OMICS Analyses Unraveling Related Gene and Protein-Driven Molecular Mechanisms Underlying PACAP 38-Induced Neurite Outgrowth in PC12 Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Genome and Proteome Analyses in PC12 Cells after Treatment with PACAP38
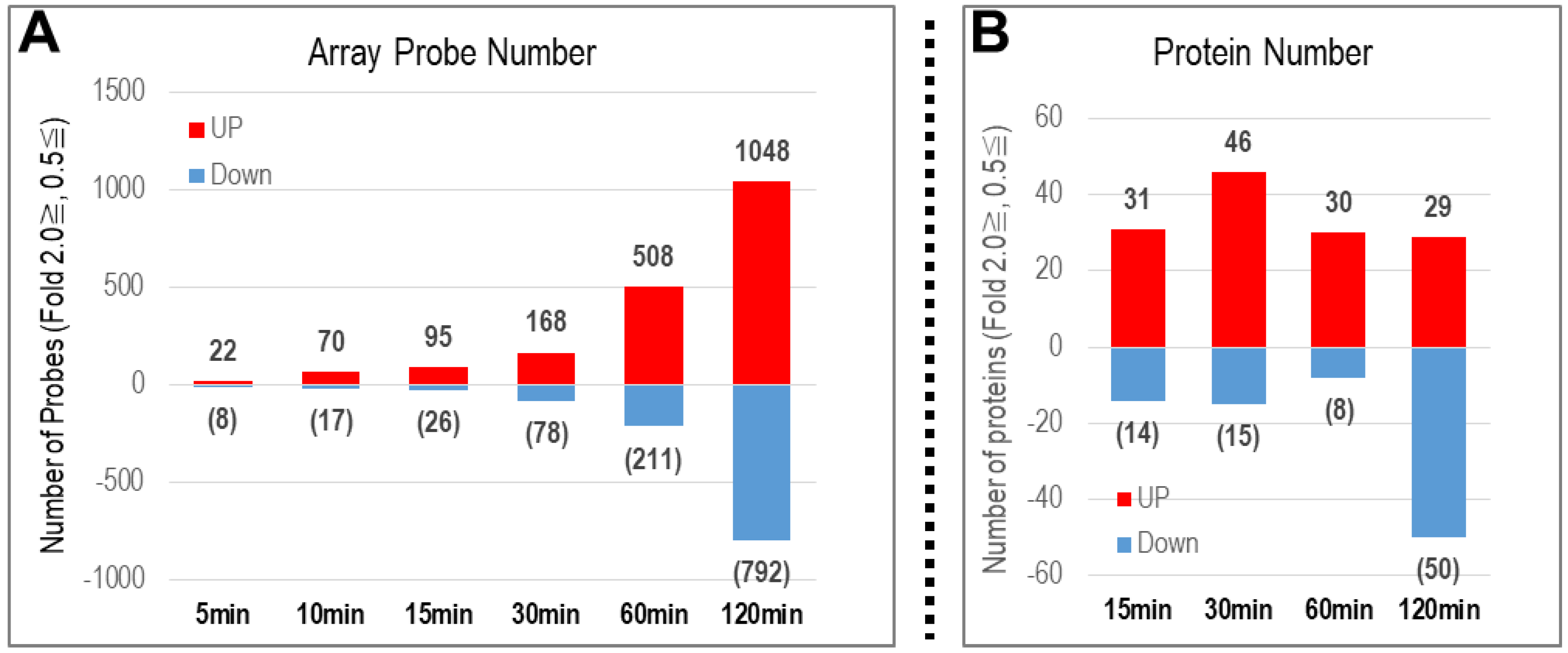
2.1.1. Number of Genes with Altered Expression
2.1.2. Number of Proteins with Altered Expression
TMT-Based Quantitative Proteomic Analysis
2.2. Results from the DNA Microarray Analysis
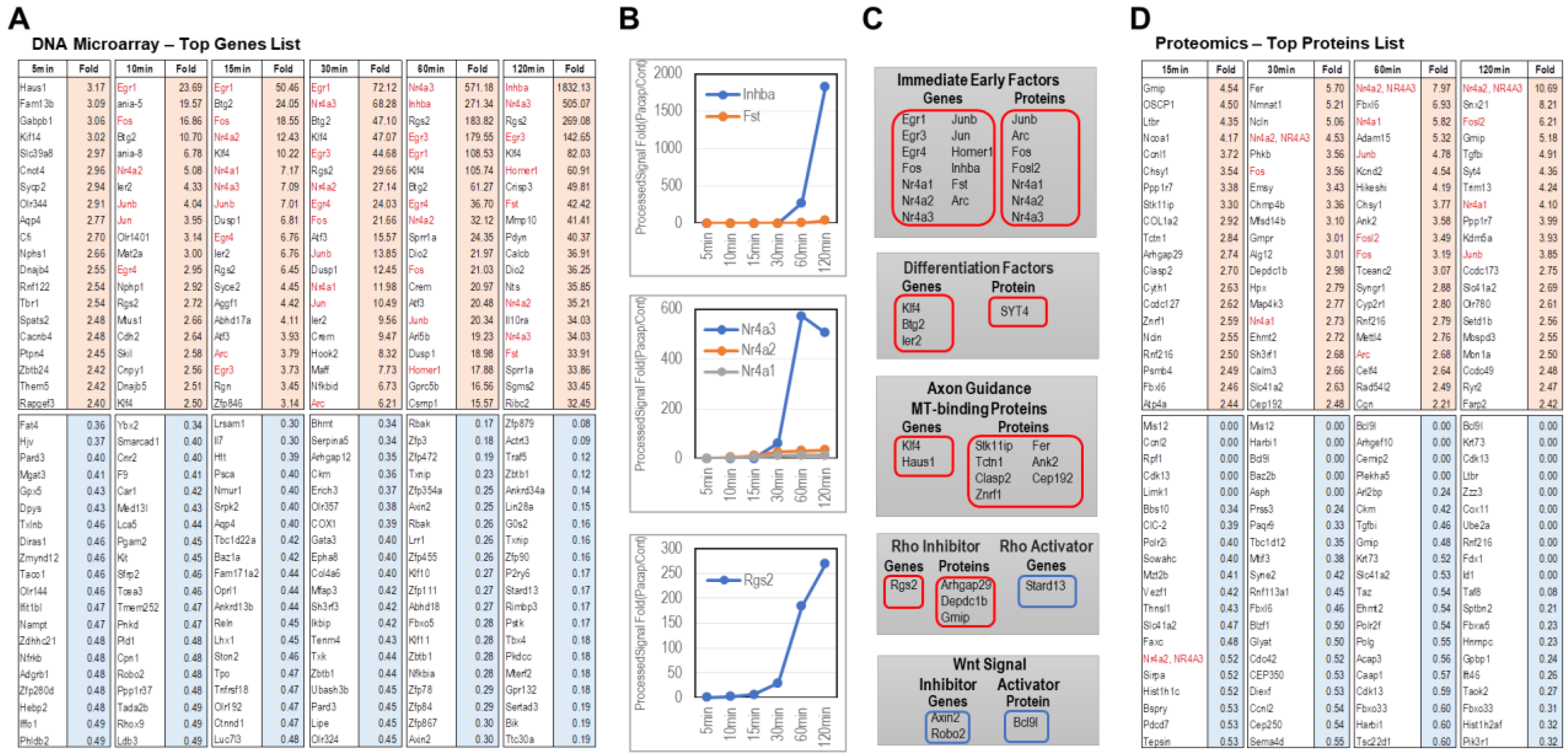
2.2.1. Genes Whose Expression Increased by PACAP
2.2.2. Genes Whose Expression Decreased by PACAP
2.3. Results from the Proteome Analysis
2.3.1. Proteins Whose Expression Increased by PACAP
2.3.2. Proteins Whose Expression Decreased by PACAP
2.4. Examination of Categories Characterized by the UP(-Regulated) and DOWN(-Regulated) in DAVID Analysis
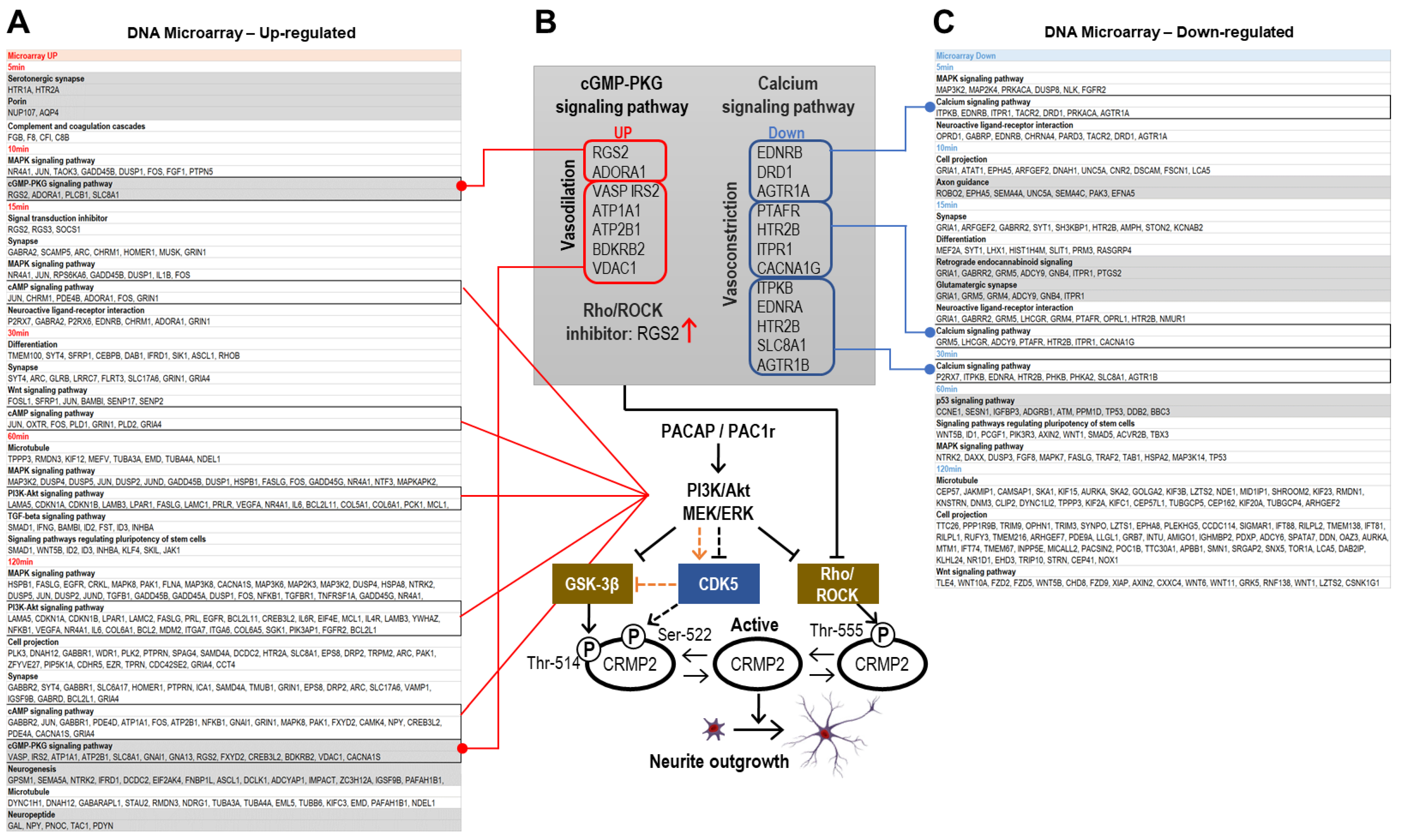
2.4.1. DNA Microarray Up-Regulated Gene Categories
2.4.2. DNA Microarray Down-Regulated Gene Categories
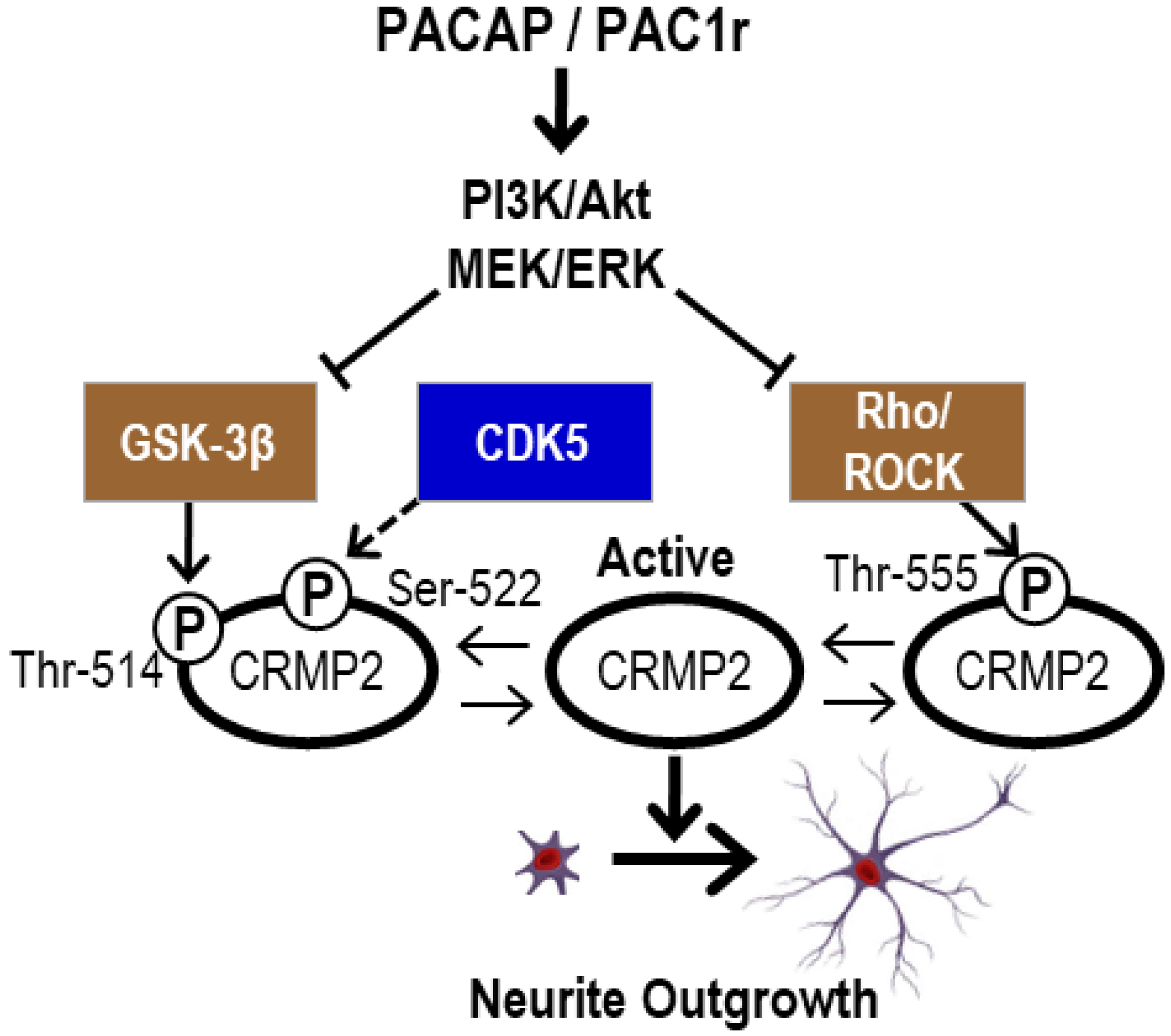
2.4.3. DAVID Analysis of Categories Involved in CRMP2 Dephosphorylation
3. Materials and Methods
3.1. Cell Culture
3.2. DNA Microarray Analysis
3.3. Proteome Analysis
3.3.1. Protein Digestion by Filter-Aided Sample Preparation (FASP)
3.3.2. Peptides Labeling with Tandem Mass Tags (TMT), Desalting, and Basic pH Reversed Phase (BPRP) Peptide Fractionation Using Stage-Tip
3.3.3. Q-Exactive MS Analysis
3.3.4. Data Processing Using MaxQuant Software and Data Analysis Using Perseus and R Program
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miyata, A.; Arimura, A.; Dahl, R.R. Isolation of a novel 38-residue hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Arimura, A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn. J. Physiol. 1998, 48, 301–331. [Google Scholar] [CrossRef]
- Miyata, A.; Jiang, L.; Dahl, R.D.; Kitada, C.; Kubo, K.; Fujino, M.; Minamino, N.; Arimura, A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem. Biophys. Res. Commun. 1990, 170, 643–648. [Google Scholar] [CrossRef]
- Köves, K.; Szabó, E.; Kántor, O.; Heinzlmann, A.; Szabó, F.; Csáki, Á. Current state of understanding of the role of PACAP in the hypothalamo-hypophyseal gonadotropin functions of mammals. Front. Endocrinol. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Shioda, S.; Nakamachi, T. PACAP as a neuroprotective factor in ischemic neuronal injuries. Peptides 2015, 72, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Toth, D.; Szabo, E.; Tamas, A.; Juhasz, T.; Horvath, G.; Fabian, E.; Opper, B.; Szabo, D.; Maugeri, G.; D’Amico, A.G.; et al. Protective effects of PACAP in peripheral organs. Front. Endocrinol. 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Harmar, A.J.; Fahrenkrug, J.; Gozes, I.; Laburthe, M.; May, V.; Pisegna, J.R.; Vaudry, D.; Vaudry, H.; Waschek, J.A.; Said, S.I. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br. J. Pharmacol. 2012, 166, 4–17. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Hori, M.; Nakamachi, T.; Rakwal, R.; Shibato, J.; Ogawa, T.; Aiuchi, T.; Tsuruyama, T.; Tamaki, K.; Shioda, S. Transcriptomics and proteomics analyses of the PACAP38 influenced ischemic brain in permanent middle cerebral artery occlusion model mice. J. Neuroinflam. 2012, 23, 256. [Google Scholar] [CrossRef]
- Hori, M.; Nakamachi, T.; Shibato, J.; Rakwal, R.; Tsuchida, M.; Shioda, S.; Numazawa, S. PACAP38 differentially effects genes and CRMP2 protein expression in ischemic core and penumbra regions of permanent middle cerebral artery occlusion model mice brain. Int. J. Mol. Sci. 2014, 15, 17014–17034. [Google Scholar] [CrossRef]
- Hori, M.; Nakamachi, T.; Rakwal, R.; Shibato, J.; Nakamura, K.; Wada, Y.; Tsuchikawa, D.; Yoshikawa, A.; Tamaki, K.; Shioda, S. Unraveling the ischemic brain transcriptome in a permanent middle cerebral artery occlusion mouse model by DNA microarray analysis. Dis. Model. Mech. 2012, 5, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Shibato, J.; Takenoya, F.; Hirabayashi, T.; Kimura, A.; Yamashita, M.; Takasaki, I.; Rakwal, R.; Shioda, S. Molecular mechanism for PACAP 38-induced neurite outgrowth in PC12 cells. Neural. Plast. 2021, 2021, 2522454. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Kawano, Y.; Arimura, N.; Kawabata, S.; Kikuchi, A.; Kaibuchi, K. GSK-3β regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 2005, 120, 137–149. [Google Scholar] [CrossRef]
- Zhang, W.; Smith, A.; Liu, J.-P.; Cheung, N.S.; Zhou, S.; Liu, K.; Li, Q.-T.; Duan, W. GSK3beta modulates PACAP-induced neuritogenesis in PC12 cells by acting downstream of Rap1 in a caveolae-dependent manner. Cell Signal. 2009, 21, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.R.; Causeret, F.; Yadirgi, G.; Hastie, C.J.; McLauchlan, H.; McManus, E.J.; Hernandez, F.; Eickholt, B.J.; Nikolic, M.; Sutherland, C. Distinct priming kinases contribute to differential regulation of collapsin response mediator proteins by glycogen synthase kinase-3 in vivo. J. Biol. Chem. 2006, 281, 16591–16598. [Google Scholar] [CrossRef] [PubMed]
- Arimura, N.; Inagaki, N.; Chihara, K.; Menager, C.; Nakamura, N.; Amano, M.; Iwamatsu, A.; Goshima, Y.; Kaibuchi, K. Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J. Biol. Chem. 2000, 275, 23973–23980. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.R.; Soutar, M.P.; Rembutsu, M.; van Aalten, L.; Hastie, C.J.; McLauchlan, H.; Peggie, M.; Balastik, M.; Lu, K.P.; Sutherland, C. Relative resistance of Cdk5-phosphorylated CRMP2 to dephosphorylation. J. Biol Chem. 2008, 283, 18227–18237. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Ohshima, T.; Sasaki, Y.; Suzuki, H.; Yanai, S.; Yamashita, N.; Nakamura, F.; Takei, K.; Ihara, Y.; Mikoshiba, K.; et al. Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3β phosphorylation of CRMP2: Implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes Cells 2005, 10, 165–179. [Google Scholar] [CrossRef]
- Arimura, N.; Menager, C.; Kawano, Y.; Yoshimura, T.; Kawabata, S.; Hattori, A.; Fukata, Y.; Amano, M.; Goshima, Y.; Inagaki, M.; et al. Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Mol. Cell Biol. 2005, 2, 9973–9984. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Hewapathirana, S.; García-Seisdedos, D.; Kamatchinathan, S.; Kundu, D.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A Hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Bandeira, N.; Sharma, V.; Perez-Riverol, Y.; Carver, J.J.; Kundu, D.J.; García-Seisdedos, D.; Jarnuczak, A.F.; Hewapathirana, S.; Pullman, B.S.; et al. The ProteomeXchange consortium in 2020: Enabling ‘big data’ approaches in proteomics. Nucleic Acids Res. 2020, 48, D1145–D1152. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kobayashi, T.; Funatsu, O.; Morita, A.; Ikekita, M. Activin A induces neuronal differentiation and survival via ALK4 in a SMAD-independent manner in a subpopulation of human neuroblastomas. Biochem. Biophys. Res. Commun. 2010, 394, 639–645. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, G.; Molina-Hernández, A.; Velasco, I. Activin A promotes neuronal differentiation of cerebrocortical neural progenitor cells. PLoS ONE 2012, 7, e43797. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Wang, Y.N.; Cui, X.L.; Fang, S.Y.; Ge, J.Y.; Sun, Y.; Liu, Z.H. The role and mechanism of action of activin A in neurite outgrowth of chicken embryonic dorsal root ganglia. J. Cell Sci. 2012, 125, 1500–1507. [Google Scholar] [PubMed]
- Zhang, W.; Zhu, X.; Liu, Y.; Chen, M.; Yan, S.; Mao, X.; Liu, Z.; Wu, W.; Chen, C.; Xu, X.; et al. Nur77 was essential for neurite outgrowth and involved in Schwann cell differentiation after sciatic nerve injury. J. Mol. Neurosci. 2015, 57, 38–47. [Google Scholar] [CrossRef]
- Maruoka, H.; Sasaya, H.; Shimamura, Y.; Nakatani, Y.; Shimoke, K.; Ikeuchi, T. Dibutyryl-cAMP up-regulates nur77 expression via histone modification during neurite outgrowth in PC12 cells. J. Biochem. 2010, 148, 93–101. [Google Scholar] [CrossRef]
- Hirano, T.; Nagasaki-Maeoka, E.; Ishizuka, Y.; Takatori, A.; Watanabe, Y.; Hoshi, R.; Yoshizawa, S.; Kawashima, H.; Uekusa, S.; Sugito, K.; et al. Forced expression of NR4A3 induced the differentiation of human neuroblastoma-derived NB1 cells. Med. Oncol. 2019, 36, 66. [Google Scholar] [CrossRef]
- Lin, S.C.; Yao, C.Y.; Hsu, C.A.; Lin, C.T.; Calkins, M.J.; Kuo, Y.Y.; Tang, J.L.; Tien, H.F.; Wu, S.J. Functional association of NR4A3 downregulation with impaired differentiation in myeloid leukemogenesis. Ann. Hematol. 2022, 101, 2209–2218. [Google Scholar] [CrossRef]
- Jennings, E.; Elliot, T.A.E.; Thawait, N.; Kanabar, S.; Yam-Puc, J.C.; Ono, M.; Toellner, K.M.; Wraith, D.C.; Anderson, G.; Bending, D. Differential Nr4a1 and Nr4a3 expression discriminates tonic from activated TCR signalling events in vivo. Cell Rep. 2020, 33, 108328. [Google Scholar] [CrossRef]
- Zakaria, S.; Mao, Y.; Kuta, A.; de Sousa, C.F.; Gaufo, G.O.; McNeill, H.; Hindges, R.; Guthrie, S.; Irvine, K.D.; Francis-West, P.H. Regulation of neuronal migration by Dchs1-Fat4 planar cell polarity. Curr. Biol. 2014, 24, 1620–1627. [Google Scholar] [CrossRef]
- Förster, E. Reelin, neuronal polarity and process orientation of cortical neurons. Neuroscience 2014, 269, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Laumonnerie, C.; Solecki, D.J. Regulation of polarity protein levels in the developing central nervous system. J. Mol. Biol. 2018, 430, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Miete, C.; Solis, G.P.; Koval, A.; Brückner, M.; Katanaev, V.L.; Behrens, J.; Bernkopf, D.B. Gαi2-induced conductin/axin2 condensates inhibit Wnt/β-catenin signaling and suppress cancer growth. Nat. Commun. 2022, 13, 674. [Google Scholar] [CrossRef] [PubMed]
- Fancy, S.P.; Harrington, E.P.; Yuen, T.J.; Silbereis, J.C.; Zhao, C.; Baranzini, S.E.; Bruce, C.C.; Otero, J.J.; Huang, E.J.; Nusse, R.; et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat. Neurosci. 2011, 14, 1009–1016. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, A.; Mohanbhai, S.J.; Tiwari, V.; Chaturvedi, R.K.; Khurana, S.; Shukla, S. Axin-2 knockdown promote mitochondrial biogenesis and dopaminergic neurogenesis by regulating Wnt/β-catenin signaling in rat model of Parkinson’s disease. Free Radic Biol. Med. 2018, 129, 73–87. [Google Scholar] [CrossRef]
- Harburg, G.; Compton, J.; Liu, W.; Iwai, N.; Zada, S.; Marlow, R.; Strickland, P.; Zeng, Y.A.; Hinck, L. SLIT/ROBO2 signaling promotes mammary stem cell senescence by inhibiting Wnt signaling. Stem Cell Rep. 2014, 3, 385–393. [Google Scholar] [CrossRef]
- Ota, H.; Hikita, T.; Sawada, M.; Nishioka, T.; Matsumoto, M.; Komura, M.; Ohno, A.; Kamiya, Y.; Miyamoto, T.; Asai, N.; et al. Speed control for neuronal migration in the postnatal brain by Gmip-mediated local inactivation of RhoA. Nat. Commun. 2014, 5, 4532. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Ito, K.; Fujita, T.; Matsuzaki, Y.; Asano, T.; Hayakawa, M.; Asano, T.; Kawakami, Y. Progression of Human Renal Cell Carcinoma via Inhibition of RhoA-ROCK Axis by PARG1. Transl. Oncol. 2017, 10, 142–152. [Google Scholar] [CrossRef]
- Marchesi, S.; Montani, F.; Deflorian, G.; D’Antuono, R.; Cuomo, A.; Bologna, S.; Mazzoccoli, C.; Bonaldi, T.; Di Fiore, P.P.; Nicassio, F. DEPDC1B coordinates de-adhesion events and cell-cycle progression at mitosis. Dev. Cell. 2014, 31, 420–433. [Google Scholar] [CrossRef]
- Jiang, S.X.; Whitehead, S.; Aylsworth, A.; Slinn, J.; Zurakowski, B.; Chan, K.; Li, J.; Hou, S.T. Neuropilin 1 directly interacts with Fer kinase to mediate semaphorin 3A-induced death of cortical neurons. J. Biol. Chem. 2010, 285, 9908–9918. [Google Scholar] [CrossRef]
- Zheng, Y.; Sethi, R.; Mangala, L.S.; Taylor, C.; Goldsmith, J.; Wang, M.; Masuda, K.; Carrami, E.M.; Mannion, D.; Miranda, F.; et al. Author Correction: Tuning microtubule dynamics to enhance cancer therapy by modulating FER-mediated CRMP2 phosphorylation. Nat. Commun. 2022, 13, 3352. [Google Scholar] [CrossRef] [PubMed]
- El Sayegh, T.Y.; Arora, P.D.; Fan, L.; Laschinger, C.A.; Greer, P.A.; McCulloch, C.A.; Kapus, A. Phosphorylation of N-cadherin-associated cortactin by Fer kinase regulates N-cadherin mobility and intercellular adhesion strength. Mol. Biol. Cell. 2005, 16, 5514–5527. [Google Scholar] [CrossRef] [PubMed]
- Akhmanova, A.; Hoogenraad, C.C.; Drabek, K.; Stepanova, T.; Dortland, B.; Verkerk, T.; Vermeulen, W.; Burgering, B.M.; De Zeeuw, C.I.; Grosveld, F.; et al. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell 2001, 104, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Lyle, K.S.; Gierke, S.; Matov, A.; Danuser, G.; Wittmann, T. GSK3beta phosphorylation modulates CLASP-microtubule association and lamella microtubule attachment. J. Cell Biol. 2009, 184, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Pei, H.Z.; Chang, H.W.; Baek, S.H. The E3 ubiquitin ligase Trim13 regulates Nur77 stability via casein kinase 2α. Sci. Rep. 2018, 8, 13895. [Google Scholar] [CrossRef]
- Zhao, G.; Oztan, A.; Ye, Y.; Schwarz, T.L. Kinetochore proteins have a post-mitotic function in neurodevelopment. Dev. Cell. 2019, 48, 873–882.e4. [Google Scholar] [CrossRef]
- Chen, H.R.; Lin, G.T.; Huang, C.K.; Fann, M.J. Cdk12 and Cdk13 regulate axonal elongation through a common signaling pathway that modulates Cdk5 expression. Exp. Neurol. 2014, 261, 10–21. [Google Scholar] [CrossRef]
- Cantù, C.; Zimmerli, D.; Hausmann, G.; Valenta, T.; Moor, A.; Aguet, M.; Basler, K. Pax6-dependent, but β-catenin-independent, function of Bcl9 proteins in mouse lens development. Genes Dev. 2014, 28, 1879–1884. [Google Scholar] [CrossRef]
- Martín-Cámara, O.; Cores, Á.; López-Alvarado, P.; Menéndez, J.C. Emerging targets in drug discovery against neurodegenerative diseases: Control of synapsis disfunction by the RhoA/ROCK pathway. Eur. J. Med. Chem. 2021, 225, 113742. [Google Scholar] [CrossRef]
- Ishima, T.; Futamura, T.; Ohgi, Y.; Yoshimi, N.; Kikuchi, T.; Hashimoto, K. Potentiation of neurite outgrowth by brexpiprazole, a novel serotonin-dopamine activity modulator: A role for serotonin 5-HT1A and 5-HT2A receptors. Eur. Neuropsychopharmacol. 2015, 25, 505–511. [Google Scholar] [CrossRef]
- Kong, H.; Fan, Y.; Xie, J.; Ding, J.; Sha, L.; Shi, X.; Sun, X.; Hu, G. QP4 knockout impairs proliferation, migration and neuronal differentiation of adult neural stem cells. J. Cell Sci. 2008, 121, 4029–4036. [Google Scholar] [CrossRef]
- Zhuang, J.; Chen, Z.; Cai, P.; Wang, R.; Yang, Q.; Li, L.; Yang, H.; Zhu, R. Targeting microRNA-125b promotes neurite outgrowth but represses cell apoptosis and inflammation via blocking PTGS2 and CDK5 in a FOXQ1-dependent way in Alzheimer disease. Front. Cell Neurosci. 2020, 14, 587747. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca, M.Q.; Khalil, R.A. Vascular endothelin receptor type B: Structure, function and dysregulation in vascular disease. Biochem. Pharmacol. 2012, 84, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, S.S.; Huang, H.L.; Song, B.J.; Liu, X.; Qi, Z. Enhanced endothelin A and B receptor expression and receptor-mediated vasoconstriction in Rat mesenteric arteries after lipopolysaccharide challenge. Mediators Inflamm. 2019, 2019, 6248197. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Kaneko, M.; Yoshioka, K.; Obara, K.; Tanaka, Y. Platelet-activating factor (PAF) strongly enhances contractile mechanical activities in guinea pig and mouse urinary bladder. Sci. Rep. 2022, 12, 2783. [Google Scholar] [CrossRef]
- Drummond, G.R.C.T. Endothelium-dependent relaxations mediated by inducible B1 and constitutive B2 kinin receptors in the bovine isolated coronary artery. Br. J. Pharmacol. 1995, 116, 2473–2481. [Google Scholar] [CrossRef]
- Turaihi, A.H.; Bakker, W.; van Hinsbergh, V.W.M.; Serné, E.H.; Smulders, Y.M.; Niessen, H.W.M.; Eringa, E.C. Insulin receptor substrate 2 controls insulin-mediated vasoreactivity and perivascular adipose tissue function in muscle. Front. Physiol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Nakashima, A.; Kawamoto, T.; Noshiro, M.; Ueno, T.; Doi, S.; Honda, K.; Maruhashi, T.; Noma, K.; Honma, S.; Masaki, T.; et al. Dec1 and CLOCK regulate Na+/K+-ATPase β1 subunit expression and blood pressure. Hypertension 2018, 72, 746–754. [Google Scholar] [CrossRef]
- Stafford, N.; Wilson, C.; Oceandy, D.; Neyses, L.; Cartwright, E.J. The plasma membrane calcium ATPases and their role as major new players in human disease. Physiol. Rev. 2017, 97, 1089–1125. [Google Scholar] [CrossRef]
- Zhou, T.; Tang, H.; Han, Y.; Fraidenburg, D.; Kim, Y.W.; Lee, D.; Choi, J.; Bang, H.; Ko, J.H. Expression profile of mitochondrial voltage-dependent anion channel-1 (VDAC1) influenced genes is associated with pulmonary hypertension. Korean J. Physiol. Pharmacol. 2017, 21, 353–360. [Google Scholar] [CrossRef]
- Phan, H.T.N.; Jackson, W.F.; Shaw, V.S.; Watts, S.W.; Neubig, R.R. Loss-of-function mutations in human regulator of GP protein signaling RGS2 differentially regulate pharmacological reactivity of resistance vasculature. Mol. Pharmacol. 2019, 96, 826–834. [Google Scholar] [CrossRef]
- Sauzeau, V.; LeJeune, H.; Cario-Toumaniantz, C.; Smolenski, A.; Lohmann, S.M.; Bertoglio, J.; Chardin, P.; Pacaud, P.; Loirand, G. Cyclic GMP-dependent protein kinase signaling pathway inhibits Rho-A-induced Ca sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 2000, 275, 21722–21729. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.; Ha, S.H.; Chae, Y.C.; Lee, S.; Oh, Y.S.; Kim, Y.H.; Kim, S.H.; Kim, J.H.; Mizoguchi, A.; Itoh, T.J.; et al. RGS2 promotes formation of neurites by stimulating microtubule polymerization. Cell Signal. 2006, 18, 2182–2192. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Min, C.W.; Hyeon, H.; Gupta, R.; Park, J.; Cheon, Y.E.; Lee, G.H.; Jang, J.W.; Ryu, H.W.; Lee, B.W.; Park, S.U.; et al. Integrated proteomics and metabolomics analysis highlights correlative metabolite-protein networks in soybean seeds subjected to warm-water soaking. J. Agric. Food Chem. 2020, 68, 8057–8067. [Google Scholar] [CrossRef]
- Min, C.W.; Park, J.; Bae, J.W.; Agrawal, G.K.; Rakwal, R.; Kim, Y.; Yang, P.; Kim, S.T.; Gupta, R. In-depth investigation of low-abundance proteins in matured and filling stages seeds of Glycine max employing a combination of protamine sulfate precipitation and TMT-based quantitative proteomic analysis. Cells 2020, 9, 1517. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Park, J.; Han, D.; Yang, J.; Kim, A.; Woo, J.; Kim, Y.; Mook-Jung, I. Molecular and functional signatures in a novel Alzheimer’s disease mouse model assessed by quantitative proteomics. Mol. Neurodegener. 2018, 13, 2. [Google Scholar] [CrossRef]
- Pajarillo, E.A.B.; Kim, S.H.; Lee, J.Y.; Valeriano, V.D.V.; Kang, D.K. Quantitative proteogenomics and the reconstruction of the metabolic pathway in Lactobacillus mucosae LM1. Korean J. Food Sci. Anim. Resour. 2015, 35, 692–702. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Plubell, D.L.; Wilmarth, P.A.; Zhao, Y.; Fenton, A.M.; Minnier, J.; Reddy, A.P.; Klimek, J.; Yang, X.; David, L.L.; Pamir, N. Extended multiplexing of tandem mass tags (TMT) labeling reveals age and high fat diet specific proteome changes in mouse epididymal adipose tissue. Mol. Cell. Proteomics 2017, 16, 873–890. [Google Scholar] [CrossRef] [PubMed]
- Toth, D.; Tamas, A.; Reglodi, D. The neuroprotective and biomarker potential of PACAP in human traumatic brain injury. Int. J. Mol. Sci. 2020, 21, 827. [Google Scholar] [CrossRef] [PubMed]
- De Vries, L.; Zheng, B.; Fischer, T.; Elenko, E.; Farquhar, M.G. The regulator of G protein signaling family. Annu Rev. Pharmacol Toxicol. 2000, 40, 235–271. [Google Scholar] [CrossRef] [PubMed]
- Connell-Crowley, L.; Le Gall, M.; Vo, D.J.; Giniger, E. The cyclin-dependent kinase Cdk5 controls multiple aspects of axon patterning in vivo. Curr. Biol. 2000, 10, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Morfini, G.; Szebenyi, G.; Brown, H.; Pant, H.C.; Pigino, G.; DeBoer, S.; Beffert, U.; Brady, S.T. A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J. 2004, 23, 2235–2245. [Google Scholar] [CrossRef]
- Engmann, O.; Giese, K.P. Crosstalk between Cdk5 and GSK3beta: Implications for Alzheimer’s disease. Front. Mol. Neurosci. 2009, 2, 2. [Google Scholar] [CrossRef]
- Schaler, A.W.; Runyan, A.M.; Clelland, C.L.; Sydney, E.J.; Fowler, S.L.; Figueroa, H.Y.; Shioda, S.; Santa-Maria, I.; Duff, K.E.; Myeku, N. PAC1 receptor-mediated clearance of tau in postsynaptic compartments attenuates tau pathology in mouse brain. Sci. Transl. Med. 2021, 13, eaba7394. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibato, J.; Takenoya, F.; Yamashita, M.; Gupta, R.; Min, C.W.; Kim, S.T.; Kimura, A.; Takasaki, I.; Hori, M.; Shioda, S.; et al. OMICS Analyses Unraveling Related Gene and Protein-Driven Molecular Mechanisms Underlying PACAP 38-Induced Neurite Outgrowth in PC12 Cells. Int. J. Mol. Sci. 2023, 24, 4169. https://doi.org/10.3390/ijms24044169
Shibato J, Takenoya F, Yamashita M, Gupta R, Min CW, Kim ST, Kimura A, Takasaki I, Hori M, Shioda S, et al. OMICS Analyses Unraveling Related Gene and Protein-Driven Molecular Mechanisms Underlying PACAP 38-Induced Neurite Outgrowth in PC12 Cells. International Journal of Molecular Sciences. 2023; 24(4):4169. https://doi.org/10.3390/ijms24044169
Chicago/Turabian StyleShibato, Junko, Fumiko Takenoya, Michio Yamashita, Ravi Gupta, Cheol Woo Min, Sun Tae Kim, Ai Kimura, Ichiro Takasaki, Motohide Hori, Seiji Shioda, and et al. 2023. "OMICS Analyses Unraveling Related Gene and Protein-Driven Molecular Mechanisms Underlying PACAP 38-Induced Neurite Outgrowth in PC12 Cells" International Journal of Molecular Sciences 24, no. 4: 4169. https://doi.org/10.3390/ijms24044169
APA StyleShibato, J., Takenoya, F., Yamashita, M., Gupta, R., Min, C. W., Kim, S. T., Kimura, A., Takasaki, I., Hori, M., Shioda, S., & Rakwal, R. (2023). OMICS Analyses Unraveling Related Gene and Protein-Driven Molecular Mechanisms Underlying PACAP 38-Induced Neurite Outgrowth in PC12 Cells. International Journal of Molecular Sciences, 24(4), 4169. https://doi.org/10.3390/ijms24044169






