IgE and IgG4 Epitopes of Dermatophagoides and Blomia Allergens before and after Sublingual Immunotherapy
Abstract
1. Introduction
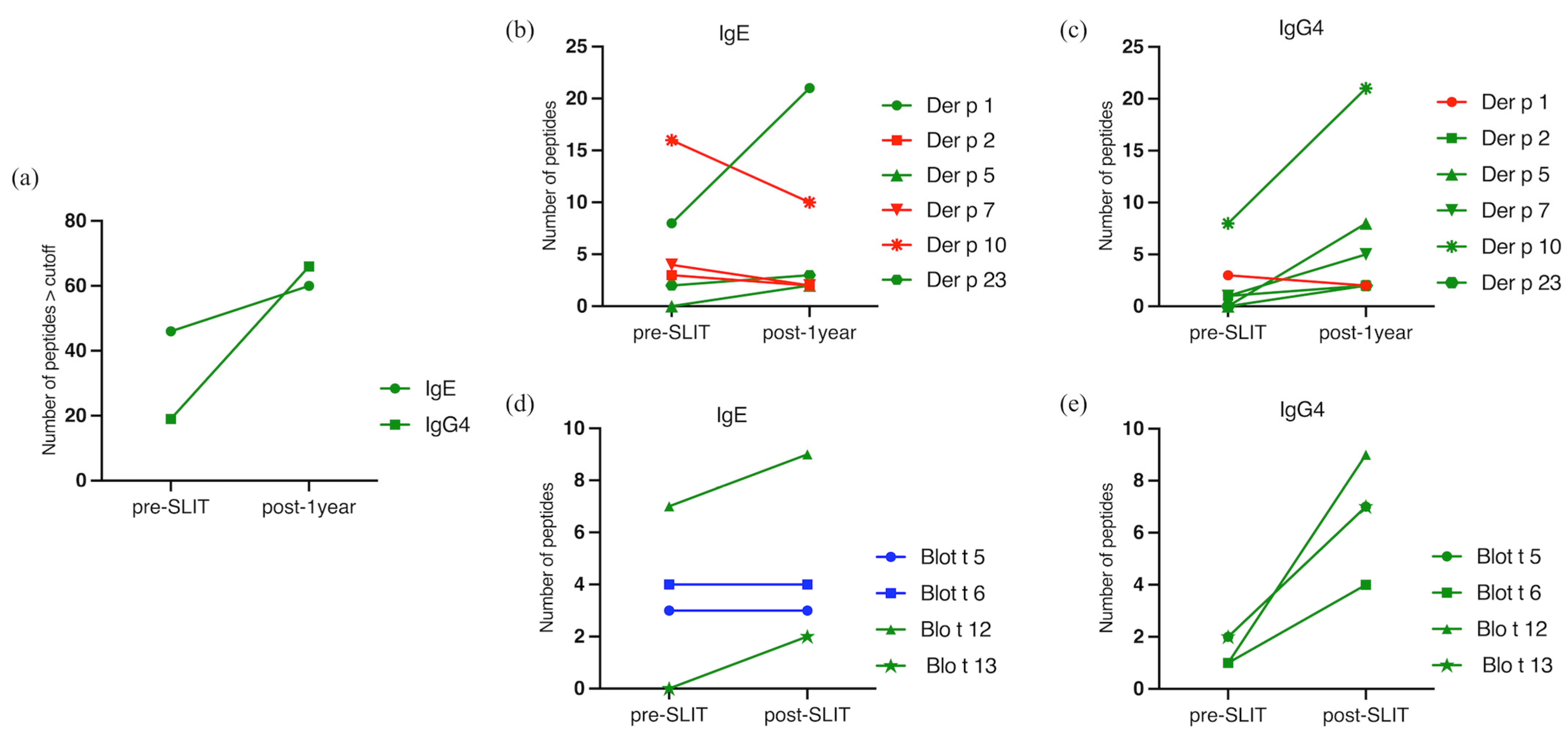
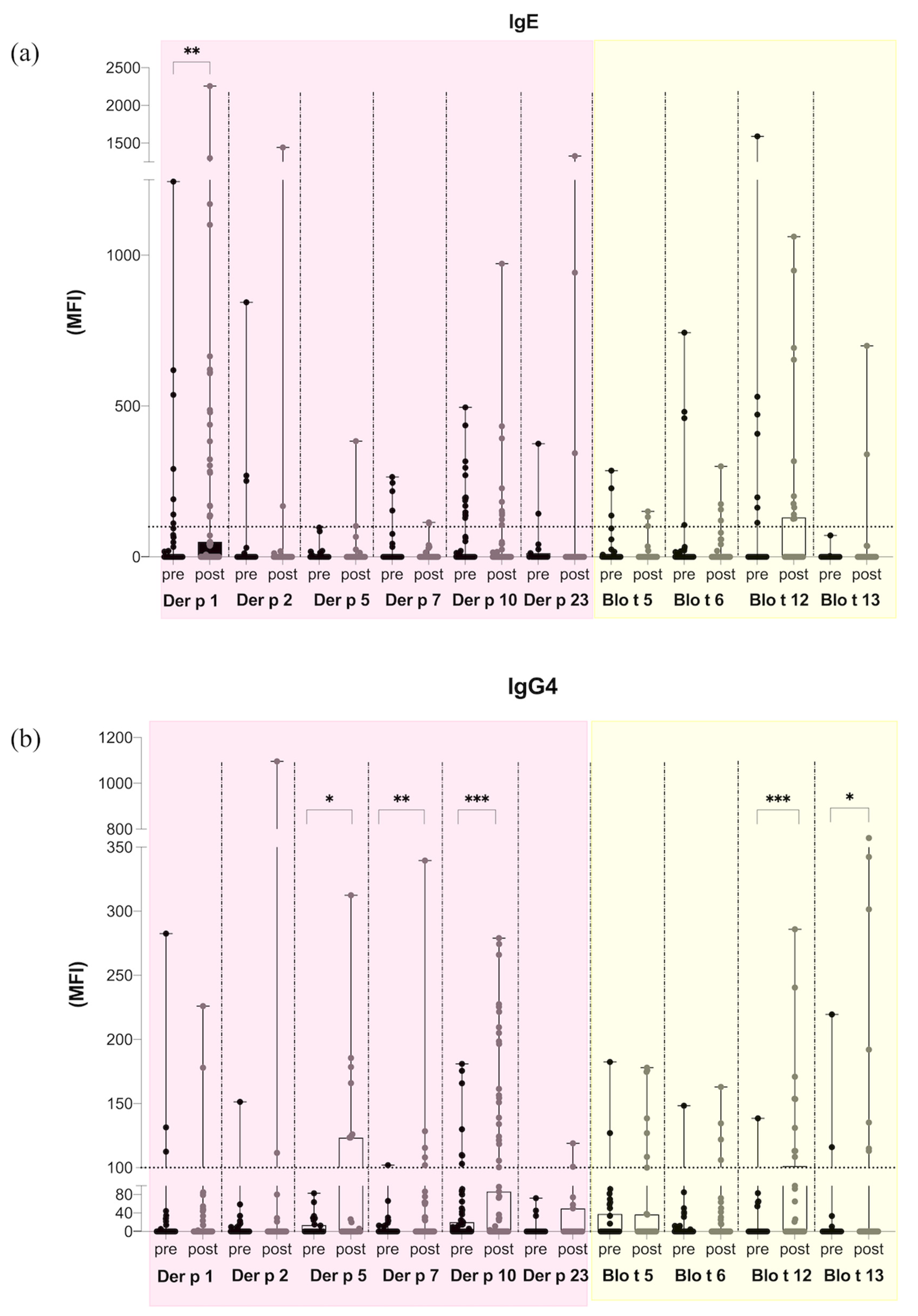
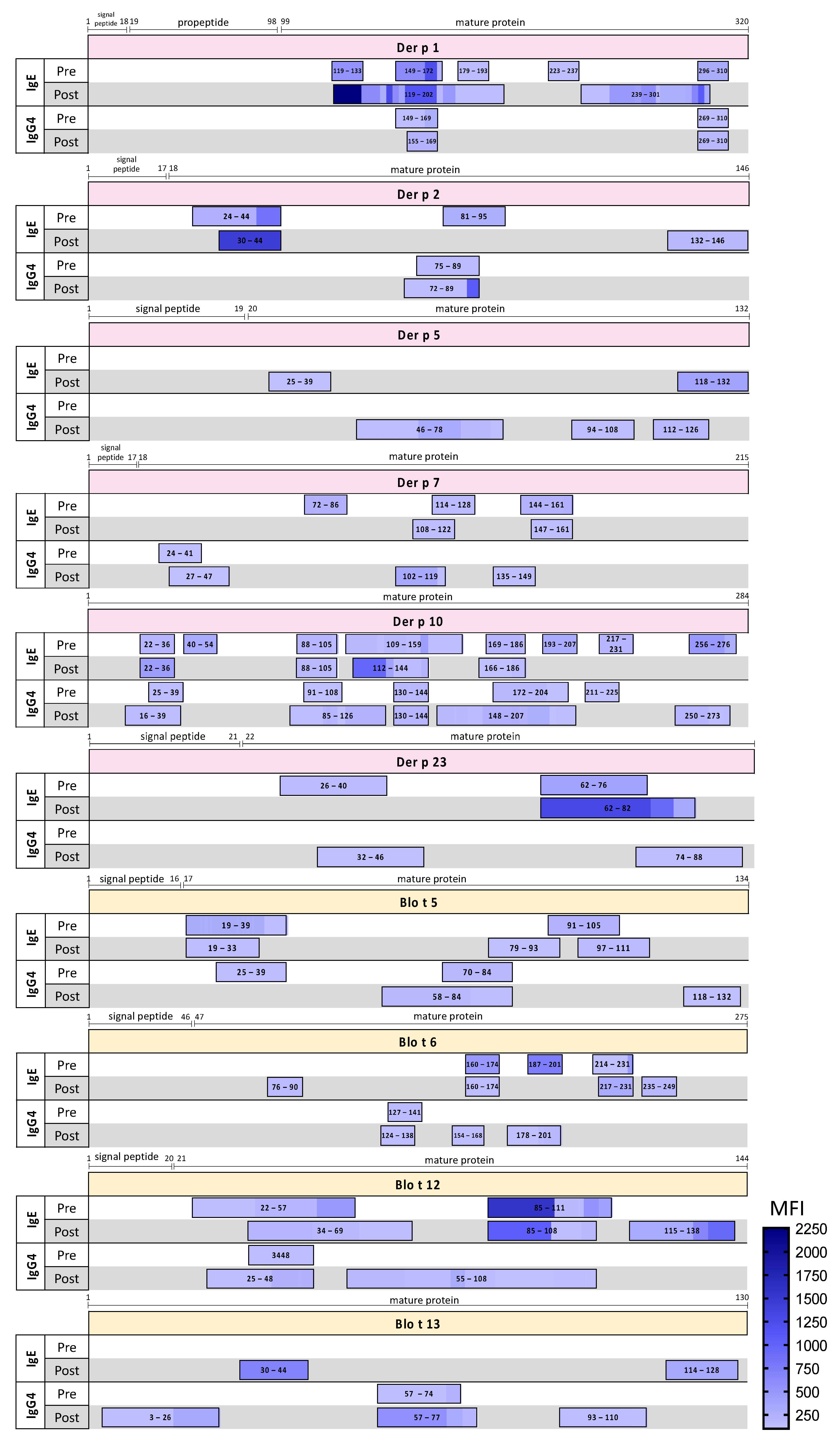
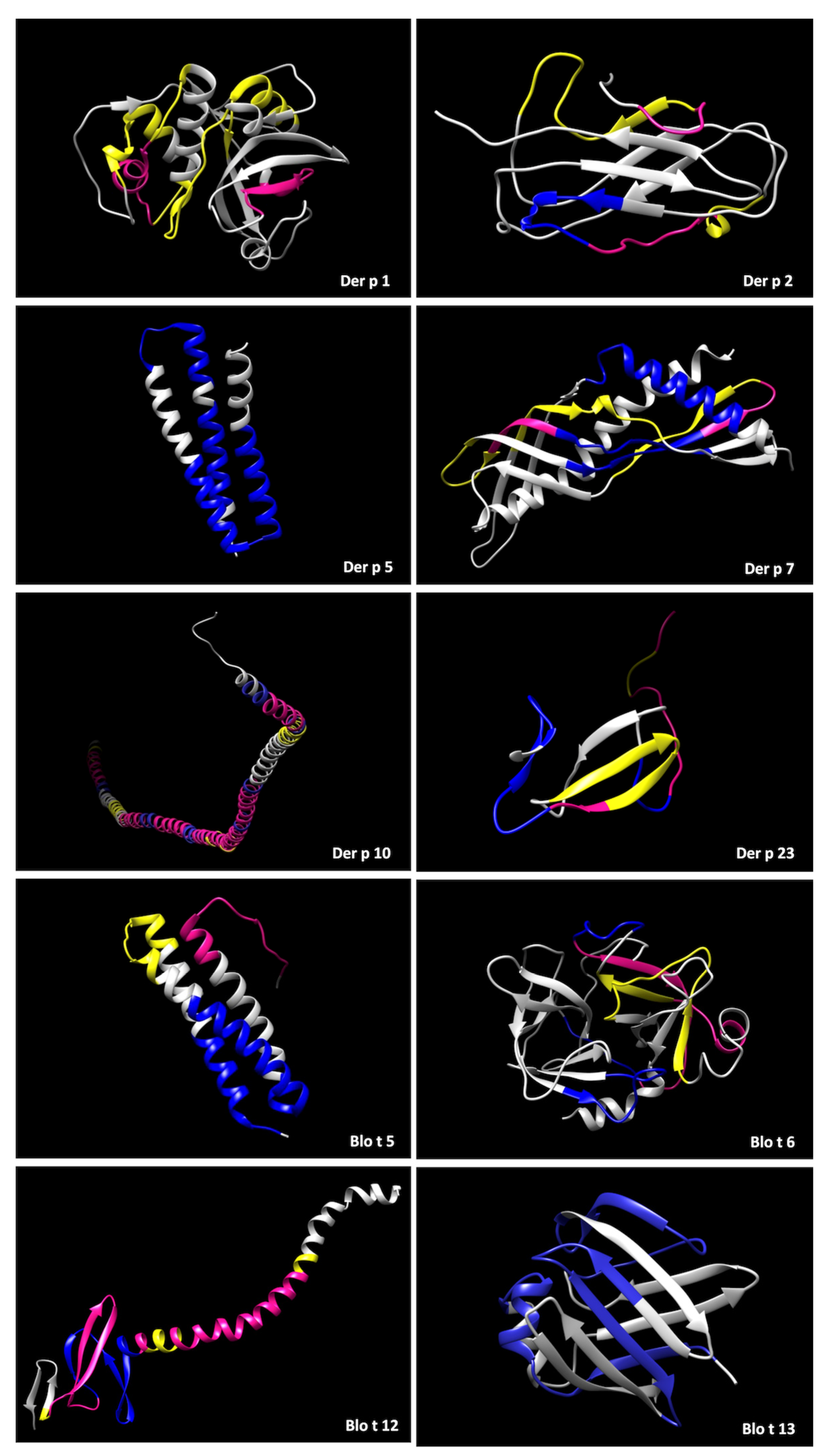
2. Results
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Epitope Microarray
4.3. Statistical Analysis
4.4. Protein Modelling
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jutel, M.; Agache, I.; Bonini, S.; Burks, A.W.; Calderon, M.; Canonica, W.; Cox, L.; Demoly, P.; Frew, A.J.; O’Hehir, R.; et al. International consensus on allergy immunotherapy. J. Allergy Clin. Immunol. 2015, 136, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Pajno, G.B.; Barberio, G.; De Luca, F.; Morabito, L.; Parmiani, S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin. Exp. Allergy 2001, 31, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, L.; Niggemann, B.; Dreborg, S.; Ferdousi, H.A.; Halken, S.; Høst, A.; Koivikko, A.; Norberg, L.A.; Valovirta, E.; The PAT investigator group; et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy 2007, 62, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Akdis, M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ. J. 2015, 8, 17. [Google Scholar] [CrossRef]
- Elenburg, S.; Blaiss, M.S. Current status of sublingual immunotherapy in the United States. World Allergy Organ. J. 2014, 7, 24. [Google Scholar] [CrossRef]
- Marogna, M.; Spadolini, I.; Massolo, A.; Canonica, G.W.; Passalacqua, G. Long-lasting effects of sublingual immunotherapy according to its duration: A 15-year prospective study. J. Allergy Clin. Immunol. 2010, 126, 969–975. [Google Scholar] [CrossRef]
- Hoffmann-Sommergruber, K.; Santos, F.A.; Breiteneder, H. The Molecular Allergology User’s Guide version 2.0. Allergy 2023. [CrossRef]
- Carnés, J.; Iraola, V.; Cho, S.H.; Esch, R.E. Mite allergen extracts and clinical practice. Ann. Allergy Asthma Immunol. 2017, 118, 249–256. [Google Scholar] [CrossRef]
- da Silva, E.S.; Asam, C.; Lackner, P.; Hofer, H.; Wallner, M.; Pinheiro, C.S.; Alcântara-Neves, N.M.; Ferreira, F. Allergens of Blomia tropicalis: An Overview of Recombinant Molecules. Int. Arch. Allergy Immunol. 2017, 172, 203–214. [Google Scholar] [CrossRef]
- Santos, A.B.R.; Chapman, M.D.; Aalberse, R.C.; Vailes, L.D.; Ferriani, V.P.; Oliver, C.; Rizzo, M.; Naspitz, C.K.; Arruda, L. Cockroach allergens and asthma in Brazil: Identification of tropomyosin as a major allergen with potential cross-reactivity with mite and shrimp allergens. J. Allergy Clin. Immunol. 1999, 104, 329–337. [Google Scholar] [CrossRef]
- Daul, C.B.; Slattery, M.; Reese, G.; Lehrer, S.B. Identification of the major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosin. Int. Arch. Allergy Immunol. 1994, 105, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, L.; Viala-Gastan, C. Blomia tropicalis: A house dust mite in the tropics. Rev. Mal. Respir. 2017, 34, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, Y.; Xu, Q.; Zhang, W.; Jiang, Q.; Li, W.; Wang, Y.; Ma, D.; Lin, X.; Sun, B.; et al. Specific IgE and IgG4 Profiles of House Dust Mite Components in Allergen-Specific Immunotherapy. Front. Immunol. 2022, 12, 786738. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; You, Y.; Zhou, Y.; Wang, Y.; Kong, W. House Dust Mite Major Allergens Contributes Significantly to Specific IgG4 Response during Allergen Immunotherapy. J. Allergy Clin. Immunol. 2016, 137, AB401. [Google Scholar] [CrossRef]
- Pomés, A.; Mueller, G.A.; Chruszcz, M. Structural Aspects of the Allergen-Antibody Interaction. Front. Immunol. 2020, 2, 2067. [Google Scholar] [CrossRef]
- Lahiani, S.; Dumez, M.-E.; Bouaziz, A.; Djenouhat, K.; Khemili, S.; Bitam, I.; Gilis, D.; Galleni, M. Immunodominant IgE Epitopes of Der p 5 Allergen. Protein. Pept. Lett. 2018, 25, 1024–1034. [Google Scholar] [CrossRef]
- Curin, M.; Huang, H.-J.; Garmatiuk, T.; Gutfreund, S.; Resch-Marat, Y.; Chen, K.-W.; Fauland, K.; Keller, W.; Zieglmayer, P.; Zieglmayer, R.; et al. IgE Epitopes of the House Dust Mite Allergen Der p 7 Are Mainly Discontinuous and Conformational. Front. Immunol. 2021, 12, 687294. [Google Scholar] [CrossRef]
- Mueller, G.A.; Glesner, J.; Daniel, J.L.; Zhang, J.; Hyduke, N.; Richardson, C.M.; Derose, E.F.; Chapman, M.D.; Peebles, R.S., Jr.; Smith, S.A.; et al. Mapping Human Monoclonal IgE Epitopes on the Major Dust Mite Allergen Der p 2. J. Immunol. 2020, 205, 1999–2007. [Google Scholar] [CrossRef]
- van’t Hof, W.; Driedijk, P.C.; van den Berg, M.; Beck-Sickinger, A.G.; Jung, G.; Aalberse, R.C. Epitope mapping of the Dermatophagoides pteronyssinus house dust mite major allergen Der p II using overlapping synthetic peptides. Mol. Immunol. 1991, 28, 1225–1232. [Google Scholar] [CrossRef]
- Li, W.; Cai, Z.; Zhang, B.; Chen, J.; Ji, K. Identification of an immunodominant IgE epitope of Der p 39, a novel allergen of Dermatophagoides pteronyssinus. World Allergy Organ. J. 2022, 15, 100651. [Google Scholar] [CrossRef]
- Ramos, J.D.A.; Cheong, N.; Lee, B.W.; Chua, K.Y. Peptide mapping of immunoglobulin E and immunoglobulin G immunodominant epitopes of an allergenic Blomia tropicalis paramyosin, Blo t 11. Clin. Exp. Allergy 2003, 33, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Lahiani, S.; Dumez, M.; Khemili, S.; Bitam, I.; Gilis, D.; Galleni, M. Cross-Reactivity between Major IgE Epitopes of Family 5 Allergens from Dermatophagoides pteronyssinus and Blomia tropicalis. Int. Arch. Allergy Immunol. 2019, 178, 10–18. [Google Scholar] [CrossRef]
- Curin, M.; Garmatiuk, T.; Resch-Marat, Y.; Chen, K.W.; Hofer, G.; Fauland, K.; Keller, W.; Hemmer, W.; Vrtala, S.; Focke-Tejkl, M.; et al. Similar localization of conformational IgE epitopes on the house dust mite allergens Der p 5 and Der p 21 despite limited IgE cross-reactivity. Allergy 2018, 73, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Múnera, M.; Martínez, D.; Labrada, A.; Caraballo, L.; Puerta, L. Identification of B Cell Epitopes of Blo t 13 Allergen and Cross-Reactivity with Human Adipocytes and Heart Fatty Acid Binding Proteins. Int. J. Mol. Sci. 2019, 20, 6107. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.C.; Cheong, N.; Shek, L.P.; Wang, D.Y.; Chua, K.Y.; Lee, B.W. Identification of shared and unique immunoglobulin E epitopes of the highly conserved tropomyosins in Blomia tropicalis and Dermatophagoides pteronyssinus. Clin. Exp. Allergy 2002, 32, 1203–1210. [Google Scholar] [CrossRef]
- Kobayashi, I.; Sakiyama, Y.; Tame, A.; Kobayashi, K.; Matsumoto, S. IgE and IgG4 antibodies from patients with mite allergy recognize different epitopes of Dermatophagoides pteronyssinus group II antigen (Der p 2). J. Allergy Clin. Immunol. 1996, 2, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Savilahti, E.M.; Kuitunen, M.; Valori, M.; Rantanen, V.; Bardina, L.; Gimenez, G.; Mäkelä, M.J.; Hautaniemi, S.; Savilahti, E.; Sampson, H.A. Use of IgE and IgG4 epitope binding to predict the outcome of oral immunotherapy in cow’s milk allergy. Pediatr. Allergy Immunol. 2014, 25, 227–235. [Google Scholar] [CrossRef]
- Vickery, B.P.; Lin, J.; Kulis, M.; Fu, Z.; Steele, P.H.; Jones, S.M.; Scurlock, A.M.; Gimenez, G.; Bardina, L.; Sampson, H.A.; et al. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J. Allergy Clin. Immunol. 2013, 131, 128–134. [Google Scholar] [CrossRef]
- Niederberger, V.; Neubauer, A.; Gevaert, P.; Zidarn, M.; Worm, M.; Aberer, W.; Malling, H.J.; Pfaar, O.; Klimek, L.; Pfützner, W.; et al. Safety and efficacy of immunotherapy with the recombinant B-cell epitope-based grass pollen vaccine BM32. J. Allergy Clin. Immunol. 2018, 142, 497–509. [Google Scholar] [CrossRef]
- Rauber, M.M.; Möbs, C.; Campana, R.; Henning, R.; Schulze-Dasbeck, M.; Greene, B.; Focke-Tejkl, M.; Weber, M.; Valenta, R.; Pfützner, W. Allergen immunotherapy with the hypoallergenic B-cell epitope-based vaccine BM32 modifies IL-10- and IL-5-secreting T cells. Allergy 2020, 75, 450–453. [Google Scholar] [CrossRef]
- Holm, J.; Willumsen, N.; Würtzen, P.A.; Christensen, L.H.; Lund, K. Facilitated antigen presentation and its inhibition by blocking IgG antibodies depends on IgE repertoire complexity. J. Allergy Clin. Immunol. 2011, 127, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Focke, M.; Swoboda, I.; Marth, K.; Valenta, R. Developments in allergen-specific immunotherapy: From allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin. Exp. Allergy 2010, 40, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Chinthrajah, R.S.; Purington, N.; Andorf, S.; Long, A.; O’Laughlin, K.L.; Lyu, S.C.; Manohar, M.; Boyd, S.D.; Tibshirani, R.; Maecker, H.; et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): A large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet 2019, 394, 10207. [Google Scholar] [CrossRef] [PubMed]
- Yukselen, A.; Kendirli, S.G.; Yilmaz, M.; Altintas, D.U.; Karakoc, G.B. Two year follow-up of clinical and inflammation parameters in children monosensitized to mites undergoing subcutaneous and sublingual immunotherapy. Asian Pac. J. Allergy Immunol. 2013, 31, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Greene, W.K.; Cyster, J.G.; Chua, K.Y.; O’Brien, R.M.; Thomas, W.R. IgE and IgG binding of peptides expressed from fragments of cDNA encoding the major house dust mite allergen Der p I. J. Immunol. 1991, 147, 3768–3773. [Google Scholar] [CrossRef]
- Nittner-Marszalska, M.; Kopeć, A.; Foks-Ciekalska, A.; Lata, A.; Bogacz-Piaseczyńska, A.; Rosiek-Biegus, M. Monitoring of molecular profiling of allergen-antibody responses in HDM-immunotherapy patients. Hum. Vaccin Immunother. 2022, 17, 2148815. [Google Scholar] [CrossRef]
- Cui, Y.; Teng, F.; Yu, L.; Zhou, Y.; Wang, N.; Zhang, C.; Yang, L. Sequential epitopes of Dermatophagoides farinae allergens identified using peptide microarray-based immunoassay. IUBMB Life 2016, 68, 792–798. [Google Scholar] [CrossRef]
- Huber, S.; Lang, R.; Steiner, M.; Aglas, L.; Ferreira, F.; Wallner, M.; Hawranek, T.; Gadermaier, G. Does clinical outcome of birch pollen immunotherapy relate to induction of blocking antibodies preventing IgE from allergen binding? A pilot study monitoring responses during first year of AIT. Clin. Transl. Allergy 2018, 8, 39. [Google Scholar] [CrossRef]
- Feng, M.; Zeng, X.; Su, Q.; Shi, X.; Xian, M.; Qin, R.; Li, J. Allergen Immunotherapy-Induced Immunoglobulin G4 Reduces Basophil Activation in House Dust Mite-Allergic Asthma Patients. Front. Cell Dev. Biol. 2020, 20, 30. [Google Scholar] [CrossRef]
- Resch, Y.; Weghofer, M.; Seiberler, S.; Horak, F.; Scheiblhofer, S.; Linhart, B.; Swoboda, I.; Thomas, W.R.; Thalhamer, J.; Valenta, R.; et al. Molecular characterization of Der p 10: A diagnostic marker for broad sensitization in house dust mite allergy. Clin. Exp. Allergy 2011, 41, 1468–1477. [Google Scholar] [CrossRef]
- Santos, A.B.R.; Rocha, G.M.; Oliver, C.; Ferriani, V.P.; Lima, R.C.; Palma, M.S.; Sales, V.S.; Aalberse, R.C.; Chapman, M.D.; Arruda, L.K. Cross-reactive IgE antibody responses to tropomyosins from Ascaris lumbricoides and cockroach. J. Allergy Clin. Immunol. 2008, 121, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Santos, A.C.A.; Moreno, A.S.; Barbosa, M.C.; Aragon, D.C.; Sales, V.S.; Arruda, L.K. Parasite Infections, Allergy and Asthma: A Role for Tropomyosin in Promoting Type 2 Immune Responses. Int. Arch. Allergy Immunol. 2020, 181, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Westritschnig, K.; Sibanda, E.; Thomas, W.; Auer, H.; Aspöck, H.; Pittner, G.; Vrtala, S.; Spitzauer, S.; Kraft, D.; Valenta, R. Analysis of the sensitization profile towards allergens in central Africa. Clin. Exp. Allergy 2003, 33, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.C.; Chua, K.Y.; Cheong, N.; Shek, L.P.; Lee, B.W. Immunoglobulin E reactivity of native Blo t 5, a major allergen of Blomia tropicalis. Clin. Exp. Allergy 2004, 34, 1762–1767. [Google Scholar] [CrossRef]
- Lombardero, M.; Heymann, P.W.; Platts-Mills, T.A.; Fox, J.W.; Chapman, M.D. Conformational stability of B cell epitopes on group I and group II Dermatophagoides spp. allergens. Effect of thermal and chemical denaturation on the binding of murine IgG and human IgE antibodies. J. Immunol. 1990, 144, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, D.; Eder, K.; Högerle, C.; Becker, S.; Martin Canis, M.; Gröger, M. De novo sensitization during subcutaneous allergen specific immunotherapy–An analysis of 51 cases of SCIT and 33 symptomatically treated controls. Sci. Rep. 2020, 10, 6048. [Google Scholar] [CrossRef]
- Movérare, R.; Elfman, L.; Vesterinen, E.; Metso, T.; Haahtela, T. Development of new IgE specificities to allergenic components in birch pollen extract during specific immunotherapy studied with immunoblotting and Pharmacia CAP System. Allergy 2002, 57, 423–430. [Google Scholar] [CrossRef]
- Mikus, M.; Zandian, A.; Sjöberg, R.; Hamsten, C.; Forsström, B.; Andersson, M.; Greiff, L.; Uhlén, M.; Levin, M.; Nilsson, P.; et al. Allergome-wide peptide microarrays enable epitope deconvolution in allergen-specific immunotherapy. J. Allergy Clin. Immunol. 2021, 147, 1077–1086. [Google Scholar] [CrossRef]
- Valenta, R.; Campana, R.; Focke-Tejkl, M.; Niederberger, V. Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: Lessons from the past and novel mechanisms of action for the future. J. Allergy Clin. Immunol. 2016, 137, 351–357. [Google Scholar] [CrossRef]
- Calzada, D.; Cremades-Jimeno, L.; López-Ramos, M.; Cárdaba, B. Peptide Allergen Immunotherapy: A New Perspective in Olive-Pollen Allergy. Pharmaceutics 2021, 13, 1007. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Nezafat, N.; Esmaeilzadeh, H.; Ghasemi, Y.; Nabavizadeh, S.H.; Alyasin, S. In silico prediction of B-cell epitopes for twenty-five mite allergens: The therapeutic potentials for immunotherapy. Mol. Cell Probes. 2019, 46, 101408. [Google Scholar] [CrossRef] [PubMed]
- Mukonyora, M. A Review of Important Discontinuous B-Cell Epitope Prediction Tools. J. Clin. Cell Immunol. 2015, 6, 1–5. [Google Scholar] [CrossRef]
- Orengo, J.M.; Radin, A.R.; Kamat, V.; Badithe, A.; Ben, L.H.; Bennett, B.L.; Zhong, S.; Birchard, D.; Limnander, A.; Rafique, A.; et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat. Commun. 2018, 9, 1421. [Google Scholar] [CrossRef] [PubMed]
- Vita, R.; Mahajan, S.; Overton, J.A.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The Immune Epitope Database (IEDB): 2018 update. Nucleic. Acids. Res. 2019, 47, D339–D343. [Google Scholar] [CrossRef] [PubMed]
- Wraith, D.C.; Krishna, M.T. Peptide allergen-specific immunotherapy for allergic airway diseases-State of the art. Clin. Exp. Allergy 2021, 51, 751–769. [Google Scholar] [CrossRef] [PubMed]
- Durham, S.R.; Shamji, M.H. Allergen immunotherapy: Past, present and future. Nat. Rev. Immunol. 2022, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Su, H.; Wang, W.; Ye, L.; Wei, H.; Peng, Z.; Anishchenko, I.; Baker, D.; Yang, J. The trRosetta server for fast and accurate protein structure prediction. Nat. Protoc. 2021, 16, 5634–5651. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
| Patient | Sex | Age (y) | Symptoms | Pre-SLIT | Post 1-Year | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IgE | IgE | IgE | Prick | Prick | IgE | IgE | IgE | ||||
| Total | Der p 1 | Blomia | DPT | Blomia | total | Der p 1 | Blomia | ||||
| (UI/mL) | (kUA/L) | (kUA/L) | (mm) | (mm) | (UI/mL) | (kUA/L) | (kUA/L) | ||||
| 4 | F | 48 | AR, conjunctivitis | 49.9 | 1.61 | 0.27 | 5 | 6 | 20.8 | 0.2 | 1.23 |
| 10 | F | 23 | AR, conjunctivitis, asthma | 2500 | >100 | >100 | 4 | 5 | 905 | 93.5 | ND |
| 16 | F | 23 | AR, conjunctivitis | 496 | 11.9 | 67 | 6 | 5 | 68.3 | 52.2 | 5.89 |
| 40 | F | 25 | AR, asthma | 19.3 | 0.26 | 11.2 | 5 | 6 | 36.2 | 0.06 | 8.11 |
| 44 | F | 43 | AR, conjunctivitis | 52.9 | 20.9 | 0.02 | 5 | 4 | 58.5 | 0.02 | 14 |
| 48 | M | 15 | AR, conjunctivitis, dermatitis | 324 | 29.9 | 16.9 | 9 | 5 | 194 | 13.9 | 23.6 |
| 51 | M | 15 | AR, conjunctivitis, asthma, dermatitis | 86.2 | 16.5 | 0.04 | 5 | 3 | 56.4 | 0.03 | 11.3 |
| 58 | F | 20 | AR | 28.3 | 90 | >100 | 4 | 6 | 35 | 0.04 | 5.88 |
| 61 | M | 13 | AR, conjunctivitis | 59.4 | 1.43 | 19.6 | 5 | 4 | 98.8 | 20.6 | 0.89 |
| 77 | M | 15 | AR, conjunctivitis | 529 | 1.52 | 1.45 | 7 | 4 | >100 | 9.77 | 11.4 |
| Unique IgG4 Epitopes | |||||||
|---|---|---|---|---|---|---|---|
| Allergen | Consent Epitope | Corresponding Aminoacid Position | |||||
| Der p 5 | ELALFYLQEQINHFEEKPTKEMKDKIVAEMDTI | 62–94 | |||||
| QRKDLDIFEQYNLEM | 110–124 | ||||||
| Der p 7 | EEINKAVDEAVAAIEKSETFD | 27–47 | |||||
| Blo t 5 | DELNENKSKELQEKIIRELDV | 58–78 | |||||
| LKDLKETEQKVKDIQ | 118–132 | ||||||
| Blo t 6 | VQHEQYDPNTIENDI | 124–138 | |||||
| Blo t 12 | KTTTEETHHSDDLIV | 70–84 | |||||
| Blo t 13 | IEGKYKLEKSDNFDKFLDELGVGF | 3–26 | |||||
| KNTEIKFKLGEEFEEDRADGK | 57–77 | ||||||
| QTQYGDKEVKIVRDFQGD | 93–110 | ||||||
| Epitopes with increased igg4:ige ratios after treatment | |||||||
| Allergen | Corresponding aminoacid position | Peptide | Ratio IgG4:IgE | Consent Epitope | Corresponding aminoacid position | ||
| pre-SLIT | post-1 year | ||||||
| Der p 1 | 149–163 | RNQSLDLAEQELVDC | 0.21 | 83.5 | RNQSLDLAEQELVDC | 149–163 | |
| 296–310 | DNGYGYFAANIDLMM | 0.39 | 3.98 | DNGYGYFAANIDLMMIEE | 296–313 | ||
| 299–313 | YGYFAANIDLMMIEE | 0.01 | 46.5 | ||||
| Der p 2 | 24–38 | DCANHEIKKVLVPGC | 0.23 | 80 | DCANHEIKKVLVPGC | 24–38 | |
| Der p 5 | 38–53 | KKHDYQNEFDFLLME | 0.05 | 26.5 | KKHDYQNEFDFLLME | 38–53 | |
| Der p 7 | 27–41 | EEINKAVDEAVAAIE | 3.09 | 102 | EEINKAVDEAVAAIE | 27–41 | |
| 103–117 | LLVGVHDDVVSMEYD | 0.01 | 339.5 | LLVGVHDDVVSMEYD | 103–117 | ||
| Der p 10 | 110–124 | TAKLEEASQSADESE | 0.12 | 221.5 | TAKLEEASQSADESE | 110–124 | |
| 146–160 | NQLKEARMMAEDADR | 0.01 | 76 | NQLKEARMMAEDADRKYDEVARKLAMVEADLERAEERAETGESKIVELEEELRVVGNNLKSL | 146–208 | ||
| 149–163 | KEARMMAEDADRKYD | 0.17 | 161.5 | ||||
| 170–184 | AMVEADLERAEERAE | 0.22 | 1.8 | ||||
| 182–196 | RAETGESKIVELEEE | 13 | 34.88 | ||||
| 194–208 | EEELRVVGNNLKSLE | 0.27 | 2.37 | ||||
| 212–226 | EKAQQREEAHEQQIR | 1.55 | 86.5 | EKAQQREEAHEQQIR | 212–226 | ||
| 257–271 | EDELVHEKEKYKSIS | 0.16 | 134 | EDELVHEKEKYKSIS | 257–271 | ||
| Der p 23 | 74–88 | KDCPGNTRWNEDEET | 1.76 | 119 | KDCPGNTRWNEDEET | 74–88 | |
| Blo t 5 | 19–33 | EHKPKKDDFRNEFDH | 0.29 | 1.32 | EHKPKKDDFRNEFDHLLIEQA | 19–39 | |
| 25–39 | DDFRNEFDHLLIEQA | 0.93 | 3.96 | ||||
| 70–84 | EKIIRELDVVCAMIE | 3.15 | 127 | EKIIRELDVVCAMIE | 70–84 | ||
| Blo t 6 | 127–141 | EQYDPNTIENDISLL | 5.39 | 31 | VQHEQYDPNTIENDISLL | 124–141 | |
| 187–201 | NLQVGELKIVSQEEC | 0.11 | 2.02 | NLQVGELKIVSQEEC | 187–201 | ||
| Blo t 12 | 22–36 | DEQTTRGRHTEPDDH | 0.48 | 92.5 | DEQTTRGRHTEPDDH | 22–36 | |
| 97–111 | EEGPIHIQEMCNKYI | 0.15 | 26.5 | EEGPIHIQEMCNKYI | 97–111 | ||
| Blo t 13 | 12–26 | SDNFDKFLDELGVGF | 11.17 | 342.25 | SDNFDKFLDELGVGF | 12–26 | |
| 96–110 | YGDKEVKIVRDFQGD | 0.01 | 3.19 | YGDKEVKIVRDFQGD | 96–110 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figo, D.D.; Cordeiro Macedo, P.R.; Gadermaier, G.; Remuzgo, C.; Castro, F.F.M.; Kalil, J.; Galvão, C.E.S.; Santos, K.S. IgE and IgG4 Epitopes of Dermatophagoides and Blomia Allergens before and after Sublingual Immunotherapy. Int. J. Mol. Sci. 2023, 24, 4173. https://doi.org/10.3390/ijms24044173
Figo DD, Cordeiro Macedo PR, Gadermaier G, Remuzgo C, Castro FFM, Kalil J, Galvão CES, Santos KS. IgE and IgG4 Epitopes of Dermatophagoides and Blomia Allergens before and after Sublingual Immunotherapy. International Journal of Molecular Sciences. 2023; 24(4):4173. https://doi.org/10.3390/ijms24044173
Chicago/Turabian StyleFigo, Daniele Danella, Priscilla Rios Cordeiro Macedo, Gabriele Gadermaier, Cesar Remuzgo, Fábio Fernandes Morato Castro, Jorge Kalil, Clovis Eduardo Santos Galvão, and Keity Souza Santos. 2023. "IgE and IgG4 Epitopes of Dermatophagoides and Blomia Allergens before and after Sublingual Immunotherapy" International Journal of Molecular Sciences 24, no. 4: 4173. https://doi.org/10.3390/ijms24044173
APA StyleFigo, D. D., Cordeiro Macedo, P. R., Gadermaier, G., Remuzgo, C., Castro, F. F. M., Kalil, J., Galvão, C. E. S., & Santos, K. S. (2023). IgE and IgG4 Epitopes of Dermatophagoides and Blomia Allergens before and after Sublingual Immunotherapy. International Journal of Molecular Sciences, 24(4), 4173. https://doi.org/10.3390/ijms24044173






