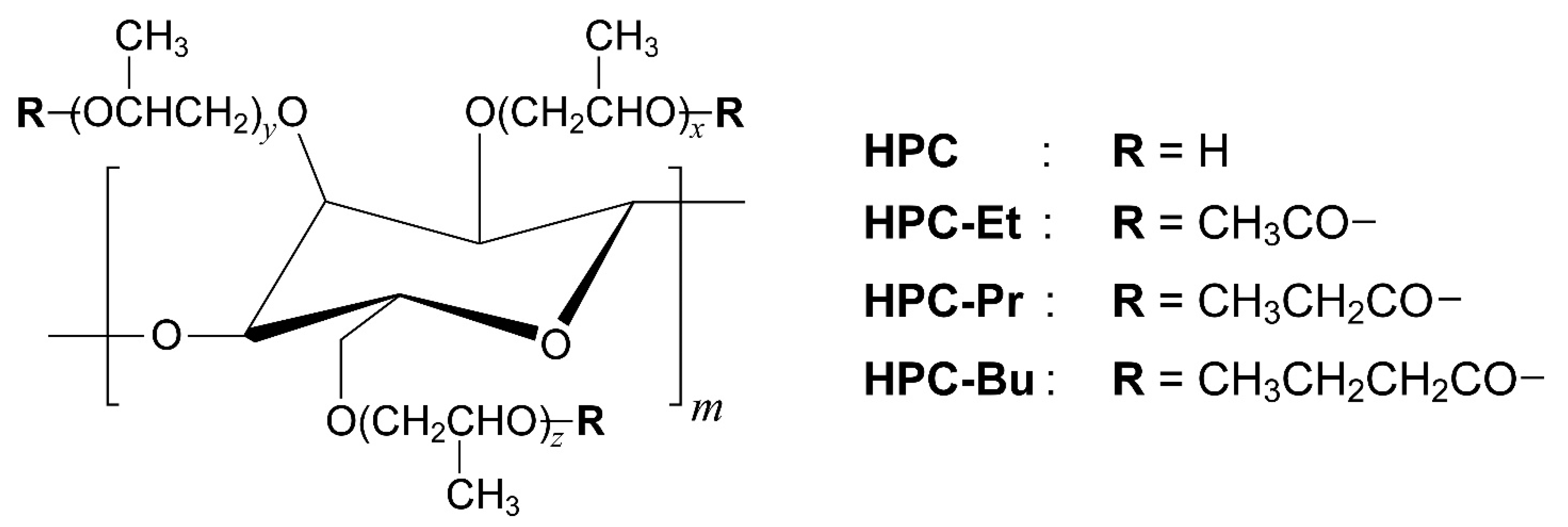
2.1. Synthesis and Characterization of HPC Ester Derivatives
Figure 1 shows the chemical structures of pristine HPC and its ester derivatives possessing alkanoyl side chains. Three kinds of HPC derivatives were synthesized by esterification of hydroxy groups in pristine HPC with alkanoyl chlorides, such as acetyl chloride, propionyl chloride, and butyryl chloride. The detailed procedures are described in
Section 3.2. Hereafter, the HPC ester derivatives possessing acetyl (ethanoyl), propionyl, or butyryl groups are defined as HPC-Et, HPC-Pr, and HPC-Bu, respectively.
After syntheses of the HPC ester derivatives,
1H-NMR and FT-IR spectral measurements confirmed that all the hydroxy groups in the side chains of HPC are completely esterified with alkanoyl groups (
Figure 2). These chemical analyses have been widely adopted for the characterization of HPC derivatives in many previous studies [
27,
28,
29]. First, the FT-IR spectra were measured before and after the esterification of HPC with alkanoyl chlorides. Before the reaction, pristine HPC showed a broad peak in the wavenumber range between 3100 and 3600 cm
−1, arising from the O-H stretching vibration of hydroxy groups in HPC (
Figure 2, left panel, indicated by the green triangle). After the esterification, the broad O-H peak of pristine HPC around 3100–3600 cm
−1 thoroughly disappeared, accompanied by the clear appearance of a sharp peak at 1700 cm
−1 (
Figure 2, left panel, indicated by the blue triangle). This sharp peak can be assigned to the C=O stretching vibration of alkanoyl groups in the side chains of HPC derivatives. Therefore, the overall FT-IR results indicated that all hydroxy groups in HPC react with alkanoyl chlorides to form ester linkages.
In another chemical analysis, the NMR spectra of HPC-Et, HPC-Pr, and HPC-Bu were measured to evaluate the stoichiometric reaction rates of hydroxy groups in HPC with alkanoyl chlorides (
Figure 2, right panel). Here, the average number of esterified hydroxy groups per HPC monomer unit is defined as the degree of esterification (
DE). The
DE values were determined by the following equation using integrated values of the peaks of the
1H-NMR spectra of HPC ester derivatives.
where
A stands for the integrated value of the peak around 5.00 ppm assigned to methine protons of the propyleneoxy termini modified with alkanoyl groups,
n is the number of protons in each alkanoyl group, and
W is the sum of the integrated values of all the peaks derived from the HPC derivatives. The average number of combined propylene oxide groups per HPC monomer unit is defined as the molecular substitution (
MS). In other words, the
MS value is equal to the sum of the
x,
y, and
z values, as depicted in
Figure 1. From the
1H-NMR spectrum of pristine HPC, the
MS value was determined to be 4.00 according to the analytic procedure reported in a previous study [
27]. The detailed numerical derivation of Equation (2) is disclosed in the
Supplementary Materials.
Considering the chemical structure of pristine HPC, the maximum value of
DE is 3.00 because the monomer unit of HPC has three hydroxy groups. In this study, the
DE values of HPC-Et, HPC-Pr, and HPC-Bu were estimated to be 3.00, 2.98, and 2.98, respectively, as shown in
Table 1. These results implied that the hydroxy groups of HPC are almost esterified with alkanoyl chlorides since all the
DE values are nearly equal to 3.00. There was almost no residual hydroxy group in these HPC ester derivatives.
The weight average molecular weight (
Mw) and its distribution with respect to the number average molecular weight (
Mw/
Mn) of a pristine HPC and its ester derivatives were determined by size exclusion chromatography (SEC) measurements using the polystyrene standards. The details are mentioned in
Section 3.2. The results of SEC measurements are also listed in
Table 1. The
Mw of each HPC ester derivative became larger than that of pristine HPC while maintaining the almost same
Mw/
Mn. This implies that the degradation of HPC hardly occurs during the esterification process.
It should be emphasized that the preparation of completely-esterified HPC derivatives is indispensable to a fair comparison of their optical and rheological properties. It has been reported that the reflection wavelength of HPC ester derivatives is remarkably affected by the
DE value. For example, the reflection peak of HPC-Bu was drastically red-shifted from 400 to 835 nm with the decrease in
DE value from 2.96 to 2.20, possibly because of residual hydroxy groups in HPC-Bu [
30]. Note that this wavelength range between 400 and 835 nm fully covers the visible wavelength region and even the near infrared region. The red-shift of the reflection peak with a decreasing
DE value was attributed to the increase in helical pitch length (
p) of the CLC structure. Such a large difference in CLC structure may also affect the rheological behavior of CLCs. This is because the rheological properties of viscoelastic materials are basically influenced by their internal structures. Taking the above-mentioned concern into account, three kinds of the completely-esterified HPC derivatives synthesized in this study are rationally suitable for the investigation of their optical and rheological properties.
2.2. Reflection Properties of HPC Ester Derivatives
The HPC ester derivatives used in this study, namely HPC-Et, HPC-Pr, and HPC-Bu, showed a thermotropic CLC phase. This is supported by polarized optical microscope (POM) images and reflection images of their CLC cells upon heating. The CLC cells were fabricated by enclosing the HPC derivatives between two glass substrates at an internal gap distance (
dg) of 0.20 mm. The fabrication procedure of the CLC cell is described in detail in
Section 3.3. The POM images showed transmitted light under crossed-Nicols, meaning the emergence of optical birefringence by liquid crystallinity (
Supplementary Materials, Figure S1A) [
31]. In addition, the emergence of the CLC phase was also evident from the visual observation of reflection colors upon changing the temperature (
Supplementary Materials, Figure S1B).
Previous reports have shown that the reflection peak wavelength of thermotropic CLCs of HPC ester derivatives can be tuned by not only the temperature but also the length of alkanoyl side chains [
5,
10]. In this study, the transmission spectra of HPC-Et, HPC-Pr, and HPC-Bu were measured as the temperature changed (
Figure 3A).
Here, the reflection peak wavelength was determined as the wavelength at which the optical transmittance became minimum. Although no reflection peak was observed in the visible wavelength range for the three kinds of HPC ester derivatives at room temperature, the subsequent heating treatment led to the appearance of the reflection peak in the visible wavelength range above ~350 nm. When HPC-Et was heated to 110 °C, the reflection peak appeared at 385 nm (
Figure 3A, upper panel). At 112 °C, HPC-Et showed a reflection peak at 405 nm, whereupon the reflection color was visualized as blue. As the temperature was elevated from 112 to 120 °C, the reflection peak was red-shifted from 405 to 460 nm, arising from the increase in the helical pitch of CLC, which corresponds to
p in Equation (1).
Similarly, the thermally induced shifting behavior of the reflection peak was also observed for the two other HPC derivatives, that is, HPC-Pr and HPC-Bu. For instance, HPC-Pr exhibited a reflection peak at 375 nm by heating it to 95 °C. As the temperature was elevated to 120 °C, the reflection peak was red-shifted to 520 nm (
Figure 3A, middle panel). For comparison, a reflection peak of HPC-Bu appeared at 380 nm by heating at 60 °C, while heating treatment at 120 °C gave rise to the red-shift of the reflection peak to 710 nm in a relatively wide wavelength range (
Figure 3A, lower panel).
In all cases, the reflection peak was reversibly blue-shifted as the temperature dropped. Notably, the reflection peak of each HPC derivative was quite unchanged with sufficient reproducibility when measured at the same temperature in the heating or cooling process (
Supplementary Materials, Figure S2A). For instance, the reflection peaks of HPC-Bu appeared at the same wavelength even after repeating the cycles of heating to 100 °C and cooling to 80 °C five times (
Supplementary Materials, Figure S2B). From the spectral results, the standard deviations of the reflection wavelength at 80 °C and 100 °C were calculated to be 1.12 nm and 1.15 nm, respectively. Those low standard deviations of the reflection wavelength indicate that the reflection properties of the HPC ester derivatives, by changing the temperature, have excellent reversibility. Such a reversible shift of the reflection peak is ascribed to the typical characteristic of thermotropic CLC.
Based on the overall spectral results, the temperature dependences of the reflection peak wavelengths of HPC-Et, HPC-Pr, and HPC-Bu are summarized in
Figure 3B. The reflection wavelengths of all the HPC ester derivatives were consecutively red-shifted with increasing temperatures (
Figure 3B, red circles for HPC-Et, blue triangles for HPC-Pr, and black squares for HPC-Bu). The length of alkanoyl side chains was found to have a remarkable influence on the reflection peak shift ranges by temperature as well as the CLC temperature ranges with visible reflection. For example, the reflection peak of HPC-Bu could be shifted throughout the full visible wavelength range between 380 and 710 nm by sweeping the temperature from 60 to 120 °C. On the other hand, the reflection peak shift ranges by temperature were quite limited for HPC-Et and HPC-Pr. Additionally, the reflection peaks of HPC ester derivatives at the same temperature, such as 110 °C, appeared at longer wavelengths in order of the length of the alkanoyl side chains. In other words, HPC-Et, HPC-Pr, and HPC-Bu exhibited blue reflection color with the wavelength at 405 nm, adjusting CLC temperatures to 112, 100, and 66 °C, respectively (
Figure 3B, dotted line). The difference in CLC temperatures probably originated from the steric hindrance on the alkanoyl side chains of HPC ester derivatives, that is, the alkanoyl chain length, implying that the CLC temperature with the same reflection wavelength is lowered as the length of the alkanoyl side chains is longer.
In addition, the isotropic phase transition temperature (
Ti) of the HPC ester derivatives decreased as the side chain length increased. The
Ti of HPC-Et was determined to be 155–165 °C by the POM observation under crossed-Nicols during the rise in temperature. In the same manner, the
Ti of HPC-Pr and HPC-Bu were observed at 150–160 °C and 140–145 °C, respectively. These differences in
Ti affect the temperature range over which the time-temperature superposition (TTS) principle can be applicable. For instance, the breakdown of the TTS principle was more likely to occur with increasing the temperature to
Ti as will be discussed in
Section 2.5. Therefore, the reference temperature (
Tr) for the construction of the master curve was defined as the temperature at which the light of 405 nm was reflected (
Figure 3A). This is because it is the lowest temperature at which each HPC derivative reflects visible light.
2.3. WAXD Results of HPC Ester Derivatives
Wide-angle X-ray diffraction (WAXD) measurements give invaluable investigation on the microscopic parameters of CLC structure, thereby leading to the twisting angle (
φ) and the layer distance (
d) in helical molecular assemblages, which are defined as the difference in azimuthal angle and distance between the neighboring nematic liquid crystalline layers in the CLC helical structures, respectively. The previous studies on the HPC ester derivatives have revealed that the
d value increases while the
φ value decreases, elongating the length of alkanoyl side chains [
32]. Thus, the relationship among the physical parameters of
λref,
d, and
φ can be determined by combining Equation (1) with Equation (3).
As derived from the equations, Equation (4) indicates that
λref is proportional to
nav,
d, and
φ−1 as follows.
First, the microscopic CLC structure can be determined by measuring the
d value from WAXD measurements. According to the previous study, the
nav values of the HPC derivatives are approximately 1.465, regardless of their chemical structures [
32]. Strictly speaking, the
nav of each HPC derivative has been reported as 1.468 for HPC-Et, 1.465 for HPC-Pr, and 1.464 for HPC-Bu. However, the
nav value can be considered a constant since these differences are quite small. Therefore, they have the same
p value as 276 nm when they reflect lights at 405 nm according to Equation (1). Furthermore, the WAXD measurements indicated that the number of layers of HPC derivatives is almost the same when they reflect light at a wavelength of 405 nm (
Supplementary Materials, Figure S3). We assigned the peaks appearing around 2
θ = 6° and halos appearing around 2
θ = 20° to the distance between piled layers and nematic structure in a layer, respectively. These peak assignments of WAXD profiles were in good agreement with those of previous studies [
32,
33,
34]. The parameters of the CLC structure determined by WAXD measurements are summarized in
Table 2.
The
d values were calculated to be 1.23 nm for HPC-Et, 1.29 nm for HPC-Pr, and 1.33 nm for HPC-Bu from their 2
θ values using Equation (5) for WAXD measurements.
where
λ means 0.154 nm as the wavelength of Cu-Kα radiation. This small increase in
d value is reasonable due to the increase in the alkanoyl side chain length of HPC ester derivatives. The
φ values were obtained by using Equation (4). We defined
NLayer as the number of layers in a periodic helical structure calculated by both Equations (1) and (6).
where the
nav value is 1.465 and
λref is 405 nm. Therefore, the
p value is estimated to be 276 nm. From Equation (6), the
NLayer values were found to be 224 for HPC-Et, 214 for HPC-Pr, and 208 for HPC-Bu, so that the HPC ester derivatives exhibited almost the same
NLayer values. Taking the overall results into account, it is plausible that three kinds of HPC ester derivatives form almost the same CLC structures at the temperatures where they reflect light at 405 nm. As a result, HPC-Et, HPC-Pr, and HPC-Bu are suitable for a fair comparison of their rheological properties. For this reason, we determined
Tr for the construction of master curves as the temperature at which HPC derivatives reflect a light of 405 nm in the following rheological measurements.
2.4. Rheological Properties of HPC Ester Derivatives: Gap Dependence of Storage and Loss Moduli
As preliminary experiments, we elucidated the dependence of the rheological properties of HPC-Pr on the gap distance (
dg), which corresponds to its thickness between the parallel plate and sample stage in the rheometer, as stated later in
Section 3.5. These experiments are necessary to examine the rheological behavior of HPC derivatives because the rheological properties of liquid crystalline polymers are greatly affected by differences in their internal structure, such as molecular orientation or domain size in general.
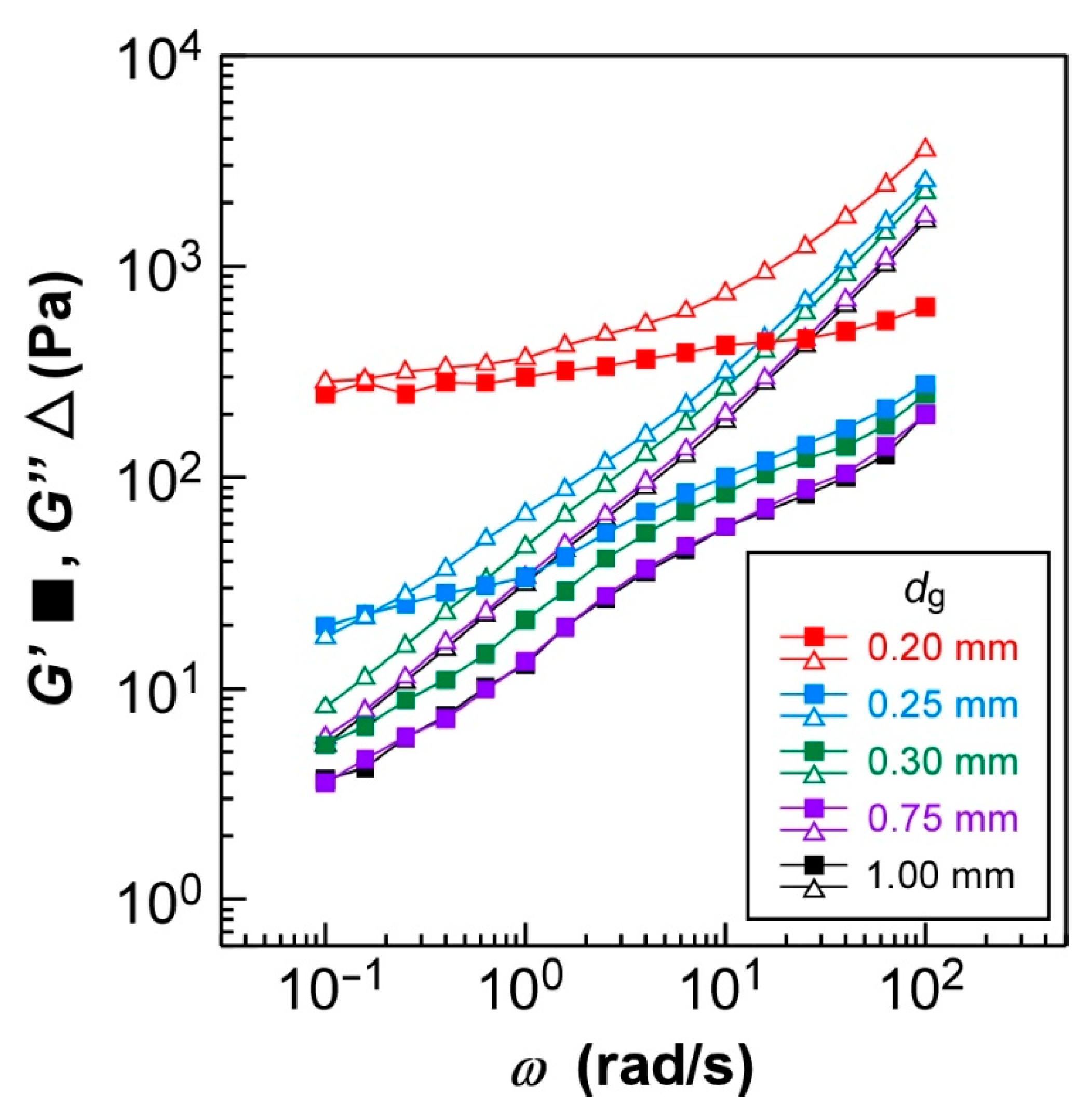
Figure 4 shows the changes in storage modulus (
G′) and loss modulus (
G″) of HPC-Pr as a function of the angular frequency (
ω). The rheological measurements were conducted in the same conditions except for the
dg value, which was adjusted to 0.20, 0.25, 0.30, 0.75, and 1.00 mm. As mentioned in
Section 3.5, the strain amplitude was tuned at 0.7%. It should be noted that the strain amplitude of 0.7% is small enough to measure the linear viscoelasticity regardless of the
dg value. This ensures that the rheological behavior of HPC derivatives is only governed by the difference in CLC structures.
As shown in
Figure 4, both
G′ and
G″ values of HPC-Pr were distinctly affected by the
dg value in the rheological measurements. However, the values of
G′ and
G″ were almost identical at the
dg values of 0.75 and 1.00 mm. Theoretically, the
G′ and
G″ values are considered independent of the
dg value in the linear rheology. In fact, the rheological profiles overlapped at
dg values between 0.75 and 1.00 mm. At this
dg range, the values of
G″ were constantly higher than those of
G′ in the entire
ω range. Furthermore, it turned out that both
G′ and
G″ values continuously decreased by reducing the
ω value. For example, at
dg = 0.75 and 1.00 mm, as
ω dropped from 10
2 to 10
−1 rad/s, the
G′ value fell from 2.0 × 10
2 to 4.0 Pa and the
G″ value also decreased from 1.8 × 10
3 to 6.0 Pa. These two trends suggest the liquid-like behavior of CLC. However, in the region below
ω = 10
1 rad/s, the slopes of both
G′ and
G″ values against
ω were nearly equal to 0.60 and 0.75, respectively. This relationship is different from those of the ideal Newtonian fluid, which would be
and
, respectively. Such a weaker
ω dependence of the
G′ and
G″ values can be attributed to the disturbance of the flow behavior caused by the CLC structure, since a similar
ω dependence can also be found for the polymer nanocomposites containing an aggregation of fillers [
35].
Additionally, both
G′ and
G″ values increased drastically when
dg was decreasingly adjusted from 0.30 mm to 0.20 mm, and the slopes of
G′ and
G″ values gradually decreased to 0. When
dg was set at 0.25 mm, both
G′ and
G″ values at
ω = 10
−1 rad/s were almost equal, approximately 20 Pa and 18 Pa, respectively (
Figure 4, blue plots). In the
ω range from 10
−1–10
0 rad/s, both
G′ and
G″ values were three times larger than those of
dg = 0.75 and 1.00 mm. The slopes also became more gradual; specifically, those of
G′ and
G″ in the
ω range below 10
0 rad/s were approximately 0.23 and 0.59, respectively. Furthermore, the plots of
G′ and
G″ were distinct from the others when the gap was further thinned to 0.20 mm. The
G′ and
G″ values in the
ω range below 10
1 rad/s became independent of
ω, and their values were approximately 250 Pa and 280 Pa, respectively, which are 60 or 45 times larger than those of
dg = 0.75 and 1.00 mm. The increase of shear moduli (
G′ and
G″) and decrease of
ω dependency indicated that the CLC showed solid-like behavior. This tendency was especially pronounced in the low
ω region, particularly below
ω = 10
1 rad/s. Such a transition in the viscoelastic properties of HPC-Pr depending on the
dg value might be caused by the orientation of CLC molecules. Since the decrease in the
dg value enhances the effect of surface anchoring from the jig interfaces of the rheometer, the HPC derivatives form more rigid structures due to the strongly planar CLC orientation, leading to the solid-like behavior of HPC-Pr. The effect of the
dg value on the CLC structure is called the wall effect in a previous study [
24].
In addition, this hypothesis can also be supported by the difference in transmission spectra of HPC-Pr with various
dg values. The transmission spectra of CLC cells, fabricated by enclosing HPC-Pr with two glass substrates in the
dg range of 0.10–1.00 mm, were measured at 120 °C (
Supplementary Materials, Figure S4). As the
dg increased, the baselines of the transmission spectrum were gradually lowered, accompanied by a broadening in the spectral widths of the reflection peaks. These results were probably caused by the light scattering due to the disordered CLC structure induced by thickening the gap. The differences in reflection peak wavelength between the CLC cells with different
dg values are probably due to the broadening of the reflection peaks. This is reasonable because the reflection wavelength is greatly affected by the enlargement of the distribution of
p accompanied by the increase in
dg as evident from Equation (1). Furthermore, the enhancement of CLC orientation with decreasing the
dg value is also supported by the reflection spectral results (
Supplementary Materials, Figure S5). We measured the changes in the reflection spectrum of HPC-Bu after applying the steady shear flow. Although the reflection spectra of HPC-Bu became broader and the reflection peaks were blue-shifted right after applying the shear stress, the reflection spectra gradually recovered due to the CLC orientation. According to previous studies, the helical axis of CLC tilts under the shear flowing force, and the oblique helical axis gradually recovers into a planar orientation after cessation of the shear flow [
21,
36]. Thus, we can estimate the CLC orientation behavior by measuring the time needed for the complete recovery in the reflection spectrum. At this time, the
dg value was set to be either 0.2 or 1.0 mm to reveal the effect of the thickness of CLC. After applying the shear stress, the reflection spectrum was recorded every 10 min until the complete recovery of the reflection spectrum using our original rheo-optics measurement system (
Supplementary Materials, Figure S5A). The result suggested that the relaxation time after the shear flow is shorter when the gap is smaller, from 105 min at
dg = 1.0 mm (
Supplementary Materials, Figure S5B) to 65 min at
dg = 0.20 mm (
Supplementary Materials, Figure S5C). The fast recovery of the reflection spectrum when
dg = 0.20 mm strongly suggests that CLC molecules of HPC ester derivatives are more likely to be oriented when the
dg value is small.
2.5. Rheological Properties of HPC Ester Derivatives: Master Curves
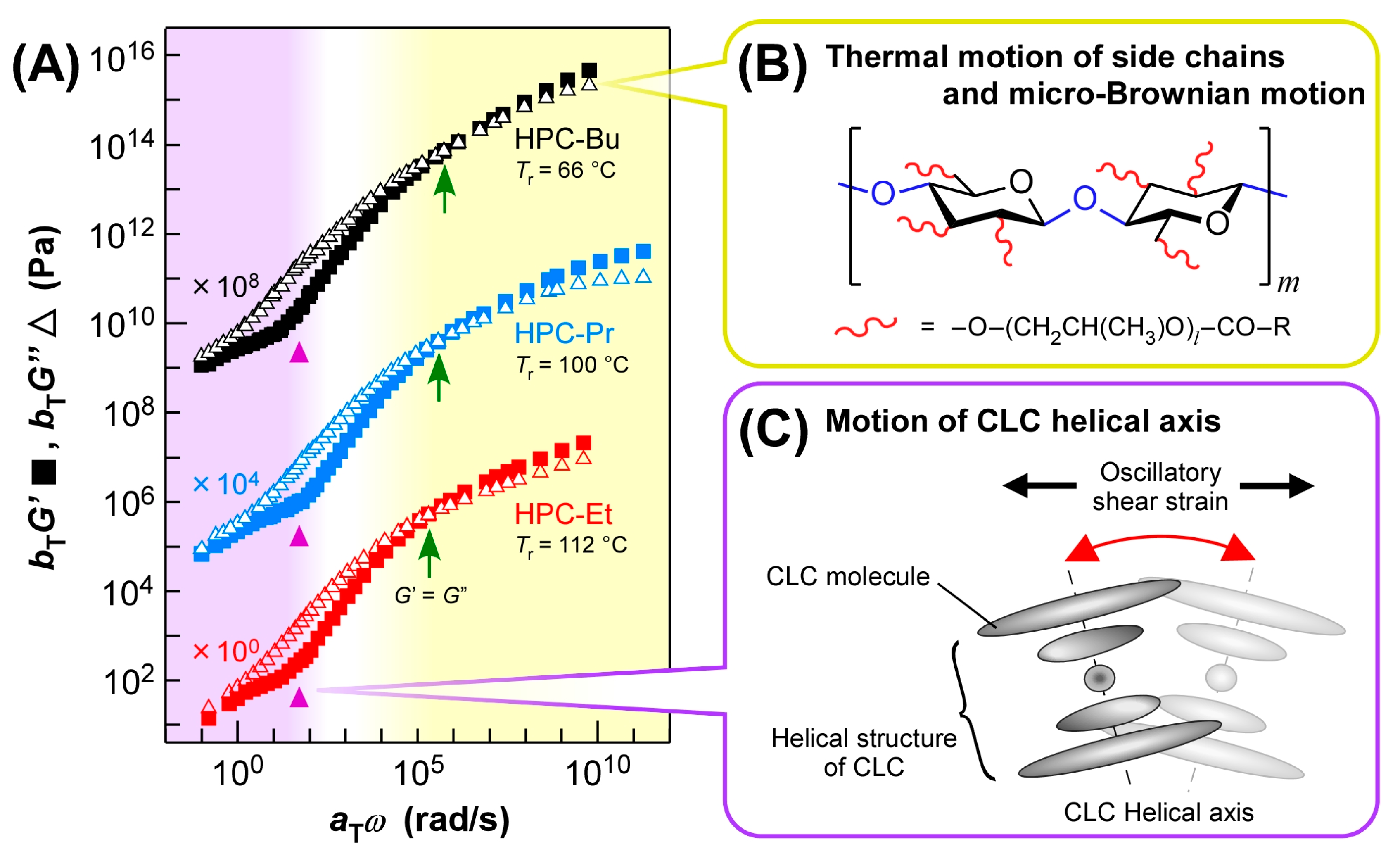
The master curves of HPC-Et, HPC-Pr, and HPC-Bu were almost identical when they were constructed with the
Tr where each derivative reflected a blue light at 405 nm (
Figure 5A, red plots for HPC-Et, blue plots for HPC-Pr, and black plots for HPC-Bu). The
Tr of HPC-Et, HPC-Pr, and HPC-Bu are 112 °C, 100 °C, and 66 °C, respectively. These
Tr values were determined from the results of optical measurements to ensure that all the HPC derivatives have the same CLC structure for a fair comparison of their rheological properties, as mentioned in
Section 2.3. To make master curves with a wide range of
ω,
ω dependence of
G′ and
G″ in the range between 10
−1 and 10
2 rad/s was measured at different temperatures and the results were combined according to the TTS principle. The TTS principle was applicable to the results of
G′ and
G″ in the temperature ranges below 112 °C for HPC-Et, 100 °C for HPC-Pr, and 80 °C for HPC-Bu, even though they exhibited the CLC phase. The validity of the TTS principle is also supported by the van Gurp-Palmen (vGP) plot, which can be obtained by plotting the phase angle (
δ) against the corresponding absolute value of the complex shear modulus (
G*) [
37].
The vGP plots of HPC derivatives are presented in
Figure S6 of the Supplementary Materials. Each curve obtained at different temperatures was overlapped to show a smooth curve, which is a proof for the validity of the TTS principle. However, the TTS principle was not applicable for the measurements at temperature ranges higher than the above-mentioned temperatures. This is probably due to the gradual transition to the isotropic phase, which may somehow destroy the CLC structure. For instance, the vGP plots of HPC-Et were well overlapped in the temperature range between 15 °C and 112 °C, but they were not overlapped when the temperature was elevated to 140 °C. When the temperature was further increased to 150 °C,
δ became nearly equal to 90° regardless of
G* (
Supplementary Materials, Figure S6, upper panel). The
δ of 90° suggests that viscoelastic behavior is dominated only by the viscosity while the sample behaves as a fluid. Such fluid-like behavior was probably caused by the disappearance of the CLC structure due to the phase transition from the CLC phase to the isotropic phase. In fact, the isotropic phase transition temperature (
Ti) of HPC-Et was determined to be 155–165 °C, as described in
Section 2.2. In the same manner, the
Ti of HPC-Pr and HPC-Bu were observed at 150–160 °C and 140–145 °C, respectively. These temperature ranges were nearly equal to those at which the breakdown of the TTS principle, that is, discontinuity of vGP plots, was observed (
Supplementary Materials, Figure S6, middle and bottom panels). From these results, we concluded that the TTS principle can be applicable for the construction of the master curves of HPC derivatives below their
Tis.
The fluid-like behavior of HPC-Pr above
Ti can also be confirmed from its temperature dependence and frequency dependence of
G′ and
G″. The temperature dependence of HPC-Pr is given in
Figure S7 of the Supplementary Materials. In this profile, a peak caused by the isotropic phase transition was observed at 150 °C, which was very close to the
Ti of HPC-Pr determined by POM observation, that is, 150–160 °C. Above this temperature,
G′ and
G″ rapidly decreased due to the fluid-like behavior of CLC in the isotropic phase. This tendency was consistent with the vGP plots shown in
Figure S6 of the Supplementary Materials. The frequency dependence at 155 °C shown in
Figure S8 of the Supplementary Materials suggests that HPC-Pr is flowing since the slopes of
G′ and
G″ are constant at 1.0. This indicates that the CLC structure seems to hinder the Newtonian flow of the molecules even at the isotropic phase. Such flow behavior of HPC-Pr above its
Ti supports the deformation of the CLC structure, which induces the breakdown of the TTS principle.
The master curves of HPC derivatives were almost identical, even though they were constructed with widely different Tr. This result strongly suggests that the rheological behavior of HPC derivatives probably depends on the reflection wavelength of the CLCs, corresponding to p. The reasons for such identification will be discussed later.
As highlighted in yellow in
Figure 5A, the higher
ω region over 10
5 rad/s is considered to be a glass region. The intersections of
G′ and
G″ appeared around
ω = 10
5–10
6 rad/s for all HPC derivatives (
Figure 5A, green arrows). Since the
G″ is always greater than
G′ on the lower
ω side of these intersections, it can be assumed that this drastic change in rheological properties may be related to the glass transition of HPC derivatives. In this region, the relaxation processes due to the thermal motion of side chains or the micro-Brownian motion of the main chain seem to be dominant, similar to those of amorphous polymers (
Figure 5B). Therefore, we synthesized HPC-Pr with different molecular weights (
Supplementary Materials, Table S1). Their master curves indicate that the micro-Brownian motion of the main chain affects their rheological phenomena in the glass region (
Supplementary Materials, Figure S9). As
Mw increased, the master curves of these HPC derivatives in the glass region tended to broaden, though their values of glass transition temperature (
Tg) remained constant at −23 °C regardless of
Mw. This tendency implies that the micro-Brownian motion from the main chain contributes to rheological behavior in the higher
ω region above 10
5 rad/s. However, side-chain motion seems to have a greater influence on the viscoelastic behavior in the glass region. As the side chain length of HPC derivatives increased, the
Tg values were significantly lowered; for instance, −8 °C for HPC-Et, −23 °C for HPC-Pr, and −37 °C for HPC-Bu, as determined by temperature-dependent rheology measurements conducted at 1.0 Hz (
Supplementary Materials, Figure S10). Although the difference in the side chain length of HPC derivatives is relatively small because the side chain of HPC consists of 1–2 hydroxypropyl groups and alkanoyl groups. However, this small difference in side chain structure dramatically affects
Tg, the influence of the side chain on the glass region seems to be dominant for the difference in this relaxation.
The master curves of each derivative were consistent in this high
ω region over 10
5 rad/s, although the side chain lengths and the
Tr values were different. This was confirmed by the fact that the intersections of
G′ and
G″ appear at approximately equal frequencies; that is,
ω = 10
5–10
6 rad/s (
Figure 5A, green arrows). In general, the viscoelastic behavior in the glass region is greatly affected by the structure of the side chain, as mentioned above. Nevertheless, the overlap of the master curves in this study is due to the offsetting effects of side chain length and
Tr values. If the
Tr values are the same, the master curves of the derivatives possessing longer side chains tend to shift to the higher
ω side when considering
Tg values and the TTS principle. On the other hand, as discussed in
Section 2.2, as the length of the side chain increased, the
Tr values, which correspond to the temperature at which the derivative reflects 405 nm blue light, were lowered, and the master curves shifted to the lower
ω side. As a result of these two opposite shift effects, these master curves seemed to be consistent in the high
ω region.
On the other hand, all the inflection points of
G′ appeared at
ω = 10
2 rad/s regardless of the length of the alkanoyl side chains (
Figure 5A, purple triangles). The appearance of inflection points is probably due to the CLC structure of HPC derivatives, which induces flattering of the slopes of
G′ and
G″ on the low
ω side, as we discussed in
Section 2.4. It should be emphasized that this is not because of the entanglement of HPC derivatives since no rubbery plateau appeared in the temperature dependence of
G′ and
G″ of HPC-Pr (
Supplementary Materials, Figure S7). This indicates that the dominant relaxation process is not due to a molecular motion but to a larger scale motion such as the tilt of the helical structure of CLC (
Figure 5C). Since the viscoelastic behavior is independent of the side chain length, the relaxation mechanism due to the tilting motion of the helical axis of the CLC, illustrated in
Figure 5C, may be dominant in this
ω region below 10
2 rad/s, as highlighted in purple in
Figure 5A. A previous study has reported that the helical axis of the CLC tilts when shear is applied to CLCs [
21]. Since each layer of the helix is uniformly tilted by shear force, it is expected to show similar viscoelastic behavior regardless of the structure of the side chain.
This proposal about the dominant molecular motion on the low
ω side is also supported by our previous work [
25]. We investigated the effect of orientation state in CLC on rheological behavior. Dynamic viscoelastic measurements of HPC-Pr were conducted after the thermal treatment in isotropic phase or shearing at a constant shear rate of 10 s
−1 for 200 s. These pre-treatments before the rheological measurements ensured that CLC would have different structures, that is, a randomly-arranged state or a shear-oriented state. As a result of the
ω dependence of
G′ and
G″ in HPC-Pr at 100 °C, the difference was apparent, especially in the
ω region below 10
1 rad/s. This result indicates that the effect of the orientation of CLC structure appears on the low
ω side, which is consistent with the result of this study.
The consistency of master curves for each derivative in the low
ω region below 10
2 rad/s is due to the same CLC structures. All the HPC derivatives had almost the same CLC structure when they reflected blue light at 405 nm, as confirmed by optical measurements and WAXD measurements, as stated in
Section 2.3. Furthermore, in this
ω region, the relaxation process caused by the CLC structure was dominant, as described in
Section 2.4. Therefore, the similar viscoelastic behavior, regardless of the length of the side chain, can be attributed to the identical structure of the CLCs.
Considering the two characteristics of the master curves mentioned above, the master curves of HPC derivatives can be divided into two regions depending on the ω. It can be assumed that the highest ω range above 105 rad/s is a glass region at which the molecular motion of CLC molecules is frozen. In the lowest ω range below 102 rad/s, large-scale motion such as tilting of the helical axis may be dominant. The intermediate ω range, that is, the ω range of 102–105 rad/s, can be estimated to be the transition region between the two regions mentioned above. In this region, the motion of the helical axial unit gradually freezes, and the motion of the side chains and main chains becomes dominant with the increase of ω.
The horizontal shift factors (
aT) used for the construction of the master curves of HPC derivatives also showed unique behavior. The profiles of
aT values for HPC-Et, HPC-Pr, and HPC-Bu are shown in
Figure S11 of the Supplementary Materials. All of them decreased logarithmically with raising the temperature to the
Tr, meaning a typical characteristic for
aT of polymers. In addition, the
aT values were almost the same for all the HPC derivatives synthesized in this study. This result suggests that the temperature dependence of the time scale of molecular motion is not affected by the length of alkanoyl side chains in HPC derivatives. Another interesting property of
aT of HPC derivatives appeared when they were fitted with the Williams-Landel-Ferry (WLF) equation shown in Equation (7) (
Supplementary Materials, Figure S11, solid lines) [
38].
The WLF parameters
c1 and
c2 for HPC derivatives are listed in
Table S2 of the Supplementary Materials. The values of
c1 and
c2 for all the derivatives were almost identical, and they are in the range of 2.38–2.53 and 126–148 K, respectively, suggesting that the values are also close to those of amorphous polymers. This tendency implies that the
aT values of the HPC derivatives show WLF-type temperature dependence.
2.6. Rheological Properties of HPC Ester Derivatives: Arrhenius Plots of Horizontal Shift Factors
The activation energies of each relaxation process (
Ea) were able to be calculated by the Arrhenius-plots of
aT values, which were used to construct the master curves of HPC-Et, HPC-Pr, and HPC-Bu in
Section 2.5 (
Figure 6). The complete explanation of the activation energies of the relaxation process is available in the
supplementary materials. The Arrhenius plot of the
aT showing the WLF-type temperature dependence is generally a curve that increases as a power function with increasing 1/
T. Note that
T is the absolute temperature in the unit of Kelvin [
39]. However, when the temperature range is narrow, the Arrhenius plot can be approximated well by a straight line, and the
Ea can be obtained by its slope. This is because
aT satisfies Equation (8) as follows:
where
R is the gas constant. Such a methodology has been adopted in many previous studies [
39,
40,
41].
The value of
Ea here means the magnitude of the energy barrier for the molecules to move. The CLC, which is in an excited state due to the application of strain, returns to the ground state by the molecular motion. In dynamic viscoelasticity measurements, the stress in the relaxation process is determined, so
Ea refers to the size of the energy barrier for the motion of the molecules when the molecules return from the excited state to the ground state. In other words,
Ea can be considered to represent the mobility of CLC in a relaxation mode in the temperature range where linear Arrhenius plots can be yielded. The complete derivation of Equation (8) is also available in the
Supplementary Materials.
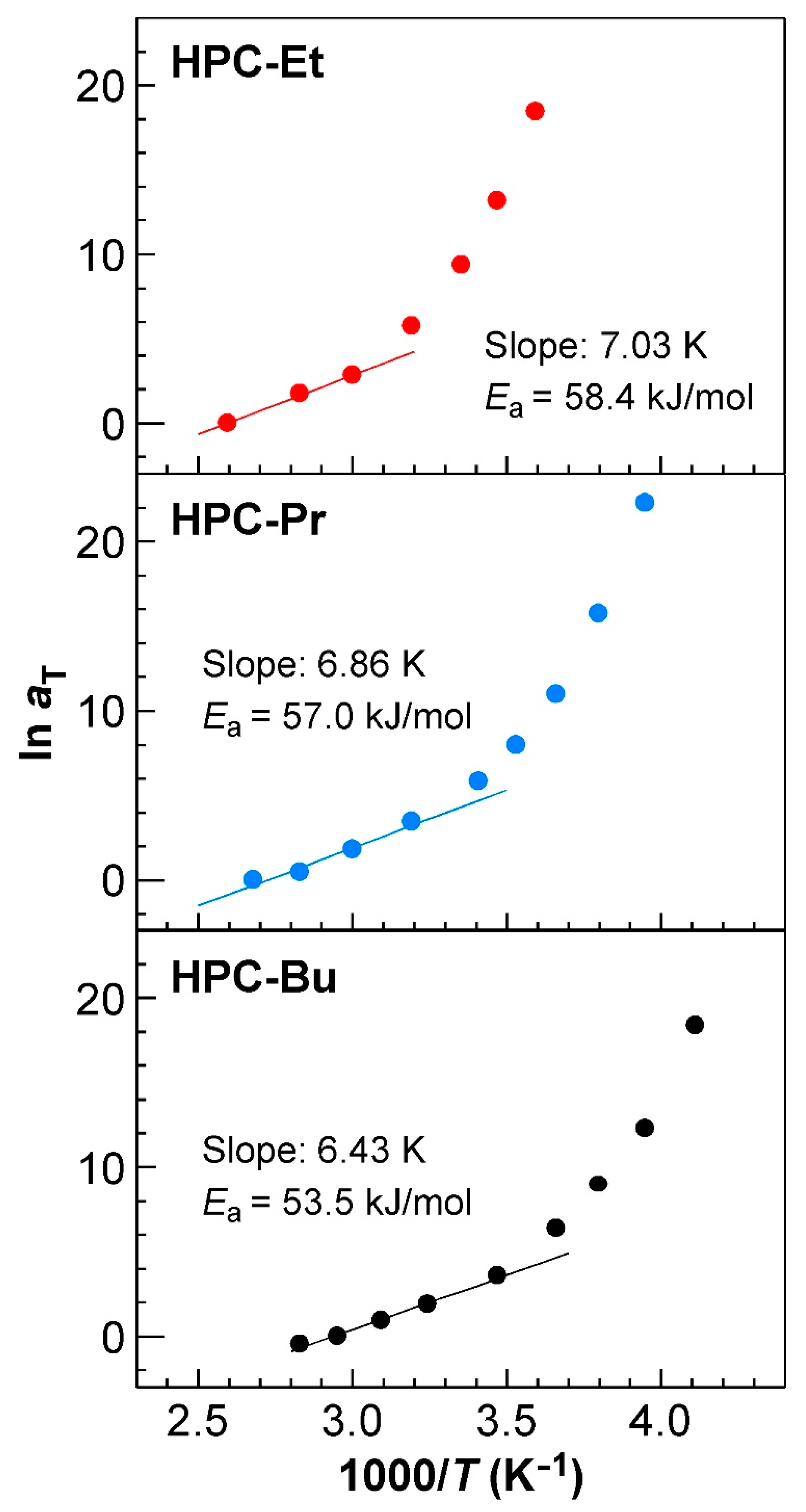
The Arrhenius plots of the three HPC derivatives investigated in this study are presented in
Figure 6. These plots were made by plotting the natural logarithm of
aT against the inverse of the temperature. As expected, the natural logarithm of
aT of HPC-Et increased in a power law fashion as the temperature decreased (
Figure 6, upper panel). Notice that the increase in 1/
T corresponds to the decrease in
T.
The value of
Ea calculated from the slope of the regression line of the Arrhenius plots in higher temperature regions may suggest the ease of orientation in the CLC structure. We fitted them with a regression line, as shown in
Figure 6. As mentioned in
Section 2.5, the master curves can be divided into two regions, and the rheological behavior in the lower
ω region, that is, the high-temperature region, is dominated by the relaxation process caused by CLC structure. Considering this result, the
Ea value from the plots in the high-temperature region represents the activation energy derived from the helical structure of CLC. The
Ea value decreased with increasing the alkanoyl side chains of HPC derivatives, that is, 58.4 kJ/mol for HPC-Et, 57.0 kJ/mol for HPC-Pr, and 53.5 kJ/mol for HPC-Bu, as shown in
Figure 6. Since the
Ea value means the mobility of molecules, as described in the
supplementary materials, the molecules become easier to move as the side chain length increases, considering the result that
Ea decreases as the side chain length increases. This may be related to the improvement of the orientation of CLCs.