Abstract
Increased adenosine A2A receptor (A2AR) expression and activation underlies a higher incidence of spontaneous calcium release in atrial fibrillation (AF). Adenosine A3 receptors (A3R) could counteract excessive A2AR activation, but their functional role in the atrium remains elusive, and we therefore aimed to address the impact of A3Rs on intracellular calcium homeostasis. For this purpose, we analyzed right atrial samples or myocytes from 53 patients without AF, using quantitative PCR, patch-clamp technique, immunofluorescent labeling or confocal calcium imaging. A3R mRNA accounted for 9% and A2AR mRNA for 32%. At baseline, A3R inhibition increased the transient inward current (ITI) frequency from 0.28 to 0.81 events/min (p < 0.05). Simultaneous stimulation of A2ARs and A3Rs increased the calcium spark frequency seven-fold (p < 0.001) and the ITI frequency from 0.14 to 0.64 events/min (p < 0.05). Subsequent A3R inhibition caused a strong additional increase in the ITI frequency (to 2.04 events/min; p < 0.01) and increased phosphorylation at s2808 1.7-fold (p < 0.001). These pharmacological treatments had no significant effects on L-type calcium current density or sarcoplasmic reticulum calcium load. In conclusion, A3Rs are expressed and blunt spontaneous calcium release at baseline and upon A2AR-stimulation in human atrial myocytes, pointing to A3R activation as a means to attenuate physiological and pathological elevations of spontaneous calcium release events.
1. Introduction
Cyclic AMP (cAMP) signaling plays a crucial role in modulating calcium regulatory proteins involved in cardiac excitation-contraction coupling, such as L-type calcium channels, the sarcoplasmic reticulum (SR) calcium channel, also named the ryanodine receptor (RyR2), and phospholamban that regulates the activity of the SR calcium pump [1,2]. Physiological and pathological modulation of cAMP signaling, in turn, involves a large number of G protein-coupled receptors (i.e., GPCRs) and phosphodiesterases [3,4]. Within GPCRs, adenosine receptors play a key role in the regulation of myocardial function and rhythm [5]. In this context, the Gi-protein coupled adenosine A1 receptor (A1R) is expected to reduce cAMP production and attenuate the sympathetic tone, and the A1R is a pharmacological target for the regulation of supraventricular arrhythmias [6]. However, excessive A1R activation can also accelerate atrial fibrillation (AF) [7] or favor its induction by shortening the refractory period via activation of the G-protein coupled inwardly rectifying potassium channel [8]. Furthermore, both the A1R and the Gi-protein coupled adenosine A3 receptor (A3R) has been attributed important roles in ischemic preconditioning and cardio protection [9,10,11]. Moreover, the adenosine A2A receptor (A2AR) and A2B receptor (A2BR) are Gs-protein coupled receptors that are expected to stimulate cAMP synthesis and favor cAMP-dependent phosphorylation of key calcium regulatory proteins. Indeed, the A2AR displays an overlapping distribution with the RyR2 and has previously been shown to selectively modulate spontaneous calcium release from the SR [12]. Moreover, A2AR expression is upregulated in patients with AF and prevention of A2AR activation normalizes spontaneous calcium release in patients with AF to levels observed in patients without AF [13], and diminishes the induction of arrhythmic responses in electrically paced myocytes from patients with AF [14]. Because the A3R is expected to inhibit adenylate cyclase, activation of this receptor would be expected to dampen spontaneous A2AR-induced calcium release and contribute to maintaining a low incidence of spontaneous calcium release at baseline. Currently, the functional role of A3Rs in atrial myocytes remains elusive, and there are notable differences in A3R expression or binding of agonists to A3Rs in atria from humans and small rodents [15]. However, since there are species-dependent differences in the expression of G-protein coupled receptors and their binding constants for A3R agonists [15,16], this study aims to determine the expression of A3Rs in human right atrial samples and the functional impact on intracellular calcium homeostasis in human right atrial myocytes.
2. Results
To determine the functional impact of A3Rs in the human atrium, we analyzed the expression and functional electrophysiological impact of A3Rs in myocytes from 53 patients without a previous history of AF. Table 1 summarizes the clinical features of the study population.

Table 1.
Clinical characteristics of the study population.
2.1. Adenosine A3R Expression
First, we aimed to determine the expression levels of A3R mRNA in comparison to the other adenosine receptors. The results shown in Figure 1 indicate that A1R mRNA is the most abundant, followed by the A2AR and the A3R. Specifically, the expression of A2AR mRNA constituted the 35 ± 5% of the A1Rs, while A3R accounted for only 9.2 ± 3.3%.
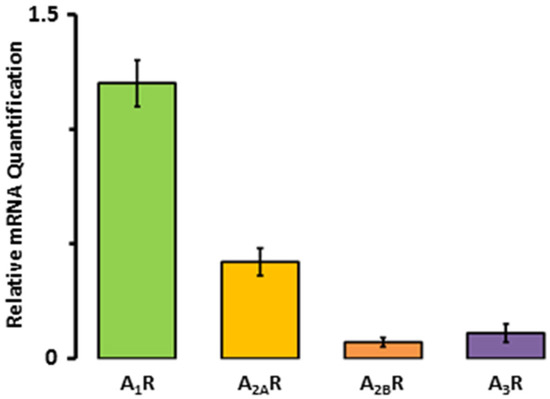
Figure 1.
Adenosine receptor mRNA expression in human right atrial tissue samples from seven patients.
2.2. Impact of Adenosine A3Rs on Calcium Homeostasis at Baseline
However, since GPCRs can be located in macromolecular clusters where they exert a strong regulation of specific molecular functions [17], we first determined how selective A3R inhibition affected calcium homeostasis at baseline. As shown in Figure 2A,B, the selective A3R antagonist MRS1191 significantly increased the incidence of ITI (Figure 2A), without affecting the caffeine releasable SR calcium load (Figure 2B). Furthermore, MRS1191 did not have a significant effect on the ICa amplitude (Figure 2C), but significantly increased the ICa inactivation (Figure 2D).
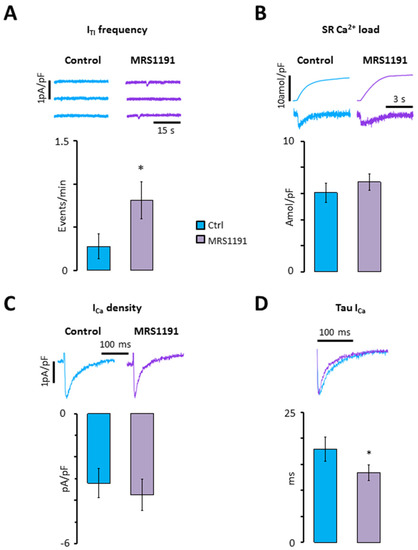
Figure 2.
Impact of A3R inhibition on calcium homeostasis at baseline. (A) ITI frequency; (B) SR calcium load; (C) ICa density; (D) tau of ICa. Data were recorded in nine myocytes from eight patients before (blue) and after perfusion of myocytes with MRS1191 (purple). Significant differences are indicated with * p < 0.05 (paired t-test).
2.3. Impact of Crosstalk between A3R and A2AR on Spontaneous Calcium Release
Since we have previously shown that A2AR activation contributes to a higher incidence of ITI in human atrial myocytes [12] and A3R activation would counteract this, we tested whether there is a crosstalk between A3Rs and A2ARs in human atrial myocytes. For this purpose, we first exposed human atrial myocytes to 200 nM CGS21680 to simultaneously stimulate A2ARs and A3Rs. As shown in Figure 3A, this significantly increased the ITI frequency four-fold. Interestingly, subsequent exposure to MRS1191 induced an additional 3-fold increase in the ITI frequency (p < 0.001), suggesting that the A3R blunts the effect of A2AR activation. Analysis of the caffeine-releasable SR calcium load revealed that the treatment with CGS21680 or CGS21680 + MRS1191 did not affect the SR calcium load significantly (Figure 3B), suggesting that the increased incidence of ITI is not caused by a higher SR calcium load. Accordingly, there was no significant correlation between SR calcium load and ITI frequency (p = 0.789; Figure 3C). Immunofluorescent labeling of the RyR2 phosphorylated at s2808 revealed that phosphorylation was significantly higher in myocytes incubated with CGS21680 + MRS1191 than in control myocytes from the same patient (Figure 3D), suggesting that s2808 phosphorylation could contribute to the higher incidence of ITI observed after exposure to CGS21680 + MRS1191.
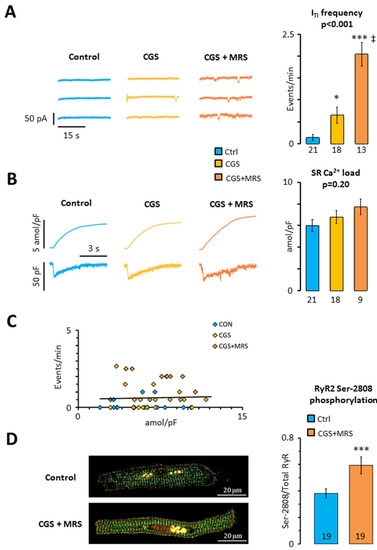
Figure 3.
Impact of crosstalk between A3R and A2AR on SR calcium homeostasis. (A) ITI frequency; (B) SR calcium load; (C) relationship between ITI frequency and SR calcium load. Data were recorded in 21 myocytes from 19 patients before (blue) and after perfusion of myocytes with CGS21680 (yellow) and CGS21680 and MRS1191 (orange); (D) RyR2 s2808 phosphorylation in 106 myocytes from 19 patients. Significant differences between the control and treatments are indicated with * p < 0.05; *** p < 0.001, ‡ p < 0.01 for CGS21680 + MRS1191 vs. CGS21680. Data in (A,B) were analyzed using ANOVA with Welch’s correction (p-values are given above the bars) and Games–Howell post-test. Unpaired t-test was used in panel (D).
To determine whether A2AR activation increases the ITI frequency by increasing the propensity of the RyR2 to open spontaneously, we analyzed the incidence of calcium sparks resulting from the opening of individual RyR2 clusters [18]. Figure 4A,B shows that both CGS21680 and CGS21680 + MRS1191 induced a dramatic increase in the calcium spark density. This concurred with a significant reduction of the calcium spark amplitude, which was most pronounced in the presence of CGS21680 + MRS1191 (Figure 4C). On the contrary, the treatments had no significant impact on the width (Figure 4D) or the decay of the calcium sparks (Figure 4E).
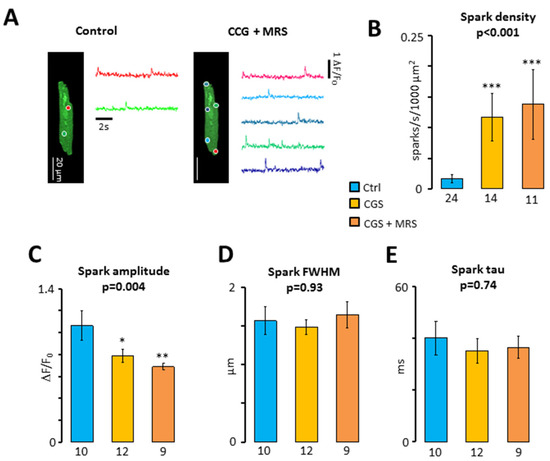
Figure 4.
Impact of the crosstalk between A3R and A2AR on calcium sparks. (A) Calcium spark recordings (colored traces) from their respective spark sites (colored circles) in a patient before and after exposure to 200 nM CGS21680 + 1 μM MRS1191; (B) spark density (sparks/s/1000 μm2). Sparks were recorded in 10 patients before (blue) and after perfusion of myocytes with CGS21680 (yellow) and CGS21680 and MRS1191 (orange). Number of cells are given below bars; (C) spark amplitude (dF/F0); (D) spark full width at half maximum (FWHM, μm); (E) tau for spark decay (ms). Significant differences between the control and treatments are indicated with* p < 0.05; ** p < 0.01; *** p < 0.001. Data were analyzed using Kruskal–Wallis (p-values are given above Bars) and Dunn’s post-test in (B) and ANOVA with Welch’s correction in panel (C–E). Number of cells with sparks are given below the bars in panel (C–E).
Table 2 summarizes the impact of the two treatments on all calcium spark features analyzed.

Table 2.
Impact of the treatment with 200 nM CGS21680 (CGS) and 1 µM MRS1191 (MRS) on the incidence of calcium sparks and their properties in human atrial myocytes. Significant differences between treatment and control are indicated with * p < 0.05, ** p < 0.01, *** p < 0.001.
2.4. Impact of Crosstalk between A3R and A2AR on L-type Calcium Current
Finally, the treatment with CSG21680 and CGS21680 + MRS1191 was used to assess the impact of A2ARs and A3Rs on ICa. Figure 5A shows that neither A2AR nor A3R activation had any impact on ICa amplitude. However, concurrent activation of A2ARs and inhibition of A3Rs with CGS21680 + MRS1191 significantly increased time-dependent inactivation of ICa (Figure 5B). Moreover, Figure 5C shows that the time constant for fast ICa inactivation (tau) was inversely correlated with the ITI frequency recorded in the same cell (p < 0.001). In contrast, Figure 5D showed only a weak correlation between tau and ICa density, suggesting that ICa inactivation by calcium influx through the proper L-type calcium channel is modest. However, Figure 5E shows that a brief transient exposure to caffeine, to eliminate SR calcium release-induced ICa inactivation, unmasks a steeper correlation between tau and the ICa density (p < 0.01). Even so, Figure 5F shows that the tau for the ICa inactivation elicited after caffeine exposure is not modified by the treatments with CGS21680 and MRS1191, suggesting that A2ARs and A3Rs have a minor impact on ICa amplitude or inactivation.
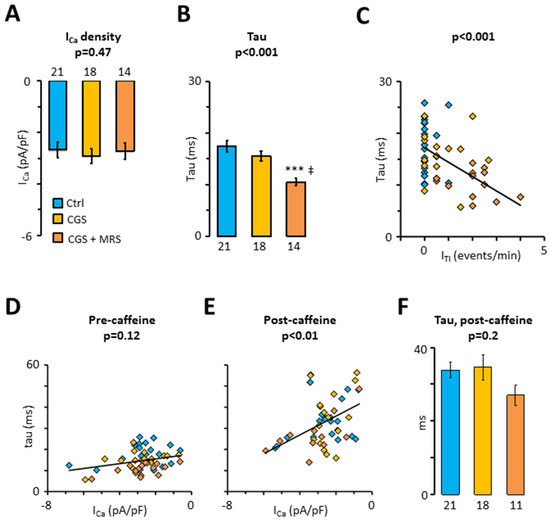
Figure 5.
Impact of the crosstalk between A3R and A2AR on L-type calcium current. (A) ICa density; (B) fast time constant, tau, for ICa decay; (C) correlation between tau and ITI frequency (from Figure 3A); (D,E) correlation between tau and ICa density before and after transient exposure to 10 mM caffeine. Currents were recorded in 19 patients before (blue) and after perfusion of myocytes with CGS21680 (yellow) and CGS21680 + MRS1191 (orange). Statistical significance was evaluated using Pearson’s product-moment correlation; (F) Time constant, tau, for the decay of the first ICa after transient exposure to caffeine. Significant differences between control and treatments are indicated with; *** p < 0.001, ‡ p < 0.01 for CGS21680 + MRS1191 vs. CGS21680. Data were analyzed using ANOVA (p-values are given above the bars) and Tukey’s post-test in (A,B,F). Number of cells are given below bars.
3. Discussion
3.1. Main Findings
While the A3R has been attributed an important role in preconditioning and cardio protection [10,19], little is known about its functional role in human atrial myocytes. Here, we analyzed the impact of the A3R on intracellular calcium homeostasis and report that even though A3R mRNA expression is modest compared to the expression of the A1R and the A2AR, endogenous activation of the A3Rs at baseline blunts the incidence of the spontaneous calcium release-induced ITI. Furthermore, crosstalk between A3R and A2AR upon activation of both receptors reduces the incidence of both calcium sparks and ITI, demonstrating that A3R activation diminishes spontaneous, A2AR-mediated, calcium release in human atrial myocytes. The findings also suggest that A3R activation could be a means of attenuating arrhythmogenic calcium release events induced by pathological elevations of the adenosine level.
3.2. Impact of the A3R on Calcium Homeostasis at Baseline
Previous electrophysiological studies in human atrial myocytes have reported a cAMP-tonus at baseline [20], which is regulated by phosphodiesterases and modulates ICa amplitude [21] as well as the incidence of spontaneous calcium release [22]. Consistent with this, we have also shown that the ruptured whole-cell patch configuration dialyses adenosine out of the cell, leading to a reduction of the ITI frequency, presumably because the endogenous adenosine level is sufficient to induce spontaneous, A2AR-mediated, calcium release at baseline [13]. In accordance with these findings, we here observe that selective inhibition of the A3R with MRS1191 increases the basal ITI frequency, suggesting that endogenous adenosine not only activates A2ARs, but also A3Rs at baseline, and that the latter attenuates A2AR-mediated activation of adenylate cyclase. Interestingly, we do not observe any significant effect of A3R inhibition on ICa density or SR calcium loading, pointing to compartmentalization of A3R-mediated signaling. This finding is similar to previous observations on the impact of pharmacological manipulation of Gs-protein coupled receptors in human atrial myocytes where acute pharmacological manipulation of A2AR or treatment of patients with β-adrenergic receptor blockers had no impact on ICa density or SR calcium load [12,13,23].
3.3. Impact of Crosstalk between A3Rs and A2ARs on Calcium Homeostasis
Since AF has previously been associated with increased A2AR expression and activation that promotes spontaneous calcium release [13], concurrent activation of the A3R would be expected to dampen A2AR-mediated stimulation of calcium release. The present findings demonstrate that when A2ARs and A3Rs are activated simultaneously, A3R activation does indeed attenuate significantly the A2AR-mediated increase in the incidence of both calcium sparks and ITI. In support of this finding, both A3R and A2AR bind adenosine with an affinity of approximately 300 nM [24]. Similar to observations at baseline, A3R inhibition did not modify the ICa density when the A2ARs and A3Rs were stimulated simultaneously with CGS21680. However, A3R inhibition did speed up ICa inactivation and this was correlated with the ITI frequency but not with the ICa amplitude recorded in the same cell. This, combined with the higher incidence of calcium sparks and increased RyR2 phosphorylation at s2808 observed upon concurrent activation of A2AR and A3R suggests that the A3R selectively targets cAMP-dependent phosphorylation of the RyR2 and that this not only leads to a higher ITI frequency, but also leads to a faster calcium-release induced ICa inactivation. Interestingly, the tau for ICa inactivation was inversely proportional to the ICa amplitude when cells had previously been exposed to caffeine to clear the SR calcium content and prevent calcium-release induced inactivation. However, even under these conditions, CGS21680 or CGS21680 + MRS1191 did not affect the ICa amplitude significantly, confirming that A2AR and A3R-dependent signaling targets the RyR2 but not the L-type calcium channel.
3.4. Study Limitations
In the present study, we have focused on the impact of A3Rs on intracellular calcium homeostasis. However, being a Gi-protein coupled receptor, it is conceivable that the A3Rs could also modulate the activity of other ion channels that are regulated by Gi-protein coupled receptors [8,9] or influence the activity of other Gs-protein coupled receptors [25]. This, in turn, would potentially influence the net impact of A3R activity on the amplitude and frequency of calcium release-induced afterdepolarizations. Similarly, the relative impact of A3Rs on A2AR-mediated signaling will depend on the spatial distribution of the A1R, the A2AR, and the A3R with respect to target proteins, such as the RyR2, L-type calcium channels, phospholamban, etc. While this issue has been addressed in non-myocardial preparations [25,26,27], such information is currently limited for atrial myocytes. In this regard, we did show that a gradual elevation of intracellular adenosine levels to pathological levels strongly increases the incidence of calcium waves and ITI and that this could be reversed by selective A2AR inhibition [13], suggesting that A2AR activation plays a prominent role in pathological elevations of the adenosine level. Moreover, this study uses human atrial myocytes that are well suited for translational studies of receptor-mediated modulation of electrophysiological function. However, we cannot rule out that our findings could potentially be affected or present variability due to variations in concurrent disease, risk factors, or pharmacological treatments among the study population. In this context, age has been shown to affect ICa density [28] and sex has been shown to have differential effects on ICa density and ITI frequency [29]. However, because this study does not compare different groups of patients, this issue is primarily expected to increase variability between measurements, rather than the effect of pharmacological treatments. Similarly, the present study was conducted in right atrial myocytes, and we cannot rule out that some of the findings may be specific to the right atrium.
3.5. Clinical Implications and Conclusion
We have previously reported that the expression of A2ARs is upregulated in AF, and underlies a higher incidence of calcium-release induced ITI and afterdepolarizations in atrial myocytes from patients with AF [13]. The relevance of these findings is further underscored by higher plasmatic adenosine levels and lower adenosine deaminase activity in patients with AF [30]. In this context, the present findings, showing that A3R activation regulates the impact of A2AR activation on RyR2 phosphorylation and spontaneous calcium release, suggest that selective activation of adenosine A3Rs might be suitable to dampen pathological elevations of the incidence of spontaneous calcium release-induced electrical activity. In particular, cardioprotective approaches targeting the A3R during ischemia, where adenosine levels are known to surge [31], could be a point of departure to explore the potential of A3Ra as a novel target to prevent induction of atrial ectopic atrial activity.
4. Materials and Methods
4.1. Myocyte Isolation
Atrial myocytes were isolated from tissue fragments collected from 53 patients, without a previous history of AF, undergoing cardiac surgery at Hospital de la Santa Creu i Sant Pau in Barcelona. Clinical and echocardiographic data of these patients are summarized in Table 1. Myocytes were isolated from right atrial samples, as previously described [28]. Each patient gave written consent to obtain blood and tissue samples that would otherwise have been discarded during surgery.
4.2. Quantitative Real-Time PCR
Total RNA was isolated from human right atrial samples using a commercially available kit. First-strand cDNA was synthesized from 1 mg of total RNA. cDNA was amplified using TaqMan master mix and primers from Thermo Fisher Scientific (Waltham, MA, USA) for the human glyceraldehyde-3-phosphate dehydrogenase (GAPDH): Hs00266705_g1; for human A1R (ADORA1): Hs00181231_m1; for human A2AR (ADORA2A): Hs00169123_m1; for human A2AB (ADORA2B): Hs00386497_m1; and for human A3R (ADORA3): Hs00181232_m1.
4.3. Patch-Clamp Technique
Isolated myocytes were subjected to the perforated patch technique using a HEKA EPC-10 amplifier (HEKA Elektronik, Lambrecht/Pfalz, Germany). Series resistance compensation was not applied. The extracellular solution contained (in mM): NaCl 127, TEA 5, HEPES 10, NaHCO3 4, NaH2PO4 0.33, glucose 10, pyruvic acid 5, CaCl2 2, and MgCl2 1.8 (pH = 7.4). The pipette solution contained (in mM): aspartic acid 109, CsCl 47, Mg2ATP 3, MgCl2 1, na2phosphocreatine 5, Li2GTP 0.42, HEPES 10 (pH = 7.2 with CsOH), and 250 µg/mL amphotericin B. ICa density and properties were measured using a 200 ms depolarization from a holding potential of −80 mV to 0 mV. A 50 ms prepulse to −45 mV was used to inactivate the Na+ current. The ICa amplitude was normalized to the cell capacitance to obtain the ICa density. The decay of the ICa was fit with a double exponential to obtain the time constants tau-1 and tau-2 for fast and slow ICa inactivation. This study focused on the fast time constant, which is modulated by calcium release from the SR and by calcium entry through the L-type calcium channel. ITI currents were recorded at a holding potential of −80 mV in 4 × 30 s intervals to determine the ITI frequency. Brief exposure (6s) to 10 mM caffeine at a holding potential of −80 mV was used to release calcium from the SR and the time integral of the resulting transient inward NCX-current was used to assess the SR calcium load. Transformation of the charge carried by the NCX-current assumed a stoichiometry of 3 Na+:1 Ca2+ for the NCX. Working solutions containing 200 nM CGS21680 and/or 1 µM MRS1191 were prepared from 1 mM stock solutions dissolved in DMSO.
4.4. Immunofluorescent Labelling
Isolated myocytes were fixed and permeabilized, as previously described [32] and non-specific sites were blocked by incubation with PBS/Tween 20, 0.2% and horse serum, 10% for 30 min. Total and ser-2808 phosphorylated RyR2 were inmunofluorescently labeled with mouse anti-RyR2 (C3-33 NR07, 1:1200; Calbiochem, San Diego, CA, USA) and rabbit anti-ser2808-P (1:1200, A010-30, Badrilla, Leeds, UK). The secondary antibodies AlexaFluor 488 anti-mouse and AlexaFluor 594 anti-rabbit were diluted 1:1000 and used to stain total RyR2 green and ser-2808 phosphorylated RyR2 red. Images were acquired with a Leica AOBS SP5 confocal microscope (Wetzlar, Germany) and a 63× glycerol immersion objective.
4.5. Confocal Imaging
Confocal calcium images (512 × 140 pixels) were recorded at 90 Hz with the Leica SP5 AOBS resonance-scanning confocal microscope in fluo-4 loaded myocytes, as described previously [32]. Experiments were carried out at room temperature. Calcium sparks were detected and clustered in 2 × 2 µm2 regions of interest, termed spark sites, using a custom-made algorithm based on continuous wavelet transform of the temporal profile at every spatial location, as described elsewhere [33]. The calcium spark frequency and the number of spark sites were normalized to the cell area to obtain the calcium spark density (sparks/s/1000 µm2) and the spark site density (spark sites/1000 µm2). In addition, we calculated the number of sparks per site (sparks/site/s). A series of morphological features were measured for each spark signal: Relative amplitude of the peak to the local baseline (F/F0), full duration at half maximum (FDHM), decay constant of an exponential fit (tau), the coefficient of determination of the exponential fit (R2), and full width at half maximum (FWHM).
4.6. Data Analysis
Experimental data were collected and analyzed without knowledge about clinical data and clinicians did not know the experimental results. Statistical analysis was carried out using RStudio 4.2.2 statistical software. Unless otherwise stated, data were averaged for each patient and results are given as mean ± s.e.m. with indication of the number of patients in each group. Fisher’s exact test was carried out for categorical data. Student’s t-test was used for paired or unpaired comparisons, and ANOVA, ANOVA with Welch correction or Kruskal–Wallis were used for comparison of multiple effects, as indicated. Tests used are indicated for each figure and statistically significant effects are indicated with p-values or *: p < 0.05, **: p < 0.01; ***: p < 0.001.
Author Contributions
Conceptualization, R.F. and L.H.-M.; methodology, C.T., V.J.-S., J.M., J.G. and L.H.-M.; formal analysis, C.T., V.J.-S., J.M. and L.H.-M.; investigation, C.T., V.J.-S. and L.H.-M.; resources, R.F., J.M. and F.C.; writing—original draft preparation, L.H.-M.; writing—review and editing, C.T., V.J.-S., R.F., J.G., F.C. and L.H.-M.; supervision, R.F. and L.H.-M.; project administration, L.H.-M.; funding acquisition, J.G., F.C. and L.H.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FEDER-EU/Ministerio de Ciencia e Innovación (PID2020-116927RB-C21 to LH-M and PID2020-118511RB-I00 to FC) Generalitat de Catalunya (2017SGR1769 to LH-M and 2017SGR1604 to FC), Fundació la Marató de TV3 (Grant 20152030/31 to LH-M/FC).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the ethics committee at Hospital de la Santa Creu i Sant Pau, Barcelona, Spain (protocol code AZAR-AF_2015, approved 30/11/2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
Acknowledgments
The collaboration of Carlos Barrera and the cardiac surgery team at Hospital de la Santa Creu i Sant Pau is greatly appreciated.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Vest, J.A.; Wehrens, X.H.; Reiken, S.R.; Lehnart, S.E.; Dobrev, D.; Chandra, P.; Danilo, P.; Ravens, U.; Rosen, M.R.; Marks, A.R. Defective Cardiac Ryanodine Receptor Regulation during Atrial Fibrillation. Circulation 2005, 111, 2025–2032. [Google Scholar] [CrossRef]
- Mattiazzi, A.; Hove-Madsen, L.; Bers, D.M. Protein Kinase Inhibitors Reduce SR Ca Transport in Permeabilized Cardiac Myocytes. Am. J. Physiol. Circ. Physiol. 1994, 267, H812–H820. [Google Scholar] [CrossRef] [PubMed]
- Hove-Madsen, L.; Méry, P.-F.; Jurevičius, J.; Skeberdis, A.V.; Fischmeister, R. Regulation of Myocardial Calcium Channels by Cyclic AMP Metabolism. Basic Res. Cardiol. 1996, 91, 1–8. [Google Scholar] [CrossRef]
- Fischmeister, R.; Castro, L.R.V.; Abi-Gerges, A.; Rochais, F.; Jurevičius, J.; Leroy, J.; Vandecasteele, G. Compartmentation of Cyclic Nucleotide Signaling in the Heart: The Role of Cyclic Nucleotide Phosphodiesterases. Circ. Res. 2006, 99, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli, L.; Shryock, J.C.; Song, Y.; Wang, D.; Srinivas, M. Ionic Basis of the Electrophysiological Actions of Adenosine on Cardiomyocytes. FASEB J. 1995, 9, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Dennis, D.M.; Raatikainen, M.J.P.; Martens, J.R.; Belardinelli, L. Modulation of Atrioventricular Nodal Function by Metabolic and Allosteric Regulators of Endogenous Adenosine in Guinea Pig Heart. Circulation 1996, 94, 2551–2559. [Google Scholar] [CrossRef] [PubMed]
- Atienza, F.; Almendral, J.; Moreno, J.; Vaidyanathan, R.; Talkachou, A.; Kalifa, J.; Arenal, A.; Villacastín, J.P.; Torrecilla, E.G.; Sánchez, A.; et al. Activation of Inward Rectifier Potassium Channels Accelerates Atrial Fibrillation in Humans: Evidence for a Reentrant Mechanism. Circulation 2006, 114, 2434–2442. [Google Scholar] [CrossRef]
- Li, N.; Csepe, T.A.; Hansen, B.J.; Sul, L.V.; Kalyanasundaram, A.; Zakharkin, S.O.; Zhao, J.; Guha, A.; Van Wagoner, D.R.; Kilic, A.; et al. Adenosine-Induced Atrial Fibrillation. Circulation 2016, 134, 486–498. [Google Scholar] [CrossRef]
- Wan, T.C.; Tampo, A.; Kwok, W.M.; Auchampach, J.A. Ability of CP-532,903 to Protect Mouse Hearts from Ischemia/Reperfusion Injury Is Dependent on Expression of A 3 Adenosine Receptors in Cardiomyoyctes. Biochem. Pharmacol. 2019, 163, 21–31. [Google Scholar] [CrossRef]
- Stambaugh, K.; Elliott, G.T.; Jacobson, K.A.; Liang, B.T. Additive Effects of Late Preconditioning Produced by Monophosphoryl Lipid A and the Early Preconditioning Mediated by Adenosine Receptors and K(ATP) Channel. Circulation 1999, 99, 3300–3307. [Google Scholar] [CrossRef]
- Carr, C.S.; Hill, R.J.; Masamune, H.; Kennedy, S.P.; Knight, D.R.; Tracey, W.R.; Yellon, D.M. Evidence for a Role for Both the Adenosine A1 and A3 Receptors in Protection of Isolated Human Atrial Muscle against Simulated Ischaemia. Cardiovasc. Res. 1997, 36, 52–59. [Google Scholar] [CrossRef]
- Hove-Madsen, L.; Prat-Vidal, C.; Llach, A.; Ciruela, F.; Casadó, V.; Lluis, C.; Bayes-Genis, A.; Cinca, J.; Franco, R. Adenosine A2A Receptors Are Expressed in Human Atrial Myocytes and Modulate Spontaneous Sarcoplasmic Reticulum Calcium Release. Cardiovasc. Res. 2006, 72, 292–302. [Google Scholar] [CrossRef]
- Llach, A.; Molina, C.E.; Prat-Vidal, C.; Fernandes, J.; Casado, V.; Ciruela, F.; Lluis, C.; Franco, R.; Cinca, J.; Hove-Madsen, L. Abnormal Calcium Handling in Atrial Fibrillation Is Linked to Up-Regulation of Adenosine A2A Receptors. Eur. Heart J. 2011, 32, 721–729. [Google Scholar] [CrossRef]
- Molina, C.E.; Llach, A.; Herraiz-Martínez, A.; Tarifa, C.; Barriga, M.; Wiegerinck, R.F.; Fernandes, J.; Cabello, N.; Vallmitjana, A.; Benitéz, R.; et al. Prevention of Adenosine A2A Receptor Activation Diminishes Beat-to-Beat Alternation in Human Atrial Myocytes. Basic Res. Cardiol. 2016, 111, 1–15. [Google Scholar] [CrossRef]
- Gao, Z.G.; Blaustein, J.B.; Gross, A.S.; Melman, N.; Jacobson, K.A. N6-Substituted Adenosine Derivatives: Selectivity, Efficacy, and Species Differences at A3 Adenosine Receptors. Biochem. Pharmacol. 2003, 65, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Langlois, M.; Fischmeister, R. 5-HT4 Receptor Ligands: Applications and New Prospects. ChemInform 2003, 34, 319–344. [Google Scholar] [CrossRef]
- Ferré, S.; Ciruela, F.; Dessauer, C.W.; González-Maeso, J.; Hébert, T.E.; Jockers, R.; Logothetis, D.E.; Pardo, L. G Protein-Coupled Receptor-Effector Macromolecular Membrane Assemblies (GEMMAs). Pharmacol. Ther. 2022, 231, 107977. [Google Scholar] [CrossRef]
- Nolla-Colomer, C.; Casabella-Ramon, S.; Jimenez-Sabado, V.; Vallmitjana, A.; Tarifa, C.; Herraiz-Martínez, A.; Llach, A.; Tauron, M.; Montiel, J.; Cinca, J.; et al. Β2-Adrenergic Stimulation Potentiates Spontaneous Calcium Release By Increasing Signal Mass and Co-Activation of Ryanodine Receptor Clusters. Acta Physiol. 2021, 234, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.T.; Jacobson, K.A. A Physiological Role of the Adenosine A3 Receptor: Sustained Cardioprotection. Proc. Natl. Acad. Sci. USA 1998, 95, 6995–6999. [Google Scholar] [CrossRef] [PubMed]
- Voigt, N.; Li, N.; Wang, Q.; Wang, W.; Trafford, A.W.; Abu-Taha, I.; Sun, Q.; Wieland, T.; Ravens, U.; Nattel, S.; et al. Enhanced Sarcoplasmic Reticulum Ca2+-Leak and Increased Na+-Ca2+ Exchanger Function Underlie Delayed Afterdepolarizations in Patients with Chronic Atrial Fibrillation. Circulation 2012, 125, 2059–2070. [Google Scholar] [CrossRef]
- Rivet-Bastide, M.; Vandecasteele, G.; Hatem, S.; Verde, I.; Bénardeau, A.; Mercadier, J.J.; Fischmeister, R. CGMP-Stimulated Cyclic Nucleotide Phosphodiesterase Regulates the Basal Calcium Current in Human Atrial Myocytes. J. Clin. Invest. 1997, 99, 2710–2718. [Google Scholar] [CrossRef]
- Molina, C.E.; Leroy, J.; Richter, W.; Xie, M.; Scheitrum, C.; Lee, I.O.; Maack, C.; Rucker-Martin, C.; Donzeau-Gouge, P.; Verde, I.; et al. Cyclic Adenosine Monophosphate Phosphodiesterase Type 4 Protects against Atrial Arrhythmias. J. Am. Coll. Cardiol. 2012, 59, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sábado, V.; Casabella-Ramón, S.; Llach, A.; Gich, I.; Casellas, S.; Ciruela, F.; Chen, S.R.W.; Guerra, J.M.; Ginel, A.; Benítez, R.; et al. Beta-Blocker Treatment of Patients with Atrial Fibrillation Attenuates Spontaneous Calcium Release-Induced Electrical Activity. Biomed. Pharmacother. 2023, 158, 114169. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Tosh, D.K.; Jain, S.; Gao, Z.G. Historical and Current Adenosine Receptor Agonists in Preclinical and Clinical Development. Front. Cell. Neurosci. 2019, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekera, P.C.; Wan, T.C.; Gizewski, E.T.; Auchampach, J.A.; Lasley, R.D. Adenosine A1 Receptors Heterodimerize with Β1- and Β2-Adrenergic Receptors Creating Novel Receptor Complexes with Altered G Protein Coupling and Signaling. Cell. Signal. 2013, 25, 736–742. [Google Scholar] [CrossRef]
- Ladera, C.; Godino, M.D.C.; Martín, R.; Luján, R.; Shigemoto, R.; Ciruela, F.; Torres, M.; Sánchez-Prieto, J. The Coexistence of Multiple Receptors in a Single Nerve Terminal Provides Evidence for Pre-Synaptic Integration. J. Neurochem. 2007, 103, 2314–2326. [Google Scholar] [CrossRef]
- Dragic, M.; Stekic, A.; Zeljkovic, M.; Zaric Kontic, M.; Mihajlovic, K.; Adzic, M.; Grkovic, I.; Nedeljkovic, N. Altered Topographic Distribution and Enhanced Neuronal Expression of Adenosine-Metabolizing Enzymes in Rat Hippocampus and Cortex from Early to Late Adulthood. Neurochem. Res. 2022, 47, 1637–1650. [Google Scholar] [CrossRef]
- Herraiz-Martínez, A.; Álvarez-García, J.; Llach, A.; Molina, C.E.; Fernandes, J.; Ferrero-Gregori, A.; Rodríguez, C.; Vallmitjana, A.; Benítez, R.; Padró, J.M.; et al. Ageing Is Associated with Deterioration of Calcium Homeostasis in Isolated Human Right Atrial Myocytes. Cardiovasc. Res. 2015, 106, 76–86. [Google Scholar] [CrossRef]
- Herraiz-Martínez, A.; Tarifa, C.; Jiménez-Sábado, V.; Llach, A.; Godoy-Marín, H.; Colino-Lage, H.; Nolla-Colomer, C.; Casabella-Ramon, S.; Izquierdo-Castro, P.; Benítez, I.; et al. Influence of Sex on Intracellular Calcium Homoeostasis in Patients with Atrial Fibrillation. Cardiovasc. Res. 2022, 118, 1033–1045. [Google Scholar] [CrossRef]
- Godoy-Marín, H.; Duroux, R.; Jacobson, K.A.; Soler, C.; Colino-Lage, H.; Jiménez-Sábado, V.; Montiel, J.; Hove-Madsen, L.; Ciruela, F. Adenosine A2A Receptors Are Upregulated in Peripheral Blood Mononuclear Cells from Atrial Fibrillation Patients. Int. J. Mol. Sci. 2021, 22, 3467. [Google Scholar] [CrossRef]
- Bertolet, B.D.; Hill, J.A.; Kerensky, R.A.; Belardinelli, L. Myocardial Infarction Related Atrial Fibrillation: Role of Endogenous Adenosine. Heart 1997, 78, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Herraiz-Martínez, A.; Llach, A.; Tarifa, C.; Gandía, J.; Jiménez-Sabado, V.; Lozano-Velasco, E.; Serra, S.A.; Vallmitjana, A.; Vázquez Ruiz De Castroviejo, E.; Benítez, R.; et al. The 4q25 Variant Rs13143308T Links Risk of Atrial Fibrillation to Defective Calcium Homoeostasis. Cardiovasc. Res. 2019, 115, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Tarifa, C.; Vallmitjana, A.; Jiménez-Sábado, V.; Marchena, M.; Llach, A.; Herraiz-Martínez, A.; Godoy-Marín, H.; Nolla-Colomer, C.; Ginel, A.; Viñolas, X.; et al. The Spatial Distribution of Calcium Sparks Determines Their Ability to Induce Afterdepolarizations in Human Atrial Myocytes. JACC Basic Transl. Sci. 2022, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
