Cationic Pullulan Derivatives Based Flocculants for Removal of Some Metal Oxides from Simulated Wastewater
Abstract
1. Introduction
2. Results and Discussion
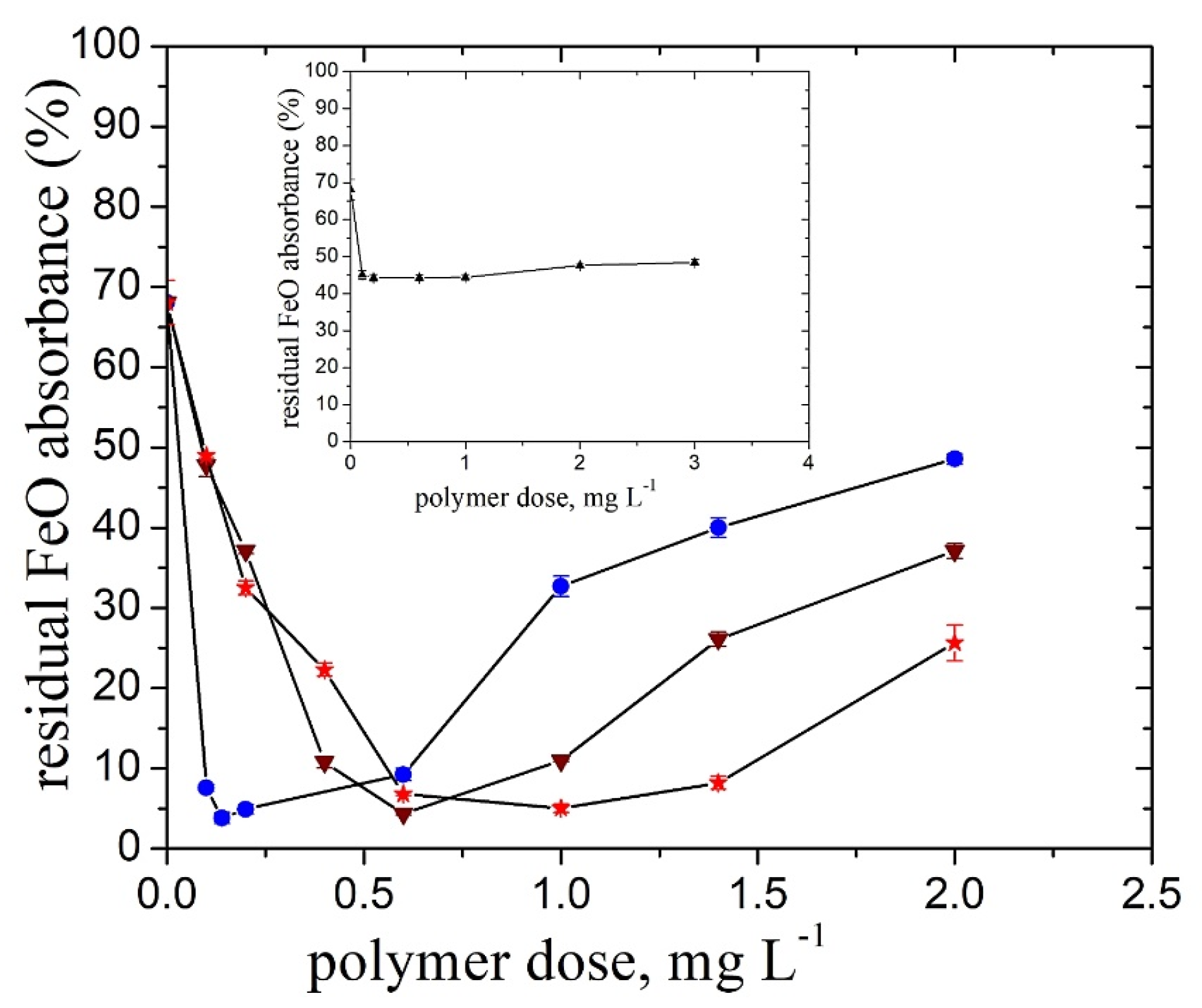
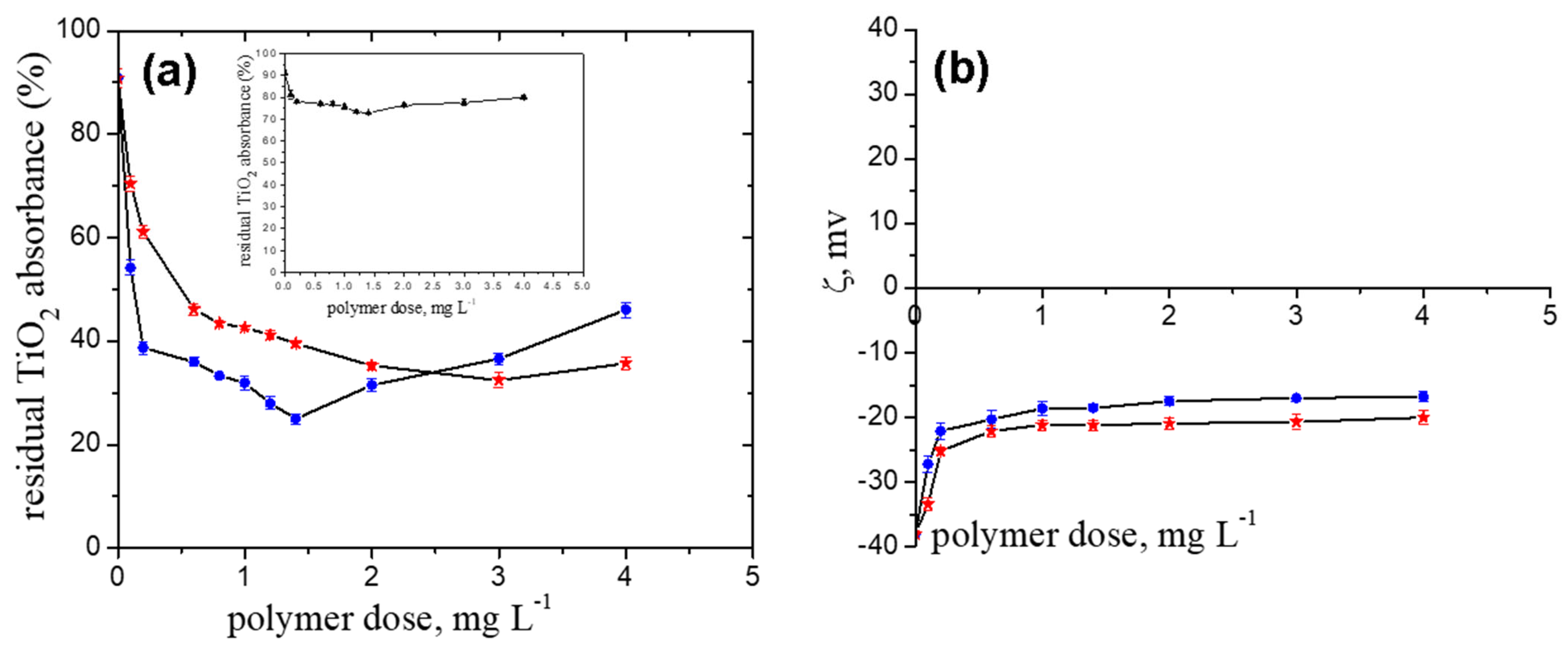
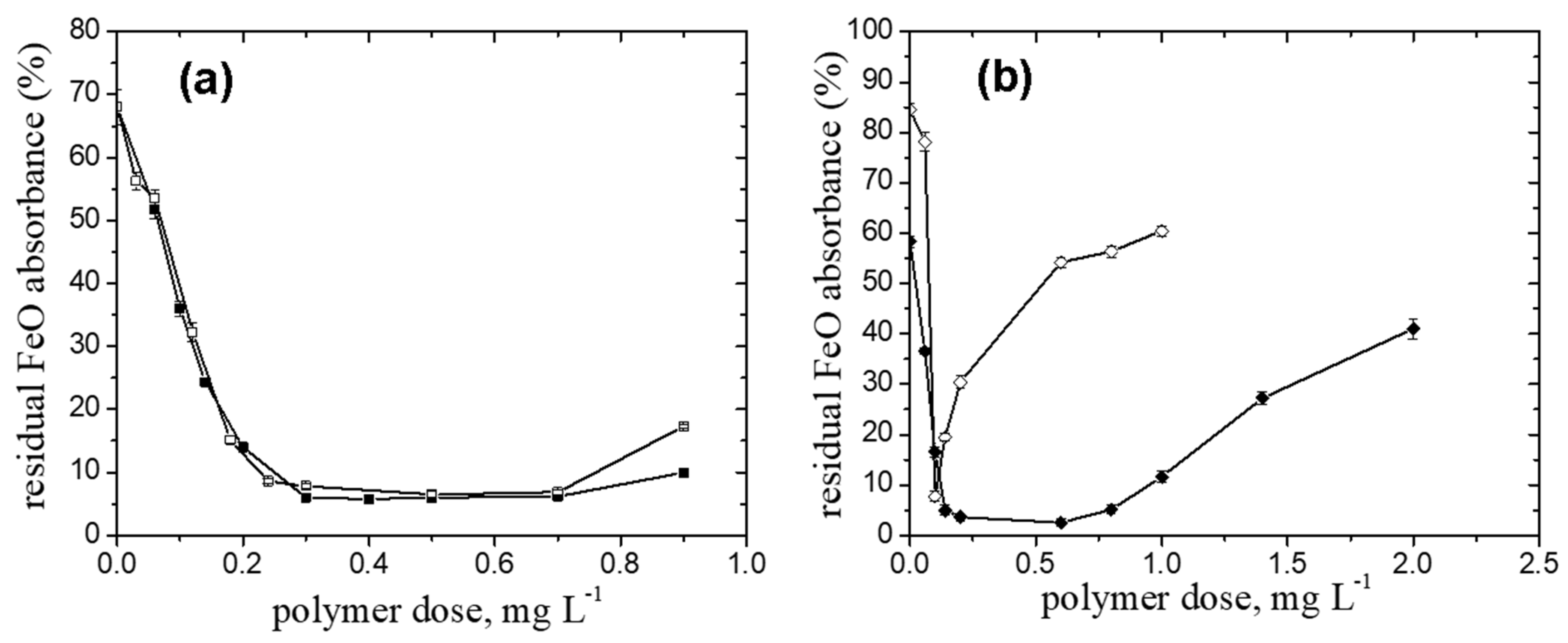
2.1. Pullulan Derivatives Ionic Group Content
- FeO suspension
- b.
- TiO2 suspension
2.2. Initial Solution Concentration of the Pullulan Derivatives (cip) and FeO Dispersion (cFeO)
2.3. Suspension pH
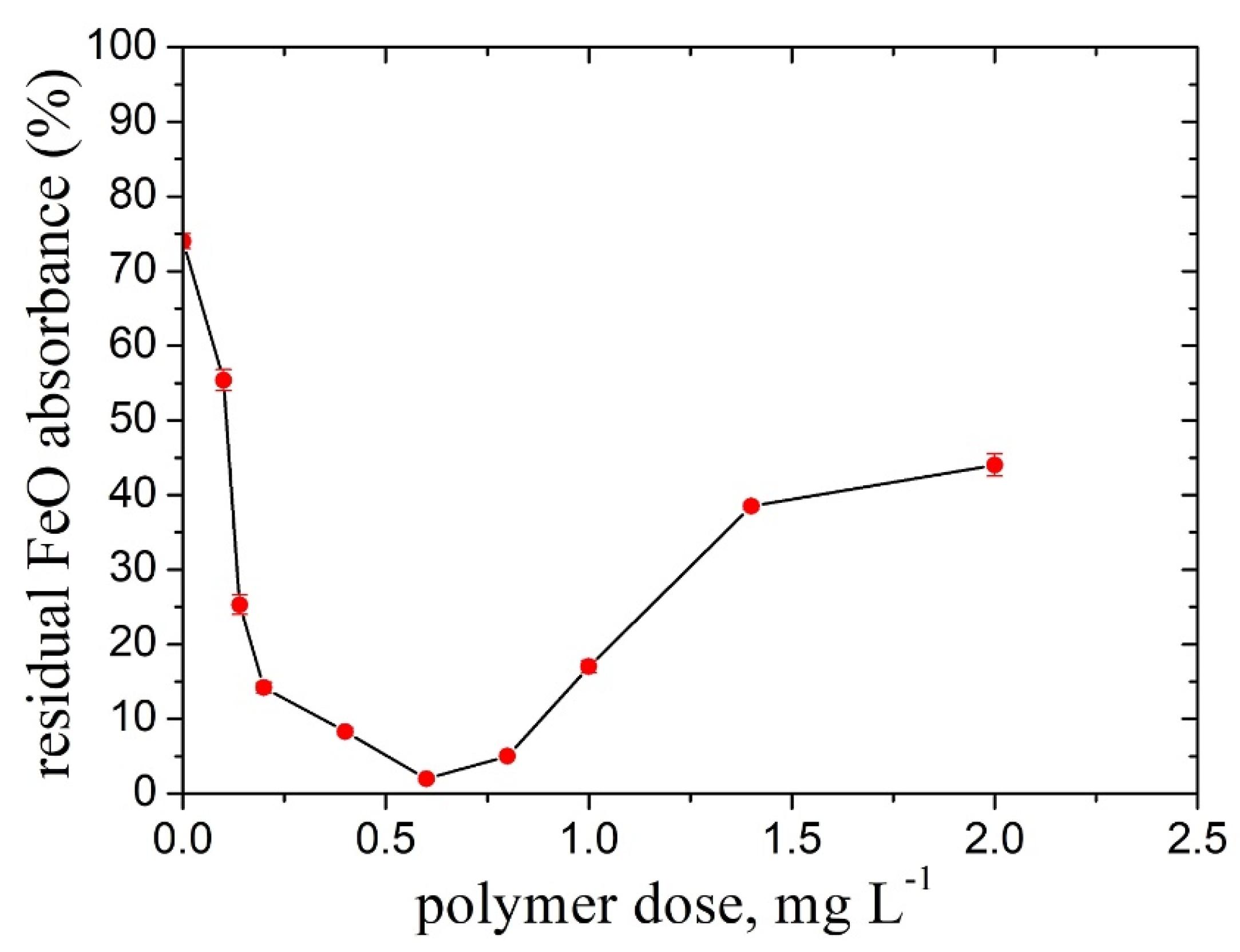
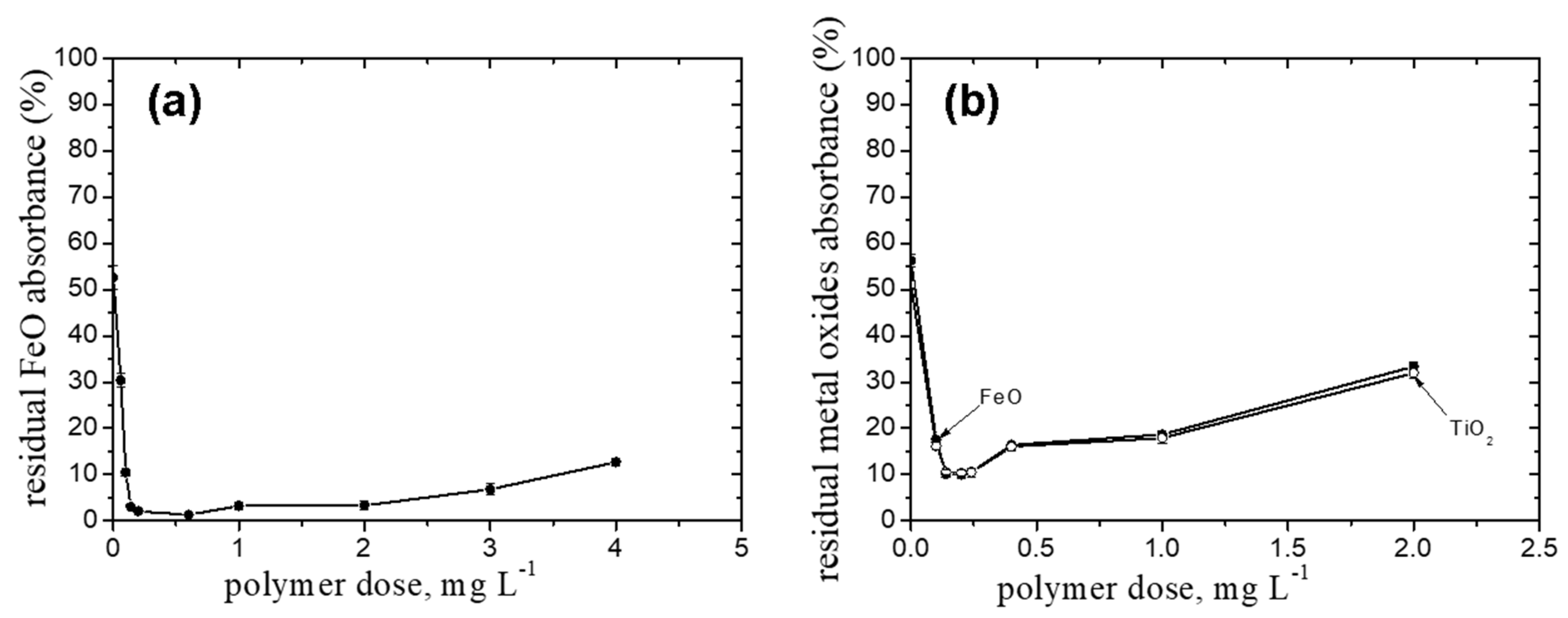
2.4. FeO Particles in Suspensions Containing Salts, Kaolin, or TiO2 Particles
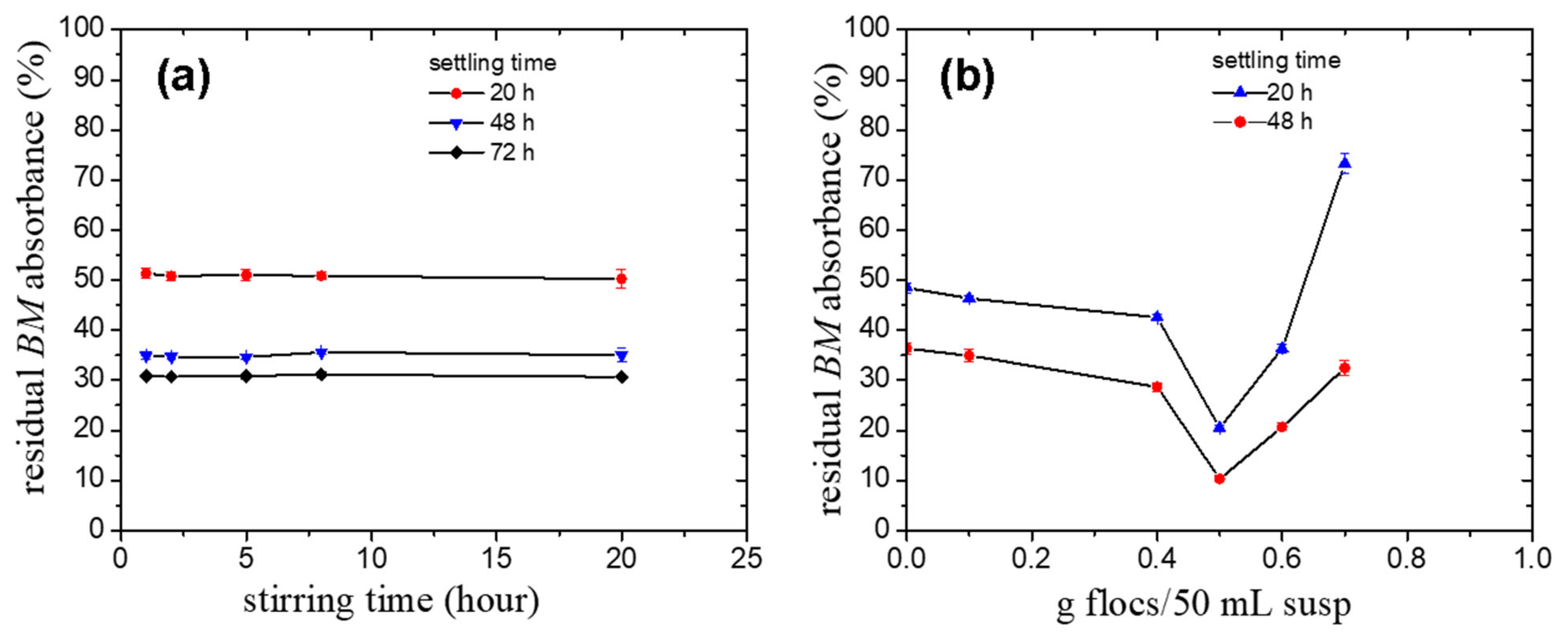
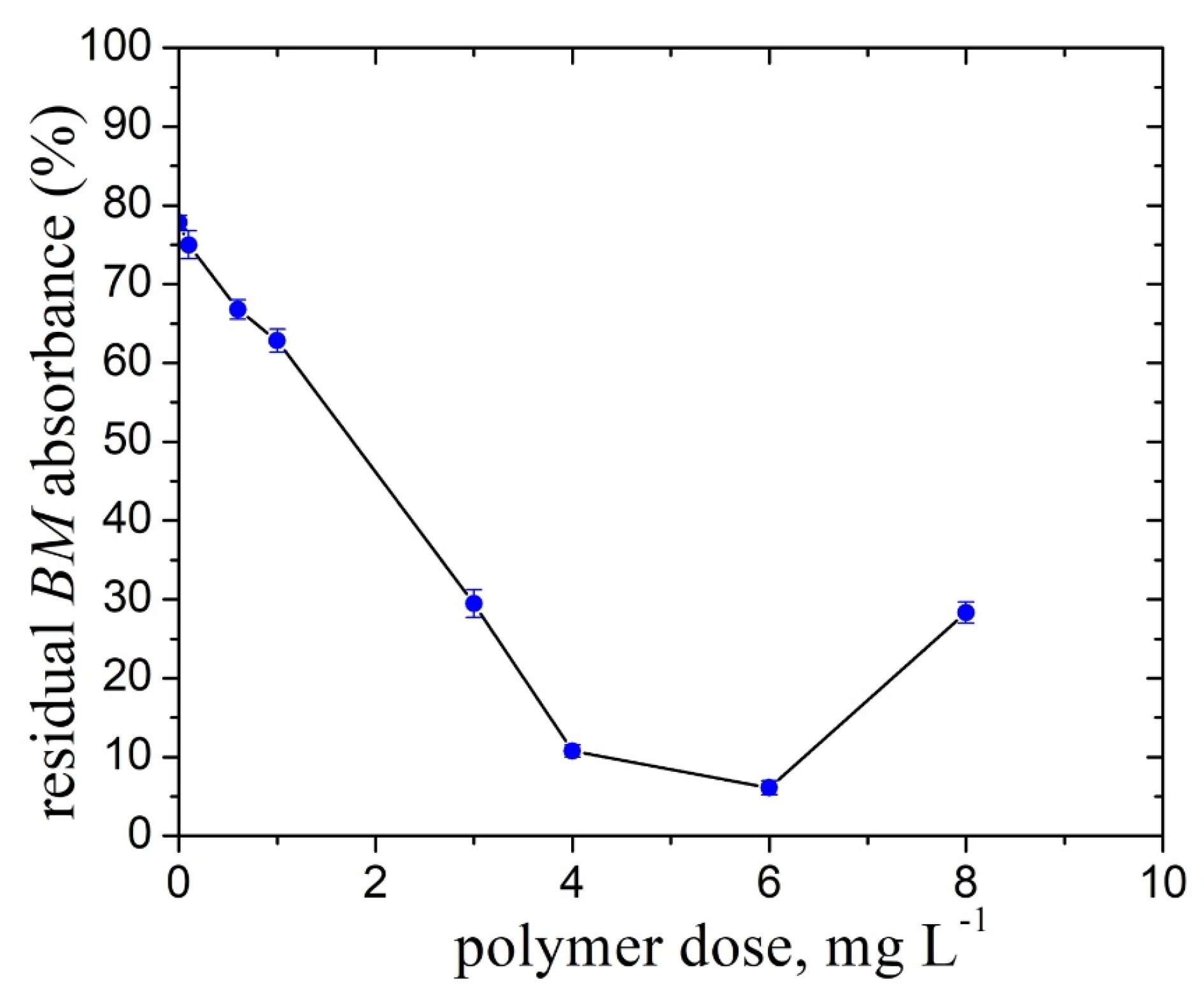
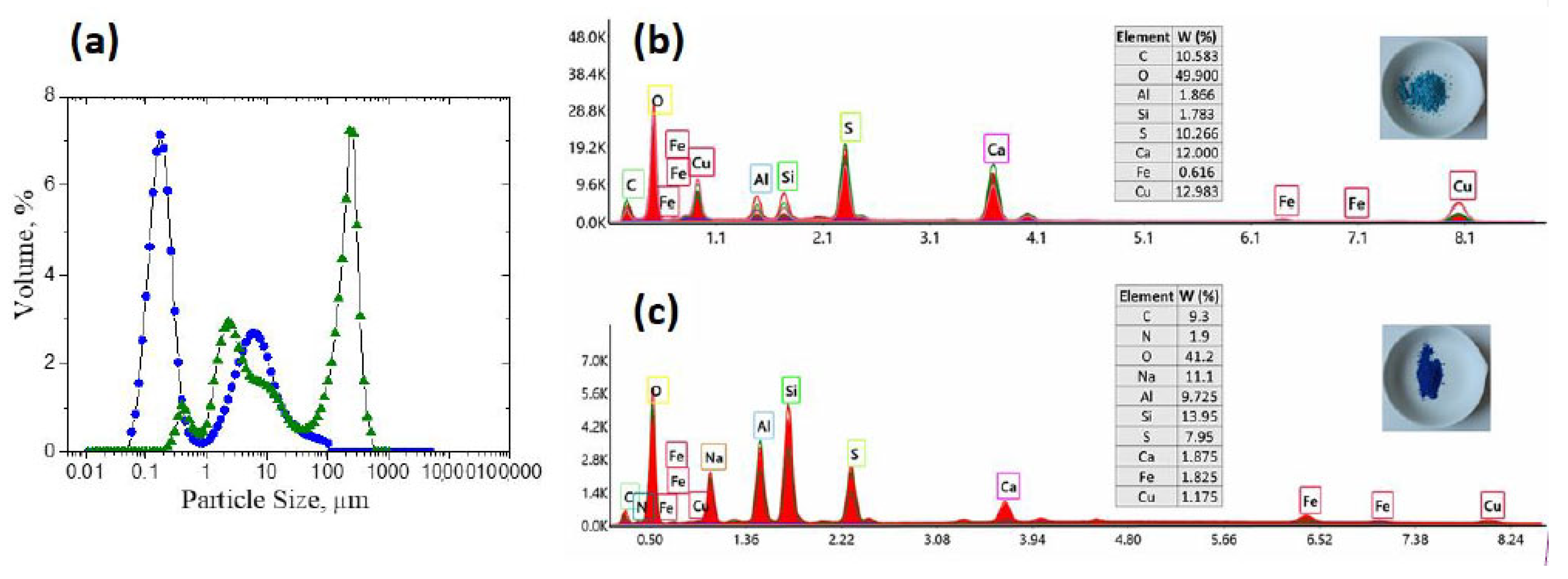
2.5. Effect of the Pullulan Derivative/FeO Flocs on the Reduction of the Fungicide Bordeaux Mixture Particles Content from Synthetic Wastewater
3. Experimental
3.1. Materials
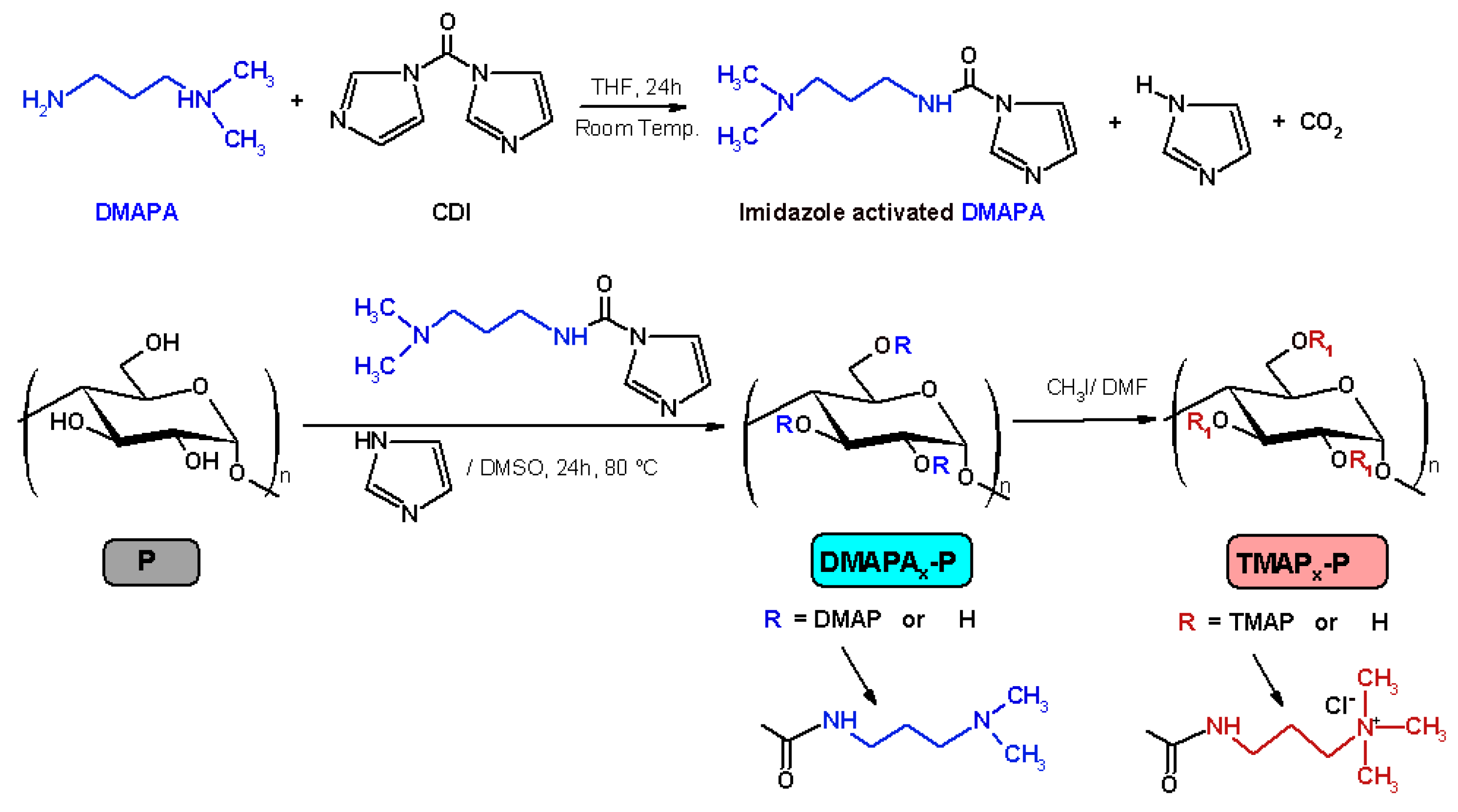
| Sample Code | P (mmol) | CDI (mmol) | DMAPA (mmol) | Molar Ratio MeI/UGU of DMAPAx-P | Degree of Substitution DS a | Amine Groups Content (meq. g−1) |
|---|---|---|---|---|---|---|
| TMAP0.2–P | 6.17 | 1.54 | 1.54 | 0.56/1 | 0.2 ± 0.029 | 1.09 ± 0.108 |
| TMAP0.4–P | 3.70 | 3.73 | 1.47/1 | 0.4 ± 0.042 | 2.14 ± 0.065 | |
| TMAP0.7–P | 6.17 | 6.19 | 2.07/1 | 0.7 ± 0.084 | 2.78 ± 0.125 |
3.2. Methods
4. Conclusions
- The residual metal oxides absorbance values changed with the addition of different doses of pullulan derivatives, irrespective of the particle′s dispersion medium composition.
- The doseop values decreased with increasing ionic group content from 1 mg L−1. (TMAP0.2–P) to 0.14 mg L−1 (TMAP0.7–P) in the case of the FeO particles and from 3 mg L−1 (TMAP0.2–P) to 1.4 mg L−1 (TMAP0.7–P) in the case of the TiO2 ones.
- For the FeO particles removal, higher doseop values were recorded for the initial polymer concentration, cip located either in the dilute regime (cip = 0.1 g L−1 − doseop= 0.5 mg L−1) or in the semidilute one (cip = 1 g L−1 − doseop = 0.6 mg L−1) than that recorded for cip close to c* (cip = 0.5 g L−1 − doseop = 0.3 mg L−1).
- A noticeable increase in the doseop (0.6 mg L−1) and optimum dose interval (0.14 mg L−1 and 0.8 mg L−1) was found for the higher pH value than that of the natural one and the high particle suspension concentration, cFeo= 0.6 g L−1, respectively.
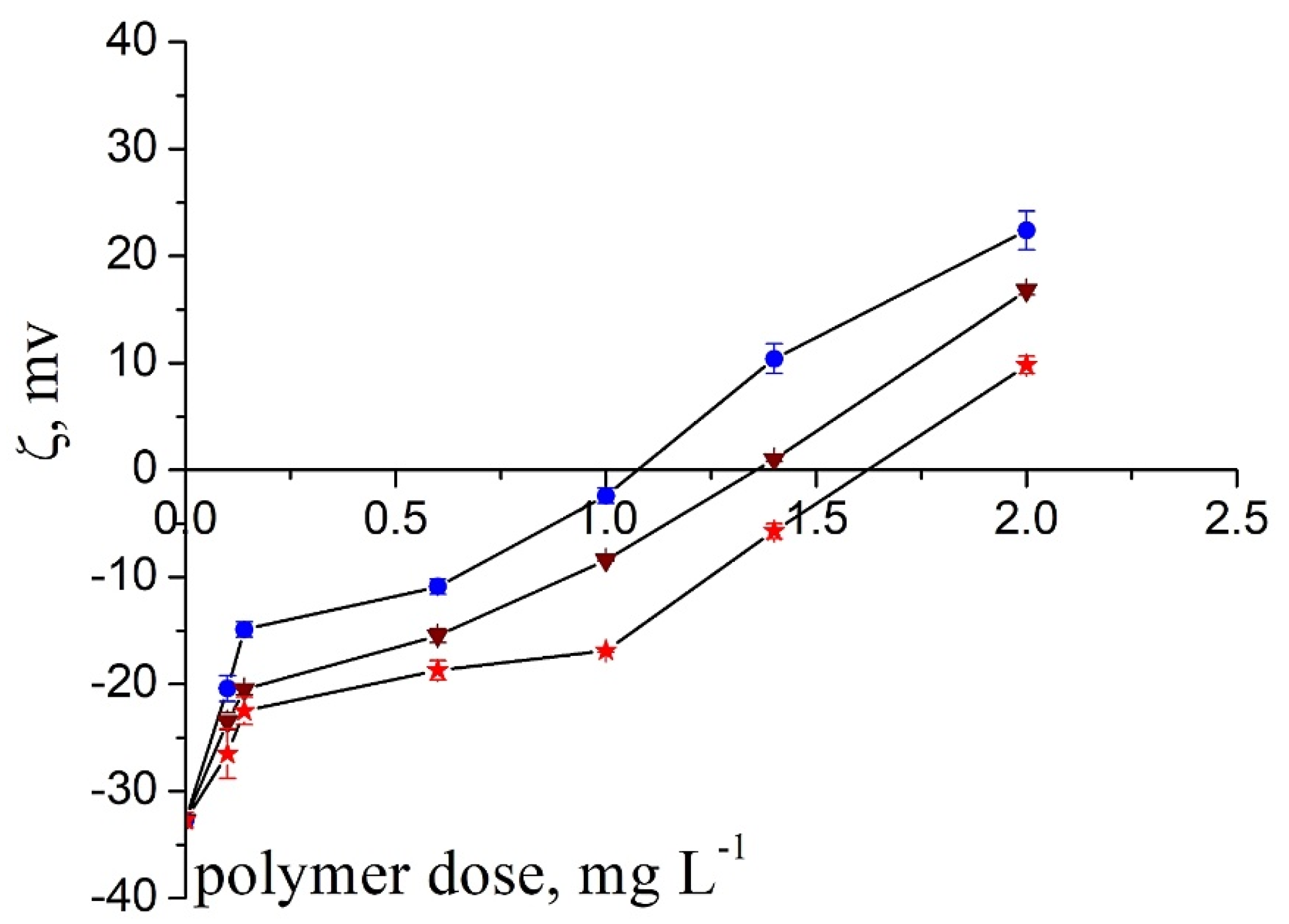
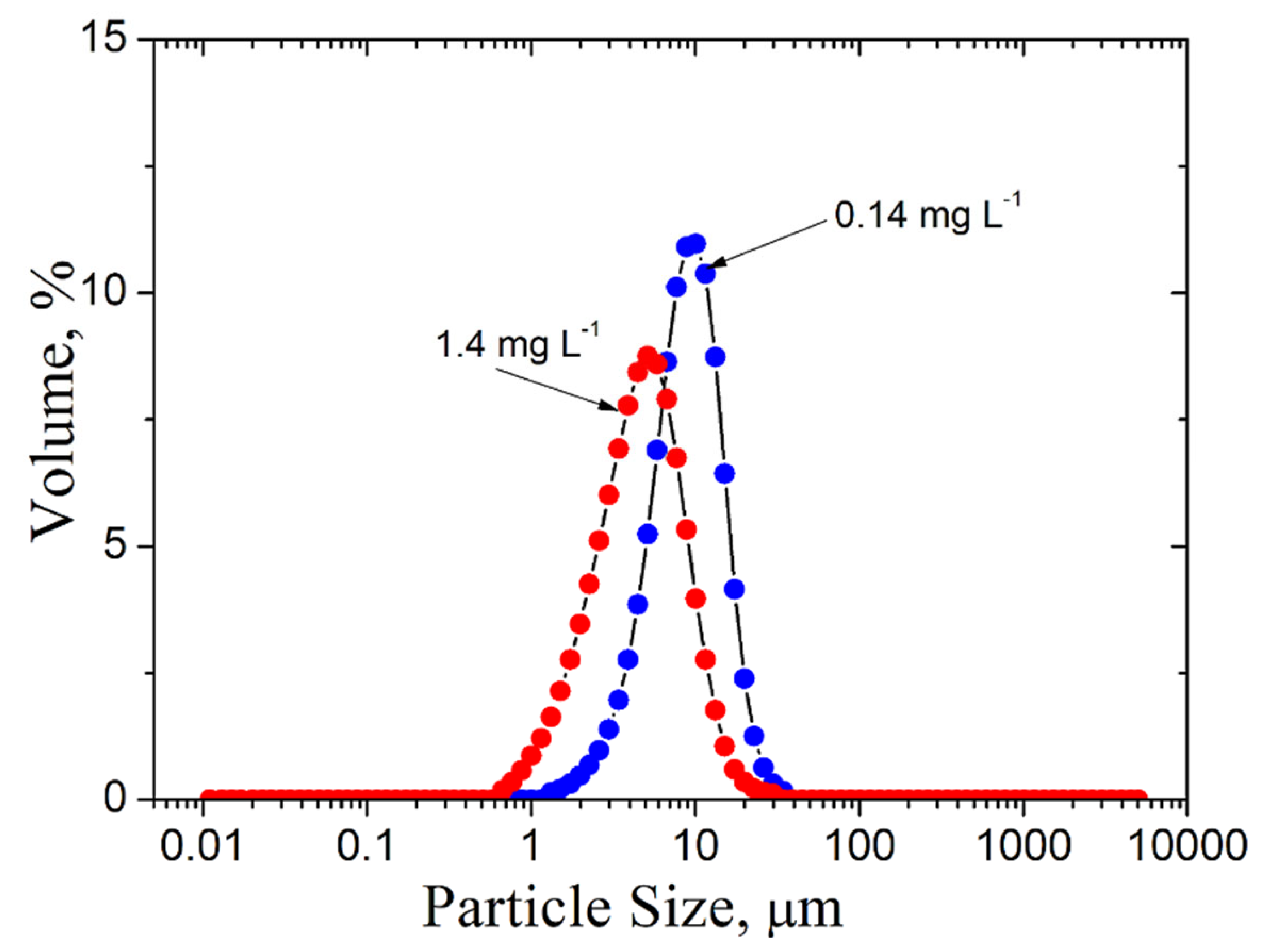
- The zeta potential and the particle aggregate size measurement results pointed to the patch flocculation mechanism.
- The pullulan derivatives proved to be good flocculants for the mixture of FeO and TiO2 particles (w/w, 1/1) as well as for the FeO particles from suspensions containing mixtures of salts and kaolin particles.
- The FeO/TMAP0.7–P flocs exhibit good performance in the removal of the fungicide Bordeaux mixture particles from simulated wastewater (residual BM content of 0.049 g L−1) at the optimum amount of particles (50 g flocs/1000 mL BM suspension).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Pullulan: Microbial sources, production and Applications. Carbohydr. Polym. 2008, 73, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Demirci, A.; Catchmark, J.M. Pullulan: Biosynthesis, production, and applications. Appl. Microbiol. Biotechnol. 2011, 92, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Salehizadeh, H.; Yan, N.; Farnood, R. Recent advances in polysaccharide bio-based flocculants. Biotechnol. Adv. 2018, 36, 92–119. [Google Scholar] [CrossRef] [PubMed]
- Bataille, I.; Meddahi-Pelle, A.; Le Visage, C.; Letourneur, D.; Chaubet, F. Pullulan for biomedical uses. In Polysaccharides in Medicinal and Pharmaceutical Applications; Popa, V., Ed.; Ismithers Rapra Publishing: Shrewsbury, UK, 2011; pp. 145–182. [Google Scholar]
- Prado, H.J.; Matulewicz, M.C. Cationization of polysaccharides:A path to greener derivatives with many industrial applications. Eur. Polym. J. 2014, 52, 53–75. [Google Scholar] [CrossRef]
- Dionísio, M.; Braz, L.; Corvo, M.; Lourenço, J.P.; Grenha, A.; Rosa da Costa, A.M. Charged pullulan derivatives for the development of nanocarriers by polyelectrolyte complexation. Int. J. Biol. Macromol. 2016, 86, 129–138. [Google Scholar] [CrossRef]
- Constantin, M.; Asmarandei, I.; Filimon, A.; Fundueanu, G. Synthesis, characterization and solution behavior of pullulan functionalized with tertiary amino groups. High Perform. Polym. 2015, 27, 625–636. [Google Scholar] [CrossRef]
- Constantin, M.; Bucatariu, S.; Ursu, L.; Butnaru, M.; Daraba, O.M.; Burlui, A.M.; Fundueanu, G. Novel cationic and hydrophobic pullulan derivatives as DNA nanoparticulate carriers. Cell. Chem. Technol. 2019, 53, 695–707. [Google Scholar] [CrossRef]
- Grigoras, A.G.; Constantin, M. Solution behaviour of new pullulan derivatives with biotechnological potential. Environ. Eng. Manag. J. 2019, 18, 301–310. [Google Scholar] [CrossRef]
- Moraes, F.C.; Antunes, J.C.; Forero Ramirez, L.M.; Aprile, P.; Franck, G.; Chauvierre, C.; Chaubet, F.; Letourneur, D. Synthesis of cationic quaternized pullulan derivatives for miRNA delivery. Int. J. Pharm. 2020, 577, 119041. [Google Scholar] [CrossRef]
- Constantin, M.; Asmarandei, I.; Harabagiu, V.; Ghimici, L.; Ascenzi, P.; Fundueanu, G. Removal of anionic dyes from aqueous solutions by an ion-exchanger based on pullulan microspheres. Carbohydr. Polym. 2013, 91, 74–84. [Google Scholar] [CrossRef]
- Ghimici, L.; Constantin, M. A review of the use of pullulan derivatives in wastewater purification. React. Funct. Polym. 2020, 149, 104510. [Google Scholar] [CrossRef]
- Ghimici, L.; Constantin, M.; Nafureanu, M.-M. Grafted Pullulan Derivatives for Reducing the Content of Some Pesticides from Simulated Wastewater. Polymers 2022, 14, 2663. [Google Scholar] [CrossRef]
- Ghimici, L.; Constantin, M. The separation of kreutzonit particles by cationic pullulan derivatives from model suspension, Environ. Chall. 2021, 5, 100352. [Google Scholar]
- Li, D.; Zhu, S.; Pelton, R.H.; Spafford, M. Flocculation of dilute titanium dioxide suspensions by graft cationic polyelectrolytes. Colloid Polym. Sci. 1999, 277, 108–114. [Google Scholar] [CrossRef]
- Tahir, S.S.; Rauf, N. Removal of Fe(II) from the wastewater of a galvanized pipe manufacturing industry by adsorption onto bentonite clay. J. Environ. Manag. 2004, 73, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Ghimici, L.; Nichifor, M. Separation of TiO2 particles from water and water/methanol mixtures by cationic dextran derivatives. Carbohydr. Polym. 2013, 98, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Divakaran, R.; Sivasankara Pillai, V.N. Mechanism of kaolinite and titanium dioxide flocculation using chitosan-assistance by fulvic acids? Water Res. 2004, 38, 2135–2143. [Google Scholar] [CrossRef]
- Ghimici, L.; Brunchi, C.E. Titanium dioxide separation from water by PEG and Pluronic type polymers. Sep. Purif. Technol. 2013, 103, 306–312. [Google Scholar] [CrossRef]
- Ghimici, L.; Suflet, D.M. Phosphorylated polysaccharide derivatives as efficient separation agents for zinc and ferric oxides particles from water. Sep. Purif. Technol. 2015, 144, 31–36. [Google Scholar] [CrossRef]
- Tang, X.; Huang, T.; Zhang, S.; Wang, W.; Zheng, H. The role of sulfonated chitosan-based flocculant in the treatment of hematite wastewater containing heavy metals. Colloids Surf. A Physicochem. Eng. Aspects 2020, 585, 124070. [Google Scholar] [CrossRef]
- Khan, J.; Lin, S.; Nizeyimana, J.C.; Wu, Y.; Wang, Q.; Liu, X. Removal of copper ions from wastewater via adsorption on modified hematite (α-Fe2O3) iron oxide coated sand. J. Clean. Prod. 2021, 319, 128687. [Google Scholar] [CrossRef]
- You, Z.; Zhao, C.; Sun, Y.; Zhuang, C. Application of PAFC/CPAM for the removal of ZnO nanoparticles by enhanced coagulation. Water Sci. Technol. 2021, 84, 484–498. [Google Scholar] [CrossRef]
- Bolto, B.; Gregory, J. Organic polyelectrolytes in water treatment. Water Res. 2007, 41, 2301–2324. [Google Scholar] [CrossRef]
- Claesson, P.M.; Dahlgren, M.A.G.; Eriksson, L. Forces between polyelectrolyte-coated surfaces: Relations between surface interaction and floc properties. Colloids Surf. A Physicochem. Eng. Aspects 1994, 93, 293–303. [Google Scholar] [CrossRef]
- Petzold, G.; Geissler, U.; Smolka, N.; Schwarz, S. Influence of humic acid on the flocculation of clay. Colloid Polym. Sci. 2004, 282, 670–676. [Google Scholar]
- Ghimici, L.; Morariu, S.; Nichifor, M. Separation of clay suspension by ionic dextran derivatives. Sep. Purif. Technol. 2009, 68, 165–171. [Google Scholar] [CrossRef]
- Bobacka, V.; Eklund, D. The influence of charge density of cationic starch on dissolved and colloidal material from peroxide bleached thermomechanical pulp. Colloids Surf. A Physicochem. Eng. Aspects 1999, 152, 285–291. [Google Scholar] [CrossRef]
- Kleimann, J.; Gehin-Delval, C.; Auweter, H.; Borkovec, M. Super-stoichiometric charge neutralization in particle-polyelectrolyte systems. Langmuir 2005, 21, 3688–3698. [Google Scholar] [CrossRef]
- Frish, H.L.; Simha, R. The Viscosity of Colloidal Suspensions and Macromolecular Solution; Academic Press: New York, NY, USA, 1956. [Google Scholar]
- Wolf, B.A. Polyelectrolytes revisited: Reliable determination of intrinsic viscosity. Macromol. Rapid Commun. 2007, 28, 164–170. [Google Scholar] [CrossRef]
- Smith, K.M.; Fowler, G.D.; Pullket, S.; Graham, N.J.D. Sewage sludge-based adsorbents: A review of their production, properties and use in water treatment applications. Water Res. 2009, 43, 2569–2594. [Google Scholar] [CrossRef]
- Roy, M.M.; Dutta, A.; Corscadden, K.; Havard, P.; Dickie, L. Review of biosolids managements options and co-incineration of a biosolid-derived fuel. Waste Manag. 2011, 31, 2228–2235. [Google Scholar] [CrossRef] [PubMed]
- Gianico, A.; Braguglia, C.M.; Gallipoli, A.; Montecchio, D.; Mininni, G. Land Application of Biosolids in Europe: Possibilities, Con-Straints and Future Perspectives. Water 2021, 13, 103. [Google Scholar] [CrossRef]
- Hanum, F.; Yuan, L.C.; Kamahara, H.; Abdul Aziz, H.; Atsuta, Y.; Yamada, T.; Daimon, H. Treatment of Sewage Sludge Using Anaerobic Digestion in Malaysia: Current State and Challenges. Front. Energy Res. 2019, 7, 19. [Google Scholar] [CrossRef]
- Babatunde, A.O.; Zhao, Y.Q. Constructive Approaches Toward Water Treatment Works Sludge Management: An International Review of Beneficial Reuses. Environm. Sci. Technol. 2007, 37, 129–164. [Google Scholar] [CrossRef]
- Devi, P.; Saroha, A.K. Utilization of sludge based adsorbents for the removal of various pollutants: A review. Sci. Total Environ. 2017, 578, 16–33. [Google Scholar] [CrossRef]
- Soliman, N.K.; Moustafa, A.F. Industrial solid waste for heavy metals adsorption features and challenges; a review. J. Mater. Res. Technol. 2020, 9, 10235–10253. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Oulego, P.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Towards the Implementation of Circular Economy in the Wastewater Sector: Challenges and Opportunities. Water 2020, 12, 1431. [Google Scholar] [CrossRef]
- Taheriyoun, M.; Memaripour, A.; Nazari-Sharabian, M. Using recycled chemical sludge as a coagulant aid in chemical wastewater treatment in Mobarakeh Steel Complex. J. Mater. Cycles Waste Manag. 2020, 22, 745–756. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Suhas, S.V.K. Adsorption of 2,4-D and carbofuran pesticides using fertilizer and steel industry wastes. J. Colloid Interface Sci. 2006, 299, 556–563. [Google Scholar] [CrossRef]
- Hu, Y.S.; Zhao, Y.Q.; Sorohan, B. Removal of glyphosate from aqueous environment by adsorption using water industrial residual. Desalination 2011, 271, 150–156. [Google Scholar] [CrossRef]
- Sousa, S.; Jiménez-Guerrero, P.; Ruiz, A.; Ratola, N.; Alves, A. Organochlorine pesticides removal from wastewater by pine bark adsorption after activated sludge treatment. Environ. Technol. 2011, 32, 673–683. [Google Scholar] [CrossRef] [PubMed]

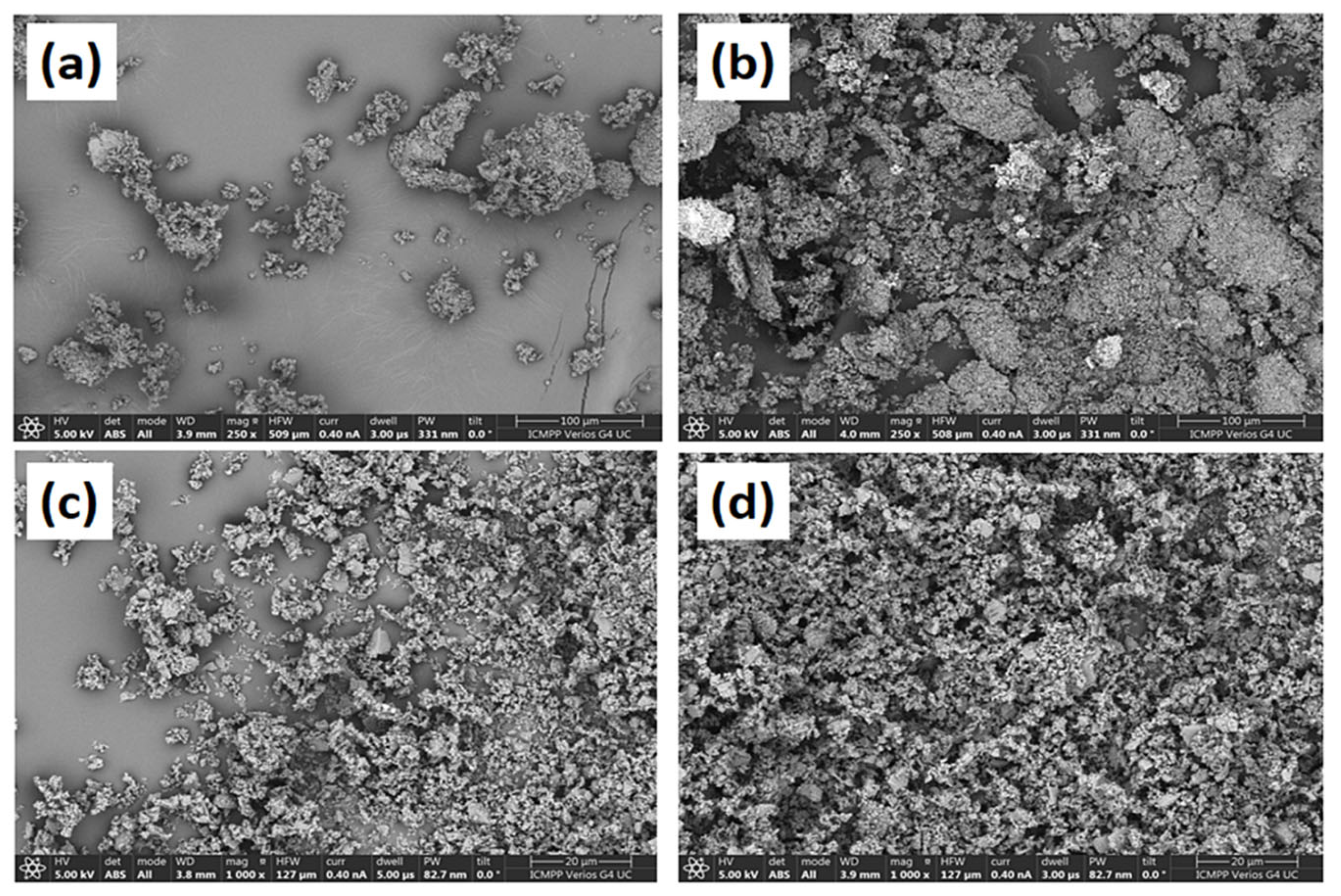
| Polymer | Mineral Oxides | Doseop | Removal Efficiency (%) | Remarks | Reference |
|---|---|---|---|---|---|
| PolyDADMAC | TiO2 | 1.25 mg polymer/g TiO2 | - | Suspension prepared in water | [15] |
| Pluronic-type polymers | TiO2 | Over the entire polymer dose | 20 | Suspension prepared in water | [19] |
| PEG | 240 mg L−1 | 56 | |||
| Chitosan | TiO2 | - | - | No flocculation in the case of the suspension prepared in distilled, but only in the tap water | [18] |
| Dextran derivatives | TiO2 | 1.2 ÷ 1.4 mg L−1 | 50 ÷ 60 | Suspension prepared in water | [17] |
| Polycation | FeO | TiO2 |
|---|---|---|
| doseop (mg L−1) | ||
| TMAP0.2–P | 1 | 3.0 |
| TMAP0.4–P | 0.6 | - |
| TMAP0.7–P | 0.14 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimici, L.; Nafureanu, M.M.; Constantin, M. Cationic Pullulan Derivatives Based Flocculants for Removal of Some Metal Oxides from Simulated Wastewater. Int. J. Mol. Sci. 2023, 24, 4383. https://doi.org/10.3390/ijms24054383
Ghimici L, Nafureanu MM, Constantin M. Cationic Pullulan Derivatives Based Flocculants for Removal of Some Metal Oxides from Simulated Wastewater. International Journal of Molecular Sciences. 2023; 24(5):4383. https://doi.org/10.3390/ijms24054383
Chicago/Turabian StyleGhimici, Luminita, Maria Magdalena Nafureanu, and Marieta Constantin. 2023. "Cationic Pullulan Derivatives Based Flocculants for Removal of Some Metal Oxides from Simulated Wastewater" International Journal of Molecular Sciences 24, no. 5: 4383. https://doi.org/10.3390/ijms24054383
APA StyleGhimici, L., Nafureanu, M. M., & Constantin, M. (2023). Cationic Pullulan Derivatives Based Flocculants for Removal of Some Metal Oxides from Simulated Wastewater. International Journal of Molecular Sciences, 24(5), 4383. https://doi.org/10.3390/ijms24054383







