Towards a New Biomarker for Diabetic Retinopathy: Exploring RBP3 Structure and Retinoids Binding for Functional Imaging of Eyes In Vivo
Abstract
1. Introduction
1.1. Diabetic Retinopathy
1.2. Eye Imaging
2. Diabetic Retinopathy
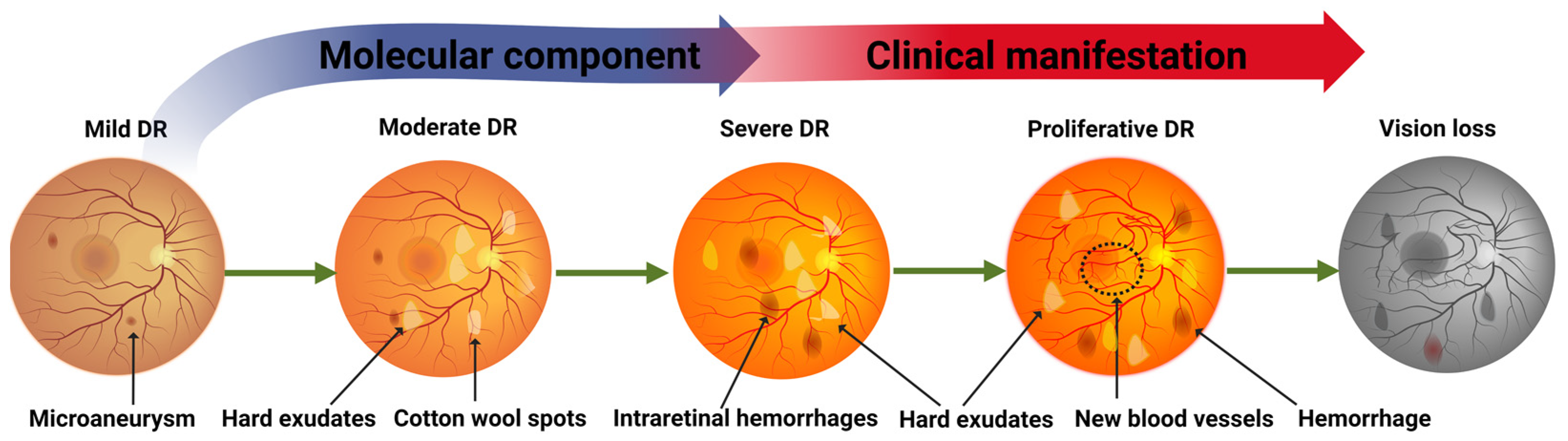
2.1. Clinical Progression
2.2. Molecular Component
2.3. Biomarkers
2.4. Non-Invasive Tracing of Disease Manifestations
3. RBP3/Two-Photon
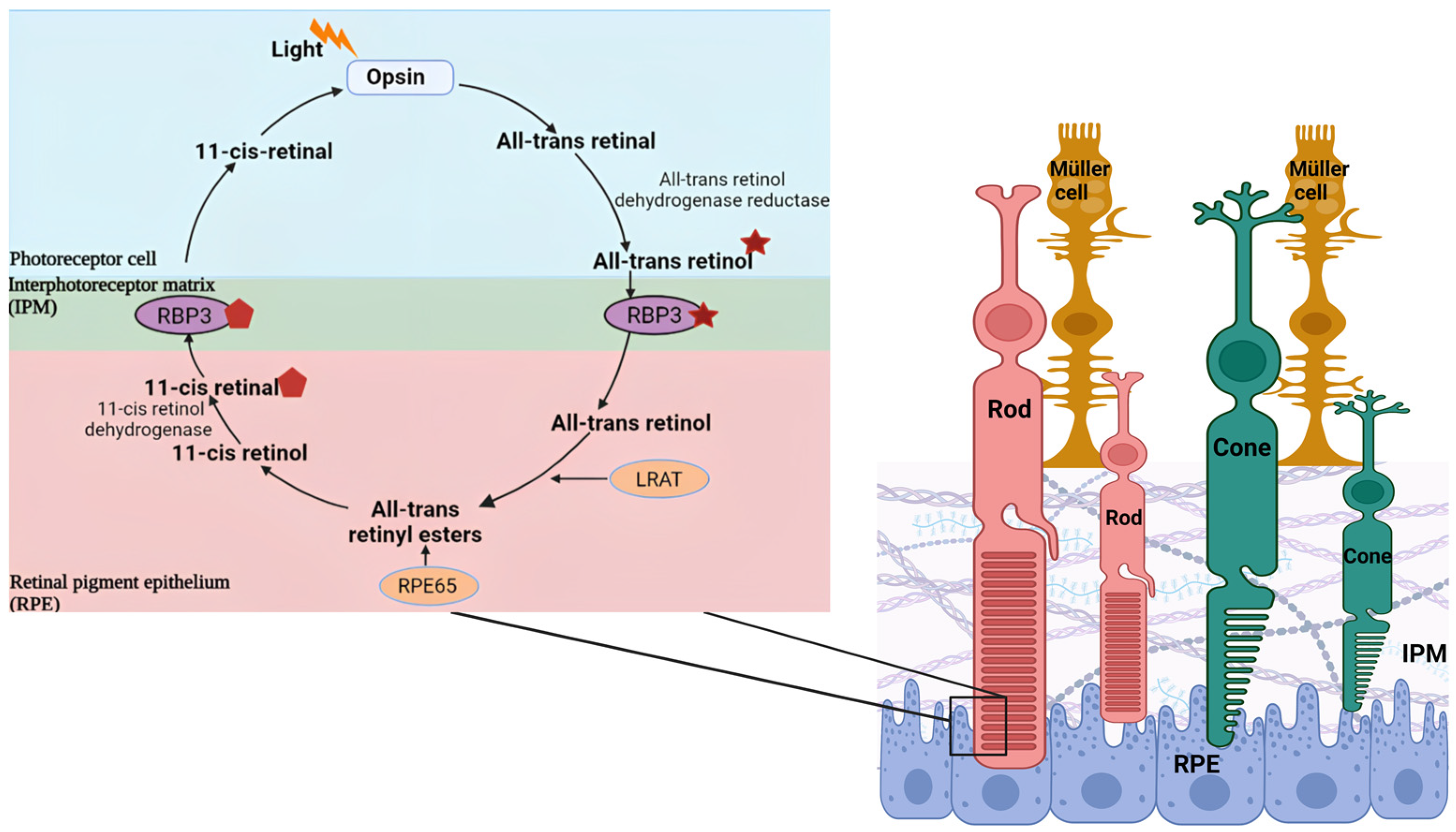
3.1. RBP3
3.1.1. Historical
3.1.2. Gene Duplication
3.1.3. Ligand Binding
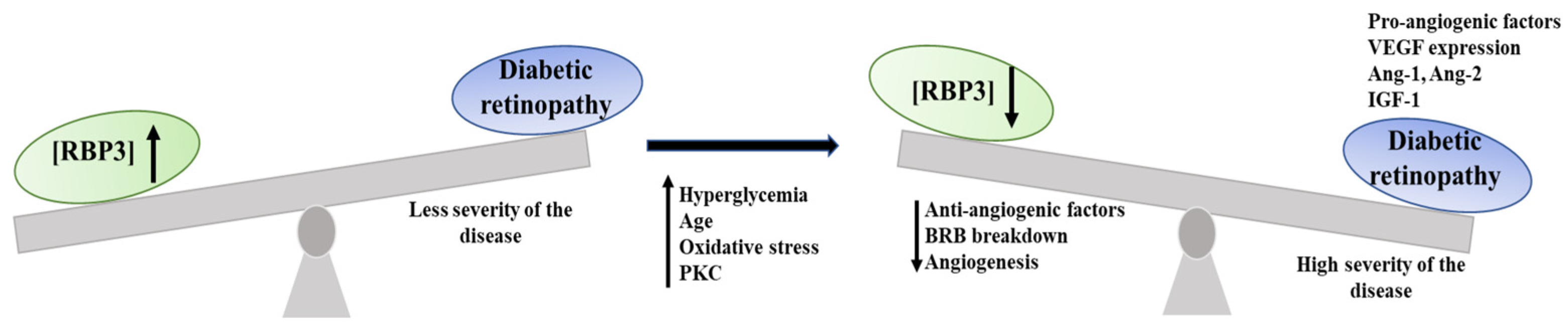
3.1.4. Link to DR
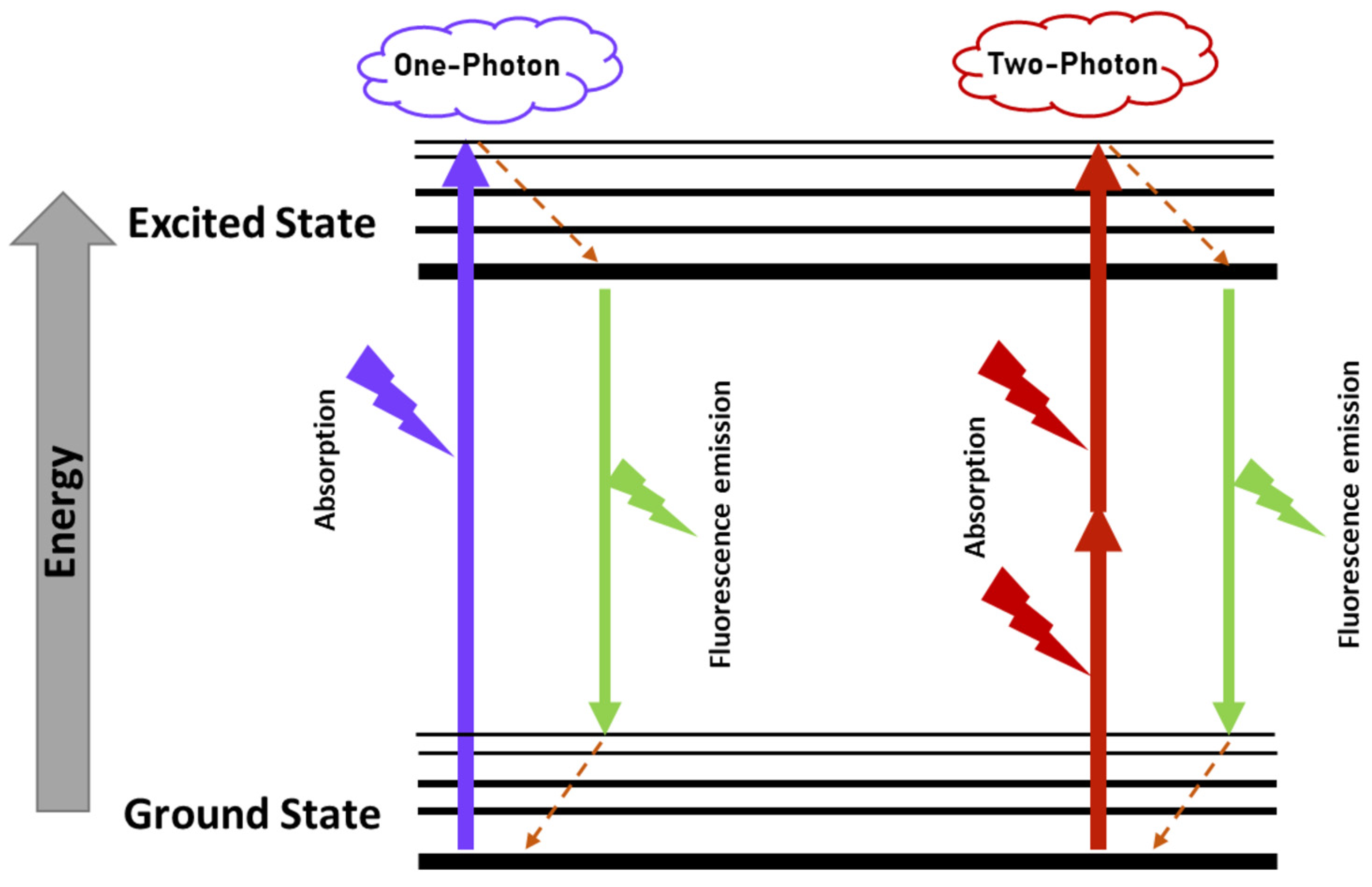
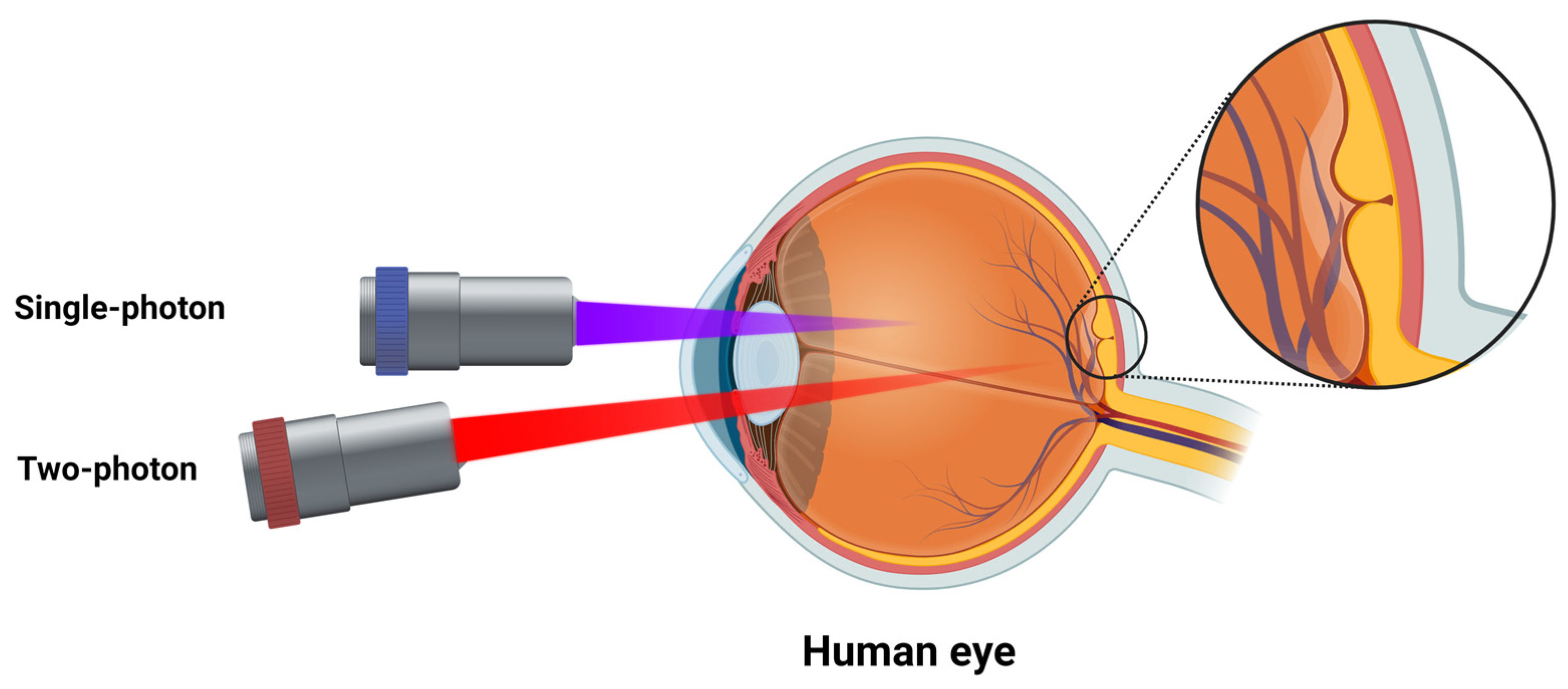
3.2. Two-Photon Excitation Fluorescence
3.2.1. Historical
3.2.2. Clinical Applications
3.2.3. Retinoids/Phasor
3.2.4. Possible Applications in a DR Context
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frudd, K.; Sivaprasad, S.; Raman, R.; Krishnakumar, S.; Revathy, Y.R.; Turowski, P. Diagnostic circulating biomarkers to detect vision-threatening diabetic retinopathy: Potential screening tool of the future? Acta Ophthalmol. 2022, 100, e648–e668. [Google Scholar] [CrossRef]
- Berkowitz, B.A. Preventing diabetic retinopathy by mitigating subretinal space oxidative stress in vivo. Vis. Neurosci. 2020, 37, E002. [Google Scholar] [CrossRef]
- Jenkins, A.J.; Joglekar, M.V.; Hardikar, A.A.; Keech, A.C.; O’Neal, D.N.; Januszewski, A.S. Biomarkers in Diabetic Retinopathy. Rev. Diabet. Stud. 2015, 12, 159–195. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; He, M.; Congdon, N. The worldwide epidemic of diabetic retinopathy. Indian J. Ophthalmol. 2012, 60, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Jampol, L.M.; Glassman, A.R.; Sun, J. Evaluation and Care of Patients with Diabetic Retinopathy. N. Engl. J. Med. 2020, 382, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.E.; Browning, D.J.; Wong, K.; Flynn, H.W., Jr.; Bhavsar, A.R. The Evolving Treatment of Diabetic Retinopathy. Clin. Ophthalmol. 2020, 14, 653–678. [Google Scholar] [CrossRef]
- Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Bressler, N.M.; Bressler, S.B.; Brucker, A.J.; Ferris, F.L.; Hampton, G.R.; Jhaveri, C.; et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology 2016, 123, 1351–1359. [Google Scholar] [CrossRef]
- Cecilia, O.M.; Jose Alberto, C.G.; Jose, N.P.; Ernesto German, C.M.; Ana Karen, L.C.; Luis Miguel, R.P.; Ricardo Raul, R.R.; Adolfo Daniel, R.C. Oxidative Stress as the Main Target in Diabetic Retinopathy Pathophysiology. J. Diabetes Res. 2019, 2019, 8562408. [Google Scholar] [CrossRef]
- Gardner, T.W.; Chew, E.Y. Future opportunities in diabetic retinopathy research. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 91–96. [Google Scholar] [CrossRef]
- Ting, D.S.; Tan, K.A.; Phua, V.; Tan, G.S.; Wong, C.W.; Wong, T.Y. Biomarkers of Diabetic Retinopathy. Curr. Diabet. Rep. 2016, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Barber, A.J.; Bronson, S.K.; Freeman, W.M.; Gardner, T.W.; Jefferson, L.S.; Kester, M.; Kimball, S.R.; Krady, J.K.; LaNoue, K.F.; et al. Diabetic retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes 2006, 55, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Safi, H.; Safi, S.; Hafezi-Moghadam, A.; Ahmadieh, H. Early detection of diabetic retinopathy. Surv. Ophthalmol. 2018, 63, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Malechka, V.V.; Moiseyev, G.; Takahashi, Y.; Shin, Y.; Ma, J.X. Impaired Rhodopsin Generation in the Rat Model of Diabetic Retinopathy. Am. J. Pathol. 2017, 187, 2222–2231. [Google Scholar] [CrossRef]
- Parisi, V.; Uccioli, L. Visual electrophysiological responses in persons with type 1 diabetes. Diabetes Metab. Res. Rev. 2001, 17, 12–18. [Google Scholar] [CrossRef]
- Bearse, M.A., Jr.; Han, Y.; Schneck, M.E.; Barez, S.; Jacobsen, C.; Adams, A.J. Local multifocal oscillatory potential abnormalities in diabetes and early diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3259–3265. [Google Scholar] [CrossRef]
- Han, Y.; Bearse, M.A., Jr.; Schneck, M.E.; Barez, S.; Jacobsen, C.H.; Adams, A.J. Multifocal electroretinogram delays predict sites of subsequent diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2004, 45, 948–954. [Google Scholar] [CrossRef]
- Adams, A.J.; Bearse, M.A., Jr. Retinal neuropathy precedes vasculopathy in diabetes: A function-based opportunity for early treatment intervention? Clin. Exp. Optom. 2012, 95, 256–265. [Google Scholar] [CrossRef]
- McAnany, J.J.; Park, J.C. Temporal Frequency Abnormalities in Early-Stage Diabetic Retinopathy Assessed by Electroretinography. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4871–4879. [Google Scholar] [CrossRef] [PubMed]
- McAnany, J.J.; Park, J.C.; Chau, F.Y.; Leiderman, Y.I.; Lim, J.I.; Blair, N.P. Amplitude Loss of the High-Frequency Flicker Electroretinogram in Early Diabetic Retinopathy. Retina 2019, 39, 2032–2039. [Google Scholar] [CrossRef]
- Daley, M.L.; Watzke, R.C.; Riddle, M.C. Early loss of blue-sensitive color vision in patients with type I diabetes. Diabetes Care 1987, 10, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Henson, D.B.; North, R.V. Dark adaptation in diabetes mellitus. Br. J. Ophthalmol. 1979, 63, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Lieth, E.; Gardner, T.W.; Barber, A.J.; Antonetti, D.A. Retinal neurodegeneration: Early pathology in diabetes. Clin. Exp. Ophthalmol. 2000, 28, 3–8. [Google Scholar] [CrossRef]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef]
- Simo, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Pusparajah, P.; Lee, L.H.; Abdul Kadir, K. Molecular Markers of Diabetic Retinopathy: Potential Screening Tool of the Future? Front. Physiol. 2016, 7, 200. [Google Scholar] [CrossRef]
- Catalani, E.; Cervia, D. Diabetic retinopathy: A matter of retinal ganglion cell homeostasis. Neural Regen. Res. 2020, 15, 1253–1254. [Google Scholar] [CrossRef]
- Potilinski, M.C.; Lorenc, V.; Perisset, S.; Gallo, J.E. Mechanisms behind Retinal Ganglion Cell Loss in Diabetes and Therapeutic Approach. Int. J. Mol. Sci. 2020, 21, 2351. [Google Scholar] [CrossRef]
- Tonade, D.; Kern, T.S. Photoreceptor cells and RPE contribute to the development of diabetic retinopathy. Prog. Retin. Eye Res. 2021, 83, 100919. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, T.; Madigan, M.C.; Fernando, N.; Aggio-Bruce, R.; Zhou, F.; Pierce, M.; Chen, Y.; Huang, L.; Natoli, R.; et al. Interphotoreceptor Retinoid-Binding Protein (IRBP) in Retinal Health and Disease. Front. Cell NeuroSci. 2020, 14, 577935. [Google Scholar] [CrossRef]
- Garcia-Ramirez, M.; Hernandez, C.; Villarroel, M.; Canals, F.; Alonso, M.A.; Fortuny, R.; Masmiquel, L.; Navarro, A.; Garcia-Arumi, J.; Simo, R. Interphotoreceptor retinoid-binding protein (IRBP) is downregulated at early stages of diabetic retinopathy. Diabetologia 2009, 52, 2633–2641. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, F.; Ghosh, D. Focus on Molecules: Interphotoreceptor retinoid-binding protein (IRBP). Exp. Eye Res. 2008, 86, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Li, S.; Nusinowitz, S.; Lloyd, M.; Hu, J.; Radu, R.A.; Bok, D.; Travis, G.H. The role of interphotoreceptor retinoid-binding protein on the translocation of visual retinoids and function of cone photoreceptors. J. NeuroSci. 2009, 29, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, T.G.; Takimoto, N.; Jin, M.; Tsuda, M. Evolution and the origin of the visual retinoid cycle in vertebrates. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2897–2910. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, F. Interphotoreceptor retinoid-binding protein--an old gene for new eyes. Vis. Res. 2003, 43, 3021–3036. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Blakeley, L.R.; Cornwall, M.C.; Crouch, R.K.; Wiggert, B.N.; Koutalos, Y. Interphotoreceptor retinoid-binding protein is the physiologically relevant carrier that removes retinol from rod photoreceptor outer segments. Biochemistry 2007, 46, 8669–8679. [Google Scholar] [CrossRef]
- Yokomizo, H.; Maeda, Y.; Park, K.; Clermont, A.C.; Hernandez, S.L.; Fickweiler, W.; Li, Q.; Wang, C.H.; Paniagua, S.M.; Simao, F.; et al. Retinol binding protein 3 is increased in the retina of patients with diabetes resistant to diabetic retinopathy. Sci. Transl. Med. 2019, 11, eaau6627. [Google Scholar] [CrossRef]
- Vujosevic, S.; Aldington, S.J.; Silva, P.; Hernandez, C.; Scanlon, P.; Peto, T.; Simo, R. Screening for diabetic retinopathy: New perspectives and challenges. Lancet Diabetes Endocrinol. 2020, 8, 337–347. [Google Scholar] [CrossRef]
- Aspelund, T.; Thornorisdottir, O.; Olafsdottir, E.; Gudmundsdottir, A.; Einarsdottir, A.B.; Mehlsen, J.; Einarsson, S.; Palsson, O.; Einarsson, G.; Bek, T.; et al. Individual risk assessment and information technology to optimise screening frequency for diabetic retinopathy. Diabetologia 2011, 54, 2525–2532. [Google Scholar] [CrossRef]
- Andersson, E.; Persson, S.; Hallen, N.; Ericsson, A.; Thielke, D.; Lindgren, P.; Steen Carlsson, K.; Jendle, J. Costs of diabetes complications: Hospital-based care and absence from work for 392,200 people with type 2 diabetes and matched control participants in Sweden. Diabetologia 2020, 63, 2582–2594. [Google Scholar] [CrossRef]
- Entenberg, D.; Oktay, M.H.; Condeelis, J.S. Intravital imaging to study cancer progression and metastasis. Nat. Rev. Cancer 2023, 23, 25–42. [Google Scholar] [CrossRef]
- Li, H.; Yu, J.; Zhang, R.; Li, X.; Zheng, W. Two-photon excitation fluorescence lifetime imaging microscopy: A promising diagnostic tool for digestive tract tumors. J. Innov. Opt. Health Sci. 2019, 12, 1930009. [Google Scholar] [CrossRef]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Noh, C.K.; Lim, C.S.; Lee, G.H.; Cho, M.K.; Lee, H.W.; Roh, J.; Kim, Y.B.; Lee, E.; Park, B.; Kim, H.M.; et al. A Diagnostic Method for Gastric Cancer Using Two-Photon Microscopy with Enzyme-Selective Fluorescent Probes: A Pilot Study. Front. Oncol. 2021, 11, 634219. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zhang, C.; Li, Y.; Yan, J.; Xu, Y.; Pan, Y.; Hu, R.; Liu, L.; Qu, J. Human serum albumin gradient in serous ovarian cancer cryosections measured by fluorescence lifetime. BioMed. Opt. Express 2021, 12, 1195–1204. [Google Scholar] [CrossRef]
- Cho, M.K.; Lim, C.S.; Sarkar, A.R.; Lee, H.W.; Choi, H.J.; Noh, C.-K.; Shin, S.J.; Kim, H.M. A two-photon ratiometric probe for detection of hNQO1 enzyme activity in human colon tissue. Sens. Actuators B Chem. 2018, 272, 203–210. [Google Scholar] [CrossRef]
- Palczewska, G.; Dong, Z.; Golczak, M.; Hunter, J.J.; Williams, D.R.; Alexander, N.S.; Palczewski, K. Noninvasive two-photon microscopy imaging of mouse retina and retinal pigment epithelium through the pupil of the eye. Nat. Med. 2014, 20, 785–789. [Google Scholar] [CrossRef]
- Maeda, A.; Palczewska, G.; Golczak, M.; Kohno, H.; Dong, Z.; Maeda, T.; Palczewski, K. Two-photon microscopy reveals early rod photoreceptor cell damage in light-exposed mutant mice. Proc. Natl. Acad. Sci. USA 2014, 111, E1428–E1437. [Google Scholar] [CrossRef]
- Wang, B.G.; Konig, K.; Halbhuber, K.J. Two-photon microscopy of deep intravital tissues and its merits in clinical research. J. Microsc. 2010, 238, 1–20. [Google Scholar] [CrossRef]
- Palczewska, G.; Stremplewski, P.; Suh, S.; Alexander, N.; Salom, D.; Dong, Z.; Ruminski, D.; Choi, E.H.; Sears, A.E.; Kern, T.S.; et al. Two-photon imaging of the mammalian retina with ultrafast pulsing laser. JCI Insight 2018, 3, e121555. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, G.; Boguslawski, J.; Stremplewski, P.; Kornaszewski, L.; Zhang, J.; Dong, Z.; Liang, X.X.; Gratton, E.; Vogel, A.; Wojtkowski, M.; et al. Noninvasive two-photon optical biopsy of retinal fluorophores. Proc. Natl. Acad. Sci. USA 2020, 117, 22532–22543. [Google Scholar] [CrossRef] [PubMed]
- Boguslawski, J.; Palczewska, G.; Tomczewski, S.; Milkiewicz, J.; Kasprzycki, P.; Stachowiak, D.; Komar, K.; Marzejon, M.J.; Sikorski, B.L.; Hudzikowski, A.; et al. In vivo imaging of the human eye using a 2-photon-excited fluorescence scanning laser ophthalmoscope. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Mahajan, N.; Arora, P.; Sandhir, R. Perturbed Biochemical Pathways and Associated Oxidative Stress Lead to Vascular Dysfunctions in Diabetic Retinopathy. Oxid. Med. Cell Longev. 2019, 2019, 8458472. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Lai, T.T.; Li, C.J. The Oxidative Stress and Mitochondrial Dysfunction during the Pathogenesis of Diabetic Retinopathy. Oxid. Med. Cell Longev. 2018, 2018, 3420187. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef]
- Brownlee, M.; Cerami, A.; Vlassara, H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N. Engl. J. Med. 1988, 318, 1315–1321. [Google Scholar] [CrossRef]
- Stitt, A.W. AGEs and diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4867–4874. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, L.J.; Yu, J.; Wang, H.J.; Zhang, F.; Liu, Q.; Wu, J. Involvement of Advanced Glycation End Products in the Pathogenesis of Diabetic Retinopathy. Cell Physiol. Biochem. 2018, 48, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Cadenas, E. Oxidants and antioxidants revisited. New concepts of oxidative stress. Free Radic. Res. 2007, 41, 951–952. [Google Scholar] [CrossRef]
- Al-Shabrawey, M.; Bartoli, M.; El-Remessy, A.B.; Ma, G.; Matragoon, S.; Lemtalsi, T.; Caldwell, R.W.; Caldwell, R.B. Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3231–3238. [Google Scholar] [CrossRef]
- Al-Shabrawey, M.; Rojas, M.; Sanders, T.; Behzadian, A.; El-Remessy, A.; Bartoli, M.; Parpia, A.K.; Liou, G.; Caldwell, R.B. Role of NADPH oxidase in retinal vascular inflammation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3239–3244. [Google Scholar] [CrossRef]
- Berkowitz, B.A.; Kern, T.S.; Bissig, D.; Patel, P.; Bhatia, A.; Kefalov, V.J.; Roberts, R. Systemic Retinaldehyde Treatment Corrects Retinal Oxidative Stress, Rod Dysfunction, and Impaired Visual Performance in Diabetic Mice. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6294–6303. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, R.B.; Bartoli, M.; Behzadian, M.A.; El-Remessy, A.E.; Al-Shabrawey, M.; Platt, D.H.; Liou, G.I.; Caldwell, R.W. Vascular endothelial growth factor and diabetic retinopathy: Role of oxidative stress. Curr. Drug Targets 2005, 6, 511–524. [Google Scholar] [CrossRef]
- Du, Y.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc. Natl. Acad. Sci. USA 2013, 110, 16586–16591. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, M.; Chan, P.S.; Kern, T.S.; Kowluru, R.A. Oxidative damage in the retinal mitochondria of diabetic mice: Possible protection by superoxide dismutase. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3805–3811. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Tang, J.; Kern, T.S. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes 2001, 50, 1938–1942. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kowluru, A.; Mishra, M.; Kumar, B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 2015, 48, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, B.A.; Gradianu, M.; Bissig, D.; Kern, T.S.; Roberts, R. Retinal ion regulation in a mouse model of diabetic retinopathy: Natural history and the effect of Cu/Zn superoxide dismutase overexpression. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; He, J.; Johnson, D.; Wei, Y.; Liu, Y.; Wang, S.; Lutty, G.A.; Duh, E.J.; Semba, R.D. Deletion of placental growth factor prevents diabetic retinopathy and is associated with Akt activation and HIF1alpha-VEGF pathway inhibition. Diabetes 2015, 64, 200–212. [Google Scholar] [CrossRef]
- Lupo, G.; Motta, C.; Giurdanella, G.; Anfuso, C.D.; Alberghina, M.; Drago, F.; Salomone, S.; Bucolo, C. Role of phospholipases A2 in diabetic retinopathy: In vitro and in vivo studies. Biochem. Pharmacol. 2013, 86, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.I.; Hykin, P.G.; Gregor, Z.J.; Boulton, M.; Cree, I.A. Angiopoietin concentrations in diabetic retinopathy. Br. J. Ophthalmol. 2005, 89, 480–483. [Google Scholar] [CrossRef]
- Rangasamy, S.; Srinivasan, R.; Maestas, J.; McGuire, P.G.; Das, A. A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3784–3791. [Google Scholar] [CrossRef]
- Li, X.; Cai, Y.; Wang, Y.S.; Shi, Y.Y.; Hou, W.; Xu, C.S.; Wang, H.Y.; Ye, Z.; Yao, L.B.; Zhang, J. Hyperglycaemia exacerbates choroidal neovascularisation in mice via the oxidative stress-induced activation of STAT3 signalling in RPE cells. PLoS ONE 2012, 7, e47600. [Google Scholar] [CrossRef]
- Liu, H.; Tang, J.; Du, Y.; Lee, C.A.; Golczak, M.; Muthusamy, A.; Antonetti, D.A.; Veenstra, A.A.; Amengual, J.; von Lintig, J.; et al. Retinylamine Benefits Early Diabetic Retinopathy in Mice. J. Biol. Chem. 2015, 290, 21568–21579. [Google Scholar] [CrossRef]
- Bailey, T.A.; Kanuga, N.; Romero, I.A.; Greenwood, J.; Luthert, P.J.; Cheetham, M.E. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 675–684. [Google Scholar] [CrossRef]
- MacGregor, L.C.; Matschinsky, F.M. Treatment with aldose reductase inhibitor or with myo-inositol arrests deterioration of the electroretinogram of diabetic rats. J. Clin. Investig. 1985, 76, 887–889. [Google Scholar] [CrossRef]
- MacGregor, L.C.; Matschinsky, F.M. Altered retinal metabolism in diabetes. II. Measurement of sodium-potassium ATPase and total sodium and potassium in individual retinal layers. J. Biol. Chem. 1986, 261, 4052–4058. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, L.C.; Matschinsky, F.M. Experimental diabetes mellitus impairs the function of the retinal pigmented epithelium. Metabolism 1986, 35, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, A.; Kowluru, R.A.; Yamazaki, A. Functional alterations of G-proteins in diabetic rat retina: A possible explanation for the early visual abnormalities in diabetes mellitus. Diabetologia 1992, 35, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Ostroy, S.E.; Frede, S.M.; Wagner, E.F.; Gaitatzes, C.G.; Janle, E.M. Decreased rhodopsin regeneration in diabetic mouse eyes. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3905–3909. [Google Scholar]
- Kern, T.S.; Engerman, R.L. Capillary lesions develop in retina rather than cerebral cortex in diabetes and experimental galactosemia. Arch. Ophthalmol. 1996, 114, 306–310. [Google Scholar] [CrossRef]
- Ostroy, S.E. Altered rhodopsin regeneration in diabetic mice caused by acid conditions within the rod photoreceptors. Curr. Eye Res. 1998, 17, 979–985. [Google Scholar] [CrossRef]
- Lahdenranta, J.; Pasqualini, R.; Schlingemann, R.O.; Hagedorn, M.; Stallcup, W.B.; Bucana, C.D.; Sidman, R.L.; Arap, W. An anti-angiogenic state in mice and humans with retinal photoreceptor cell degeneration. Proc. Natl. Acad. Sci. USA 2001, 98, 10368–10373. [Google Scholar] [CrossRef]
- Colantuoni, A.; Longoni, B.; Marchiafava, P.L. Retinal photoreceptors of Syrian hamsters undergo oxidative stress during streptozotocin-induced diabetes. Diabetologia 2002, 45, 121–124. [Google Scholar] [CrossRef]
- Phipps, J.A.; Fletcher, E.L.; Vingrys, A.J. Paired-flash identification of rod and cone dysfunction in the diabetic rat. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4592–4600. [Google Scholar] [CrossRef]
- Wong, V.H.; Vingrys, A.J.; Bui, B.V. Glial and neuronal dysfunction in streptozotocin-induced diabetic rats. J. Ocul. Biol. Dis. Infor. 2011, 4, 42–50. [Google Scholar] [CrossRef]
- Berkowitz, B.A.; Grady, E.M.; Khetarpal, N.; Patel, A.; Roberts, R. Oxidative stress and light-evoked responses of the posterior segment in a mouse model of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 606–615. [Google Scholar] [CrossRef]
- Du, Y.; Cramer, M.; Lee, C.A.; Tang, J.; Muthusamy, A.; Antonetti, D.A.; Jin, H.; Palczewski, K.; Kern, T.S. Adrenergic and serotonin receptors affect retinal superoxide generation in diabetic mice: Relationship to capillary degeneration and permeability. FASEB J. 2015, 29, 2194–2204. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S.; Berkowitz, B.A. Photoreceptors in diabetic retinopathy. J. Diabetes Investig. 2015, 6, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Samuels, I.S.; Bell, B.A.; Pereira, A.; Saxon, J.; Peachey, N.S. Early retinal pigment epithelium dysfunction is concomitant with hyperglycemia in mouse models of type 1 and type 2 diabetes. J. NeuroPhysiol. 2015, 113, 1085–1099. [Google Scholar] [CrossRef]
- Berkowitz, B.A.; Bissig, D.; Roberts, R. MRI of rod cell compartment-specific function in disease and treatment in vivo. Prog. Retin. Eye Res. 2016, 51, 90–106. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S. Do photoreceptor cells cause the development of retinal vascular disease? Vis. Res. 2017, 139, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kern, T.S.; Song, B.; Stuebe, C. Mechanistic Insights into Pathological Changes in the Diabetic Retina: Implications for Targeting Diabetic Retinopathy. Am J. Pathol. 2017, 187, 9–19. [Google Scholar] [CrossRef]
- Tarchick, M.J.; Cutler, A.H.; Trobenter, T.D.; Kozlowski, M.R.; Makowski, E.R.; Holoman, N.; Shao, J.; Shen, B.; Anand-Apte, B.; Samuels, I.S. Endogenous insulin signaling in the RPE contributes to the maintenance of rod photoreceptor function in diabetes. Exp. Eye Res. 2019, 180, 63–74. [Google Scholar] [CrossRef]
- de Gooyer, T.E.; Stevenson, K.A.; Humphries, P.; Simpson, D.A.; Gardiner, T.A.; Stitt, A.W. Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5561–5568. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, Y.S.; Noh, H.S.; Kang, S.S.; Cheon, E.W.; Park, S.K.; Lee, B.J.; Choi, W.S.; Cho, G.J. Changes in rhodopsin kinase and transducin in the rat retina in early-stage diabetes. Exp. Eye Res. 2005, 80, 753–760. [Google Scholar] [CrossRef]
- Liu, H.; Tang, J.; Du, Y.; Saadane, A.; Samuels, I.; Veenstra, A.; Kiser, J.Z.; Palczewski, K.; Kern, T.S. Transducin1, Phototransduction and the Development of Early Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1538–1546. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, F.; Sung, D.; Haswell, K.M.; Tsin, A.; Ghosh, D. Thiol-dependent antioxidant activity of interphotoreceptor retinoid-binding protein. Exp. Eye Res. 2014, 120, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Muller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Nelson, K.C.; Wu, M.; Sternberg, P., Jr.; Jones, D.P. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 2000, 19, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Plafker, S.M.; O’Mealey, G.B.; Szweda, L.I. Mechanisms for countering oxidative stress and damage in retinal pigment epithelium. Int. Rev. Cell Mol. Biol. 2012, 298, 135–177. [Google Scholar] [CrossRef]
- Giordano, C.R.; Roberts, R.; Krentz, K.A.; Bissig, D.; Talreja, D.; Kumar, A.; Terlecky, S.R.; Berkowitz, B.A. Catalase therapy corrects oxidative stress-induced pathophysiology in incipient diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3095–3102. [Google Scholar] [CrossRef]
- Muir, E.R.; Chandra, S.B.; De La Garza, B.H.; Velagapudi, C.; Abboud, H.E.; Duong, T.Q. Layer-Specific Manganese-Enhanced MRI of the Diabetic Rat Retina in Light and Dark Adaptation at 11.7 Tesla. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4006–4012. [Google Scholar] [CrossRef]
- Saliba, A.; Du, Y.; Liu, H.; Patel, S.; Roberts, R.; Berkowitz, B.A.; Kern, T.S. Photobiomodulation Mitigates Diabetes-Induced Retinopathy by Direct and Indirect Mechanisms: Evidence from Intervention Studies in Pigmented Mice. PLoS ONE 2015, 10, e0139003. [Google Scholar] [CrossRef]
- Berkowitz, B.A.; Podolsky, R.H.; Lins-Childers, K.M.; Li, Y.; Qian, H. Outer Retinal Oxidative Stress Measured In Vivo Using QUEnch-assiSTed (QUEST) OCT. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1566–1570. [Google Scholar] [CrossRef]
- Zhang, T.; Gillies, M.; Wang, Y.; Shen, W.; Bahrami, B.; Zeng, S.; Zhu, M.; Yao, W.; Zhou, F.; Murray, M.; et al. Simvastatin protects photoreceptors from oxidative stress induced by all-trans-retinal, through the up-regulation of interphotoreceptor retinoid binding protein. Br. J. Pharmacol. 2019, 176, 2063–2078. [Google Scholar] [CrossRef]
- Lee, M.; Li, S.; Sato, K.; Jin, M. Interphotoreceptor Retinoid-Binding Protein Mitigates Cellular Oxidative Stress and Mitochondrial Dysfunction Induced by All-trans-Retinal. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1553–1562. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, X.; Zhang, K.; Zhou, F.; Zhu, L. Neuroprotective effect of tetramethylpyrazine against all-trans-retinal toxicity in the differentiated Y-79 cells via upregulation of IRBP expression. Exp. Cell Res. 2017, 359, 120–128. [Google Scholar] [CrossRef] [PubMed]
- van Veen, T.; Katial, A.; Shinohara, T.; Barrett, D.J.; Wiggert, B.; Chader, G.J.; Nickerson, J.M. Retinal photoreceptor neurons and pinealocytes accumulate mRNA for interphotoreceptor retinoid-binding protein (IRBP). FEBS Lett. 1986, 208, 133–137. [Google Scholar] [CrossRef]
- Wisard, J.; Faulkner, A.; Chrenek, M.A.; Waxweiler, T.; Waxweiler, W.; Donmoyer, C.; Liou, G.I.; Craft, C.M.; Schmid, G.F.; Boatright, J.H.; et al. Exaggerated eye growth in IRBP-deficient mice in early development. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5804–5811. [Google Scholar] [CrossRef]
- Markand, S.; Baskin, N.L.; Chakraborty, R.; Landis, E.; Wetzstein, S.A.; Donaldson, K.J.; Priyadarshani, P.; Alderson, S.E.; Sidhu, C.S.; Boatright, J.H.; et al. IRBP deficiency permits precocious ocular development and myopia. Mol. Vis. 2016, 22, 1291–1308. [Google Scholar] [PubMed]
- Liou, G.I.; Fei, Y.; Peachey, N.S.; Matragoon, S.; Wei, S.; Blaner, W.S.; Wang, Y.; Liu, C.; Gottesman, M.E.; Ripps, H. Early onset photoreceptor abnormalities induced by targeted disruption of the interphotoreceptor retinoid-binding protein gene. J. NeuroSci. 1998, 18, 4511–4520. [Google Scholar] [CrossRef] [PubMed]
- Ooi, X.Y.; Khan, R.; Choudhury, A.; Elisarraras, F.X.; Grigsby, J.; Obregon, B.; Tsin, A. Recent advances on visual cycle protein research and progress on clinical translation. Arch Clin. Exp. Ophthalmol. 2020, 2, 73–76. [Google Scholar] [CrossRef]
- Ishikawa, M.; Sawada, Y.; Yoshitomi, T. Structure and function of the interphotoreceptor matrix surrounding retinal photoreceptor cells. Exp. Eye Res. 2015, 133, 3–18. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, F.; Lippincott, J.; Powell, A.; Coleman, T. Rat CRISPR/Cas9 knockout of Interphotoreceptor retinoid-binding protein (IRBP, RBP3) suggests role in maintaining lamellar disc outer segment stability. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1528. [Google Scholar]
- Wald, G. Molecular basis of visual excitation. Science 1968, 162, 230–239. [Google Scholar] [CrossRef]
- Kiser, P.D.; Golczak, M.; Palczewski, K. Chemistry of the retinoid (visual) cycle. Chem. Rev. 2014, 114, 194–232. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Haswell, K.M.; Sprada, M.; Gonzalez-Fernandez, F. Structure of zebrafish IRBP reveals fatty acid binding. Exp. Eye Res. 2015, 140, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Saari, J.C.; Noy, N. Interactions of all-trans-retinol and long-chain fatty acids with interphotoreceptor retinoid-binding protein. Biochemistry 1993, 32, 11311–11318. [Google Scholar] [CrossRef] [PubMed]
- Marmorstein, A.D.; Marmorstein, L.Y.; Sakaguchi, H.; Hollyfield, J.G. Spectral profiling of autofluorescence associated with lipofuscin, Bruch’s Membrane, and sub-RPE deposits in normal and AMD eyes. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2435–2441. [Google Scholar]
- Radu, R.A.; Mata, N.L.; Nusinowitz, S.; Liu, X.; Sieving, P.A.; Travis, G.H. Treatment with isotretinoin inhibits lipofuscin accumulation in a mouse model of recessive Stargardt’s macular degeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 4742–4747. [Google Scholar] [CrossRef]
- Wiggert, B.; van Veen, T.; Kutty, G.; Lee, L.; Nickerson, J.; Si, J.S.; Nilsson, S.E.; Chader, G.J.; Narfstrom, K. An early decrease in interphotoreceptor retinoid-binding protein gene expression in Abyssinian cats homozygous for hereditary rod-cone degeneration. Cell Tissue Res. 1994, 278, 291–298. [Google Scholar] [CrossRef]
- Narfstrom, K.; Nilsson, S.E.; Wiggert, B.; Lee, L.; Chader, G.J.; van Veen, T. Reduced level of interphotoreceptor retinoid-binding protein (IRBP), a possible cause for retinal degeneration in the Abyssinian cat. Cell Tissue Res. 1989, 257, 631–639. [Google Scholar] [CrossRef]
- Arno, G.; Hull, S.; Robson, A.G.; Holder, G.E.; Cheetham, M.E.; Webster, A.R.; Plagnol, V.; Moore, A.T. Lack of Interphotoreceptor Retinoid Binding Protein Caused by Homozygous Mutation of RBP3 Is Associated with High Myopia and Retinal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2358–2365. [Google Scholar] [CrossRef]
- Li, S.; Yang, Z.; Hu, J.; Gordon, W.C.; Bazan, N.G.; Haas, A.L.; Bok, D.; Jin, M. Secretory defect and cytotoxicity: The potential disease mechanisms for the retinitis pigmentosa (RP)-associated interphotoreceptor retinoid-binding protein (IRBP). J. Biol. Chem. 2013, 288, 11395–11406. [Google Scholar] [CrossRef]
- den Hollander, A.I.; McGee, T.L.; Ziviello, C.; Banfi, S.; Dryja, T.P.; Gonzalez-Fernandez, F.; Ghosh, D.; Berson, E.L. A homozygous missense mutation in the IRBP gene (RBP3) associated with autosomal recessive retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1864–1872. [Google Scholar] [CrossRef]
- Zheng, Q.; Ren, Y.; Tzekov, R.; Hua, S.; Li, M.; Pang, J.; Qu, J.; Li, W. iTRAQ-Based Proteomic Analysis of Visual Cycle-Associated Proteins in RPE of rd12 Mice before and after RPE65 Gene Delivery. J. Ophthalmol. 2015, 2015, 918473. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Ulyanova, T.; Organisciak, D.T.; Bennett, S.; Lakins, J.; Arnold, J.M.; Kutty, R.K.; Tenniswood, M.; vanVeen, T.; Darrow, R.M.; et al. Expression of multiple forms of clusterin during light-induced retinal degeneration. Curr. Eye Res. 2001, 23, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.J.; Severin, K.M. Proteins of the bovine interphotoreceptor matrix: Tissues of origin. Exp. Eye Res. 1981, 32, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.I.; Bridges, C.D.; Fong, S.L.; Alvarez, R.A.; Gonzalez-Fernandez, F. Vitamin A transport between retina and pigment epithelium--an interstitial protein carrying endogenous retinol (interstitial retinol-binding protein). Vis. Res. 1982, 22, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.J.; Evans, C.D. Rapid isolation of bovine interphotoreceptor retinol-binding protein. Biochim. Biophys. Acta 1983, 761, 217–222. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, F. Interphotoreceptor retinoid binding protein; myths and mysteries. J. Ophthalmic. Vis. Res. 2012, 7, 100–104. [Google Scholar] [PubMed]
- Nickerson, J.M.; Frey, R.A.; Ciavatta, V.T.; Stenkamp, D.L. Interphotoreceptor retinoid-binding protein gene structure in tetrapods and teleost fish. Mol. Vis. 2006, 12, 1565–1585. [Google Scholar]
- Fei, Y.; Matragoon, S.; Smith, S.B.; Overbeek, P.A.; Chen, S.; Zack, D.J.; Liou, G.I. Functional dissection of the promoter of the interphotoreceptor retinoid-binding protein gene: The cone-rod-homeobox element is essential for photoreceptor-specific expression in vivo. J. Biochem. 1999, 125, 1189–1199. [Google Scholar] [CrossRef]
- Nishida, A.; Furukawa, A.; Koike, C.; Tano, Y.; Aizawa, S.; Matsuo, I.; Furukawa, T. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat. NeuroSci. 2003, 6, 1255–1263. [Google Scholar] [CrossRef]
- Wang, J.S.; Kefalov, V.J. An alternative pathway mediates the mouse and human cone visual cycle. Curr. Biol. 2009, 19, 1665–1669. [Google Scholar] [CrossRef]
- Sato, S.; Kefalov, V.J. cis Retinol oxidation regulates photoreceptor access to the retina visual cycle and cone pigment regeneration. J. Physiol. 2016, 594, 6753–6765. [Google Scholar] [CrossRef]
- Mata, N.L.; Radu, R.A.; Clemmons, R.C.; Travis, G.H. Isomerization and oxidation of vitamin a in cone-dominant retinas: A novel pathway for visual-pigment regeneration in daylight. Neuron 2002, 36, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.H.; Kono, M.; Koutalos, Y.; Ablonczy, Z.; Crouch, R.K. New insights into retinoid metabolism and cycling within the retina. Prog. Retin. Eye Res. 2013, 32, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.S.; Fong, S.L.; Bridges, C.D. Retinoids bound to interstitial retinol-binding protein during light and dark-adaptation. Vis. Res. 1989, 29, 1699–1709. [Google Scholar] [CrossRef]
- Saari, J.C.; Teller, D.C.; Crabb, J.W.; Bredberg, L. Properties of an interphotoreceptor retinoid-binding protein from bovine retina. J. Biol. Chem. 1985, 260, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.L.; Bazan, N.G. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc. Natl. Acad. Sci. USA 1989, 86, 2903–2907. [Google Scholar] [CrossRef]
- Jastrzebska, B.; Debinski, A.; Filipek, S.; Palczewski, K. Role of membrane integrity on G protein-coupled receptors: Rhodopsin stability and function. Prog. Lipid. Res. 2011, 50, 267–277. [Google Scholar] [CrossRef]
- Semenova, E.M.; Converse, C.A. Comparison between oleic acid and docosahexaenoic acid binding to interphotoreceptor retinoid-binding protein. Vis. Res. 2003, 43, 3063–3067. [Google Scholar] [CrossRef]
- Putilina, T.; Sittenfeld, D.; Chader, G.J.; Wiggert, B. Study of a fatty acid binding site of interphotoreceptor retinoid-binding protein using fluorescent fatty acids. Biochemistry 1993, 32, 3797–3803. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Li, G.R.; Takizawa, N.; Si, J.S.; Gross, E.A.; Richardson, K.; Nickerson, J.M. Structure-function relationships in interphotoreceptor retinoid-binding protein (IRBP). Mol. Vis. 1997, 3, 17. [Google Scholar]
- Uehara, F.; Matthes, M.T.; Yasumura, D.; LaVail, M.M. Light-evoked changes in the interphotoreceptor matrix. Science 1990, 248, 1633–1636. [Google Scholar] [CrossRef]
- Hollyfield, J.G.; Fliesler, S.J.; Rayborn, M.E.; Fong, S.L.; Landers, R.A.; Bridges, C.D. Synthesis and secretion of interstitial retinol-binding protein by the human retina. Investig. Ophthalmol. Vis. Sci. 1985, 26, 58–67. [Google Scholar]
- Huang, B.; Karwoski, C.J. Light-evoked expansion of subretinal space volume in the retina of the frog. J. NeuroSci. 1992, 12, 4243–4252. [Google Scholar] [CrossRef]
- Yamamoto, F.; Steinberg, R.H. Effects of intravenous acetazolamide on retinal pH in the cat. Exp. Eye Res. 1992, 54, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Govardovskii, V.I.; Li, J.D.; Dmitriev, A.V.; Steinberg, R.H. Mathematical model of TMA+ diffusion and prediction of light-dependent subretinal hydration in chick retina. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2712–2724. [Google Scholar]
- Li, J.D.; Gallemore, R.P.; Dmitriev, A.; Steinberg, R.H. Light-dependent hydration of the space surrounding photoreceptors in chick retina. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2700–2711. [Google Scholar]
- Wolfensberger, T.J.; Dmitriev, A.V.; Govardovskii, V.I. Inhibition of membrane-bound carbonic anhydrase decreases subretinal pH and volume. Doc. Ophthalmol. 1999, 97, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Bissig, D.; Berkowitz, B.A. Light-dependent changes in outer retinal water diffusion in rats in vivo. Mol. Vis. 2012, 18, 2561. [Google Scholar]
- Li, Y.; Fariss, R.N.; Qian, J.W.; Cohen, E.D.; Qian, H. Light-Induced Thickening of Photoreceptor Outer Segment Layer Detected by Ultra-High Resolution OCT Imaging. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT105–OCT111. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.; Roggan, A.; Schweitzer, D.; Muller, G. Optical properties of ocular fundus tissues--an in vitro study using the double-integrating-sphere technique and inverse Monte Carlo simulation. Phys. Med. Biol. 1995, 40, 963–978. [Google Scholar] [CrossRef]
- Imanishi, Y.; Batten, M.L.; Piston, D.W.; Baehr, W.; Palczewski, K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J. Cell Biol. 2004, 164, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, Y.; Gerke, V.; Palczewski, K. Retinosomes: New insights into intracellular managing of hydrophobic substances in lipid bodies. J. Cell Biol. 2004, 166, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Golczak, M.; Imanishi, Y.; Kuksa, V.; Maeda, T.; Kubota, R.; Palczewski, K. Lecithin:retinol acyltransferase is responsible for amidation of retinylamine, a potent inhibitor of the retinoid cycle. J. Biol. Chem. 2005, 280, 42263–42273. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Schwarz, C.; Hunter, J.J.; Palczewska, G.; Palczewski, K.; Williams, D.R. Formation and Clearance of All-Trans-Retinol in Rods Investigated in the Living Primate Eye with Two-Photon Ophthalmoscopy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kiser, P.D.; Badiee, M.; Palczewska, G.; Dong, Z.; Golczak, M.; Tochtrop, G.P.; Palczewski, K. Molecular pharmacodynamics of emixustat in protection against retinal degeneration. J. Clin. Investig. 2015, 125, 2781–2794. [Google Scholar] [CrossRef] [PubMed]
- Kiser, P.D.; Palczewski, K. Retinoids and Retinal Diseases. Annu. Rev. Vis. Sci. 2016, 2, 197–234. [Google Scholar] [CrossRef]
- Fereidouni, F.; Bader, A.N.; Gerritsen, H.C. Spectral phasor analysis allows rapid and reliable unmixing of fluorescence microscopy spectral images. Opt. Express 2012, 20, 12729–12741. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaushik, V.; Gessa, L.; Kumar, N.; Fernandes, H. Towards a New Biomarker for Diabetic Retinopathy: Exploring RBP3 Structure and Retinoids Binding for Functional Imaging of Eyes In Vivo. Int. J. Mol. Sci. 2023, 24, 4408. https://doi.org/10.3390/ijms24054408
Kaushik V, Gessa L, Kumar N, Fernandes H. Towards a New Biomarker for Diabetic Retinopathy: Exploring RBP3 Structure and Retinoids Binding for Functional Imaging of Eyes In Vivo. International Journal of Molecular Sciences. 2023; 24(5):4408. https://doi.org/10.3390/ijms24054408
Chicago/Turabian StyleKaushik, Vineeta, Luca Gessa, Nelam Kumar, and Humberto Fernandes. 2023. "Towards a New Biomarker for Diabetic Retinopathy: Exploring RBP3 Structure and Retinoids Binding for Functional Imaging of Eyes In Vivo" International Journal of Molecular Sciences 24, no. 5: 4408. https://doi.org/10.3390/ijms24054408
APA StyleKaushik, V., Gessa, L., Kumar, N., & Fernandes, H. (2023). Towards a New Biomarker for Diabetic Retinopathy: Exploring RBP3 Structure and Retinoids Binding for Functional Imaging of Eyes In Vivo. International Journal of Molecular Sciences, 24(5), 4408. https://doi.org/10.3390/ijms24054408






