelli, J.; MacKinnon, A.C.; Aslanis, V.; Li, F.; O’Connor, R.; Boz, C.; et al. An Inhaled Galectin-3 Inhibitor in COVID-19 Pneumonitis: A Phase Ib/IIa Randomized Controlled Clinical Trial (DEFINE). Am. J. Respir. Crit. Care Med. 2023, 207, 138–149. [Google Scholar] [CrossRef]
- Stasenko, M.; Smith, E.; Yeku, O.; Park, K.J.; Laster, I.; Lee, K.; Walderich, S.; Spriggs, E.; Rueda, B.; Weigelt, B.; et al. Targeting galectin-3 with a high-affinity antibody for inhibition of high-grade serous ovarian cancer and other MUC16/CA-125-expressing malignancies. Sci. Rep. 2021, 11, 3718. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mangala, L.S.; Rodriguez-Aguayo, C.; Kong, X.; Lopez-Berestein, G.; Sood, A.K. RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 2018, 37, 107–124. [Google Scholar] [CrossRef]
- Dings, R.P.M.; Miller, M.C.; Griffin, R.J.; Mayo, K.H. Galectins as Molecular Targets for Therapeutic Intervention. Int. J. Mol. Sci. 2018, 19, 905. [Google Scholar] [CrossRef] [PubMed]
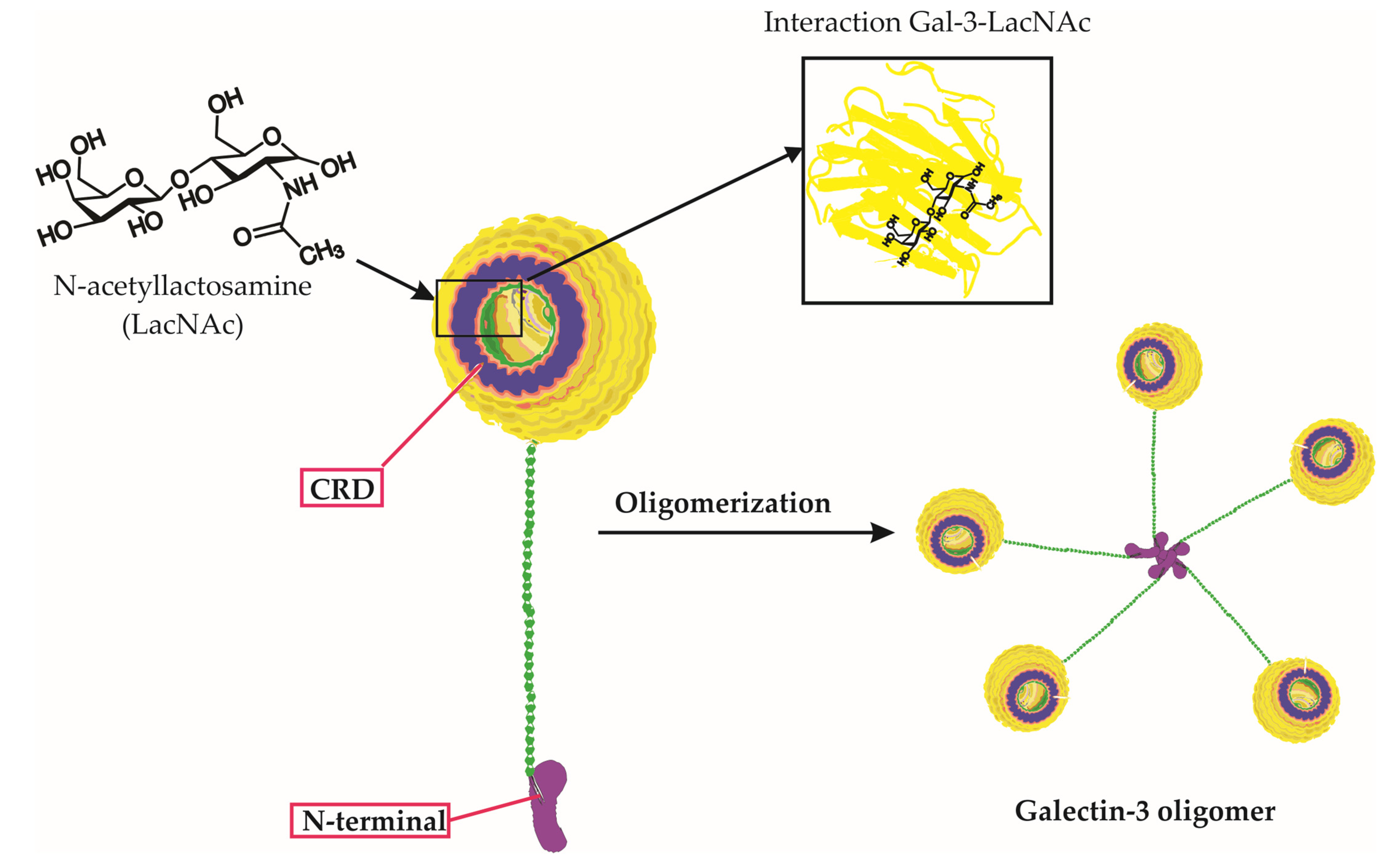
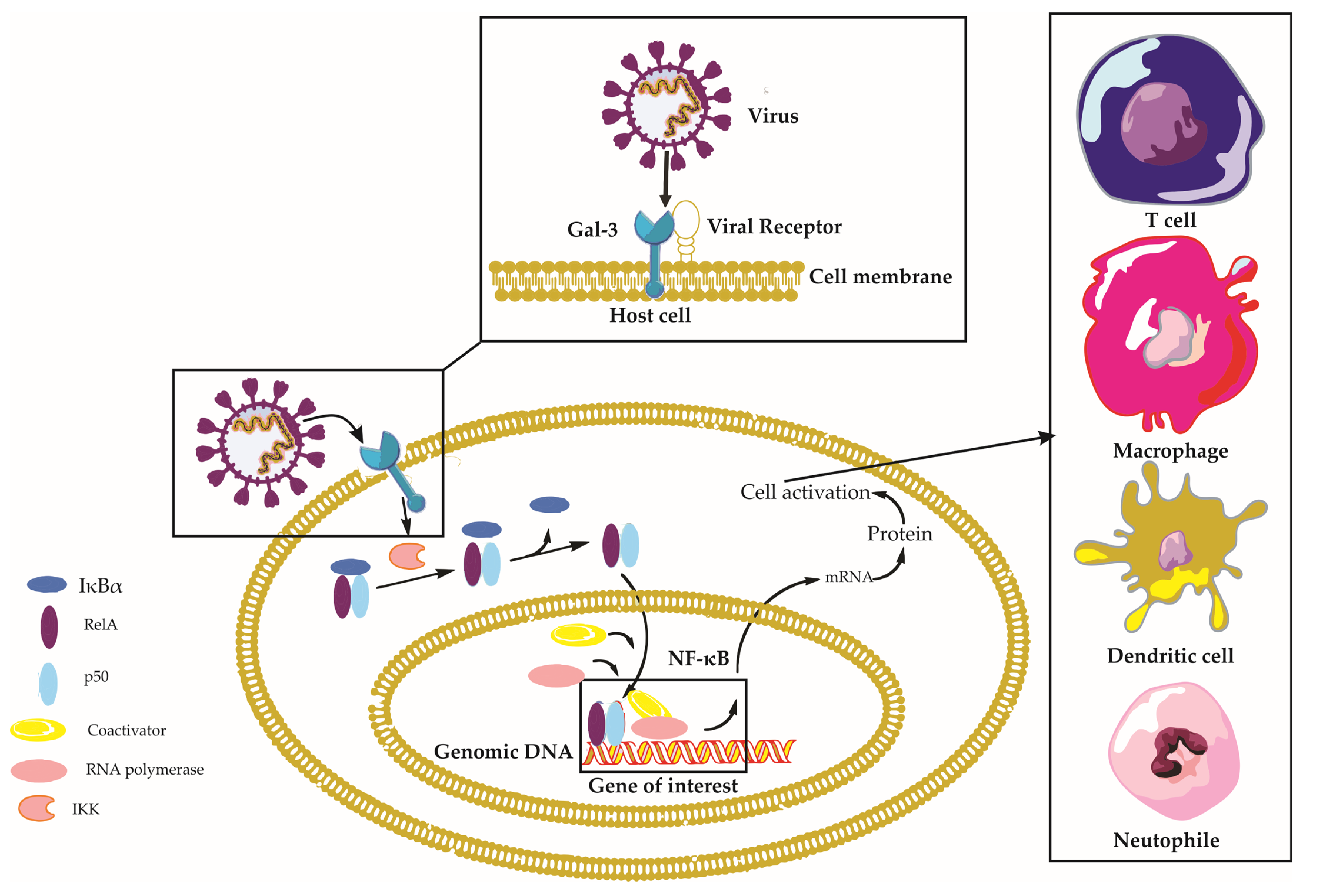
| Role | Description | Examples of Viruses Involved |
|---|---|---|
| Viral entry | Facilitates virus attachment and entry into host cells through binding to viral and host cell surface glycoproteins | HIV [15], SARS-CoV-2 [16], HSV [17], Influenza [18] |
| Viral replication | Modulates key cellular signaling pathways that influence viral replication processes | Influenza [19], Enterovirus [20] |
| Immune response modulation | Regulates both innate and adaptive immune responses, influencing the balance between pro-inflammatory and anti-inflammatory responses | Influenza [21], SARS-CoV-2 [22], HBV [23] |
| Potential therapeutic target | Development of novel Gal-3 inhibitors and their application in antiviral therapy | SARS-CoV-2 [24] |
| Viral Infection | Sample Type | Galectin-3 Expression Pattern | Clinical Implications |
|---|---|---|---|
| Influenza | Serum, bronchoalveolar lavage fluid | Elevated levels during infection | Prognosis, disease severity |
| Human immunodeficiency virus | Plasma, peripheral blood mononuclear cells (PBMCs) | Elevated levels in patients | Disease progression, immune dysfunction |
| Hepatitis C virus | Serum, liver tissue | Elevated levels in patients | Liver fibrosis, inflammation |
| Severe acute respiratory syndrome coronavirus 2 | Serum, plasma, bronchoalveolar lavage fluid, lung tissue | Elevated levels in patients | Disease severity, prognosis, potential therapeutic target |
| Research Area | Description |
|---|---|
| Mechanisms of Gal-3-mediated viral entry | Elucidate the molecular mechanisms through which Gal-3 facilitates virus attachment and entry into host cells |
| Role in viral latency | Investigate the potential involvement of Gal-3 in maintaining viral latency and reactivation processes |
| Immune response regulation | Unravel the complex regulatory mechanisms through which Gal-3 modulates host immune responses to viral infections |
| Development of novel Gal-3 inhibitors | Design, synthesis, and evaluation of new Gal-3 inhibitors with improved potency and selectivity for antiviral therapy |
| Clinical trials | Conduct clinical trials to assess the safety, efficacy, and potential side effects of Gal-3 inhibitors in antiviral therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanovic, B.S.; Stojanovic, B.; Milovanovic, J.; Arsenijević, A.; Dimitrijevic Stojanovic, M.; Arsenijevic, N.; Milovanovic, M. The Pivotal Role of Galectin-3 in Viral Infection: A Multifaceted Player in Host–Pathogen Interactions. Int. J. Mol. Sci. 2023, 24, 9617. https://doi.org/10.3390/ijms24119617
Stojanovic BS, Stojanovic B, Milovanovic J, Arsenijević A, Dimitrijevic Stojanovic M, Arsenijevic N, Milovanovic M. The Pivotal Role of Galectin-3 in Viral Infection: A Multifaceted Player in Host–Pathogen Interactions. International Journal of Molecular Sciences. 2023; 24(11):9617. https://doi.org/10.3390/ijms24119617
Chicago/Turabian StyleStojanovic, Bojana S., Bojan Stojanovic, Jelena Milovanovic, Aleksandar Arsenijević, Milica Dimitrijevic Stojanovic, Nebojsa Arsenijevic, and Marija Milovanovic. 2023. "The Pivotal Role of Galectin-3 in Viral Infection: A Multifaceted Player in Host–Pathogen Interactions" International Journal of Molecular Sciences 24, no. 11: 9617. https://doi.org/10.3390/ijms24119617
APA StyleStojanovic, B. S., Stojanovic, B., Milovanovic, J., Arsenijević, A., Dimitrijevic Stojanovic, M., Arsenijevic, N., & Milovanovic, M. (2023). The Pivotal Role of Galectin-3 in Viral Infection: A Multifaceted Player in Host–Pathogen Interactions. International Journal of Molecular Sciences, 24(11), 9617. https://doi.org/10.3390/ijms24119617








