The Biological Behaviors of Neural Stem Cell Affected by Microenvironment from Host Organotypic Brain Slices under Different Conditions
Abstract
1. Introduction
2. Results
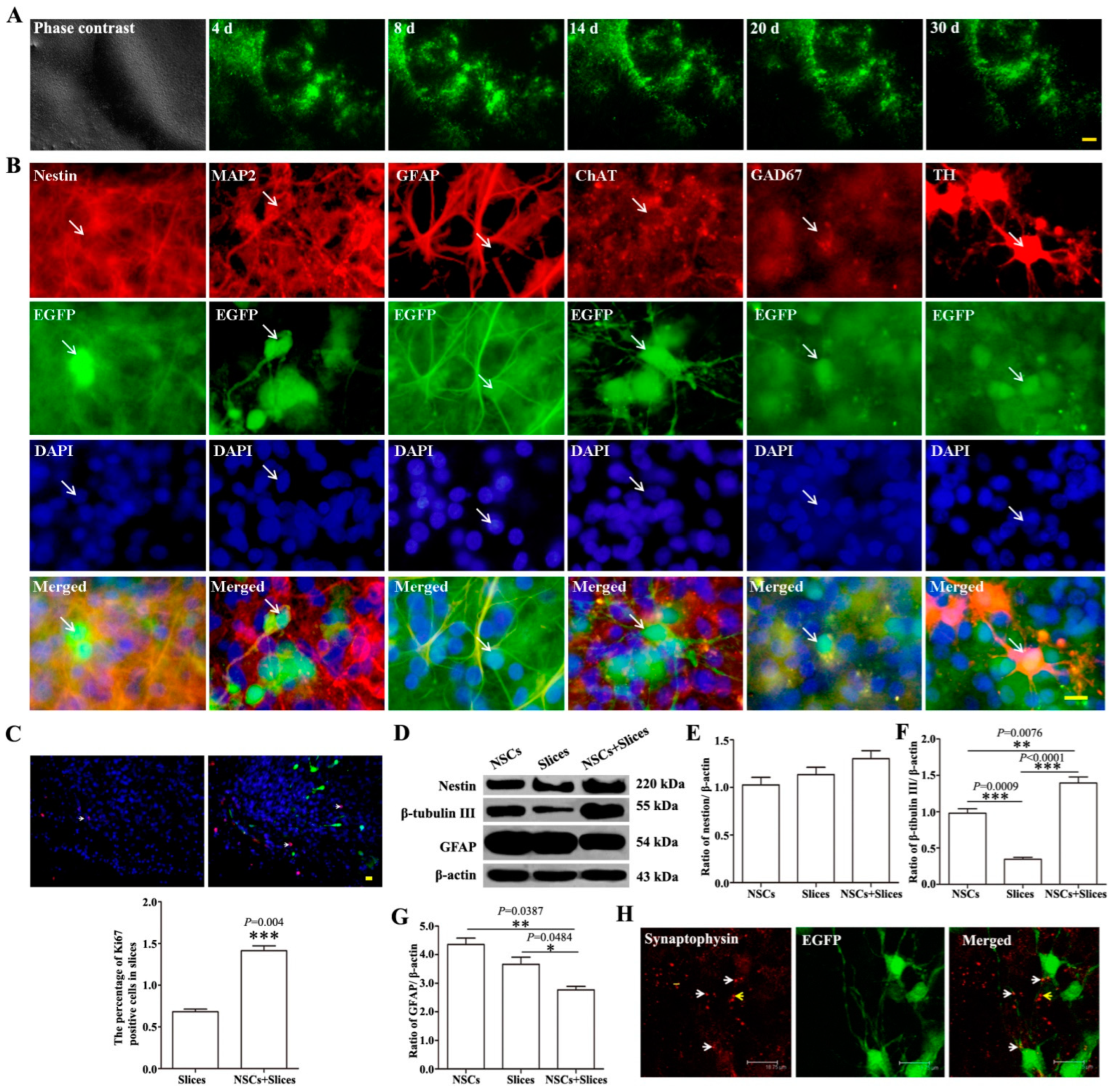
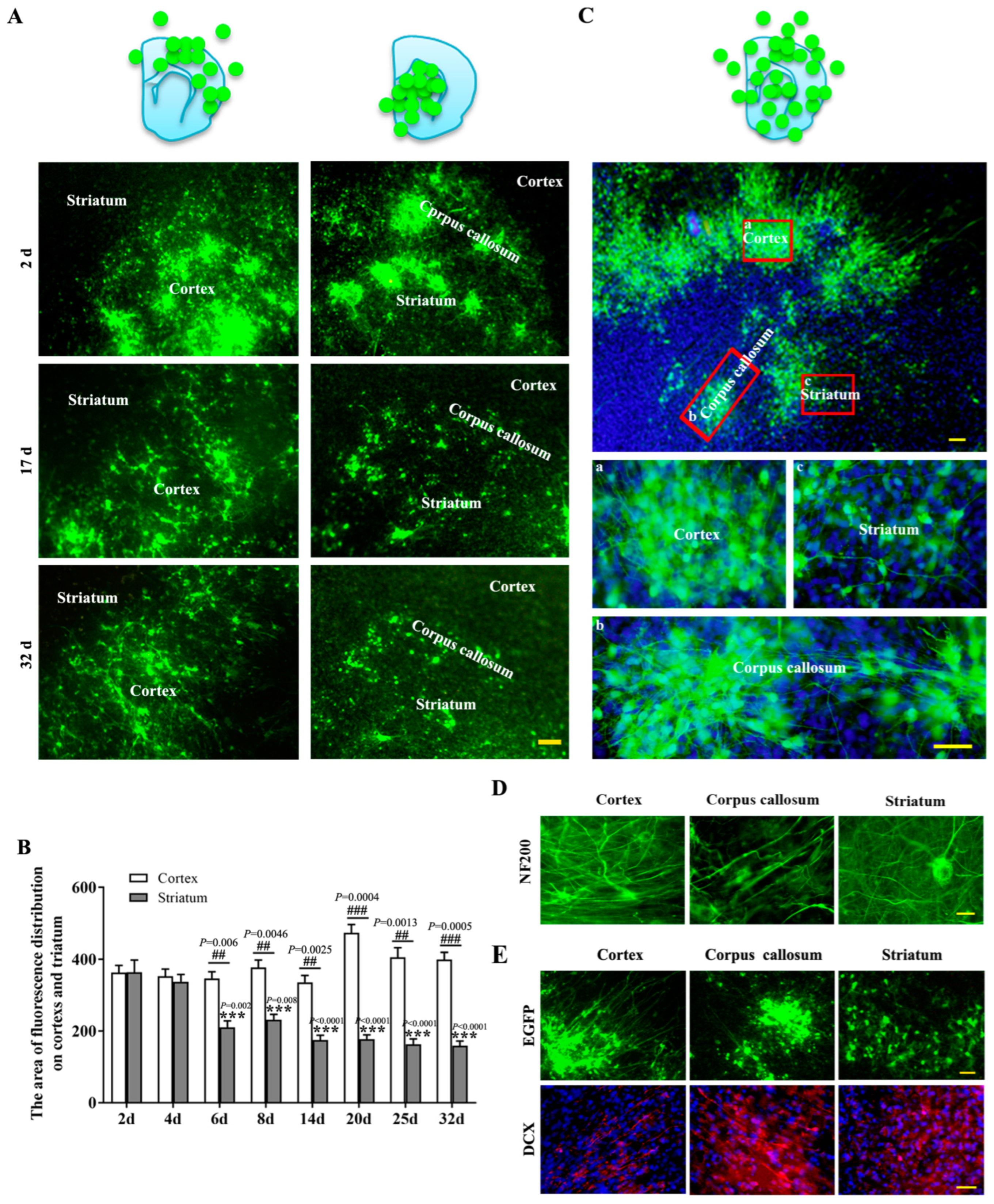
2.1. The Biological Behaviors of NSCs-EGFP after Transplantation onto Brain Slices
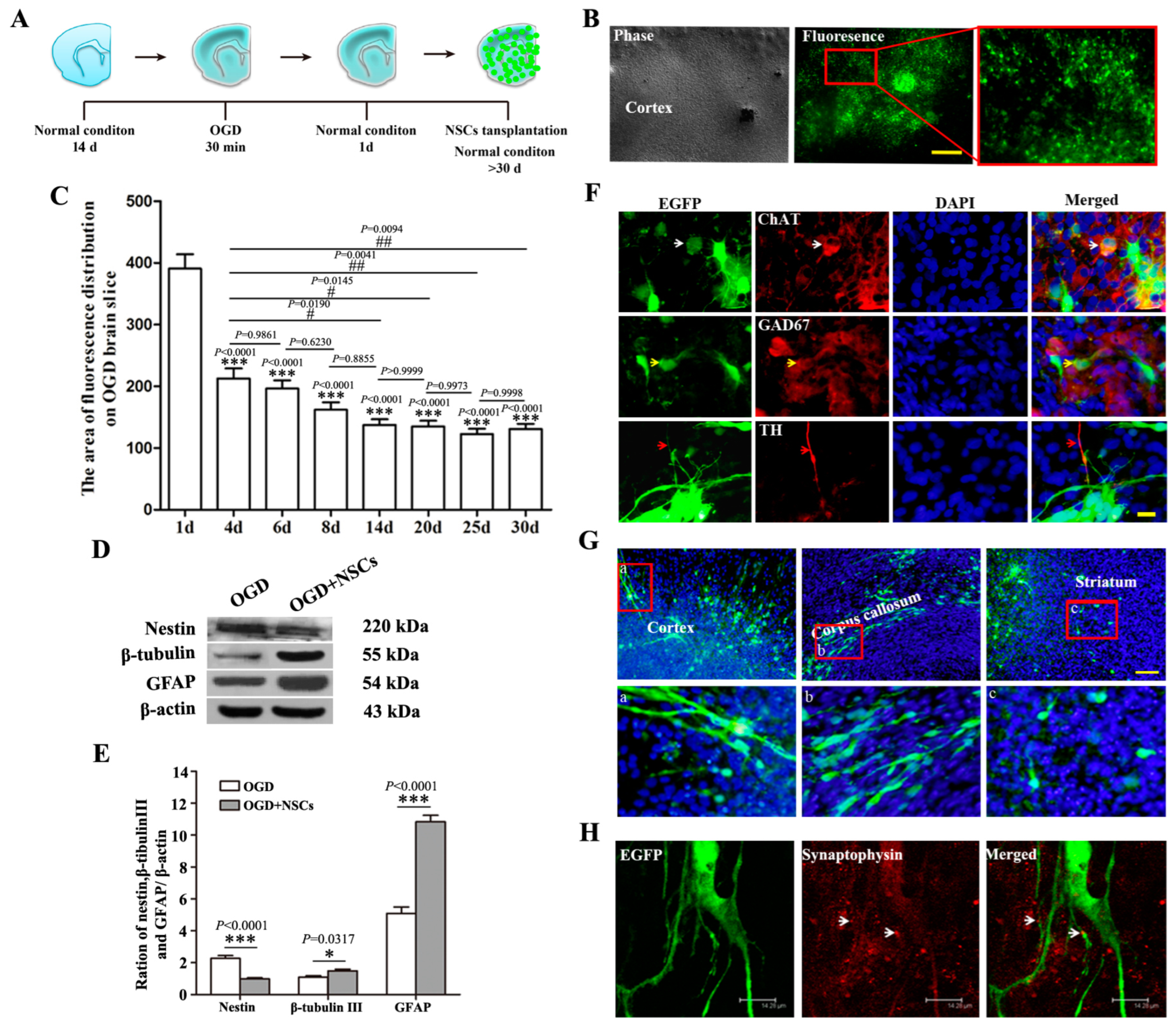
2.2. The Biological Behaviors of Transplanted NSCs on OGD-Damaged Brain Slices
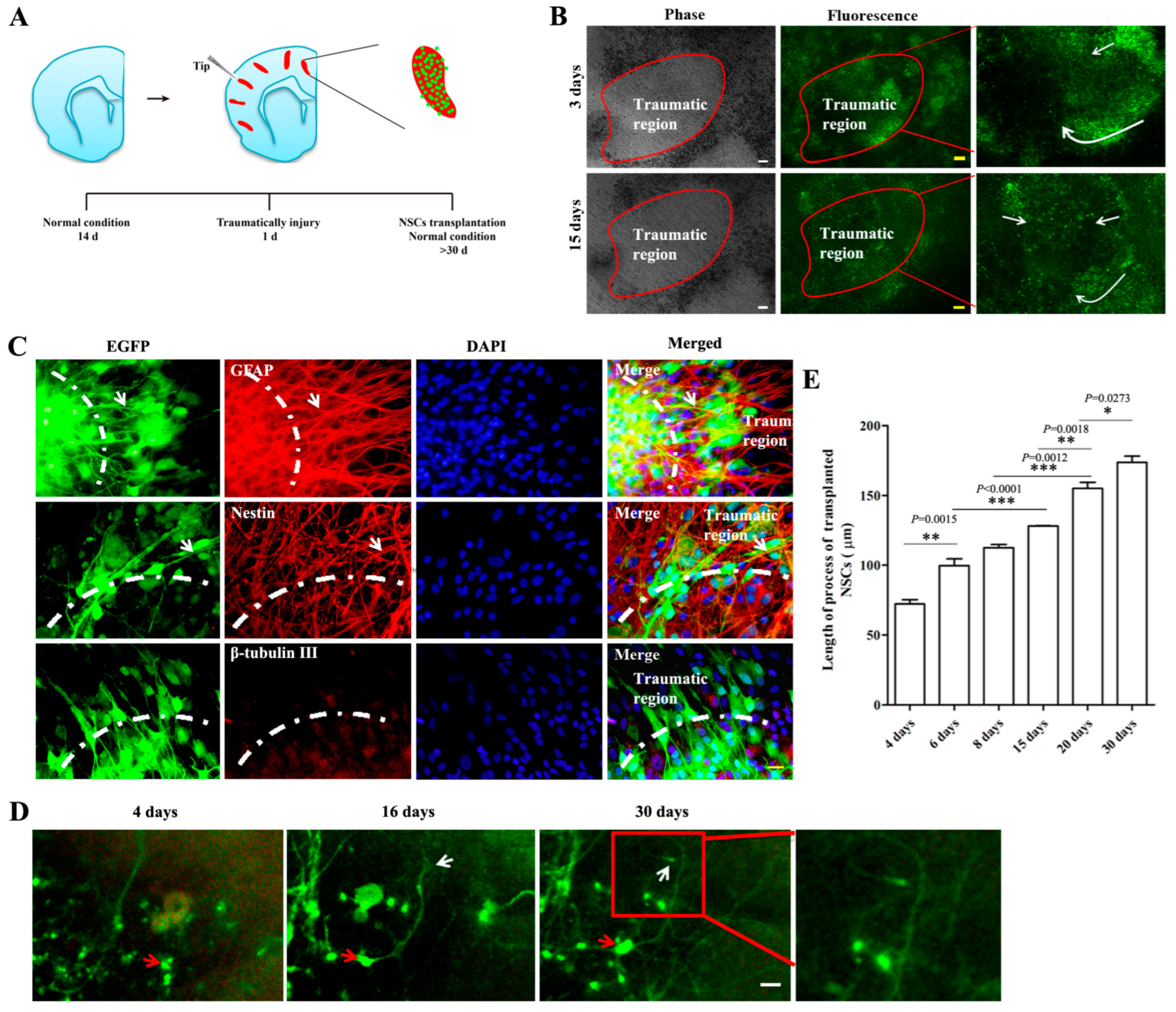
2.3. The Biological Behaviors of Transplanted NSCs on Mechanically Injured Organotypic Brain Slices
3. Discussion
4. Material and Methods
4.1. Prepare of NSCs
4.2. Culture of Organotypic Brain Slice
4.3. Brain Injury Models
4.4. NSCs Transplantation
4.5. Immunostaining
4.6. Western Blot Analysis
4.7. Quantification and Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Dai, J.; Li, S.Q.; Qiu, Y.M.; Xiong, W.H.; Yin, Y.H.; Jia, F.; Jiang, J.Y. Migration of neural stem cells to ischemic brain regions in ischemic stroke in rats. Neurosci. Lett. 2013, 552, 124–128. [Google Scholar] [CrossRef]
- Jensen, M.B.; Yan, H.; Krishnaney-Davison, R.; Al Sawaf, A.; Zhang, S.C. Survival and differentiation of transplanted neural stem cells derived from human induced pluripotent stem cells in a rat stroke model. J. Stroke Cerebrovasc. Dis. 2013, 22, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Darsalia, V.; Allison, S.J.; Cusulin, C.; Monni, E.; Kuzdas, D.; Kallur, T.; Lindvall, O.; Kokaia, Z. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J. Cereb. Blood Flow Metab. 2011, 31, 235–242. [Google Scholar] [CrossRef]
- Andres, R.H.; Horie, N.; Slikker, W.; Keren-Gill, H.; Zhan, K.; Sun, G.; Manley, N.C.; Pereira, M.P.; Sheikh, L.A.; McMillan, E.L.; et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 2011, 134 Pt 6, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, K.S.; Kim, E.J.; Choi, H.B.; Lee, K.H.; Park, I.H.; Ko, Y.; Jeong, S.W.; Kim, S.U. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells 2007, 25, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.K.; Bonaguidi, M.A.; Ming, G.L.; Song, H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009, 19, 672–682. [Google Scholar] [CrossRef]
- Decimo, I.; Bifari, F.; Krampera, M.; Fumagalli, G. Neural stem cell niches in health and diseases. Curr. Pharm. Des. 2012, 18, 1755–1783. [Google Scholar] [CrossRef] [PubMed]
- Faigle, R.; Song, H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta 2013, 1830, 2435–2448. [Google Scholar] [CrossRef] [PubMed]
- Kjell, J.; Fischer-Sternjak, J.; Thompson, A.J.; Friess, C.; Sticco, M.J.; Salinas, F.; Cox, J.; Martinelli, D.C.; Ninkovic, J.; Franze, K.; et al. Defining the Adult Neural Stem Cell Niche Proteome Identifies Key Regulators of Adult Neurogenesis. Cell Stem Cell 2020, 26, 277–293.e8. [Google Scholar] [CrossRef]
- Miller, F.D.; Gauthier-Fisher, A. Home at last: Neural stem cell niches defined. Cell Stem Cell 2009, 4, 507–510. [Google Scholar] [CrossRef]
- Fuentealba, L.C.; Obernier, K.; Alvarez-Buylla, A. Adult neural stem cells bridge their niche. Cell Stem Cell 2012, 10, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Tao, Y.; Gao, Q.; Feng, B.; Yan, W.; Zhou, Y.; Kotsonis, T.A.; Yuan, T.; You, Z.; Wu, Z.; et al. Human Stem Cell-Derived Neurons Repair Circuits and Restore Neural Function. Cell Stem Cell 2021, 28, 112–126.e6. [Google Scholar] [CrossRef] [PubMed]
- Kahroba, H.; Ramezani, B.; Maadi, H.; Sadeghi, M.R.; Jaberie, H.; Ramezani, F. The role of Nrf2 in neural stem/progenitors cells: From maintaining stemness and self-renewal to promoting differentiation capability and facilitating therapeutic application in neurodegenerative disease. Ageing Res. Rev. 2021, 65, 101211. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Yoon, S.J.; Tran, S.S.; Makinson, C.D.; Park, J.Y.; Andersen, J.; Valencia, A.M.; Horvath, S.; Xiao, X.; Huguenard, J.R.; et al. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat. Neurosci. 2021, 24, 331–342. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, Y.H.; Hebisch, M.; Sliwinski, C.; Lee, S.; D’Avanzo, C.; Chen, H.; Hooli, B.; Asselin, C.; Muffat, J.; et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 2014, 515, 274–278. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Long, X.; Xu, T. Three-dimensional-engineered bioprinted in vitro human neural stem cell self-assembling culture model constructs of Alzheimer’s disease. Bioact. Mater. 2022, 11, 192–205. [Google Scholar] [CrossRef]
- Park, K.I.; Teng, Y.D.; Snyder, E.Y. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat. Biotechnol. 2002, 20, 1111–1117. [Google Scholar] [CrossRef]
- Peruzzotti-Jametti, L.; Bernstock, J.D.; Willis, C.M.; Manferrari, G.; Rogall, R.; Fernandez-Vizarra, E.; Williamson, J.C.; Braga, A.; van den Bosch, A.; Leonardi, T.; et al. Neural stem cells traffic functional mitochondria via extracellular vesicles. PLoS Biol. 2021, 19, e3001166. [Google Scholar] [CrossRef]
- Gahwiler, B.H.; Capogna, M.; Debanne, D.; McKinney, R.A.; Thompson, S.M. Organotypic slice cultures: A technique has come of age. Trends Neurosci. 1997, 20, 471–477. [Google Scholar] [CrossRef]
- Pena, F. Organotypic cultures as tool to test long-term effects of chemicals on the nervous system. Curr. Med. Chem. 2010, 17, 987–1001. [Google Scholar] [CrossRef]
- Del Turco, D.; Deller, T. Organotypic entorhino-hippocampal slice cultures—A tool to study the molecular and cellular regulation of axonal regeneration and collateral sprouting in vitro. Methods Mol. Biol. 2007, 399, 55–66. [Google Scholar] [PubMed]
- Sundstrom, L.; Morrison, B., 3rd; Bradley, M.; Pringle, A. Organotypic cultures as tools for functional screening in the CNS. Drug Discov. Today 2005, 10, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.J.; Liedmann, A.; Hubner, R.; Hovakimyan, M.; Rolfs, A.; Frech, M.J. Human neural progenitor cells show functional neuronal differentiation and regional preference after engraftment onto hippocampal slice cultures. Stem Cells Dev. 2012, 21, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Jaderstad, L.M.; Jaderstad, J.; Herlenius, E. Graft and host interactions following transplantation of neural stem cells to organotypic striatal cultures. Regen. Med. 2010, 5, 901–917. [Google Scholar] [CrossRef]
- Hofer, S.; Magloire, V.; Streit, J.; Leib, S.L. Grafted neuronal precursor cells differentiate and integrate in injured hippocampus in experimental pneumococcal meningitis. Stem Cells 2012, 30, 1206–1215. [Google Scholar] [CrossRef]
- Novozhilova, E.; Olivius, P.; Siratirakun, P.; Lundberg, C.; Englund-Johansson, U. Neuronal differentiation and extensive migration of human neural precursor cells following co-culture with rat auditory brainstem slices. PLoS ONE 2013, 8, e57301. [Google Scholar] [CrossRef]
- Scheffler, B.; Schmandt, T.; Schroder, W.; Steinfarz, B.; Husseini, L.; Wellmer, J.; Seifert, G.; Karram, K.; Beck, H.; Blumcke, I.; et al. Functional network integration of embryonic stem cell-derived astrocytes in hippocampal slice cultures. Development 2003, 130, 5533–5541. [Google Scholar] [CrossRef]
- Lagos-Cabre, R.; Burgos-Bravo, F.; Avalos, A.M.; Leyton, L. Connexins in Astrocyte Migration. Front. Pharmacol. 2019, 10, 1546. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Chai, X.; Forster, E.; Zhao, S.; Bock, H.H.; Frotscher, M. Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3. J. Neurosci. 2009, 29, 288–299. [Google Scholar] [CrossRef]
- Iafrati, J.; Orejarena, M.J.; Lassalle, O.; Bouamrane, L.; Chavis, P. Reelin, an extracellular matrix protein linked to early onset psychiatric diseases, drives postnatal development of the prefrontal cortex via GluN2B-NMDARs and the mTOR pathway. Mol. Psychiatry 2013, 19, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Forster, E.; Jossin, Y.; Zhao, S.; Chai, X.; Frotscher, M.; Goffinet, A.M. Recent progress in understanding the role of Reelin in radial neuronal migration, with specific emphasis on the dentate gyrus. Eur. J. Neurosci. 2006, 23, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Dere, E.; Zlomuzica, A. The role of gap junctions in the brain in health and disease. Neurosci. Biobehav. Rev. 2012, 36, 206–217. [Google Scholar] [CrossRef]
- Jin, Y.; Sura, K.; Fischer, I. Differential effects of distinct central nervous system regions on cell migration and axonal extension of neural precursor transplants. J. Neurosci. Res. 2012, 90, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Oishi, K.; Tabata, H.; Torii, K.; Nakajima, K. Segregation and pathfinding of callosal axons through EphA3 signaling. J. Neurosci. 2011, 31, 16251–16260. [Google Scholar] [CrossRef]
- Mendes, S.W.; Henkemeyer, M.; Liebl, D.J. Multiple Eph receptors and B-class ephrins regulate midline crossing of corpus callosum fibers in the developing mouse forebrain. J. Neurosci. 2006, 26, 882–892. [Google Scholar] [CrossRef]
- Di Pietro, V.; Amin, D.; Pernagallo, S.; Lazzarino, G.; Tavazzi, B.; Vagnozzi, R.; Pringle, A.; Belli, A. Transcriptomics of traumatic brain injury: Gene expression and molecular pathways of different grades of insult in a rat organotypic hippocampal culture model. J. Neurotrauma 2010, 27, 349–359. [Google Scholar] [CrossRef]
- Yu, Z.; Elkin, B.S.; Morrison, B. Quantification of functional alterations after in vitro traumatic brain injury. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 2009, 1135–1138. [Google Scholar]
- Liu, X.R.; Luo, M.; Yan, F.; Zhang, C.C.; Li, S.J.; Zhao, H.P.; Ji, X.M.; Luo, Y.M. Ischemic postconditioning diminishes matrix metalloproteinase 9 expression and attenuates loss of the extracellular matrix proteins in rats following middle cerebral artery occlusion and reperfusion. CNS Neurosci. Ther. 2012, 18, 855–863. [Google Scholar] [CrossRef]
- Jeter, C.B.; Hergenroeder, G.W.; Ward, N.H., 3rd; Moore, A.N.; Dash, P.K. Human traumatic brain injury alters circulating L-arginine and its metabolite levels: Possible link to cerebral blood flow, extracellular matrix remodeling, and energy status. J. Neurotrauma 2012, 29, 119–127. [Google Scholar] [CrossRef]
- Kwok, J.C.; Dick, G.; Wang, D.; Fawcett, J.W. Extracellular matrix and perineuronal nets in CNS repair. Dev. Neurobiol. 2011, 71, 1073–1089. [Google Scholar] [CrossRef]
- Zechariah, A.; ElAli, A.; Hermann, D.M. Combination of tissue-plasminogen activator with erythropoietin induces blood-brain barrier permeability, extracellular matrix disaggregation, and DNA fragmentation after focal cerebral ischemia in mice. Stroke 2010, 41, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Frotscher, M. Role for Reelin in stabilizing cortical architecture. Trends Neurosci. 2010, 33, 407–414. [Google Scholar] [CrossRef]
- Powell, E.M.; Geller, H.M. Dissection of astrocyte-mediated cues in neuronal guidance and process extension. Glia 1999, 26, 73–83. [Google Scholar] [CrossRef]
- Liu, G.; Dwyer, T. Microtubule dynamics in axon guidance. Neurosci. Bull. 2014, 30, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sterne, G.R.; Ye, B. Regulatory mechanisms underlying the differential growth of dendrites and axons. Neurosci. Bull. 2014, 30, 557–568. [Google Scholar] [CrossRef]
- Wiese, S.; Karus, M.; Faissner, A. Astrocytes as a source for extracellular matrix molecules and cytokines. Front. Pharmacol. 2012, 3, 120. [Google Scholar] [CrossRef]
- Diaz-Coranguez, M.; Segovia, J.; Lopez-Ornelas, A.; Puerta-Guardo, H.; Ludert, J.; Chavez, B.; Meraz-Cruz, N.; Gonzalez-Mariscal, L. Transmigration of neural stem cells across the blood brain barrier induced by glioma cells. PLoS ONE 2013, 8, e60655. [Google Scholar] [CrossRef]
- Chen, S.Y.; Cheng, H.J. Functions of axon guidance molecules in synapse formation. Curr. Opin. Neurobiol. 2009, 19, 471–478. [Google Scholar] [CrossRef]
- Gage, F.H.; Coates, P.W.; Palmer, T.D.; Kuhn, H.G.; Fisher, L.J.; Suhonen, J.O.; Peterson, D.A.; Suhr, S.T.; Ray, J. Survival and differentiation of adult neuronal progenitor cells transsplanted to the adult brain. Proc. Natl. Acad. Sci. USA 1995, 92, 11879–11883. [Google Scholar] [CrossRef]
- Jiao, Q.; Li, X.; An, J.; Zhang, Z.; Chen, X.; Tan, J.; Zhang, P.; Lu, H.; Liu, Y. Cell-Cell Connection Enhances Proliferation and Neuronal Differentiation of Rat Embryonic Neural Stem/Progenitor Cells. Front. Cell Neurosci. 2017, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Zhang, H.X.; Lv, H.X.; Liu, Y.; Li, J.L. Organotypic slice culture of neonatal rat cortex and induced neural stem cell differentiation. Nan Fang Yi Ke Da Xue Xue Bao 2011, 31, 1318–1322. [Google Scholar] [PubMed]
- Gong, B.; Jiao, L.; Du, X.; Li, Y.; Bi, M.; Jiao, Q.; Jiang, H. Ghrelin promotes midbrain neural stem cells differentiation to dopaminergic neurons through Wnt/beta-catenin pathway. J. Cell Physiol. 2020, 235, 8558–8570. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Q.; Wang, L.; Zhang, Z.; Chen, X.; Lu, H.; Liu, Y. The Biological Behaviors of Neural Stem Cell Affected by Microenvironment from Host Organotypic Brain Slices under Different Conditions. Int. J. Mol. Sci. 2023, 24, 4182. https://doi.org/10.3390/ijms24044182
Jiao Q, Wang L, Zhang Z, Chen X, Lu H, Liu Y. The Biological Behaviors of Neural Stem Cell Affected by Microenvironment from Host Organotypic Brain Slices under Different Conditions. International Journal of Molecular Sciences. 2023; 24(4):4182. https://doi.org/10.3390/ijms24044182
Chicago/Turabian StyleJiao, Qian, Li Wang, Zhichao Zhang, Xinlin Chen, Haixia Lu, and Yong Liu. 2023. "The Biological Behaviors of Neural Stem Cell Affected by Microenvironment from Host Organotypic Brain Slices under Different Conditions" International Journal of Molecular Sciences 24, no. 4: 4182. https://doi.org/10.3390/ijms24044182
APA StyleJiao, Q., Wang, L., Zhang, Z., Chen, X., Lu, H., & Liu, Y. (2023). The Biological Behaviors of Neural Stem Cell Affected by Microenvironment from Host Organotypic Brain Slices under Different Conditions. International Journal of Molecular Sciences, 24(4), 4182. https://doi.org/10.3390/ijms24044182






