The rs1883832 Polymorphism (CD40-1C>T) Affects the Intensity of IgA Responses after BNT162b2 Vaccination
Abstract
1. Introduction
2. Results
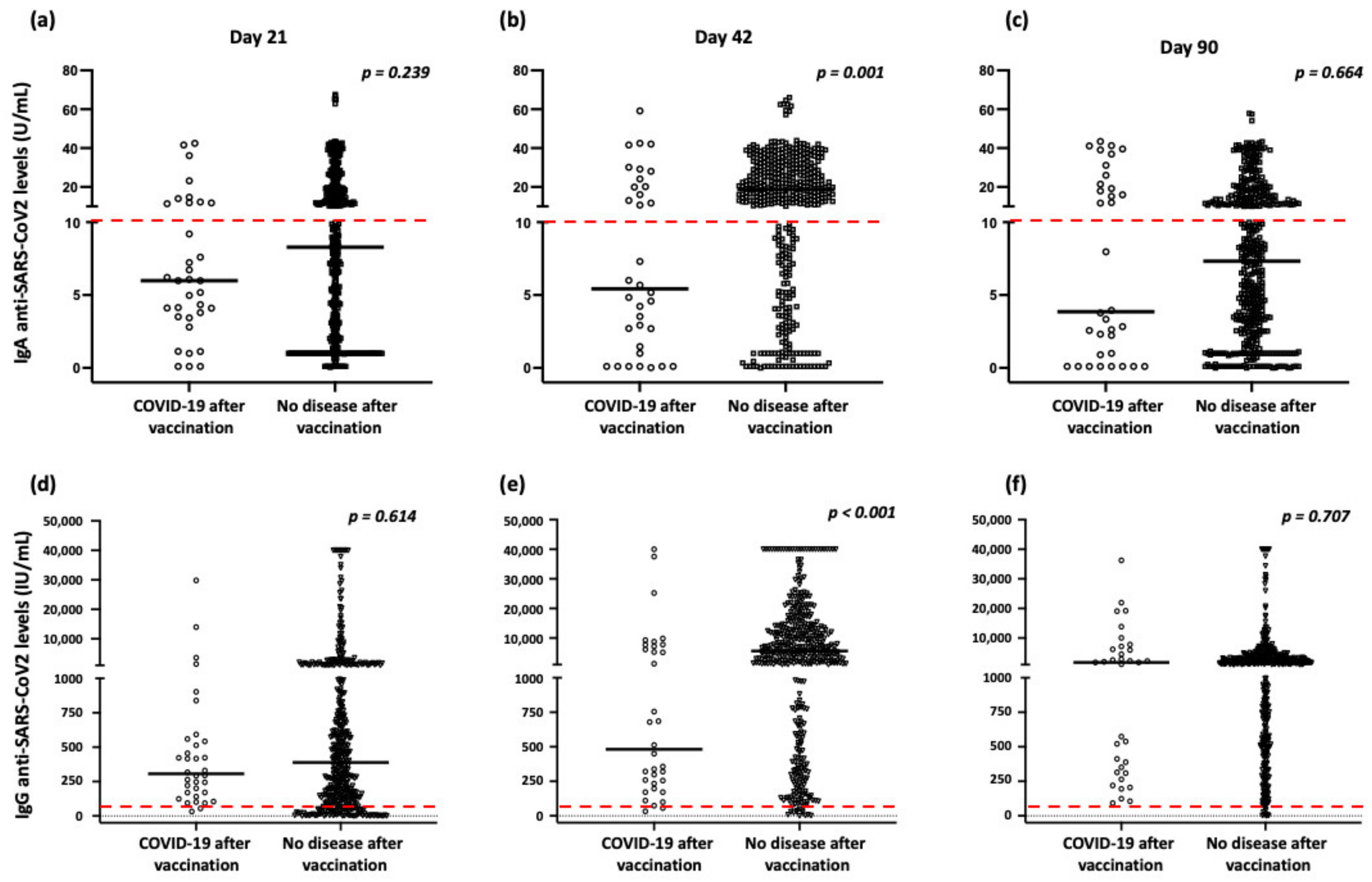
2.1. COVID-19 and Humoral Responses after Vaccination
2.2. Distribution of rs1883832 Polymorphism Frequency in the Vaccinated Participants of the Study
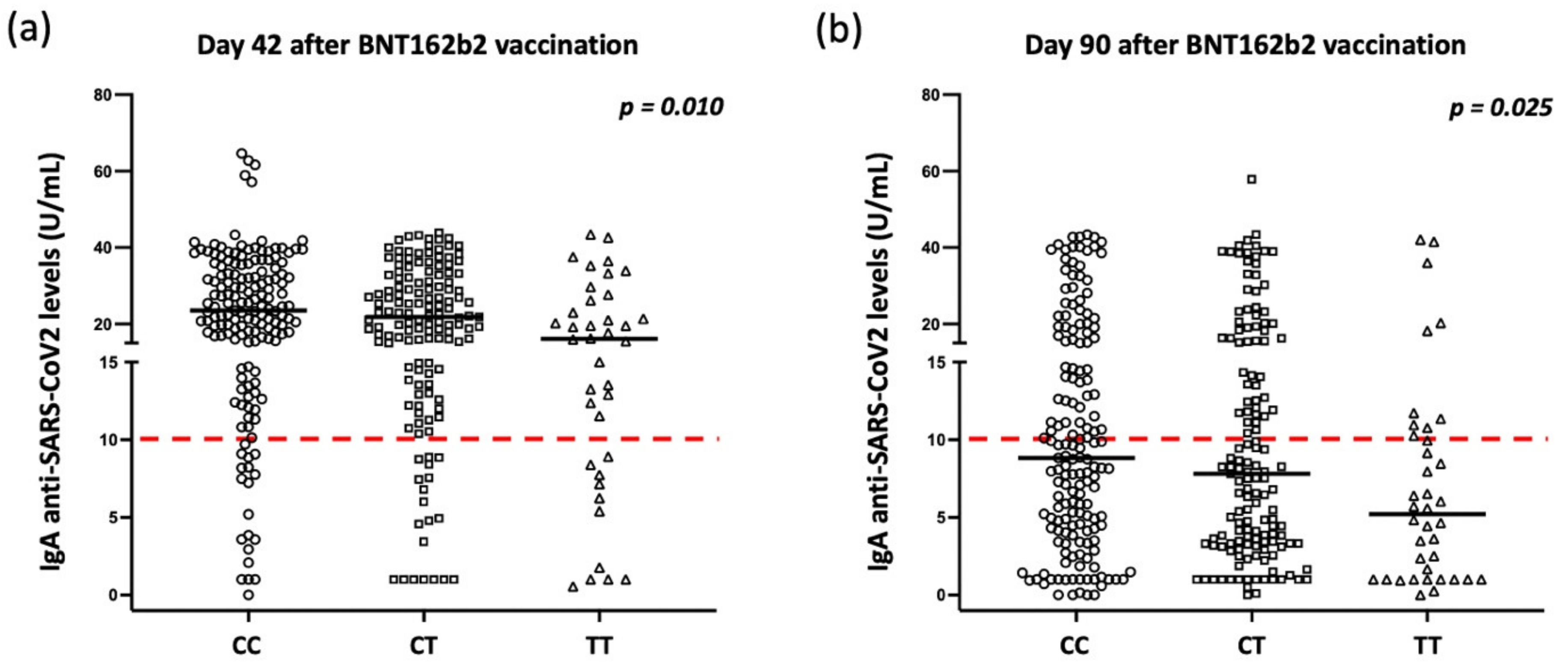
2.3. Correlations of rs1883832 Polymorphism with Humoral Responses after Vaccination
2.4. Correlation of Humoral Responses and rs1883832 Polymorphism with COVID-19 Following Vaccination
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Humoral Serum Responses to Vaccination
4.3. IgA Saliva Responses to Vaccination
4.4. Molecular Techniques
4.5. Immunophenotyping Studies
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, Y.; Yangm, H.; Jim, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Rotshild, V.; Hirsh-Raccah, B.; Miskin, I.; Muszkat, M.; Matok, I. Comparing the clinical efficacy of COVID-19 vaccines: A systematic review and network meta-analysis. Sci. Rep. 2021, 11, 22777. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, F.A. Vaccines in patients with primary immune deficiency. Immunol. Allergy Clin. N. Am. 2020, 40, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Hatchette, T.; Yue, F.; Liu, J.; Song, H.; Zhao, H.; Betschel, S.; Ostrowski, M. Impaired memory B-cell response to influenza immunization in patients with common variable immunodeficiency (CVID). Pathog. Immun. 2021, 6, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Ovsyannikova, I.G.; Jacobson, R.M.; Smith, D.I. Heterogeneity in vaccine immune response: The role of immunogenetics and the emerging field of vaccinomics. Clin. Pharmacol. Ther. 2007, 82, 653–664. [Google Scholar] [CrossRef]
- Yucesoy, B.; Johnson, V.J.; Fluharty, K.; Kashon, M.L.; Slaven, J.E.; Wilson, N.W.; Weissman, D.N.; Biagini, R.E.; Germolec, D.R.; Luster, M.I. Influence of cytokine gene variations on immunization to childhood vaccines. Vaccine 2009, 27, 6991–6997. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.; Ovsyannikova, I.G.; Vierkant, R.A.; Ryan, J.E.; Pankratz, V.S.; Jacobson, R.M.; Poland, G.A. Associations between SNPs in toll-like receptors and related intracellular signaling molecules and immune responses to measles vaccine: Preliminary results. Vaccine 2008, 26, 1731–1736. [Google Scholar] [CrossRef]
- Moore, C.; Hennig, B.J.; Perrett, K.; Hoe, J.C.; Lee, S.J.; Fletcher, H.; Brocklebank, D.; O’Connor, D.; Snape, M.; Hall, A.J.; et al. Single nucleotide polymorphisms in the Toll-like receptor 3 and CD44 genes are associated with persistence of vaccine-induced immunity to the serogroup C meningococcal conjugate vaccine. Clin. Vaccine Immunol. 2012, 19, 295–303. [Google Scholar] [CrossRef]
- Ovsyannikova, I.G.; Dhiman, N.; Haralambieva, I.H.; Vierkant, R.A.; O’Byrne, M.M.; Jacobson, R.M.; Poland, G.A. Rubella vaccine-induced cellular immunity: Evidence of associations with polymorphisms in the Toll-like, vitamin A and D receptors, and innate immune response genes. Hum. Genet. 2010, 127, 207–221. [Google Scholar] [CrossRef]
- Höhler, T.; Reuss, E.; Freitag, C.M.; Schneider, P.M. A functional polymorphism in the IL-10 promoter influences the response after vaccination with HBsAg and hepatitis A. Hepatology 2005, 42, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Bishop, G.A.; Hostager, B.S. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev. 2003, 14, 297–309. [Google Scholar] [CrossRef]
- Qin, J.; Xing, J.; Liu, R.; Chen, B.; Chen, Y.; Zhuang, X. Association between CD40 rs1883832 and immune-related diseases susceptibility: A meta-analysis. Oncotarget 2017, 8, 102235–102243. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.M.; Concepcion, E.; Oashi, T.; Tomer, Y. A Graves’ disease-associated Kozak sequence single-nucleotide polymorphism enhances the efficiency of CD40 gene translation: A case for translational pathophysiology. Endocrinology 2005, 146, 2684–2691. [Google Scholar] [CrossRef]
- Skibola, C.F.; Nieters, A.; Bracci, P.M.; Curry, J.D.; Agana, L.; Skibola, D.R.; Hubbard, A.; Becker, N.; Smith, M.T.; Holly, E.A. A functional TNFRSF5 gene variant is associated with risk of lymphoma. Blood 2008, 111, 4348–4354. [Google Scholar] [CrossRef]
- Li, M.; Sun, H.; Liu, S.; Yu, J.; Li, Q.; Liu, P.; Shen, H.; Sun, D. CD40 C/T-1 polymorphism plays different roles in Graves’ disease and Hashimoto’s thyroiditis: A meta-analysis. Endocr. J. 2012, 59, 1041–1050. [Google Scholar] [CrossRef]
- Jacobson, E.M.; Huber, A.K.; Akeno, N.; Sivak, M.; Li, C.W.; Concepcion, E.; Ho, K.; Tomer, Y. A CD40 Kozak sequence polymorphism and susceptibility to antibody-mediated autoimmune conditions: The role of CD40 tissue-specific expression. Genes Immun. 2007, 8, 205–214. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Hu, J.; Xiao, X.-F.; Peng, Y.; Zhao, S.-P.; Xiao, X.-Z.; Yang, M.-S. The CD40 rs1883832 polymorphism affects sepsis susceptibility and sCD40L levels. Biomed. Res. Int. 2018, 2018, 7497314. [Google Scholar] [CrossRef]
- Cassiano, G.C.; Furini, A.A.C.; Capobianco, M.P.; Storti-Melo, L.M.; Cunha, M.G.; Kano, F.S.; Carvalho, L.H.; Soares, I.D.S.; Santos, S.E.; Póvoa, M.M.; et al. Polymorphisms in B cell co-stimulatory genes are associated with IgG antibody responses against blood-stage proteins of plasmodium vivax. PLoS ONE 2016, 11, e0149581. [Google Scholar] [CrossRef]
- Speletas, M.; Voulgaridi, I.; Sarrou, S.; Dadouli, A.; Mouchtouri, V.A.; Nikoulis, D.J.; Tsakona, M. Intensity and dynamics of anti-SARS-CoV-2 immune responses after BNT162b2 mRNA vaccination: Implications for public health vaccination strategies. Vaccines 2022, 10, 316. [Google Scholar] [CrossRef]
- Dimitrakopoulos, F.D.; Antonacopoulou, A.G.; Kottorou, A.E.; Kalofonou, M.; Panagopoulos, N.; Dougenis, D.; Makatsoris, T. Genetic variations of CD40and LTβR genes are associated with increased susceptibility and clinical outcome of non-small-cell carcinoma patients. Front. Oncol. 2021, 11, 721577. [Google Scholar] [CrossRef] [PubMed]
- İnal, E.E.; Rüstemoğlu, A.; İnanır, A.; Ekinci, D.; Gül, Ü.; Yiğit, S.; Ateş, Ö. Associations of rs4810485 and rs1883832 polymorphisms of CD40 gene with susceptibility and clinical findings of Behçet’s disease. Rheumatol. Int. 2015, 35, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Pineda, B.; Tarín, J.J.; Hermenegildo, C.; Laporta, P.; Cano, A.; García-Pérez, M.Á. Gene-gene interaction between CD40 and CD40L reduces bone mineral density and increases osteoporosis risk in women. Osteoporos. Int. 2011, 22, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Corthésy, B. Role of secretory immunoglobulin A and secretory component in the protection of mucosal surfaces. Future Microbiol. 2010, 5, 817–829. [Google Scholar] [CrossRef] [PubMed]
- De Sousa-Pereira, P.; Woof, J.M. IgA: Structure, function, and developability. Antibodies 2019, 8, 57. [Google Scholar] [CrossRef]
- Pilette, C.; Ouadrhiri, Y.; Godding, V.; Vaerman, J.P.; Sibille, Y. Lung mucosal immunity: Immunoglobulin-A revisited. Eur. Respir. J. 2001, 18, 571–588. [Google Scholar] [CrossRef]
- Sheikh-Mohamed, S.; Sanders, E.C.; Gommerman, J.L.; Tal, M.C. Guardians of the oral and nasopharyngeal galaxy: IgA and protection against SARS-CoV-2 infection. Immunol. Rev. 2022, 309, 75–85. [Google Scholar] [CrossRef]
- Hennings, V.; Thörn, K.; Albinsson, S.; Lingblom, C.; Andersson, K.; Andersson, C.; Järbur, K. The presence of serum anti-SARS-CoV-2 IgA appears to protect primary health care workers from COVID-19. Eur. J. Immunol. 2022, 52, 800–809. [Google Scholar] [CrossRef]
- Barzegar-Amini, M.; Mahmoudi, M.; Dadgarmoghaddam, M.; Farzad, F.; Najafabadi, A.Q.; Jabbari-Azad, F. Comparison of serum total IgA levels in severe and mild COVID-19 patients and control group. J. Clin. Immunol. 2022, 42, 10–18. [Google Scholar] [CrossRef]
- Adjobimey, T.; Meyer, J.; Sollberg, L.; Bawolt, M.; Berens, C.; Kovačević, P.; Trudić, A.; Parcina, M.; Hoerauf, A. Comparison of IgA, IgG, and neutralizing antibody responses following immunization with Moderna, BioNTech, AstraZeneca, Sputnik-V, Johnson and Johnson, and Sinopharm’s COVID-19 vaccines. Front. Immunol. 2022, 13, 917905. [Google Scholar] [CrossRef]
- Voulgaridi, I.; Sarrou, S.; Dadouli, A.; Peristeri, A.-M.; Nasika, A.; Onoufriadis, I.; Kyritsi, M.A.; Anagnostopoulos, L.; Theodoridou, A.; Avakian, I.; et al. Intensity of humoral immune responses, adverse reactions, and post-vaccination morbidity after adenovirus vector-based and mRNA anti-COVID-19 vaccines. Vaccines 2022, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Mohamed, S.; Isho, B.; Chao, G.Y.C.; Zuo, M.; Cohen, C.; Lustig, Y.; Nahass, G.R.; Salomon-Shulman, R.E.; Blacker, G.; Fazel-Zarandi, M.; et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal Immunol. 2022, 15, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Giliani, S.; Insalaco, A.; Al-Ghonaium, A.; Soresina, A.R.; Loubser, M.; Avanzini, M.A.; Marconi, M.; Badolato, R.; Ugazio, A.G.; et al. Mutations of CD40 gene cause an autosomal recessive form of immunodeficiency with hyper IgM. Proc. Natl. Acad. Sci. USA 2001, 98, 12614–12619. [Google Scholar] [CrossRef] [PubMed]
- Lougaris, V.; Badolato, R.; Ferrari, S.; Plebani, A. Hyper immunoglobulin M syndrome due to CD40 deficiency: Clinical, molecular, and immunological features. Immunol. Rev. 2005, 203, 48–66. [Google Scholar] [CrossRef]
- Speletas, M.; Kyritsi, M.A.; Vontas, A.; Theodoridou, A.; Chrysanthidis, T.; Hatzianastasiou, S.; Petinaki, E. Evaluation of two chemiluminescent and three ELISA immunoassays for the detection of SARS-CoV-2 IgG antibodies: Implications for disease diagnosis and patients’ management. Front. Immunol. 2020, 11, 609242. [Google Scholar] [CrossRef]
| Participants with Genotype | ||||
|---|---|---|---|---|
| CC (wt) | CT (het) | TT (hom) | p * | |
| BNT162b2 vaccine (Pfizer-BioNTech) (No 342) | ||||
| No (%) | 161 (47.1) | 143 (41.8) | 38 (11.1) | |
| Day 21 (median, range) | 9.6, 0.0–64.9 | 7.8, 0.4–43.5 | 6.2, 1.0–43.4 | 0.159 |
| Day 42 (median, range) | 23.5, 0.0–64.6 | 21.9, 1.0–43.9 | 16.1, 0.6–43.4 | 0.010 |
| Day 90 (median, range) | 8.8, 0.0–43.4 | 7.8, 0.0–57.9 | 5.2, 0.0–42.1 | 0.028 |
| Adenovirus-based vector vaccines (No 134) | ||||
| No (%) | 55 (41.1) | 65 (48.5) | 14 (10.4) | |
| Day 21 (median, range) | 6.7, 0.1–67.6 | 6.8, 0.1–65.4 | 7.4, 0.1–41.6 | 0.665 |
| Day 42 (median, range) | 5.3, 0.0–66.0 | 2.86, 0.1–62.5 | 3.0, 0.1–41.6 | 0.078 |
| Day 90 (median, range) | 4.8, 0.1–57.5 | 2.69, 0.1–41.1 | 7.6, 0.1–41.3 | 0.296 |
| Total (No 476) | ||||
| No (%) | 216 (45.4) | 208 (43.7) | 52 (10.9) | |
| Day 21 (median, range) | 9.0, 0.0–67.6 | 7.3, 0.1–65.4 | 6.4, 0.1–43.4 | 0.125 |
| Day 42 (median, range) | 20.6, 0.0–66.0 | 16.2, 0.1–62.5 | 13.1, 0.1–43.4 | 0.005 |
| Day 90 (median, range) | 8.2, 0.0–57.5 | 6.6, 0.0–57.9 | 5.6, 0.0–42.1 | 0.053 |
| Participants with Genotype | ||||
|---|---|---|---|---|
| CC (wt) | CT (het) | TT (hom) | p * | |
| BNT162b2 vaccine (Pfizer-BioNTech) (No 342) | ||||
| No (%) | 161 (47.1) | 143 (41.8) | 38 (11.1) | |
| Day 21 (median, range) | 438.9, 0.0–40,000.0 | 467.9, 0.0–40,000.0 | 293.1, 0.5–40,000.0 | 0.563 |
| Day 42 (median, range) | 9107.3, 0.2–40,000.0 | 9314.6, 1.6–40,000.0 | 7024.3, 8.2–40,000.0 | 0.571 |
| Day 90 (median, range) | 2097.2, 3.2–40,000.0 | 2303.5, 0.0–40,000.0 | 1757.4, 6.4–40,000.0 | 0.677 |
| Adenovirus-based vector vaccines (No 134) | ||||
| No (%) | 55 (41.1) | 65 (48.5) | 14 (10.4) | |
| Day 21 (median, range) | 336.0, 7.1–33,994.2 | 285.0, 7.8–13,966.7 | 292.9, 135.7–29,806.1 | 0.508 |
| Day 42 (median, range) | 364.1, 29.6–26,246.5 | 298.2, 22.4–11,215.5 | 315.0, 85.7–9827.3 | 0.244 |
| Day 90 (median, range) | 839.7, 93.5–40,000.0 | 713.6, 43.2–36,241.2 | 677.2, 276.2–19,093.0 | 0.371 |
| Total (No 476) | ||||
| No (%) | 216 (45.4) | 208 (43.7) | 52 (10.9) | |
| Day 21 (median, range) | 414.5, 0.0–40,000.0 | 362.5, 0.0–40,000.0 | 293.1, 0.5–40,000.0 | 0.559 |
| Day 42 (median, range) | 6173.1, 0.2–40,000.0 | 4579.7, 1.6–40,000.0 | 4857.8, 8.2–40,000.0 | 0.223 |
| Day 90 (median, range) | 1783.1, 3.2–40,000.0 | 1628.9, 0.0–40,000.0 | 1601.7, 6.4–40,000.0 | 0.531 |
| Parameter | Coefficient, 95% CI | p |
|---|---|---|
| Day 42 after vaccination | ||
| Sex (female/male) | −0.54, −2.69–1.60 | 0.617 |
| Age | −0.12, −0.18–−0.07 | <0.001 |
| History of COVID-19 before vaccination | 18.07, 14.70–21.43 | <0.001 |
| History of COVID-19 after vaccination | −0.68, −4.87–3.52 | 0.752 |
| rs1883832 polymorphism (het and hom vs. wt) | −4.00, −6.06–−1.94 | <0.001 |
| Vaccination type (BNT162b2 vs. adenovirus-based vector vaccines) | 6.93, 5.71–8.15 | <0.001 |
| Day 90 after vaccination | ||
| Sex (female/male) | −1.39, −3.21–0.43 | 0.135 |
| Age | −0.02, −0.07–0.03 | 0.443 |
| History of COVID-19 before vaccination | 21.78, 18.92–24.64 | <0.001 |
| History of COVID-19 after vaccination | 4.59, 1.02–8.16 | 0.012 |
| rs1883832 polymorphism (het and hom vs. wt) | −2.43, −4.18–−0.88 | 0.007 |
| Vaccination type (BNT162b2 vs. adenovirus-based vector vaccines) | 1.40, 0.36–2.44 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speletas, M.; Bakaros, E.; Peristeri, A.-M.; Voulgaridi, I.; Sarrou, S.; Paliatsa, V.; Nasika, A.; Tseroni, M.; Anagnostopoulos, L.; Theodoridou, K.; et al. The rs1883832 Polymorphism (CD40-1C>T) Affects the Intensity of IgA Responses after BNT162b2 Vaccination. Int. J. Mol. Sci. 2022, 23, 14056. https://doi.org/10.3390/ijms232214056
Speletas M, Bakaros E, Peristeri A-M, Voulgaridi I, Sarrou S, Paliatsa V, Nasika A, Tseroni M, Anagnostopoulos L, Theodoridou K, et al. The rs1883832 Polymorphism (CD40-1C>T) Affects the Intensity of IgA Responses after BNT162b2 Vaccination. International Journal of Molecular Sciences. 2022; 23(22):14056. https://doi.org/10.3390/ijms232214056
Chicago/Turabian StyleSpeletas, Matthaios, Evangelos Bakaros, Athanasia-Marina Peristeri, Ioanna Voulgaridi, Styliani Sarrou, Vassiliki Paliatsa, Asimina Nasika, Maria Tseroni, Lemonia Anagnostopoulos, Kalliopi Theodoridou, and et al. 2022. "The rs1883832 Polymorphism (CD40-1C>T) Affects the Intensity of IgA Responses after BNT162b2 Vaccination" International Journal of Molecular Sciences 23, no. 22: 14056. https://doi.org/10.3390/ijms232214056
APA StyleSpeletas, M., Bakaros, E., Peristeri, A.-M., Voulgaridi, I., Sarrou, S., Paliatsa, V., Nasika, A., Tseroni, M., Anagnostopoulos, L., Theodoridou, K., Kalala, F., Theodoridou, A., Mouchtouri, B. A., Tsiodras, S., Eibel, H., & Hadjichristodoulou, C. (2022). The rs1883832 Polymorphism (CD40-1C>T) Affects the Intensity of IgA Responses after BNT162b2 Vaccination. International Journal of Molecular Sciences, 23(22), 14056. https://doi.org/10.3390/ijms232214056











