Using Optogenetics to Model Cellular Effects of Alzheimer’s Disease
Abstract
1. Introduction
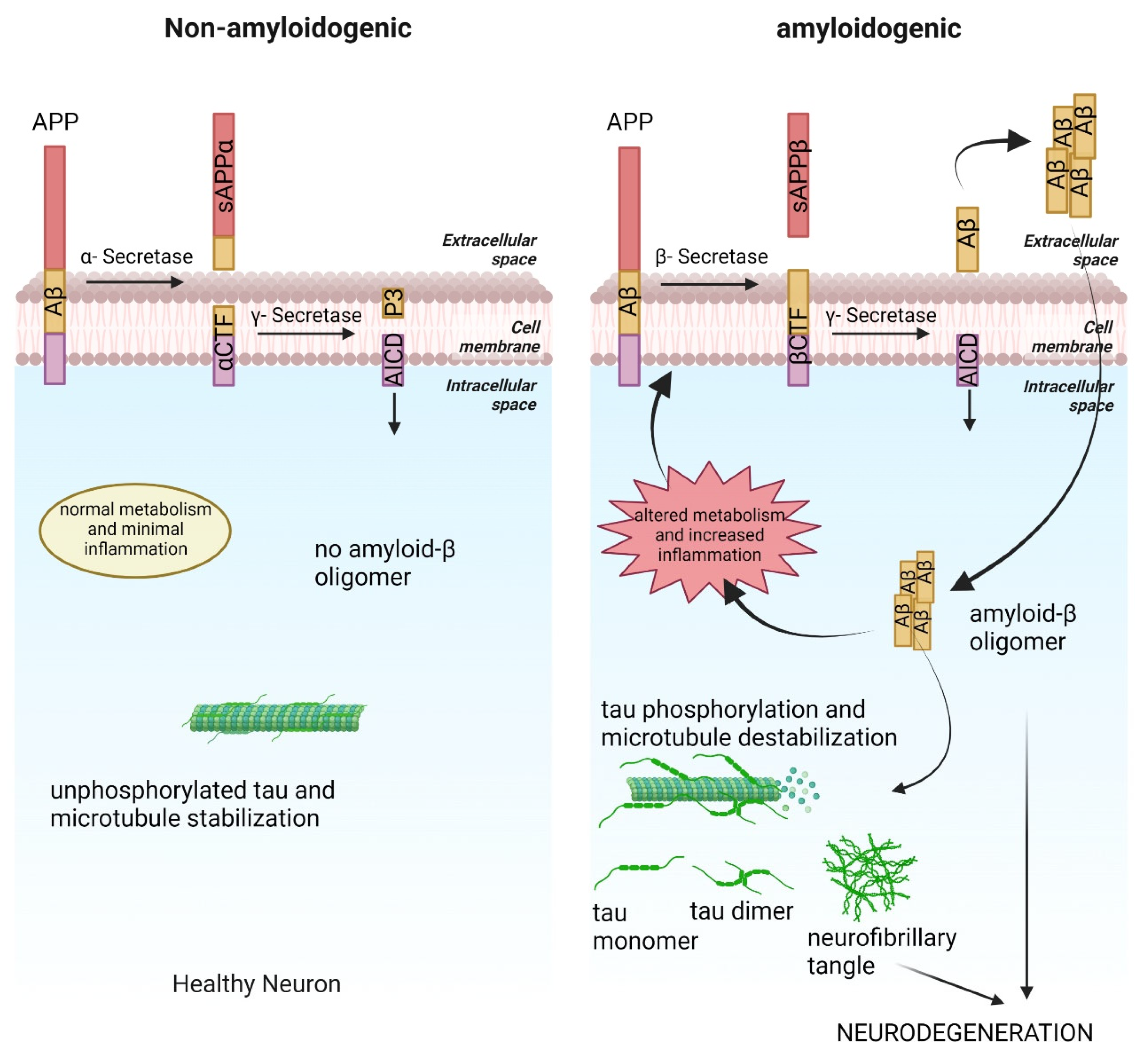
2. Amyloid Cascade and AD
3. Tauopathy in AD
4. Metabolic Alteration and AD
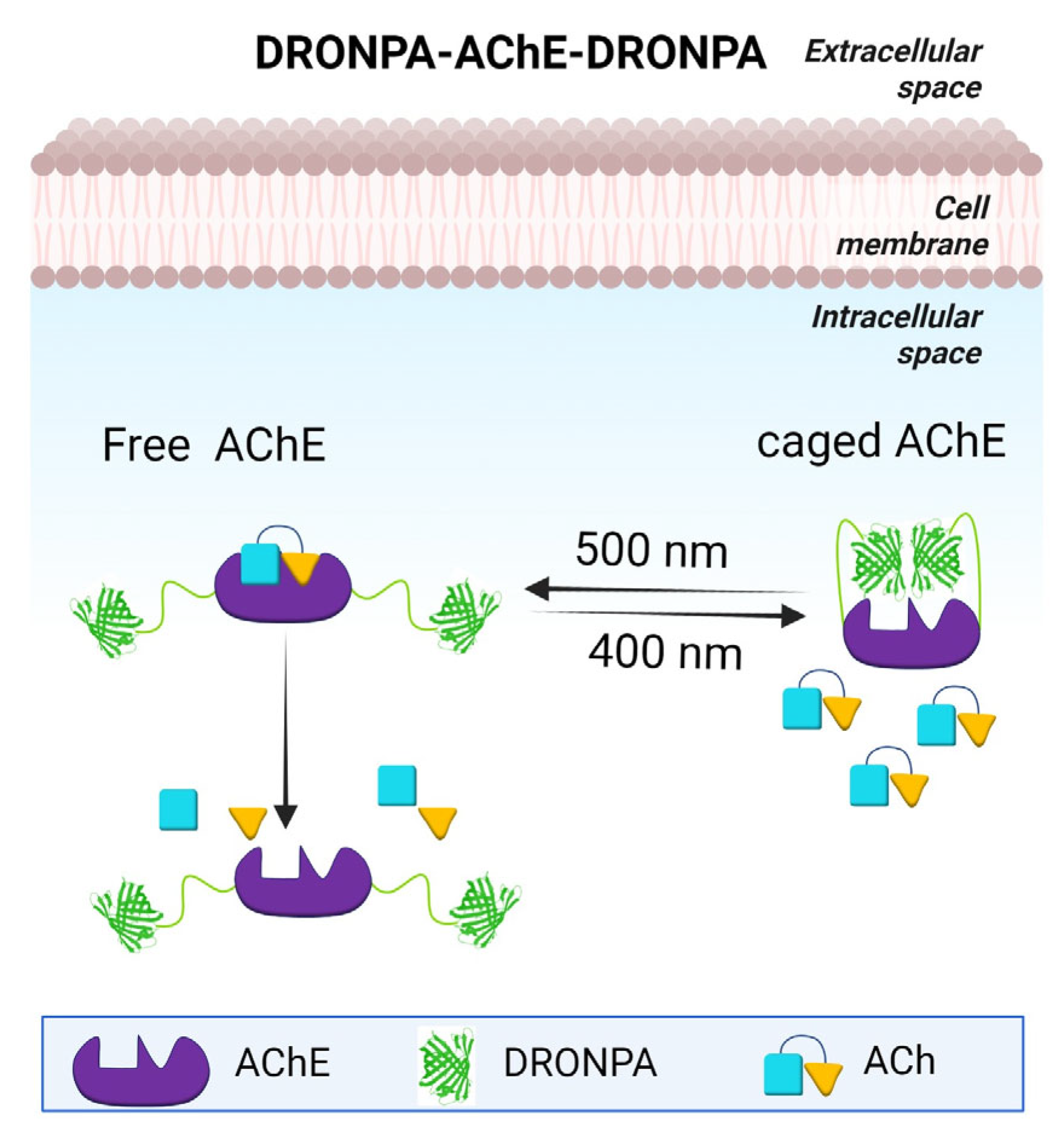
5. Cholinergic Hypothesis
6. Endoplasmic Reticulum Stress
7. Inflammation and AD
8. Amyloid-β Interaction with Signaling Pathways
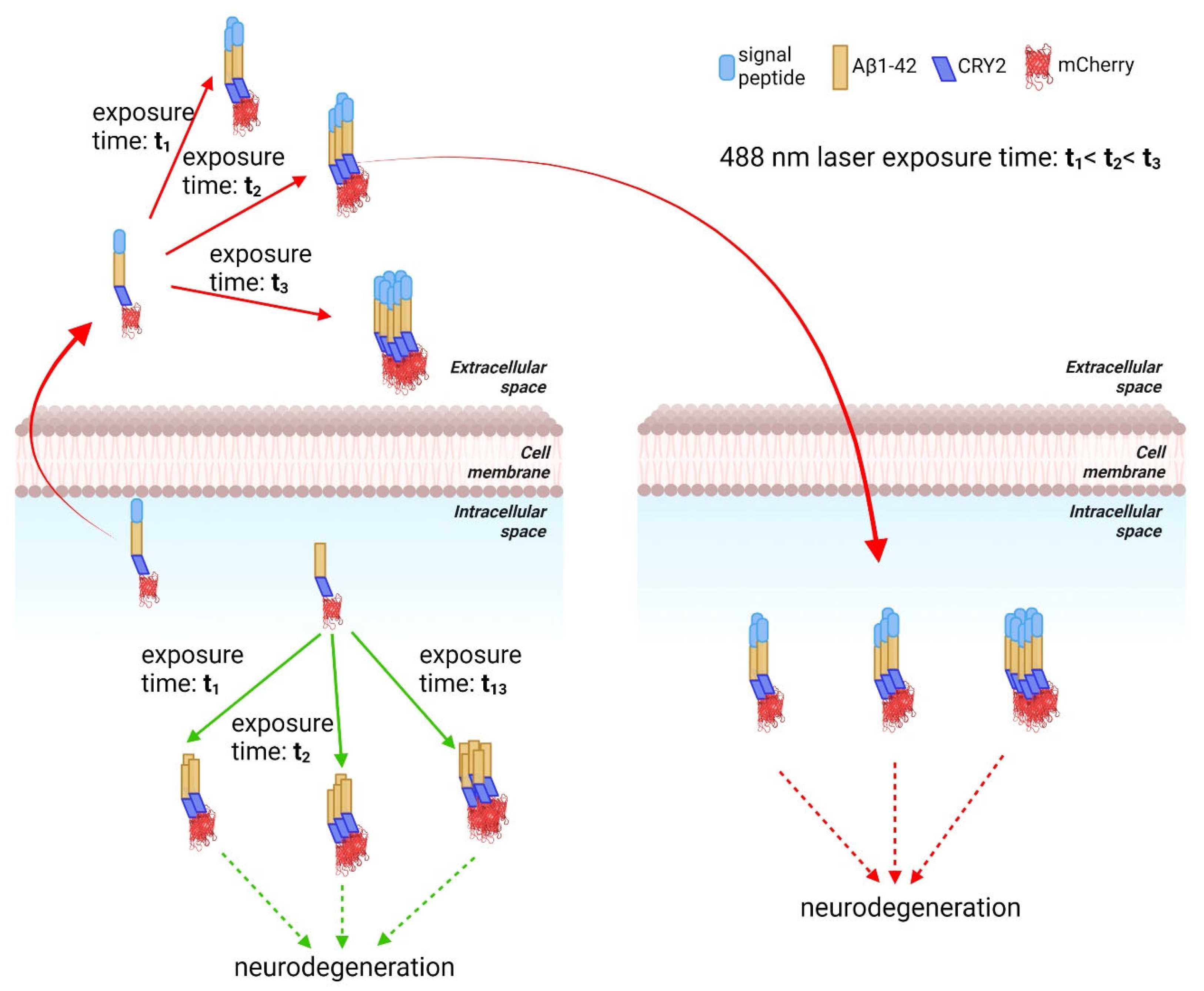
9. Optogenetics as a Method to Study the Effect of Aβ Aggregation
10. Opto-Tau
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, P.P.; Xie, Y.; Meng, X.Y.; Kang, J.S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target. Ther. 2019, 4, 29. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Ashe, K.H. The biogenesis and biology of amyloid beta oligomers in the brain. Alzheimer’s Dement. 2020, 16, 1561–1567. [Google Scholar] [CrossRef]
- Friedrich, R.P.; Tepper, K.; Ronicke, R.; Soom, M.; Westermann, M.; Reymann, K.; Kaether, C.; Fandrich, M. Mechanism of amyloid plaque formation suggests an intracellular basis of abeta pathogenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 1942–1947. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Sakono, M.; Zako, T. Amyloid oligomers: Formation and toxicity of abeta oligomers. FEBS J. 2010, 277, 1348–1358. [Google Scholar] [CrossRef]
- Mohandas, E.; Rajmohan, V.; Raghunath, B. Neurobiology of Alzheimer’s disease. Indian J. Psychiatry 2009, 51, 55–61. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Lindholm, D.; Ren, J.; Pratico, D. Er stress and upr in Alzheimer’s disease: Mechanisms, pathogenesis, treatments. Cell Death Dis. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Chen, S.; Townsend, K.; Goldberg, T.E.; Davies, P.; Conejero-Goldberg, C. Mapt isoforms: Differential transcriptional profiles related to 3r and 4r splice variants. J. Alzheimer’s Dis. 2010, 22, 1313–1329. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Aman, Y.; Ng, C.T.; Chau, W.H.; Zhang, Z.; Yue, M.; Bohm, C.; Jia, Y.; Li, S.; et al. Amyloid-beta toxicity modulates tau phosphorylation through the pax6 signalling pathway. Brain 2021, 144, 2759–2770. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-beta and tau: The trigger and bullet in alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Sharma, N.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Felemban, S.G.; Alsubayiel, A.M.; et al. “Aducanumab” making a comeback in Alzheimer’s disease: An old wine in a new bottle. Biomed. Pharmacother. 2022, 148, 112746. [Google Scholar] [CrossRef]
- Terao, I.; Honyashiki, M.; Inoue, T. Comparative efficacy of lithium and aducanumab for cognitive decline in patients with mild cognitive impairment or Alzheimer’s disease: A systematic review and network meta-analysis. Ageing Res. Rev. 2022, 81, 101709. [Google Scholar] [CrossRef] [PubMed]
- Chopade, P.; Chopade, N.; Zhao, Z.; Mitragotri, S.; Liao, R.; Chandran Suja, V. Alzheimer’s and parkinson’s disease therapies in the clinic. Bioeng. Transl. Med. 2023, 8, e10367. [Google Scholar] [CrossRef]
- Demetrius, L.A.; Driver, J. Alzheimer’s as a metabolic disease. Biogerontology 2013, 14, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Teo, E.; Ravi, S.; Barardo, D.; Kim, H.S.; Fong, S.; Cazenave-Gassiot, A.; Tan, T.Y.; Ching, J.; Kovalik, J.P.; Wenk, M.R.; et al. Metabolic stress is a primary pathogenic event in transgenic caenorhabditis elegans expressing pan-neuronal human amyloid beta. Elife 2019, 8, e50069. [Google Scholar] [CrossRef]
- Steen, E.; Terry, B.M.; Rivera, E.J.; Cannon, J.L.; Neely, T.R.; Tavares, R.; Xu, X.J.; Wands, J.R.; de la Monte, S.M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J. Alzheimer’s Dis. 2005, 7, 63–80. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Giau, V.V. Type 3 diabetes and its role implications in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 3165. [Google Scholar] [CrossRef]
- Mittal, K.; Mani, R.J.; Katare, D.P. Type 3 diabetes: Cross talk between differentially regulated proteins of type 2 diabetes mellitus and Alzheimer’s disease. Sci. Rep. 2016, 6, 25589. [Google Scholar] [CrossRef]
- de la Monte, S.M. Type 3 diabetes is sporadic alzheimer’s disease: Mini-review. Eur. Neuropsychopharmacol. 2014, 24, 1954–1960. [Google Scholar] [CrossRef]
- Snowden, S.G.; Ebshiana, A.A.; Hye, A.; An, Y.; Pletnikova, O.; O’Brien, R.; Troncoso, J.; Legido-Quigley, C.; Thambisetty, M. Association between fatty acid metabolism in the brain and alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study. PLoS Med. 2017, 14, e1002266. [Google Scholar] [CrossRef]
- Batra, R.; Arnold, M.; Worheide, M.A.; Allen, M.; Wang, X.; Blach, C.; Levey, A.I.; Seyfried, N.T.; Ertekin-Taner, N.; Bennett, D.A.; et al. The landscape of metabolic brain alterations in Alzheimer’s disease. Alzheimer’s Dement. 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Canevari, L.; Duchen, M.R. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of nadph oxidase. J. Neurosci. 2004, 24, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.; Parbo, P.; Madsen, L.S.; Hansen, A.K.; Hansen, K.V.; Schaldemose, J.L.; Kjeldsen, P.L.; Stokholm, M.G.; Gottrup, H.; Eskildsen, S.F.; et al. The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: A longitudinal pet study. J. Neuroinflamm. 2020, 17, 151. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Kumar, P.; Fu, Q.; Rosen, K.M.; Querfurth, H.W. The insulin/akt signaling pathway is targeted by intracellular beta-amyloid. MBoC 2009, 20, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.; Mirzaei, N.; Christian, M.; Sastre, M. Activation of the wnt/beta-catenin pathway represses the transcription of the beta-amyloid precursor protein cleaving enzyme (bace1) via binding of t-cell factor-4 to bace1 promoter. FASEB J. 2015, 29, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Kirouac, L.; Rajic, A.J.; Cribbs, D.H.; Padmanabhan, J. Activation of ras-erk signaling and gsk-3 by amyloid precursor protein and amyloid beta facilitates neurodegeneration in Alzheimer’s disease. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Bayer, T.A.; Wirths, O. Intracellular accumulation of amyloid-beta—A predictor for synaptic dysfunction and neuron loss in Alzheimer’s disease. Front. Aging Neurosci. 2010, 2, 8. [Google Scholar] [CrossRef]
- Aizenstein, H.J.; Nebes, R.D.; Saxton, J.A.; Price, J.C.; Mathis, C.A.; Tsopelas, N.D.; Ziolko, S.K.; James, J.A.; Snitz, B.E.; Houck, P.R.; et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 2008, 65, 1509–1517. [Google Scholar] [CrossRef]
- Ackley, S.F.; Zimmerman, S.C.; Brenowitz, W.D.; Tchetgen Tchetgen, E.J.; Gold, A.L.; Manly, J.J.; Mayeda, E.R.; Filshtein, T.J.; Power, M.C.; Elahi, F.M.; et al. Effect of reductions in amyloid levels on cognitive change in randomized trials: Instrumental variable meta-analysis. BMJ 2021, 372, n156. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Zhu, L.; Gabelle, A.; Gafson, A.R.; Platt, R.W.; Galvin, J.E.; Krolak-Salmon, P.; Rubino, I.; de Moor, C.; Belachew, S.; et al. Effect of reduction in brain amyloid levels on change in cognitive and functional decline in randomized clinical trials: An instrumental variable meta-analysis. Alzheimer’s Dement. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.H.; Kaur, P.; Teo, E.; Lam, V.Y.M.; Zhu, F.; Kibat, C.; Gruber, J.; Mathuru, A.S.; Tolwinski, N.S. Application of optogenetic amyloid-beta distinguishes between metabolic and physical damages in neurodegeneration. Elife 2020, 9, e52589. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Kibat, C.; Teo, E.; Gruber, J.; Mathuru, A.; Tolwinski, A.N.S. Use of optogenetic amyloid-beta to monitor protein aggregation in drosophila melanogaster, danio rerio and caenorhabditis elegans. Bio. Protoc. 2020, 10, e3856. [Google Scholar] [CrossRef] [PubMed]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of tau as a microtubule-associated protein: Structural and functional aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef]
- Gomez, G.; Beason-Held, L.L.; Bilgel, M.; An, Y.; Wong, D.F.; Studenski, S.; Ferrucci, L.; Resnick, S.M. Metabolic syndrome and amyloid accumulation in the aging brain. J. Alzheimer’s Dis. 2018, 65, 629–639. [Google Scholar] [CrossRef]
- De la Monte, S.M.; Wands, J.R. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef]
- Cleland, N.R.W.; Al-Juboori, S.I.; Dobrinskikh, E.; Bruce, K.D. Altered substrate metabolism in neurodegenerative disease: New insights from metabolic imaging. J. Neuroinflamm. 2021, 18, 248. [Google Scholar] [CrossRef]
- Canevari, L.; Abramov, A.Y.; Duchen, M.R. Toxicity of amyloid beta peptide: Tales of calcium, mitochondria, and oxidative stress. Neurochem. Res. 2004, 29, 637–650. [Google Scholar] [CrossRef]
- Yan, X.; Hu, Y.; Wang, B.; Wang, S.; Zhang, X. Metabolic dysregulation contributes to the progression of Alzheimer’s disease. Front. Neurosci. 2020, 14, 530219. [Google Scholar] [CrossRef]
- Mosconi, L. Glucose metabolism in normal aging and Alzheimer’s disease: Methodological and physiological considerations for pet studies. Clin. Transl. Imaging 2013, 1, 217–233. [Google Scholar] [CrossRef]
- Craft, S.; Claxton, A.; Baker, L.D.; Hanson, A.J.; Cholerton, B.; Trittschuh, E.H.; Dahl, D.; Caulder, E.; Neth, B.; Montine, T.J.; et al. Effects of regular and long-acting insulin on cognition and Alzheimer’s disease biomarkers: A pilot clinical trial. J. Alzheimer’s Dis. 2017, 57, 1325–1334. [Google Scholar] [CrossRef]
- Babic, T. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 67, 558. [Google Scholar] [CrossRef] [PubMed]
- H Ferreira-Vieira, T.; M Guimaraes, I.; R Silva, F.; M Ribeiro, F. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic-Pasti, T.; Leskovac, A.; Momic, T.; Petrovic, S.; Vasic, V. Modulators of acetylcholinesterase activity: From Alzheimer’s disease to anti-cancer drugs. Curr. Med. Chem. 2017, 24, 3283–3309. [Google Scholar] [CrossRef] [PubMed]
- Monczor, M. Diagnosis and treatment of Alzheimer’s disease. Curr. Med. Chem. Cent. Nerv. Syst. Agents 2005, 5, 5–13. [Google Scholar] [CrossRef]
- Trang, A.; Khandhar, P.B. Physiology, acetylcholinesterase. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- García-Ayllón, M.-S.; Silveyra, M.-X.; Sáez-Valero, J. Association between acetylcholinesterase and beta-amyloid peptide in Alzheimer’s cerebrospinal fluid. Chem. Biol. Interact. 2008, 175, 209–215. [Google Scholar] [CrossRef]
- García-Ayllón, M.-S.; Small, D.H.; Avila, J.; Saez-Valero, J. Revisiting the role of acetylcholinesterase in Alzheimer’s disease: Cross-talk with p-tau and β-amyloid. Front. Mol. Neurosci. 2011, 4, 22. [Google Scholar] [CrossRef]
- Lazarevic-Pasti, T. Side effects of Alzheimer’s disease treatment. Curr. Med. Chem. 2023. [Google Scholar] [CrossRef]
- Patel, A.L.; Yeung, E.; McGuire, S.E.; Wu, A.Y.; Toettcher, J.E.; Burdine, R.D.; Shvartsman, S.Y. Optimizing photoswitchable mek. Proc. Natl. Acad. Sci. USA 2019, 116, 25756–25763. [Google Scholar] [CrossRef]
- Leopold, A.V.; Chernov, K.G.; Verkhusha, V.V. Optogenetically controlled protein kinases for regulation of cellular signaling. Chem. Soc. Rev. 2018, 47, 2454–2484. [Google Scholar] [CrossRef]
- Shaaya, M.; Fauser, J.; Zhurikhina, A.; Conage-Pough, J.E.; Huyot, V.; Brennan, M.; Flower, C.T.; Matsche, J.; Khan, S.; Natarajan, V.; et al. Light-regulated allosteric switch enables temporal and subcellular control of enzyme activity. Elife 2020, 9, e60647. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Yu, W.S.; Lim, L.W. Exploring er stress response in cellular aging and neuroinflammation in Alzheimer’s disease. Ageing Res. Rev. 2021, 70, 101417. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Er stress activates immunosuppressive network: Implications for aging and Alzheimer’s disease. J. Mol. Med. 2020, 98, 633–650. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Itagaki, S.; Tago, H.; McGeer, E.G. Reactive microglia in patients with senile dementia of the alzheimer type are positive for the histocompatibility glycoprotein hla-dr. Neurosci. Lett. 1987, 79, 195–200. [Google Scholar] [CrossRef]
- Dani, M.; Wood, M.; Mizoguchi, R.; Fan, Z.; Walker, Z.; Morgan, R.; Hinz, R.; Biju, M.; Kuruvilla, T.; Brooks, D.J.; et al. Microglial activation correlates in vivo with both tau and amyloid in Alzheimer’s disease. Brain 2018, 141, 2740–2754. [Google Scholar] [CrossRef]
- Onyango, I.G.; Jauregui, G.V.; Carna, M.; Bennett, J.P., Jr.; Stokin, G.B. Neuroinflammation in Alzheimer’s disease. Biomedicines 2021, 9, 524. [Google Scholar] [CrossRef]
- Lee, H.T.; Lee, K.I.; Chen, C.H.; Lee, T.S. Genetic deletion of soluble epoxide hydrolase delays the progression of Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 267. [Google Scholar] [CrossRef]
- Ghosh, A.; Comerota, M.M.; Wan, D.; Chen, F.; Propson, N.E.; Hwang, S.H.; Hammock, B.D.; Zheng, H. An epoxide hydrolase inhibitor reduces neuroinflammation in a mouse model of Alzheimer’s disease. Sci. Transl. Med. 2020, 12, eabb1206. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Chua, E.H.Z.; Lim, W.K.; Liu, J.; Harmston, N.; Tolwinski, N.S. Wnt signaling rescues amyloid beta-induced gut stem cell loss. Cells 2022, 11, 281. [Google Scholar] [CrossRef]
- Rivers-Auty, J.; Mather, A.E.; Peters, R.; Lawrence, C.B.; Brough, D. Anti-inflammatories in Alzheimer’s disease-potential therapy or spurious correlate? Brain Commun. 2020, 2, fcaa109. [Google Scholar] [CrossRef] [PubMed]
- Sofola-Adesakin, O.; Castillo-Quan, J.I.; Rallis, C.; Tain, L.S.; Bjedov, I.; Rogers, I.; Li, L.; Martinez, P.; Khericha, M.; Cabecinha, M.; et al. Lithium suppresses abeta pathology by inhibiting translation in an adult drosophila model of Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 190. [Google Scholar] [CrossRef]
- Godoy, J.A.; Rios, J.A.; Zolezzi, J.M.; Braidy, N.; Inestrosa, N.C. Signaling pathway cross talk in Alzheimer’s disease. Cell Commun. Signal. 2014, 12, 23. [Google Scholar] [CrossRef]
- Teo, E.; Fong, S.; Tolwinski, N.; Gruber, J. Drug synergy as a strategy for compression of morbidity in a caenorhabditis elegans model of Alzheimer’s disease. Geroscience 2020, 42, 849–856. [Google Scholar] [CrossRef]
- Suresh, J.; Khor, I.W.; Kaur, P.; Heng, H.L.; Torta, F.; Dawe, G.S.; Tai, E.S.; Tolwinski, N.S. Shared signaling pathways in Alzheimer’s and metabolic disease may point to new treatment approaches. FEBS J. 2021, 288, 3855–3873. [Google Scholar] [CrossRef] [PubMed]
- Dazert, E.; Hall, M.N. Mtor signaling in disease. Curr. Opin. Cell Biol. 2011, 23, 744–755. [Google Scholar] [CrossRef]
- Castillo-Quan, J.I.; Tain, L.S.; Kinghorn, K.J.; Li, L.; Gronke, S.; Hinze, Y.; Blackwell, T.K.; Bjedov, I.; Partridge, L. A triple drug combination targeting components of the nutrient-sensing network maximizes longevity. Proc. Natl. Acad. Sci. USA 2019, 116, 20817–20819. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, A.; Reddy, P.; Martinez-Redondo, P.; Platero-Luengo, A.; Hatanaka, F.; Hishida, T.; Li, M.; Lam, D.; Kurita, M.; Beyret, E.; et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 2016, 167, 1719–1733.e12. [Google Scholar] [CrossRef]
- Kaur, P.; Otgonbaatar, A.; Ramamoorthy, A.; Chua, E.H.Z.; Harmston, N.; Gruber, J.; Tolwinski, N.S. Combining stem cell rejuvenation and senescence targeting to synergistically extend lifespan. Aging 2022, 14, 8270–8291. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Tolwinski, N.S. Optogenetics as a tool to study neurodegeneration and signal transduction. In Opsin-Free Optogenetics; CRC Press: Boca Raton, FL, USA, 2023; pp. 111–122. [Google Scholar]
- Lim, W.K.; Kaur, P.; Huang, H.; Jo, R.S.; Ramamoorthy, A.; Ng, L.F.; Suresh, J.; Maisha, F.I.; Mathuru, A.S.; Tolwinski, N.S. Optogenetic approaches for understanding homeostatic and degenerative processes in drosophila. Cell. Mol. Life Sci. 2021, 78, 5865–5880. [Google Scholar] [CrossRef]
- Bunnag, N.; Tan, Q.H.; Kaur, P.; Ramamoorthy, A.; Sung, I.C.H.; Lusk, J.; Tolwinski, N.S. An optogenetic method to study signal transduction in intestinal stem cell homeostasis. J. Mol. Biol. 2020, 432, 3159–3176. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Saunders, T.E.; Tolwinski, N.S. Coupling optogenetics and light-sheet microscopy, a method to study wnt signaling during embryogenesis. Sci. Rep. 2017, 7, 16636. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, N.A.; Colosimo, P.F.; Liu, X.; Tolwinski, N.S. Complex interactions between gsk3 and apkc in drosophila embryonic epithelial morphogenesis. PLoS ONE 2011, 6, e18616. [Google Scholar] [CrossRef]
- Mazanetz, M.P.; Fischer, P.M. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat. Rev. Drug Discov. 2007, 6, 464–479. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Albarrati, A.; Albratty, M.; Najmi, A.; Meraya, A.M.; Bungau, S. The road to precision medicine: Eliminating the “one size fits all” approach in Alzheimer’s disease. Biomed. Pharmacother. 2022, 153, 113337. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, P.; Tolwinski, N.S. Using Optogenetics to Model Cellular Effects of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4300. https://doi.org/10.3390/ijms24054300
Tiwari P, Tolwinski NS. Using Optogenetics to Model Cellular Effects of Alzheimer’s Disease. International Journal of Molecular Sciences. 2023; 24(5):4300. https://doi.org/10.3390/ijms24054300
Chicago/Turabian StyleTiwari, Prabhat, and Nicholas S. Tolwinski. 2023. "Using Optogenetics to Model Cellular Effects of Alzheimer’s Disease" International Journal of Molecular Sciences 24, no. 5: 4300. https://doi.org/10.3390/ijms24054300
APA StyleTiwari, P., & Tolwinski, N. S. (2023). Using Optogenetics to Model Cellular Effects of Alzheimer’s Disease. International Journal of Molecular Sciences, 24(5), 4300. https://doi.org/10.3390/ijms24054300







