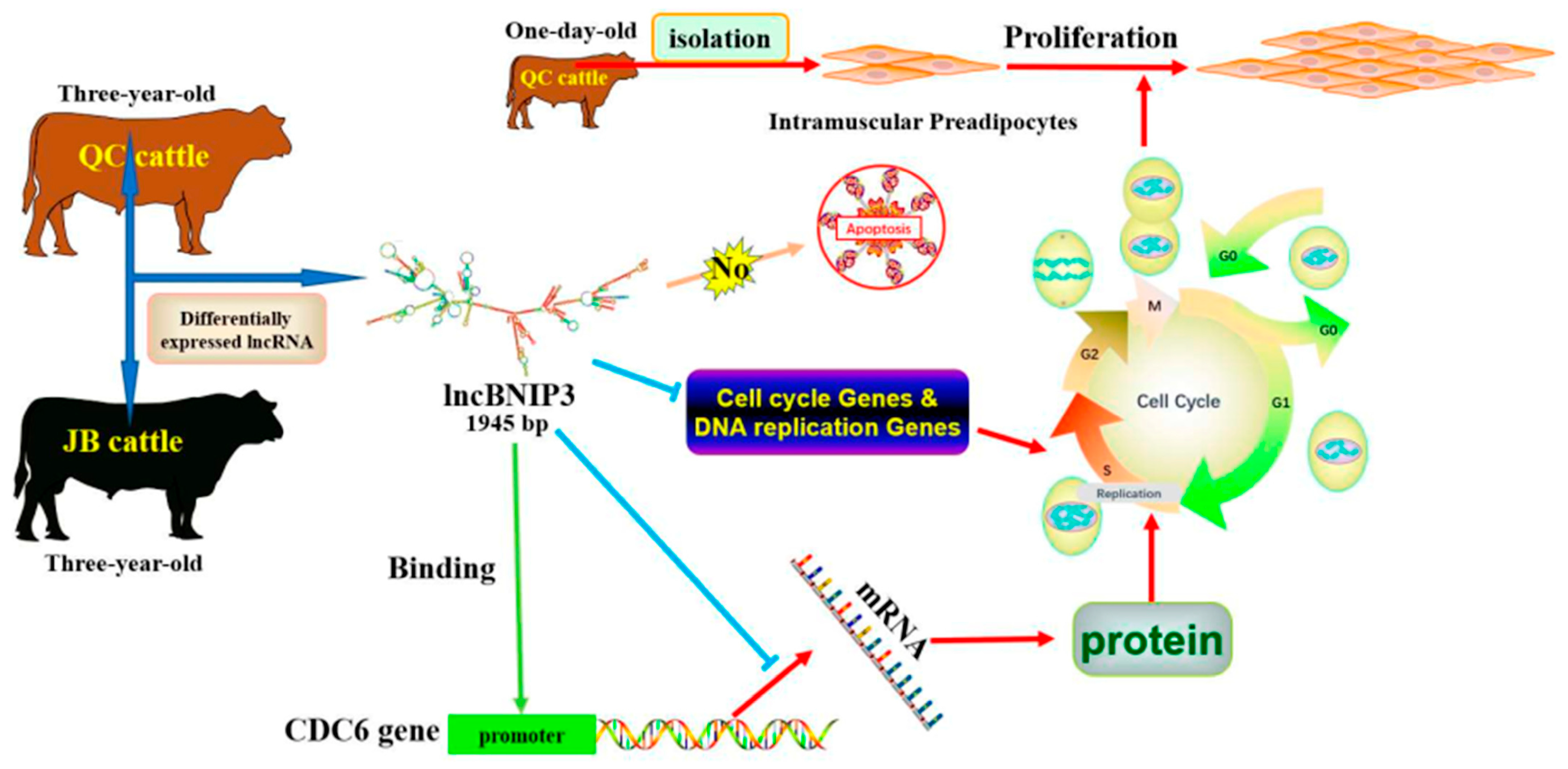
Long Non-Coding RNA BNIP3 Inhibited the Proliferation of Bovine Intramuscular Preadipocytes via Cell Cycle
Abstract
1. Introduction
2. Results
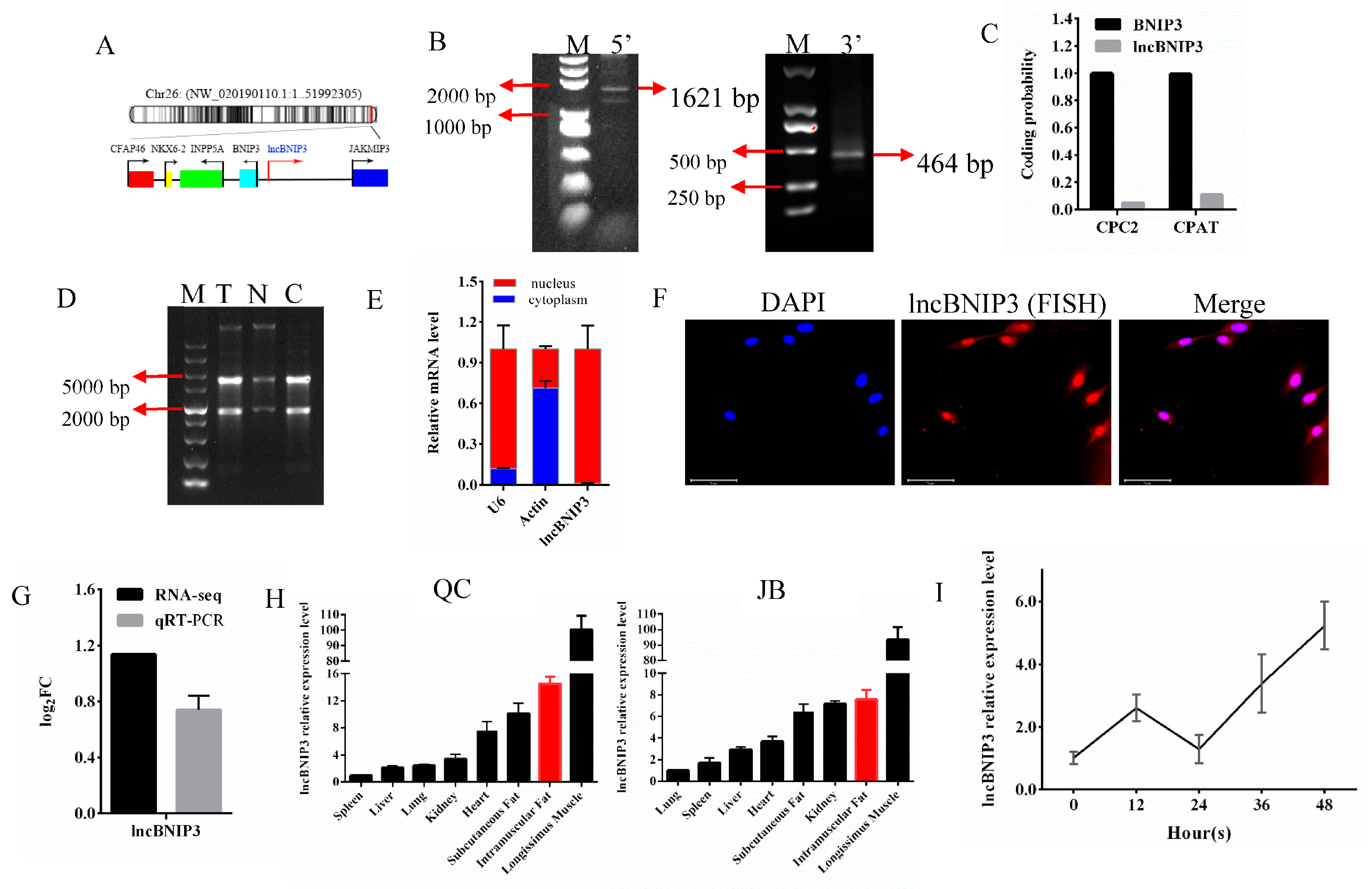
2.1. The Biological Characteristics and Expression Profile Analysis of lncBNIP3
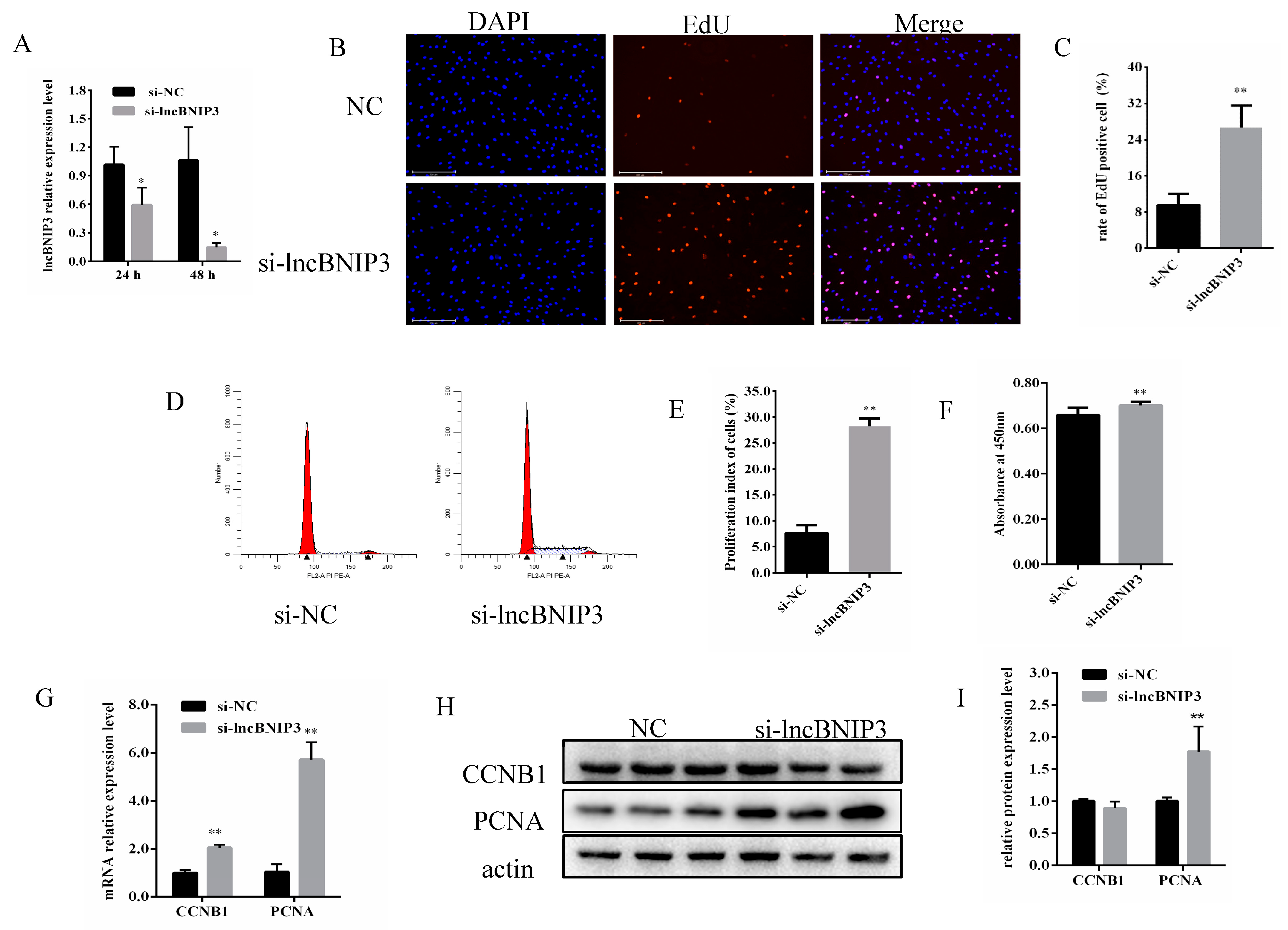
2.2. Interference with lncBNIP3 Promoted the Proliferation of Bovine Intramuscular Preadipocytes
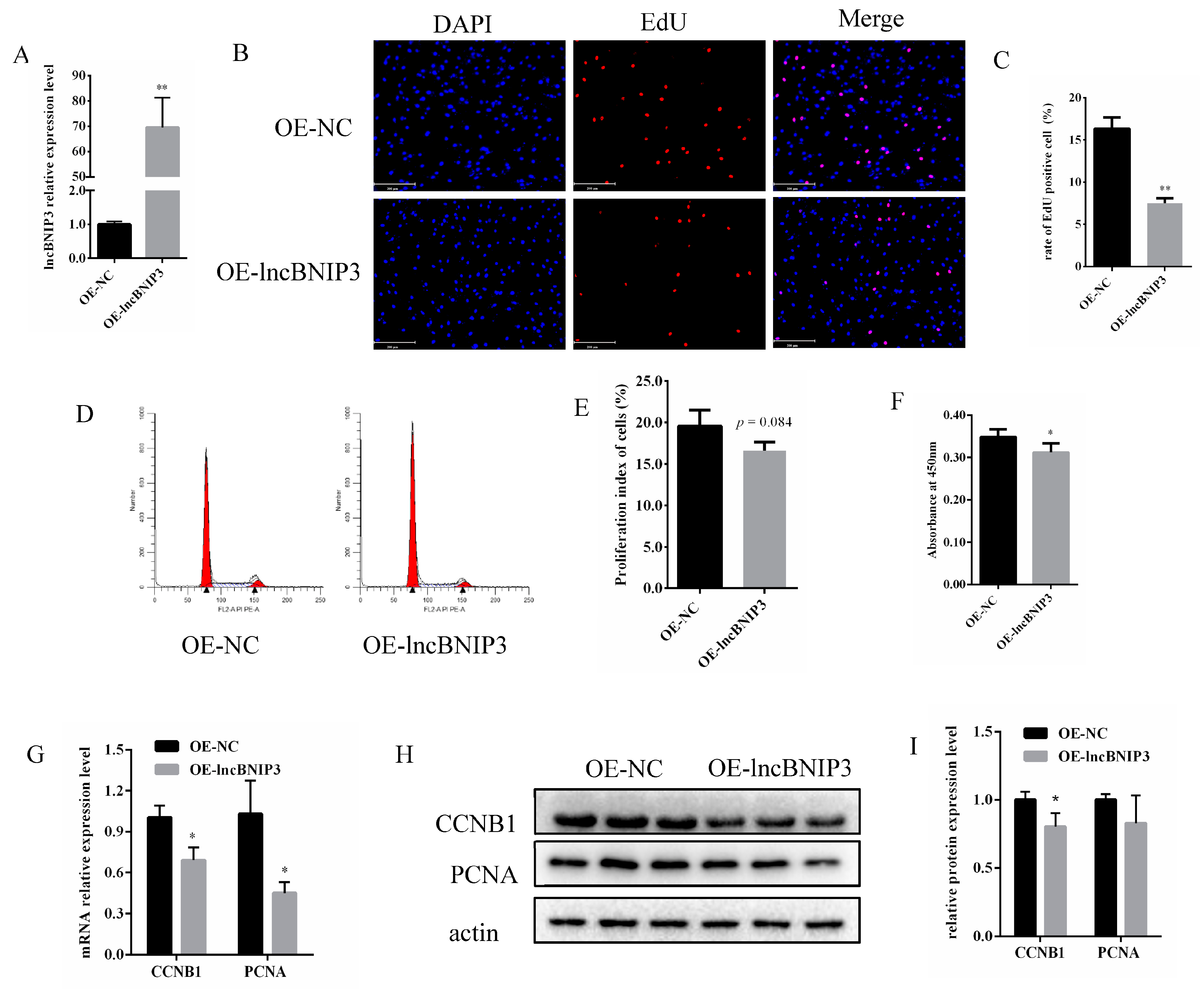
2.3. Overexpression of lncBNIP3 Inhibited the Proliferation of Bovine Intramuscular Preadipocytes
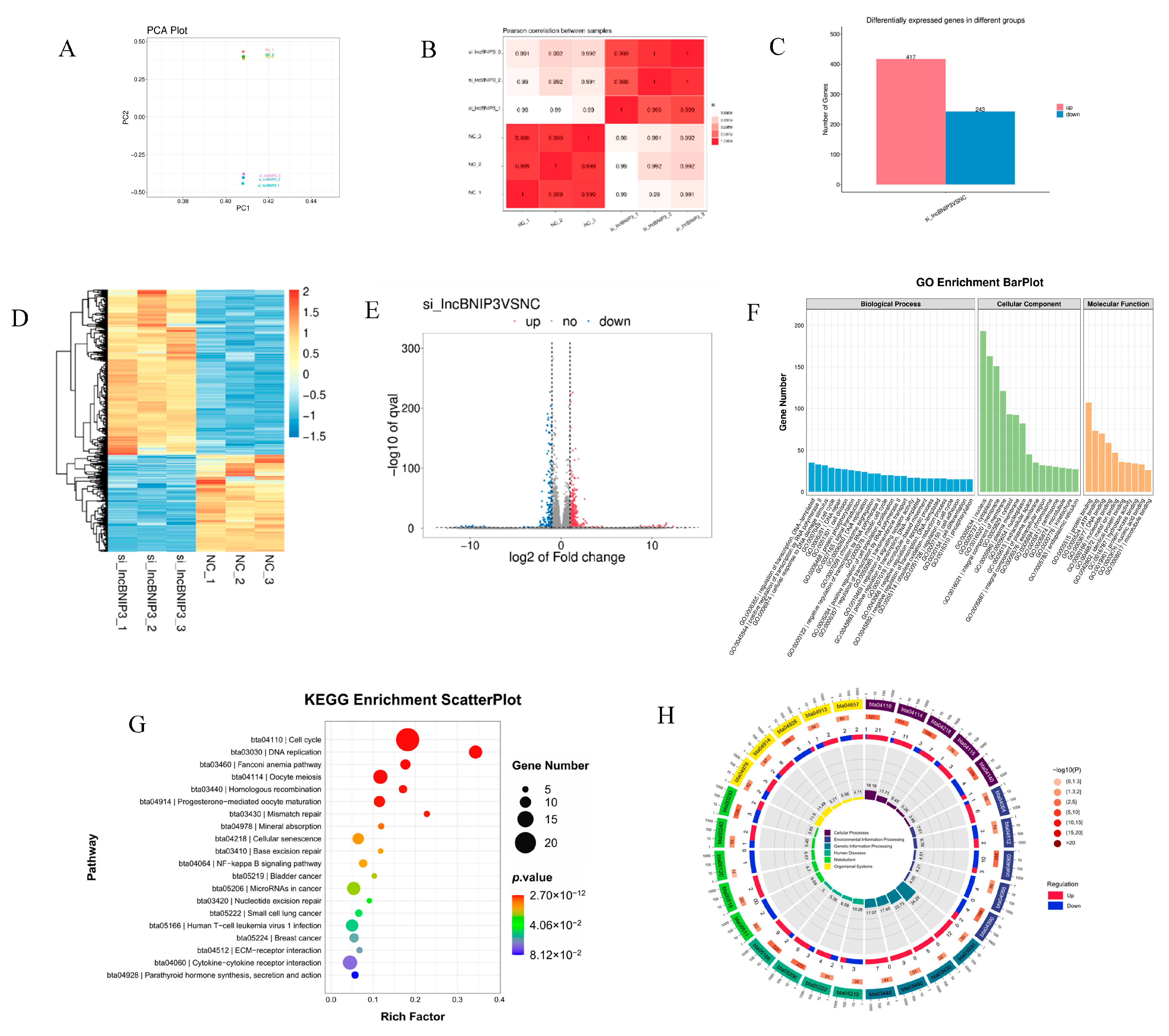
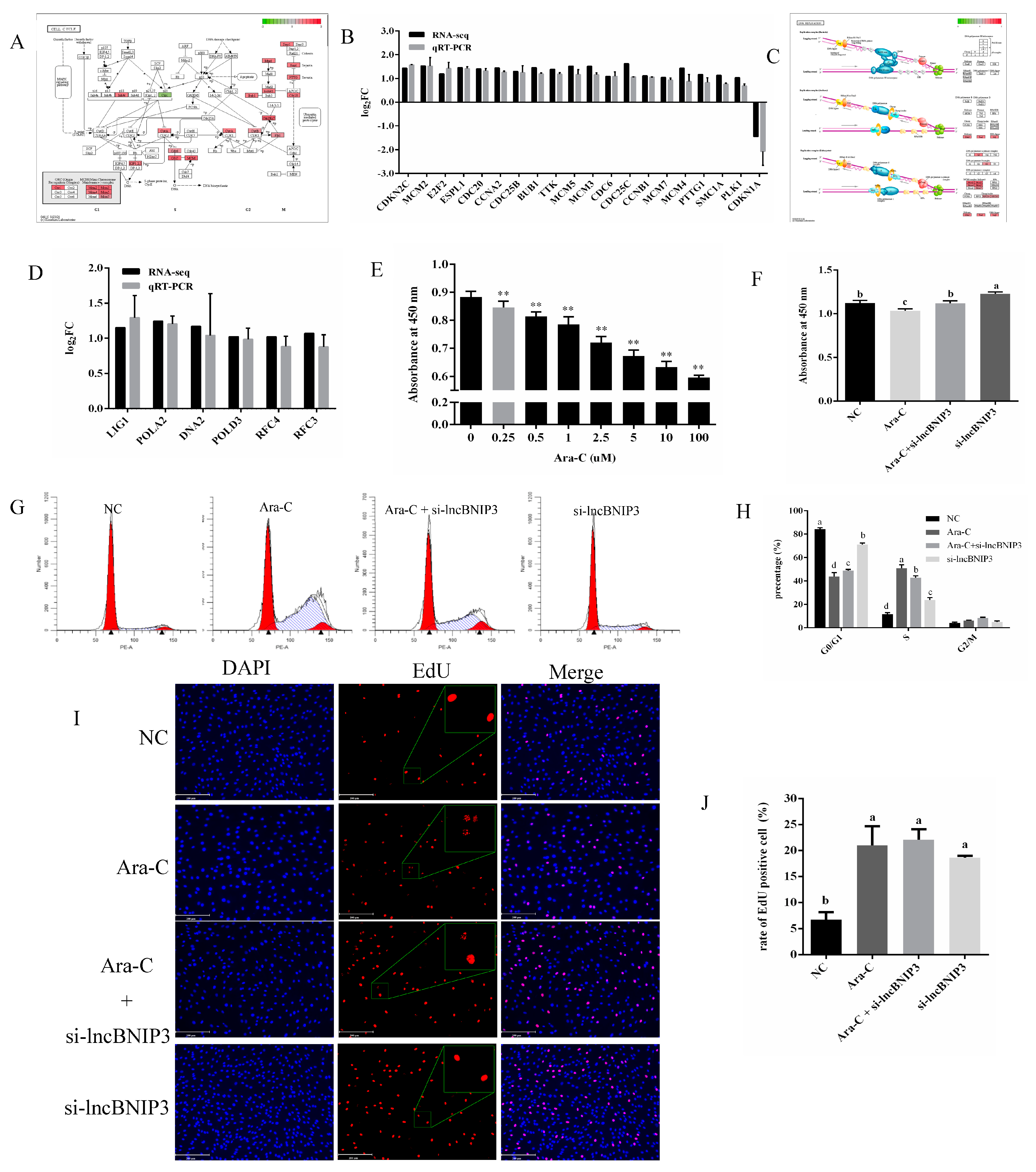
2.4. Identification and Enrichment Analyses of DEGs Associated with lncBNIP3
2.5. Interference with lncBNIP3 Promoted the Cell Cycle and Rescued the Defective Cell Cycle Caused by Ara-C
2.6. Interference with lncBNIP3 Promoted the Promoter Activity and Expression of CDC6
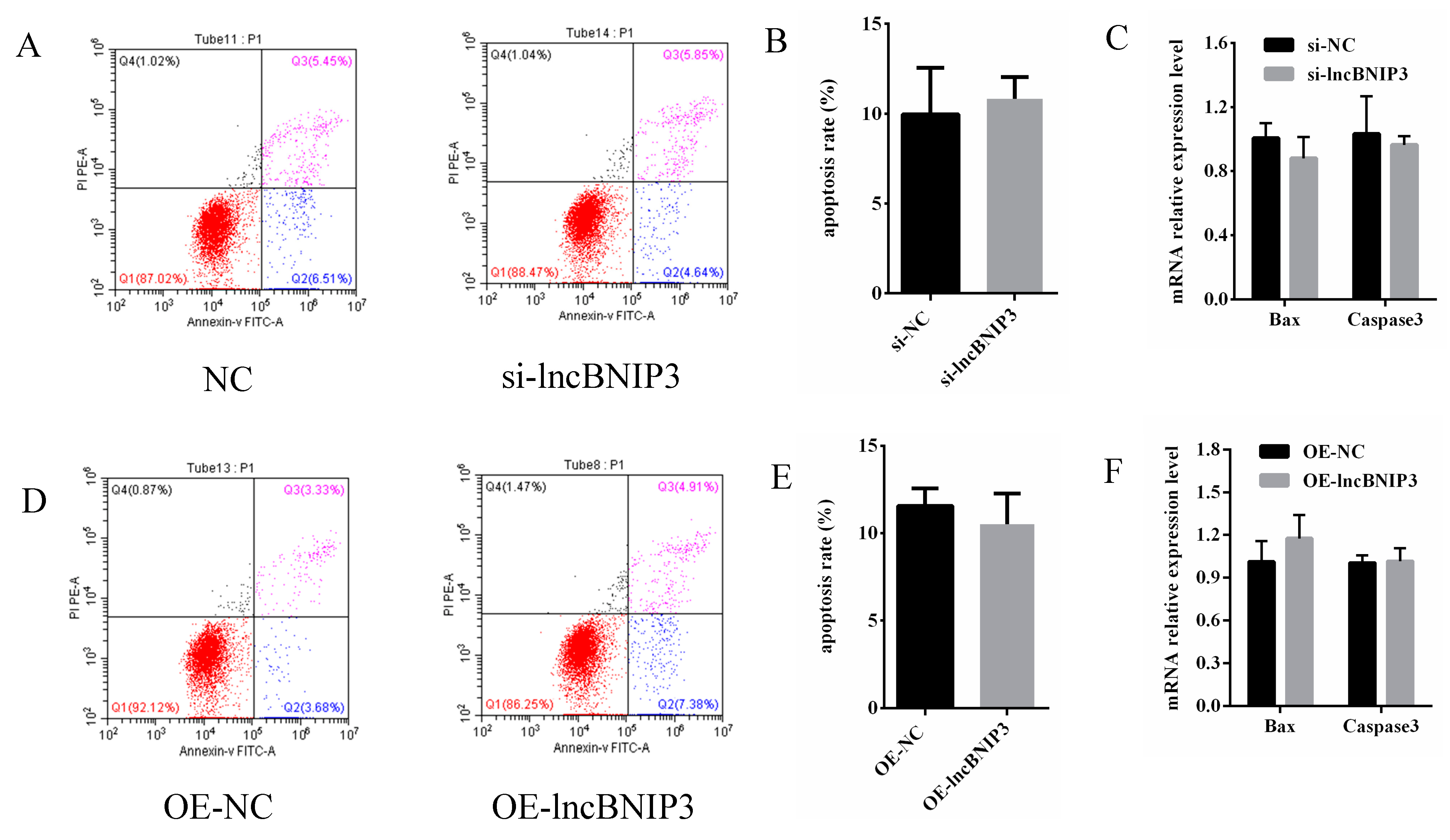
2.7. LncBNIP3 Had No Significant Effect on the Apoptosis of Bovine Intramuscular Preadipocytes
3. Discussion
4. Materials and Methods
4.1. Animal, Samples and Cell Culture
4.2. Fragment Synthesis and Recombinant Vector Construction
4.3. Cell Transfection
4.4. 5′ and 3′ Rapid Amplification of cDNA Ends (RACE)
4.5. Cytoplasmic and Nuclear RNA Separation
4.6. Fluorescence In Situ Hybridization (FISH)
4.7. Cell Proliferation and Apoptosis Analysis
4.8. Real-Time Quantitative PCR (RT-qPCR)
4.9. Western Blot Analysis
4.10. RNA Isolation, Library Construction, and Sequencing
4.11. Raw Data Quality Control and Data Analysis
4.12. Chromatin Isolation by RNA Purification (ChIRP)
4.13. Luciferase Reporter Assays
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Fu, X.; Yang, G.; Du, M. Review: Enhancing intramuscular fat development via targeting fibro-adipogenic progenitor cells in meat animals. Animal 2020, 14, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Ngapo, T.M.; Varela, D.B.; Lozano, M.S.R. Mexican consumers at the point of meat purchase. Beef choice. Meat Sci. 2017, 134, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Hausman, G.J.; Basu, U.; Du, M.; Fernyhough-Culver, M.; Dodson, M.V. Intermuscular and intramuscular adipose tissues: Bad vs. good adipose tissues. Adipocyte 2014, 3, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T. Intramuscular adipogenesis in cattle: Effects of body fat distribution and macrophage infiltration. Anim. Sci. J. 2022, 93, 6. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.Q.; Smith, S.B.; Lee, H.G. Vitamin A regulates intramuscular adipose tissue and muscle development: Promoting high-quality beef production. J. Anim. Sci. Biotechnol. 2021, 12, 10. [Google Scholar] [CrossRef]
- Nojima, T.; Proudfoot, N.J. Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat. Rev. Mol. Cell Biol. 2022, 23, 853. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef]
- Geng, H.; Bu, H.F.; Liu, F.Y.; Wu, L.T.; Pfeifer, K.; Chou, P.M.; Wang, X.; Sun, J.R.; Lu, L.; Pandey, A.; et al. In Inflamed Intestinal Tissues and Epithelial Cells, Interleukin 22 Signaling Increases Expression of H19 Long Noncoding RNA, Which Promotes Mucosal Regeneration. Gastroenterology 2018, 155, 144–155. [Google Scholar] [CrossRef]
- Wang, Z.L.; Chen, X.W.; Liu, N.; Shi, Y.; Liu, Y.T.; Ouyang, L.L.; Tam, S.; Xiao, D.S.; Liu, S.; Wen, F.Q.; et al. A Nuclear Long Non-Coding RNA LINC00618 Accelerates Ferroptosis in a Manner Dependent upon Apoptosis. Mol. Ther. 2021, 29, 263–274. [Google Scholar] [CrossRef]
- Kosinska-Selbi, B.; Mielczarek, M.; Szyda, J. Review: Long non-coding RNA in livestock. Animal 2020, 14, 2003–2013. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.L.; Chen, G.T.; Li, J.X.; Li, M.X.; Zou, C.; Fang, C.C.; Li, C.C. Transcriptome Analysis Suggests the Roles of Long Intergenic Non-coding RNAs in the Growth Performance of Weaned Piglets. Front. Genet. 2019, 10, 11. [Google Scholar] [CrossRef]
- Billerey, C.; Boussaha, M.; Esquerre, D.; Rebours, E.; Djari, A.; Meersseman, C.; Klopp, C.; Gautheret, D.; Rocha, D. Identification of large intergenic non-coding RNAs in bovine muscle using next-generation transcriptomic sequencing. BMC Genom. 2014, 15, 10. [Google Scholar] [CrossRef]
- Cai, B.L.; Li, Z.H.; Ma, M.T.; Wang, Z.J.; Han, P.G.; Abdalla, B.A.; Nie, Q.H.; Zhang, X.Q. LncRNA-Six1 Encodes a Micropeptide to Activate Six1 in Cis and Is Involved in Cell Proliferation and Muscle Growth. Front. Physiol. 2017, 8, 13. [Google Scholar] [CrossRef]
- Miao, Z.G.; Wang, S.; Zhang, J.Z.; Wei, P.P.; Guo, L.P.; Liu, D.Y.; Wang, Y.M.; Shi, M.Y. Identification and comparison of long non-conding RNA in Jinhua and Landrace pigs. Biochem. Biophys. Res. Commun. 2018, 506, 765–771. [Google Scholar] [CrossRef]
- Choi, J.Y.; Shin, D.; Lee, H.J.; Oh, J.D. Comparison of long noncoding RNA between muscles and adipose tissues in Hanwoo beef cattle. Anim. Cells Syst. 2019, 23, 50–58. [Google Scholar] [CrossRef]
- Li, D.H.; Li, F.; Jiang, K.R.; Zhang, M.; Han, R.L.; Jiang, R.R.; Li, Z.J.; Tian, Y.D.; Yan, F.B.; Kang, X.T.; et al. Integrative analysis of long noncoding RNA and mRNA reveals candidate lncRNAs responsible for meat quality at different physiological stages in Gushi chicken. PLoS ONE 2019, 14, 17. [Google Scholar] [CrossRef]
- Ran, M.L.; Chen, B.; Li, Z.; Wu, M.S.; Liu, X.C.; He, C.Q.; Zhang, S.W.; Li, Z.H. Systematic Identification of Long Noncoding RNAs in Immature and Mature Porcine Testes. Biol. Reprod. 2016, 94, 9. [Google Scholar] [CrossRef]
- Peng, Y.D.; Chang, L.; Wang, Y.Q.; Wang, R.N.; Hu, L.L.; Zhao, Z.Y.; Geng, L.Y.; Liu, Z.Z.; Gong, Y.F.; Li, J.S.; et al. Genome-wide differential expression of long noncoding RNAs and mRNAs in ovarian follicles of two different chicken breeds. Genomics 2019, 111, 1395–1403. [Google Scholar] [CrossRef]
- Fang, M.X.; Yang, Y.; Wang, N.D.; Wang, A.B.; He, Y.F.; Wang, J.S.; Jiang, Y.; Deng, Z.B. Genome-wide analysis of long non-coding RNA expression profile in porcine circovirus 2-infected intestinal porcine epithelial cell line by RNA sequencing. PeerJ 2019, 7, 20. [Google Scholar] [CrossRef]
- Hu, X.M.; Chen, S.H.; Jia, C.X.; Xue, S.L.; Dou, C.F.; Dai, Z.Q.; Xu, H.; Sun, Z.; Geng, T.Y.; Cui, H.M. Gene expression profile and long non-coding RNA analysis, using RNA-Seq, in chicken embryonic fibroblast cells infected by avian leukosis virus. J. Arch. Virol. 2018, 163, 639–647. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, J.C.; Zhang, C.F.; Chai, Z.X.; Cao, H.W.; Wang, J.K.; Zhu, J.J.; Wang, J.B.; Ji, Q.M. The whole-transcriptome landscape of muscle and adipose tissues reveals the ceRNA regulation network related to intramuscular fat deposition in yak. BMC Genom. 2020, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.R.; Ma, X.H.; Mei, C.G.; Zan, L.S. A genome-wide landscape of mRNAs, lncRNAs, circRNAs and miRNAs during intramuscular adipogenesis in cattle. BMC Genom. 2022, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, C.; Skotheim, J.M.; de Bruin, R.A.M. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Polo, S.E.; Almouzni, G. Histone metabolic pathways and chromatin assembly factors as proliferation markers. Cancer Lett. 2005, 220, 1–9. [Google Scholar] [CrossRef]
- Hanzlikova, H.; Caldecott, K.W. Perspectives on PARPs in S Phase. Trends Genet. 2019, 35, 412–422. [Google Scholar] [CrossRef]
- Ayad, N.G. CDKs give Cdc6 a license to drive into S phase. Cell 2005, 122, 825–827. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, F.; Zhang, L.; Xiong, L.; Mao, Q.; Liu, Y.H.; Qiu, X.G.; Wang, X.; Shui, L.; Chen, X.; et al. CDC6 is a prognostic biomarker and correlated with immune infiltrates in glioma. Mol. Cancer 2022, 21, 9. [Google Scholar] [CrossRef]
- Guil, S.; Esteller, M. Cis-acting noncoding RNAs: Friends and foes. Nat. Struct. Mol. Biol. 2012, 19, 1068–1075. [Google Scholar] [CrossRef]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef]
- Yao, R.W.; Wang, Y.; Chen, L.L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019, 21, 542–551. [Google Scholar] [CrossRef]
- Frank, D.; Ball, A.; Hughes, J.; Krishnamurthy, R.; Piyasiri, U.; Stark, J.; Watkins, P.; Warner, R. Sensory and Flavour Chemistry Characteristics of Australian Beef: Influence of Intramuscular Fat, Feed, and Breed. J. Agric. Food Chem. 2016, 64, 4299–4311. [Google Scholar] [CrossRef]
- Saloura, V.; Vougiouklakis, T.; Zewde, M.; Kiyotani, K.; Park, J.H.; Gao, G.M.; Karrison, T.; Lingen, M.; Nakamura, Y.; Hamamoto, R. WHSC1L1 drives cell cycle progression through transcriptional regulation of CDC6 and CDK2 in squamous cell carcinoma of the head and neck. Oncotarget 2016, 7, 42527–42538. [Google Scholar] [CrossRef]
- Sun, Y.M.; Cai, R.; Wang, Y.Q.; Zhao, R.; Qin, J.; Pang, W.J. A Newly Identified LncRNA LncIMF4 Controls Adipogenesis of Porcine Intramuscular Preadipocyte through Attenuating Autophagy to Inhibit Lipolysis. Animals 2020, 10, 15. [Google Scholar] [CrossRef]
- Sun, Y.M.; Chen, X.C.; Qin, J.; Liu, S.G.; Zhao, R.; Yu, T.Y.; Chu, G.Y.; Yang, G.S.; Pang, W.J. Comparative Analysis of Long Noncoding RNAs Expressed during Intramuscular Adipocytes Adipogenesis in Fat-Type and Lean-Type Pigs. J. Agric. Food Chem. 2018, 66, 12122–12130. [Google Scholar] [CrossRef]
- Burton, T.R.; Gibson, S.B. The role of Bcl-2 family member BNIP3 in cell death and disease: NIPping at the heels of cell death. Cell Death Differ. 2009, 16, 515–523. [Google Scholar] [CrossRef]
- Schwartz, G.K. Development of cell cycle active drugs for the treatment of gastrointestinal cancers: A new approach to cancer therapy. J. Clin. Oncol. 2005, 23, 4499–4508. [Google Scholar] [CrossRef]
- Ubhi, T.; Brown, G.W. Exploiting DNA Replication Stress for Cancer Treatment. Cancer Res. 2019, 79, 1730–1739. [Google Scholar] [CrossRef]
- Sun, F.Z.; Li, N.N.; Tong, X.L.; Zeng, J.; He, S.Z.; Gai, T.T.; Bai, Y.M.; Liu, L.L.; Lu, K.P.; Shen, J.H.; et al. Ara-c induces cell cycle G1/S arrest by inducing upregulation of the INK4 family gene or directly inhibiting the formation of the cell cycle-dependent complex CDK4/cyclin D1. Cell Cycle 2019, 18, 2293–2306. [Google Scholar] [CrossRef]
- Lim, N.; Townsend, P.A. Cdc6 as a novel target in cancer: Oncogenic potential, senescence and subcellular localisation. Int. J. Cancer 2020, 147, 1528–1534. [Google Scholar] [CrossRef]
- Borlado, L.R.; Mendez, J. CDC6: From DNA replication to cell cycle checkpoints and oncogenesis. Carcinogenesis 2008, 29, 237–243. [Google Scholar] [CrossRef]
- Kong, X.L.; Duan, Y.; Sang, Y.T.; Li, Y.M.; Zhang, H.W.; Liang, Y.R.; Liu, Y.; Zhang, N.; Yang, Q.F. LncRNA-CDC6 promotes breast cancer progression and function as ceRNA to target CDC6 by sponging microRNA-215. J. Cell. Physiol. 2019, 234, 9105–9117. [Google Scholar] [CrossRef]
- Wei, S.J.; Zhang, M.M.; Zheng, Y.Y.; Yan, P.S. ZBTB16 Overexpression Enhances White Adipogenesis and Induces Brown-Like Adipocyte Formation of Bovine White Intramuscular Preadipocytes. Cell. Physiol. Biochem. 2018, 48, 2528–2538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Z.; Sun, B.; Zhao, Y.Q.; Raza, S.H.A.; Li, Y.S.; Wang, J.F.; Ma, X.H.; Almohaimeed, H.M.; Shaheen, S.; Al-Sarraj, F.; et al. Proliferation of bovine myoblast by LncPRRX1 via regulation of the miR-137/CDC42 axis. Int. J. Biol. Macromol. 2022, 220, 33–42. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Landmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357-U121. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, 12. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.Z.; Huang, J.J.; Ding, Y.; Wu, J.M.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L.P. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wang, J.; Li, B.; Sun, B.; Yu, S.; Wang, X.; Zan, L. Long Non-Coding RNA BNIP3 Inhibited the Proliferation of Bovine Intramuscular Preadipocytes via Cell Cycle. Int. J. Mol. Sci. 2023, 24, 4234. https://doi.org/10.3390/ijms24044234
Zhang W, Wang J, Li B, Sun B, Yu S, Wang X, Zan L. Long Non-Coding RNA BNIP3 Inhibited the Proliferation of Bovine Intramuscular Preadipocytes via Cell Cycle. International Journal of Molecular Sciences. 2023; 24(4):4234. https://doi.org/10.3390/ijms24044234
Chicago/Turabian StyleZhang, Wenzhen, Jianfang Wang, Bingzhi Li, Bing Sun, Shengchen Yu, Xiaoyu Wang, and Linsen Zan. 2023. "Long Non-Coding RNA BNIP3 Inhibited the Proliferation of Bovine Intramuscular Preadipocytes via Cell Cycle" International Journal of Molecular Sciences 24, no. 4: 4234. https://doi.org/10.3390/ijms24044234
APA StyleZhang, W., Wang, J., Li, B., Sun, B., Yu, S., Wang, X., & Zan, L. (2023). Long Non-Coding RNA BNIP3 Inhibited the Proliferation of Bovine Intramuscular Preadipocytes via Cell Cycle. International Journal of Molecular Sciences, 24(4), 4234. https://doi.org/10.3390/ijms24044234






