The APC/C Activator Cdh1p Plays a Role in Mitochondrial Metabolic Remodelling in Yeast
Abstract
1. Introduction
2. Results
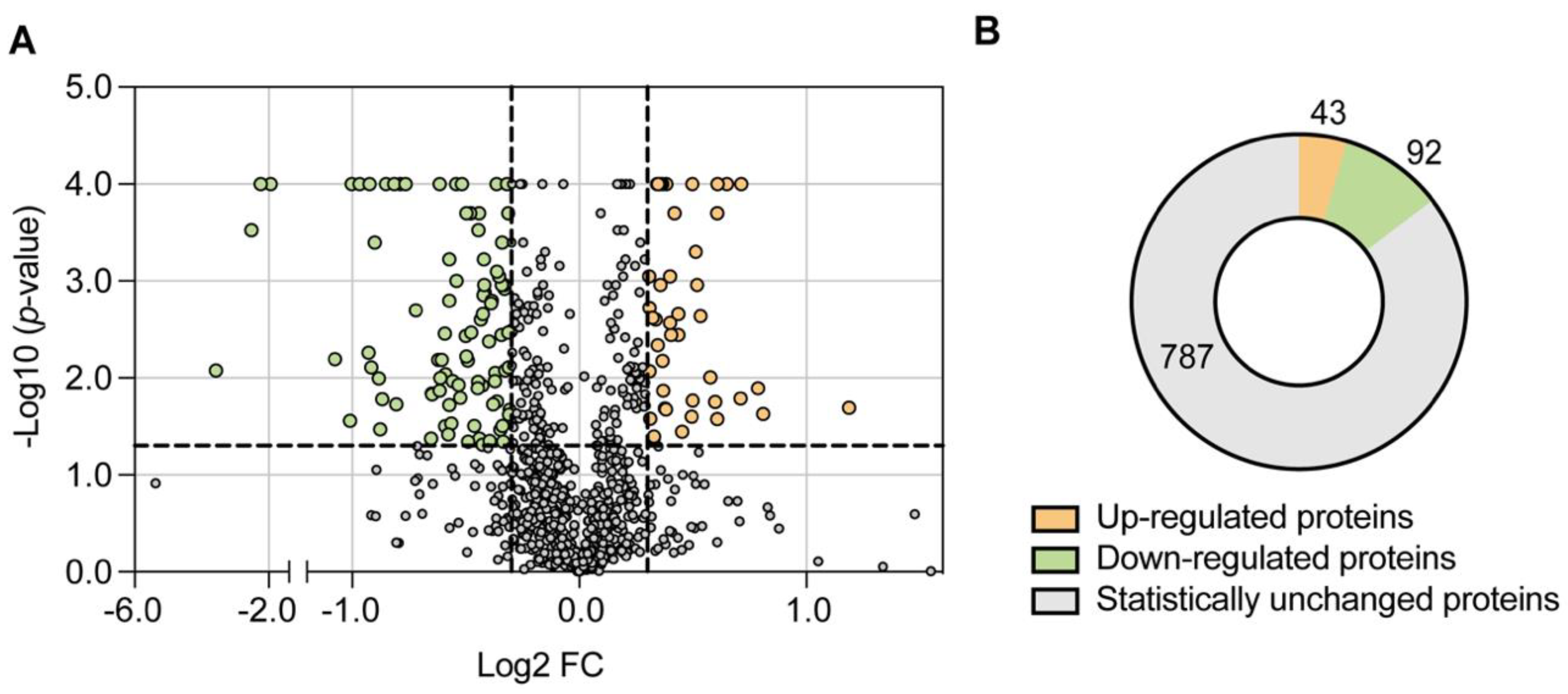
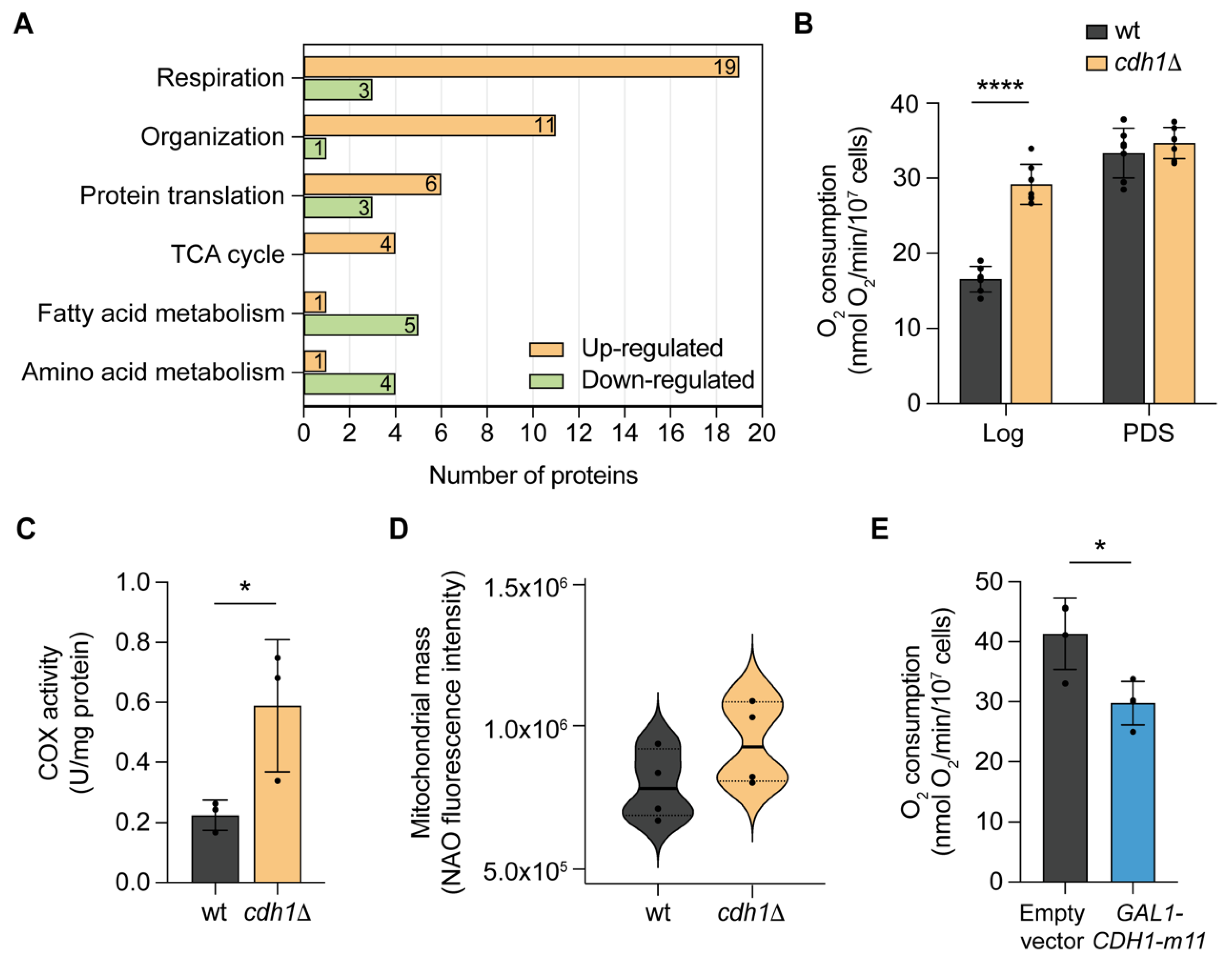
2.1. CDH1 Deletion Leads to a Remodelling of the Mitochondrial Proteome and Promotes Mitochondrial Respiration
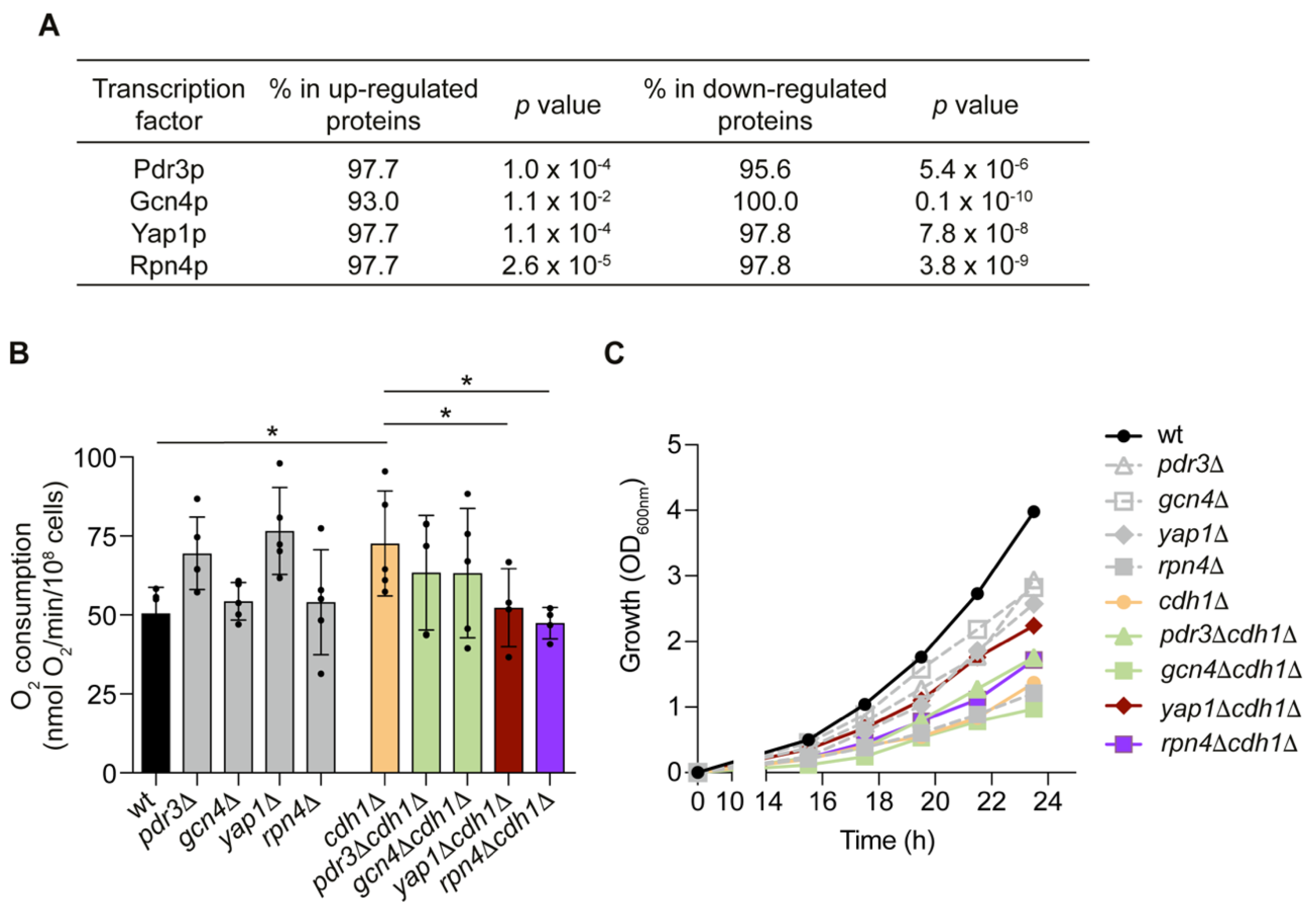
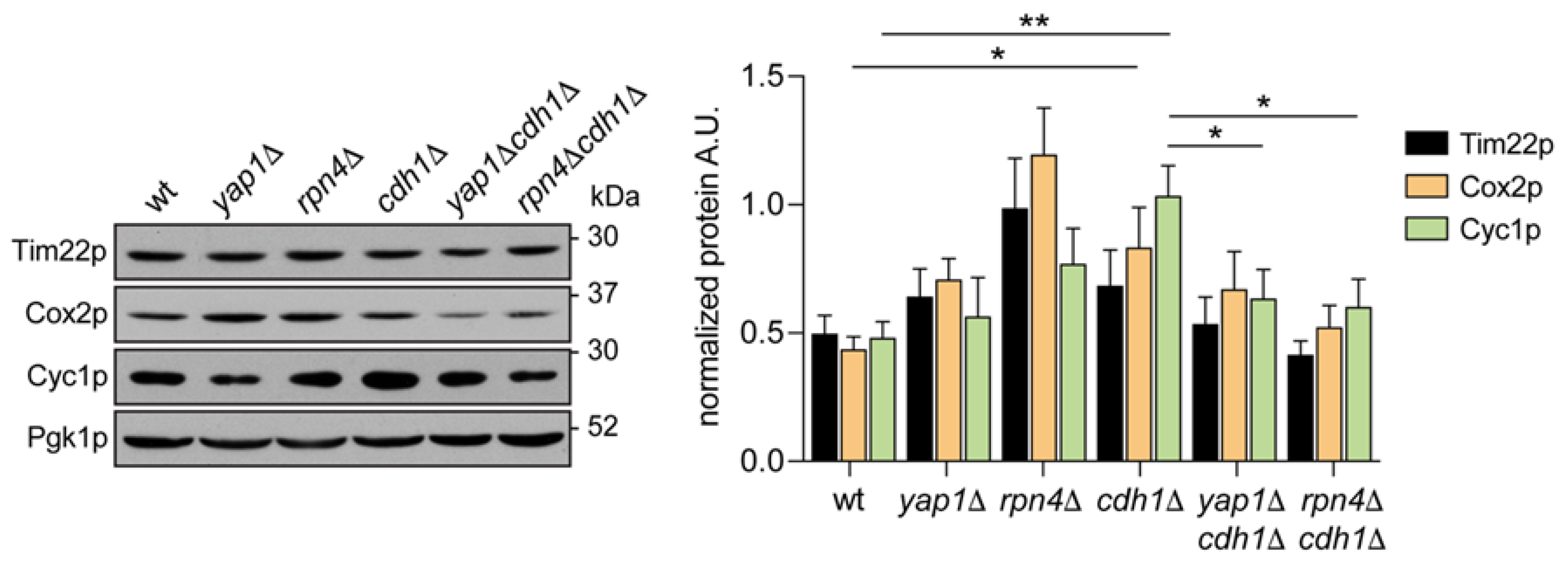
2.2. The Transcription Factors Yap1p and Rpn4p Mediate the Induction of Mitochondrial Respiration in cdh1Δ Cells
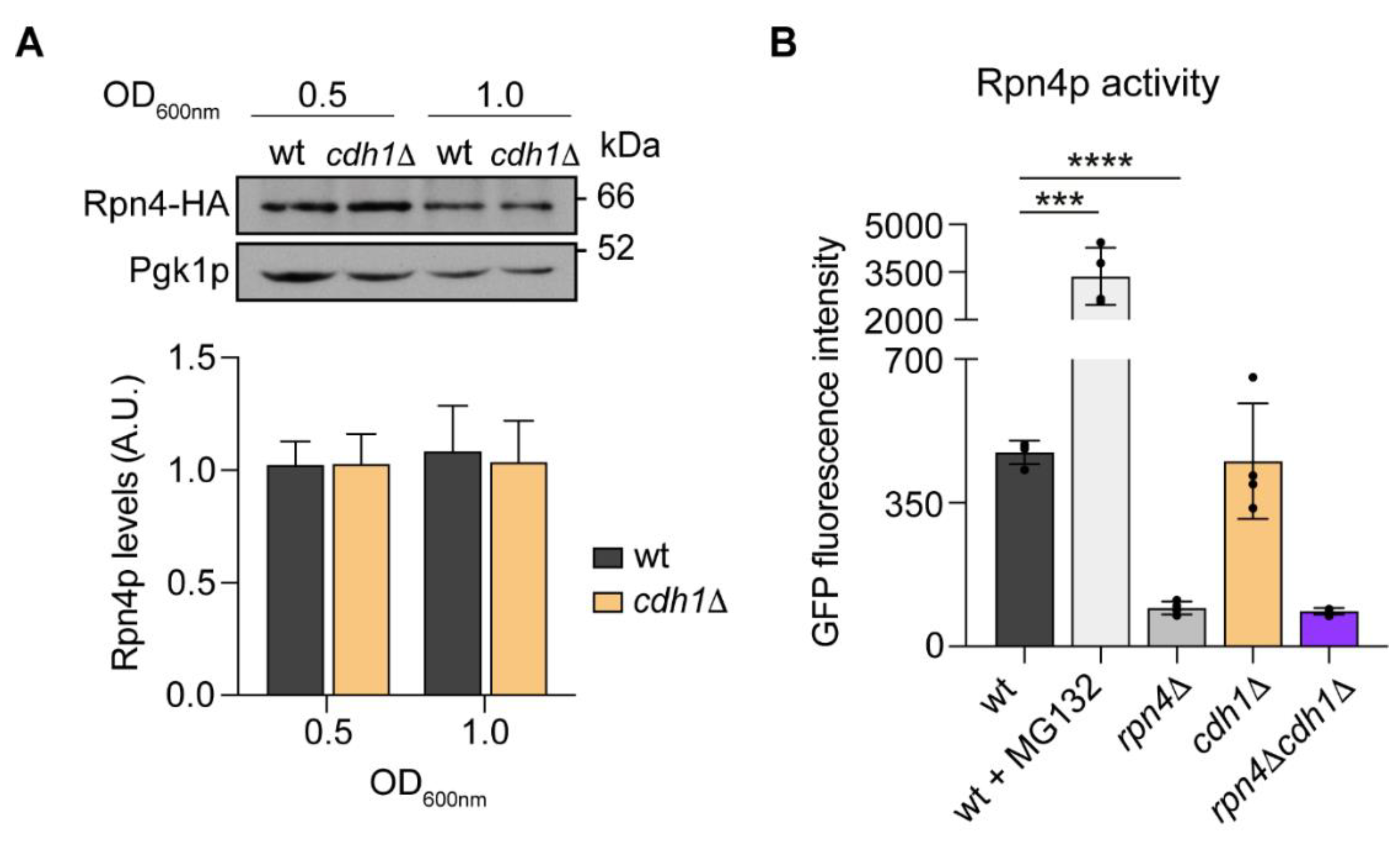
2.3. CDH1 Deletion Does Not Impact on Rpn4p Activity
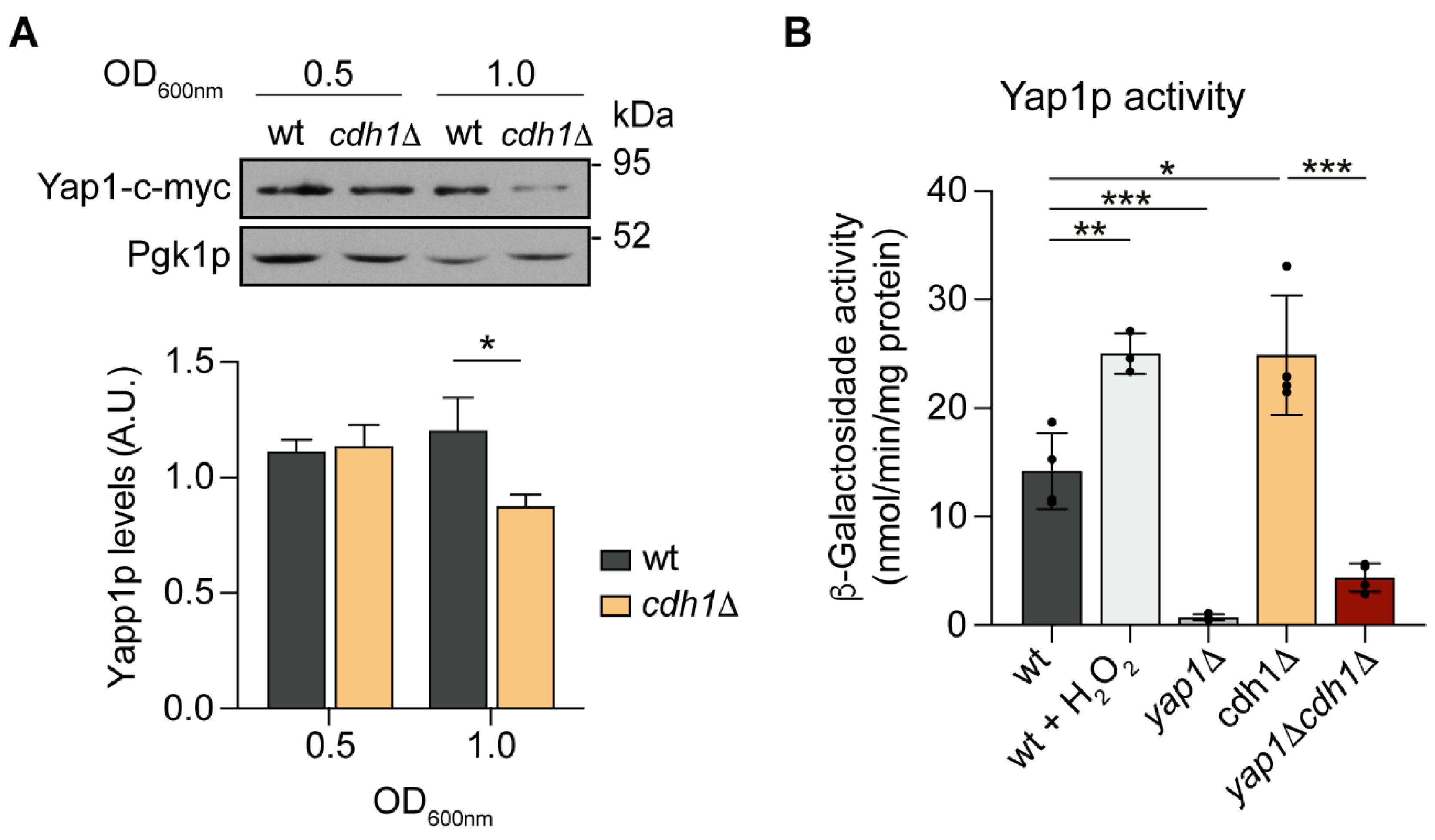
2.4. Yap1p Is More Active in cdh1∆ Cells
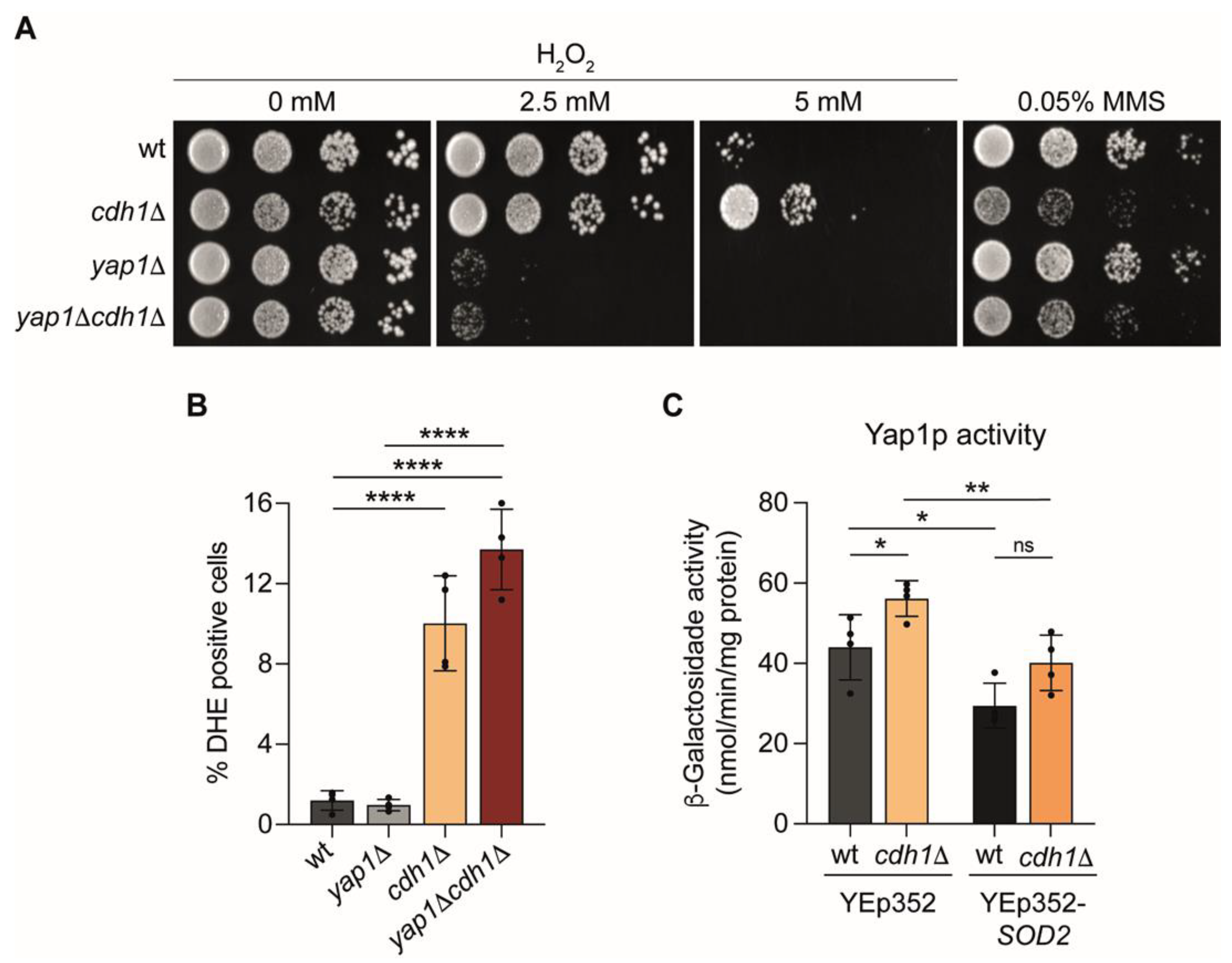
2.5. Yap1p Mediate the Oxidative Stress Resistance of cdh1∆ Cells
3. Discussion
4. Materials and Methods
4.1. Yeast Strains and Growth Conditions
4.2. Mitochondrial Isolation
4.3. Protein Identification by HPLC-MS/MS
4.4. Mitochondrial Mass Analysis
4.5. Oxygen Consumption Rate and COX Activity
4.6. SDS-PAGE and Western Blot
4.7. Fluorescent Reporter Assay Measurements
4.8. β-Galactosidase Assay
4.9. Oxidative Stress and DNA Damage Sensitivity
4.10. ROS Levels
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ulery, T.L.; Jang, S.H.; Jaehning, J.A. Glucose repression of yeast mitochondrial transcription: Kinetics of derepression and role of nuclear genes. Mol. Cell Biol. 1994, 14, 1160–1170. [Google Scholar] [CrossRef]
- Brauer, M.J.; Saldanha, A.J.; Dolinski, K.; Botstein, D. Homeostatic adjustment and metabolic remodeling in glucose-limited yeast cultures. Mol. Biol. Cell 2005, 16, 2503–2517. [Google Scholar] [CrossRef]
- Di Bartolomeo, F.; Malina, C.; Campbell, K.; Mormino, M.; Fuchs, J.; Vorontsov, E.; Gustafsson, C.M.; Nielsen, J. Absolute yeast mitochondrial proteome quantification reveals trade-off between biosynthesis and energy generation during diauxic shift. Proc. Natl. Acad. Sci. USA 2020, 117, 7524–7535. [Google Scholar] [CrossRef]
- Ohlmeier, S.; Kastaniotis, A.J.; Hiltunen, J.K.; Bergmann, U. The yeast mitochondrial proteome, a study of fermentative and respiratory growth. J. Biol. Chem. 2004, 279, 3956–3979. [Google Scholar] [CrossRef]
- Renvoisé, M.; Bonhomme, L.; Davanture, M.; Valot, B.; Zivy, M.; Lemaire, C. Quantitative variations of the mitochondrial proteome and phosphoproteome during fermentative and respiratory growth in Saccharomyces cerevisiae. J. Proteom. 2014, 106, 140–150. [Google Scholar] [CrossRef]
- Kayikci, Ö.; Nielsen, J. Glucose repression in Saccharomyces cerevisiae. FEMS Yeast Res. 2015, 15, fov068. [Google Scholar] [CrossRef]
- Chevtzoff, C.; Vallortigara, J.; Avéret, N.; Rigoulet, M.; Devin, A. The yeast cAMP protein kinase Tpk3p is involved in the regulation of mitochondrial enzymatic content during growth. Biochim. Biophys. Acta Bioenerg. 2005, 1706, 117–125. [Google Scholar] [CrossRef]
- Dejean, L.; Beauvoit, B.; Bunoust, O.; Guérin, B.; Rigoulet, M. Activation of Ras cascade increases the mitochondrial enzyme content of respiratory competent yeast. Biochem. Biophys. Res. Commun. 2002, 293, 1383–1388. [Google Scholar] [CrossRef]
- Bonawitz, N.D.; Chatenay-Lapointe, M.; Pan, Y.; Shadel, G.S. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007, 5, 265–277. [Google Scholar] [CrossRef]
- Pan, Y.; Shadel, G.S. Extension of chronological life span by reduced TOR signaling requires down-regulation of Sch9p and involves increased mitochondrial OXPHOS complex density. Aging (Albany NY) 2009, 1, 131–145. [Google Scholar] [CrossRef]
- Jablonka, W.; Guzmán, S.; Ramírez, J.; Montero-Lomelí, M. Deviation of carbohydrate metabolism by the SIT4 phosphatase in Saccharomyces cerevisiae. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Pereira, A.T.; Osório, H.; Moradas-Ferreira, P.; Costa, V. Sit4p-mediated dephosphorylation of Atp2p regulates ATP synthase activity and mitochondrial function. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.C.; Martins, T.S.; Campos, A.; Costa, V.; Pereira, C. Phosphoregulation of the ATP synthase beta subunit stimulates mitochondrial activity for G2/M progression. Adv. Biol. Regul. 2022, 85, 100905. [Google Scholar] [CrossRef] [PubMed]
- King, R.W.; Peters, J.M.; Tugendreich, S.; Rolfe, M.; Hieter, P.; Kirschner, M.W. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 1995, 81, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Sudakin, V.; Ganoth, D.; Dahan, A.; Heller, H.; Hershko, J.; Luca, F.C.; Ruderman, J.V.; Hershko, A. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol. Biol. Cell 1995, 6, 185–197. [Google Scholar] [CrossRef]
- Visintin, R.; Prinz, S.; Amon, A. CDC20 and CDH1: A family of substrate-specific activators of APC-dependent proteolysis. Science 1997, 278, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.; Neutzner, M.; Möcker, D.; Seufert, W. Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. EMBO J. 2001, 20, 5165–5175. [Google Scholar] [CrossRef]
- Burton, J.L.; Solomon, M.J. D box and KEN box motifs in budding yeast Hsl1p are required for APC-mediated degradation and direct binding to Cdc20p and Cdh1p. Genes Dev. 2001, 15, 2381–2395. [Google Scholar] [CrossRef]
- Simpson-Lavy, K.J.; Sajman, J.; Zenvirth, D.; Brandeis, M. APC/CCdh1 specific degradation of Hsl1 and Clb2 is required for proper stress responses of S. cerevisiae. Cell Cycle 2009, 8, 3003–3009. [Google Scholar] [CrossRef]
- Thornton, B.R.; Toczyski, D.P. Securin and B-cyclin/CDK are the only essential targets of the APC. Nat. Cell Biol. 2003, 5, 1090–1094. [Google Scholar] [CrossRef]
- Li, M.; Zhang, P. The function of APC/CCdh1 in cell cycle and beyond. Cell Div. 2009, 4, 2. [Google Scholar] [CrossRef]
- Morgenstern, M.; Stiller, S.B.; Lübbert, P.; Peikert, C.D.; Dannenmaier, S.; Drepper, F.; Weill, U.; Höß, P.; Feuerstein, R.; Gebert, M.; et al. Definition of a high-confidence mitochondrial proteome at quantitative scale. Cell Rep. 2017, 19, 2836–2852. [Google Scholar] [CrossRef]
- Vögtle, F.N.; Burkhart, J.M.; Gonczarowska-Jorge, H.; Kücükköse, C.; Taskin, A.A.; Kopczynski, D.; Ahrends, R.; Mossmann, D.; Sickmann, A.; Zahedi, R.P.; et al. Landscape of submitochondrial protein distribution. Nat. Commun. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Casanovas, A.; Sprenger, R.R.; Tarasov, K.; Ruckerbauer, D.E.; Hannibal-Bach, H.K.; Zanghellini, J.; Jensen, O.N.; Ejsing, C.S. Quantitative analysis of proteome and lipidome dynamics reveals functional regulation of global lipid metabolism. Chem. Biol. 2015, 22, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Zachariae, W.; Schwab, M.; Nasmyth, K.; Seufert, W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science 1998, 282, 1721–1724. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Viana, R.; Palma, M.; Oliveira, J.; Galocha, M.; Mota, M.N.; Couceiro, D.; Pereira, M.G.; Antunes, M.; Costa, I.V.; et al. YEASTRACT+: A portal for the exploitation of global transcription regulation and metabolic model data in yeast biotechnology and pathogenesis. Nucleic Acids Res. 2022, 51, gkac1041. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.A.; Lightcap, E.S.; Sadis, S.; Thoroddsen, V.; Bulawa, C.E.; Blackman, R.K. Complementary whole-genome technologies reveal the cellular response to proteasome inhibition by PS-341. Proc. Natl. Acad. Sci. USA 2002, 99, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Owsianik, G.; Balzi, L.; Ghislain, M. Control of 26S proteasome expression by transcription factors regulating multidrug resistance in Saccharomyces cerevisiae. Mol. Microbiol. 2002, 43, 1295–1308. [Google Scholar] [CrossRef]
- Mannhaupt, G.; Schnall, R.; Karpov, V.; Vetter, I.; Feldmann, H. Rpn4p acts as a transcription factor by binding to PACE, a nonamer box found upstream of 26S proteasomal and other genes in yeast. FEBS Lett. 1999, 450, 27–34. [Google Scholar] [CrossRef]
- Xie, Y.; Varshavsky, A. RPN4 is a ligand, substrate, and transcriptional regulator of the 26S proteasome: A negative feedback circuit. Proc. Natl. Acad. Sci. USA 2001, 98, 3056–3061. [Google Scholar] [CrossRef] [PubMed]
- Dohmen, R.J.; Willers, I.; Marques, A.J. Biting the hand that feeds: Rpn4-dependent feedback regulation of proteasome function. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 1599–1604. [Google Scholar] [CrossRef]
- Work, J.J.; Brandman, O. Adaptability of the ubiquitin-proteasome system to proteolytic and folding stressors. J. Cell Biol. 2020, 220, e201912041. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, A.; Pflieger, D.; Barrault, M.-B.; Vinh, J.; Toledano, M.B. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 2002, 111, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Wemmie, J.A.; Steggerda, S.M.; Moye-Rowley, W.S. The Saccharomyces cerevisiae AP-1 protein discriminates between oxidative stress elicited by the oxidants H2O2 and diamide. J. Biol. Chem. 1997, 272, 7908–7914. [Google Scholar] [CrossRef] [PubMed]
- Kuge, S.; Jones, N.; Nomoto, A. Regulation of yAP-1 nuclear localization in response to oxidative stress. EMBO J. 1997, 16, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.T.; Epping, E.A.; Steggerda, S.M.; Moye-Rowley, W.S. Yap1p activates gene transcription in an oxidant-specific Fashion. Mol. Cell Biol. 1999, 19, 8302–8313. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bertram, S.; Kaplan, J.; Jia, X.; Ward, D.M. The mitochondrial iron exporter genes MMT1 and MMT2 in yeast are transcriptionally regulated by Aft1 and Yap1. J. Biol. Chem. 2020, 295, 1716–1726. [Google Scholar] [CrossRef]
- Zyrina, A.N.; Smirnova, E.A.; Markova, O.V.; Severin, F.F.; Knorre, D.A. Mitochondrial superoxide dismutase and Yap1p act as a signaling module contributing to ethanol tolerance of the yeast Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2017, 83, e02759–e02716. [Google Scholar] [CrossRef]
- Jun, H.; Kieselbach, T.; Jönsson, L.J. Comparative proteome analysis of Saccharomyces cerevisiae: A global overview of in vivo targets of the yeast activator protein 1. BMC Genom. 2012, 13, 230. [Google Scholar] [CrossRef]
- Maeta, K.; Izawa, S.; Okazaki, S.; Kuge, S.; Inoue, Y. Activity of the Yap1 transcription factor in Saccharomyces cerevisiae is modulated by methylglyoxal, a metabolite derived from glycolysis. Mol. Cell Biol. 2004, 24, 8753–8764. [Google Scholar] [CrossRef] [PubMed]
- Gulshan, K.; Thommandru, B.; Moye-Rowley, W.S. Proteolytic degradation of the Yap1 transcription factor is regulated by subcellular localization and the E3 ubiquitin ligase Not4. J. Biol. Chem. 2012, 287, 26796–26805. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.A.; Stanley, B.A.; Sivakumaran, V.; Kembro, J.M.; O’Rourke, B.; Paolocci, N.; Cortassa, S. Glutathione/thioredoxin systems modulate mitochondrial H2O2 emission: An experimental-computational study. J. Gen. Physiol. 2012, 139, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Vilaça, R.; Silva, E.; Nadais, A.; Teixeira, V.; Matmati, N.; Gaifem, J.; Hannun, Y.A.; Miranda, M.C.S.; Costa, V. Sphingolipid signaling mediates mitochondrial dysfunctions and reduced chronological lifespan in the yeast model of Niemann-Pick type C1. Mol. Microbiol. 2014, 91, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.R.; Thomenius, M.J.; Johnson, E.S.; Freel, C.D.; Wu, J.Q.; Coloff, J.L.; Yang, C.-S.; Tang, W.; An, J.; Ilkayeva, O.R.; et al. Regulation of mitochondrial morphology by APC/CCdh1-mediated control of Drp1 stability. Mol. Biol. Cell 2011, 22, 1207–1216. [Google Scholar] [CrossRef]
- Lambhate, S.; Bhattacharjee, D.; Jain, N. APC/C CDH1 ubiquitinates IDH2 contributing to ROS increase in mitosis. Cellular Signal. 2021, 86, 110087. [Google Scholar] [CrossRef]
- Pfleger, C.M.; Kirschner, M.W. The KEN box: An APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000, 14, 655–665. [Google Scholar] [CrossRef]
- Yokoyama, H.; Mizunuma, M.; Okamoto, M.; Yamamoto, J.; Hirata, D.; Miyakawa, T. Involvement of calcineurin-dependent degradation of Yap1p in Ca2+- induced G2 cell-cycle regulation in Saccharomyces cerevisiae. EMBO Rep. 2006, 7, 519–524. [Google Scholar] [CrossRef]
- Harbauer, A.B.; Opalińska, M.; Gerbeth, C.; Herman, J.S.; Rao, S.; Schönfisch, B.; Guiard, B.; Schmidt, O.; Pfanner, N.; Meisinger, C. Cell cycle–dependent regulation of mitochondrial preprotein translocase. Science 2014, 346, 1109–1113. [Google Scholar] [CrossRef]
- Boy-Marcotte, E.; Perrot, M.; Bussereau, F.; Boucherie, H.; Jacquet, M. Msn2p and Msn4p control a large number of genes induced at the diauxic transition which are repressed by cyclic AMP in Saccharomyces cerevisiae. J. Bacteriol. 1998, 180, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Haurie, V.; Perrot, M.; Mini, T.; Jenö, P.; Sagliocco, F.; Boucherie, H. The transcriptional activator Cat8p provides a major contribution to the reprogramming of carbon metabolism during the diauxic shift in Saccharomyces cerevisiae. J. Biol. Chem. 2001, 276, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Vincent, O.; Carlson, M. Sip4, a Snf1 kinase-dependent transcriptional activator, binds to the carbon source-responsive element of gluconeogenic genes. EMBO J. 1998, 17, 7002–7008. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, D.J. Saccharomyces cerevisiae has distinct adaptive responses to both hydrogen peroxide and menadione. J. Bacteriol. 1992, 174, 6678–6681. [Google Scholar] [CrossRef]
- Stephen, D.W.S.; Rivers, S.L.; Jamieson, D.J. The role of the YAP1 and YAP2 genes in the regulation of the adaptive oxidative stress responses of Saccharomyces cerevisiae. Mol. Microbiol. 1995, 16, 415–423. [Google Scholar] [CrossRef]
- Chen, Z.; Odstrcil, E.A.; Tu, B.P.; McKnight, S.L. Restriction of DNA replication to the reductive phase of the metabolic cycle protects genome integrity. Science 2007, 316, 1916–1919. [Google Scholar] [CrossRef] [PubMed]
- Flattery-O′Brien, J.A.; Dawes, I.W. Hydrogen peroxide causes RAD9-dependent cell cycle arrest in G2 in Saccharomyces cerevisiae whereas menadione causes G1 arrest independent of RAD9 function. J. Biol. Chem. 1998, 273, 8564–8571. [Google Scholar] [CrossRef] [PubMed]
- Ostapenko, D.; Burton, J.L.; Solomon, M.J. Identification of anaphase promoting complex substrates in S. cerevisiae. PLoS ONE 2012, 7, e45895. [Google Scholar] [CrossRef]
- Gietz, R.D.; Schiestl, R.H. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2007, 2, 31–34. [Google Scholar] [CrossRef]
- Lange, H.; Kispal, G.; Lill, R. Mechanism of iron transport to the site of heme synthesis inside yeast mitochondria. J. Biol. Chem. 1999, 274, 18989–18996. [Google Scholar] [CrossRef]
- Hughes, C.S.; Moggridge, S.; Müller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef]
- Osório, H.; Silva, C.; Ferreira, M.; Gullo, I.; Máximo, V.; Barros, R.; Mendonça, F.; Oliveira, C.; Carneiro, F. Proteomics analysis of gastric cancer patients with Diabetes Mellitus. J. Clin. Med. 2021, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.V.; Samaddar, M.; Sinha, D.; Purushotham, J.; D’Silva, P. Enhanced J-protein interaction and compromised protein stability of mtHsp70 variants lead to mitochondrial dysfunction in Parkinson’s disease. Hum. Mol. Genet. 2012, 21, 3317–3332. [Google Scholar] [CrossRef]
- Poyton, R.O.; Goehring, B.; Droste, M.; Sevarino, K.A.; Allen, L.A.; Zhao, X.-J. Cytochrome-c oxidase from Saccharomyces cerevisiae. In Methods in Enzymology; Giuseppe, M.A., Anne, C., Eds.; Academic Press: Cambridge, MA, USA, 1995; Volume 260, pp. 97–116. [Google Scholar]
- Almeida, T.; Marques, M.; Mojzita, D.; Amorim, M.A.; Silva, R.D.; Almeida, B.; Rodrigues, P.; Ludovico, P.; Hohmann, S.; Moradas-Ferreira, P.; et al. Isc1p plays a key role in hydrogen peroxide resistance and chronological lifespan through modulation of iron levels and apoptosis. Mol. Biol. Cell 2007, 19, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Hewapathirana, S.; García-Seisdedos, D.; Kamatchinathan, S.; Kundu, D.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A Hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, A.C.; Barbedo, M.; Costa, V.; Pereira, C. The APC/C Activator Cdh1p Plays a Role in Mitochondrial Metabolic Remodelling in Yeast. Int. J. Mol. Sci. 2023, 24, 4111. https://doi.org/10.3390/ijms24044111
Leite AC, Barbedo M, Costa V, Pereira C. The APC/C Activator Cdh1p Plays a Role in Mitochondrial Metabolic Remodelling in Yeast. International Journal of Molecular Sciences. 2023; 24(4):4111. https://doi.org/10.3390/ijms24044111
Chicago/Turabian StyleLeite, Ana Cláudia, Maria Barbedo, Vítor Costa, and Clara Pereira. 2023. "The APC/C Activator Cdh1p Plays a Role in Mitochondrial Metabolic Remodelling in Yeast" International Journal of Molecular Sciences 24, no. 4: 4111. https://doi.org/10.3390/ijms24044111
APA StyleLeite, A. C., Barbedo, M., Costa, V., & Pereira, C. (2023). The APC/C Activator Cdh1p Plays a Role in Mitochondrial Metabolic Remodelling in Yeast. International Journal of Molecular Sciences, 24(4), 4111. https://doi.org/10.3390/ijms24044111







