Norepinephrine and Vasopressin in Hemorrhagic Shock: A Focus on Renal Hemodynamics
Abstract
1. Introduction
2. Pathophysiology of Hemorrhagic Shock: Why Could We Need Vasopressor?
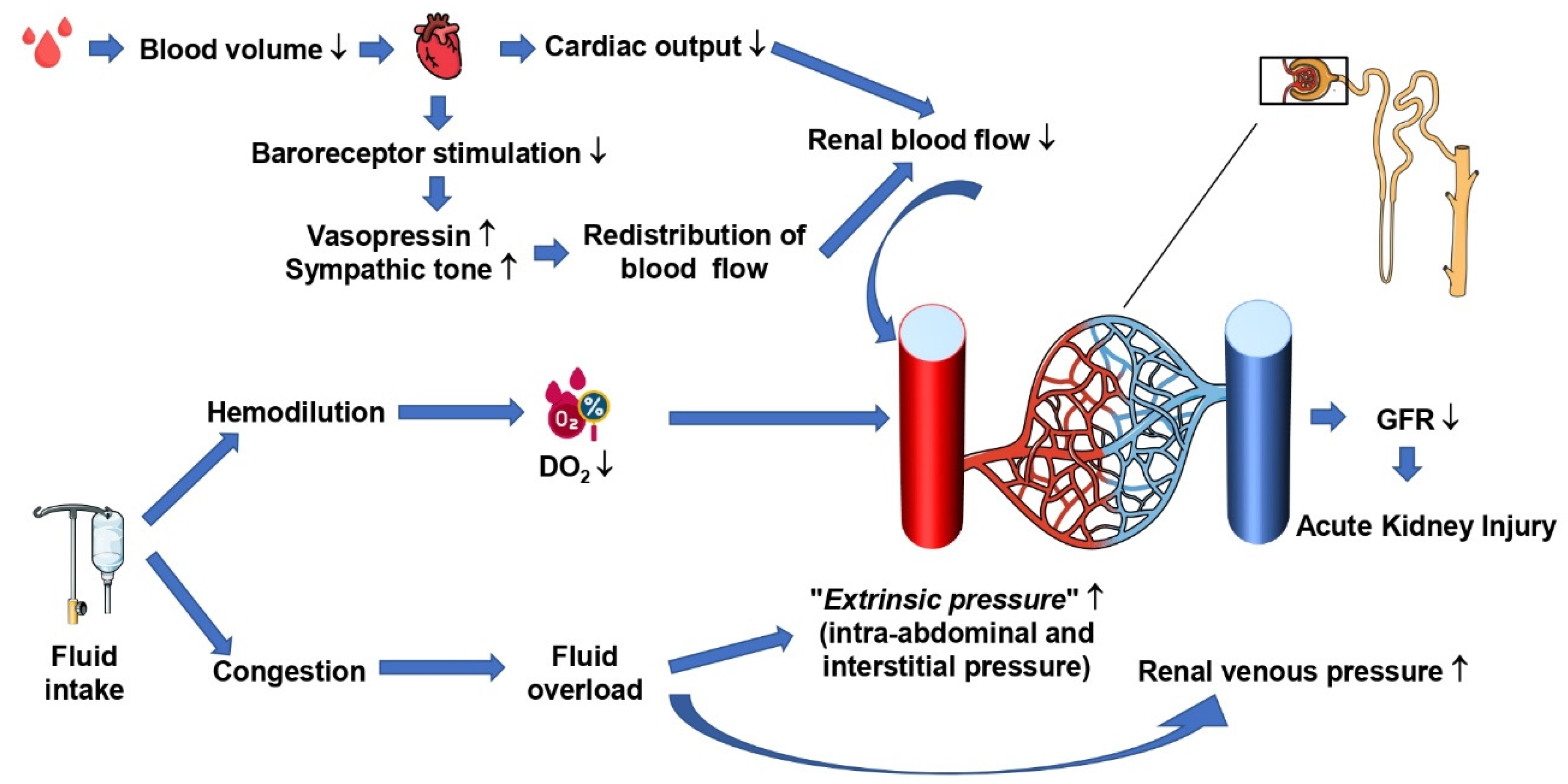
3. Hemorrhagic Shock and Acute Kidney Injury
4. Renal Hemodynamics and Autoregulation
5. Rationale for the Use of Norepinephrine in Patients with Hemorrhagic Shock
6. Renal Hemodynamic Effects of Norepinephrine during Hemorrhagic Shock
7. Rationale for the Use of Vasopressin in Patients with Hemorrhagic Shock
8. Renal Hemodynamic Effects of Vasopressin during Hemorrhagic Shock
9. Potential Adverse Effect of Vasopressor during Hemorrhagic Shock
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | Acute kidney injury |
| CO | Cardiac output |
| DO2 | Oxygen delivery |
| DO2R | Renal oxygen delivery |
| GFR | Glomerular filtration rate |
| I/R | Ischemia/Reperfusion |
| MAP | Mean arterial pressure |
| PμvO2 | Microvascular O2 partial pressure |
| RBF | Renal blood flow |
| SAP | Systolic arterial pressure |
| VO2R | Renal oxygen consumption |
References
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Asfar, P.; May, C. A Rationale for the Use of Norepinephrine after the Control of Bleeding in Hemorrhagic Shock? Am. J. Respir. Crit. Care Med. 2022, 206, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Schadt, J.C.; Ludbrook, J. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am. J. Physiol.-Heart Circ. Physiol. 1991, 260 Pt 2, H305–H318. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.; Fritz, C.; Tahon, E.; Jacquot, A.; Auchet, T.; Kimmoun, A. Vasoplegia treatments: The past, the present, and the future. Crit. Care 2018, 22, 52. [Google Scholar] [CrossRef] [PubMed]
- Douzinas, E.E.; Livaditi, O.; Tasoulis, M.-K.; Prigouris, P.; Bakos, D.; Goutas, N.; Vlachodimitropoulos, D.; Andrianakis, I.; Betrosian, A.; Tsoukalas, G.D. Nitrosative and Oxidative Stresses Contribute to Post-Ischemic Liver Injury Following Severe Hemorrhagic Shock: The Role of Hypoxemic Resuscitation. PLoS ONE 2012, 7, e32968. [Google Scholar] [CrossRef]
- Baker, T.A.; Romero, J.; Bach, H.H.; Strom, J.A.; Gamelli, R.L.; Majetschak, M. Systemic release of cytokines and heat shock proteins in porcine models of polytrauma and hemorrhage. Crit. Care Med. 2012, 40, 876–885. [Google Scholar] [CrossRef]
- Vatner, S.F.; Braunwald, E. Cardiovascular Control Mechanisms in the Conscious State. N. Engl. J. Med. 1975, 293, 970–976. [Google Scholar] [CrossRef]
- Legrand, M.; Mik, E.G.; Balestra, G.M.; Lutter, R.; Pirracchio, R.; Payen, D.; Ince, C. Fluid resuscitation does not improve renal oxygenation during hemorrhagic shock in rats. Anesthesiology 2010, 112, 119–127. [Google Scholar] [CrossRef]
- Ergin, B.; Kapucu, A.; Guerci, P.; Ince, C. The role of bicarbonate precursors in balanced fluids during haemorrhagic shock with and without compromised liver function. Br. J. Anaesth. 2016, 117, 521–528. [Google Scholar] [CrossRef]
- Nelimarkka, O.; Halkola, L.; Niinikoski, J. Renal hypoxia and lactate metabolism in hemorrhagic shock in dogs. Crit. Care Med. 1984, 12, 656–660. [Google Scholar] [CrossRef]
- Mayeur, N.; Minville, V.; Jaafar, A.; Allard, J.; al Saati, T.; Guilbeau-Frugier, C.; Fourcade, O.; Girolami, J.P.; Schaak, S.; Tack, I. Morphologic and functional renal impact of acute kidney injury after prolonged hemorrhagic shock in mice. Crit. Care Med. 2011, 39, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Rector, J.B.; Stein, J.H.; Bay, W.H.; Osgood, R.W.; Ferris, T.F. Effect of hemorrhage and vasopressor agents on distribution of renal blood flow. Am. J. Physiol.-Leg. Content 1972, 222, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Van Bommel, J.; Siegemund, M.; Henny, C.P.; Ince, C. Heart, kidney, and intestine have different tolerances for anemia. Transl. Res. 2008, 151, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Johannes, T.; Mik, E.G.; Nohé, B.; Unertl, K.E.; Ince, C. Acute decrease in renal microvascular Po2 during acute normovolemic hemodilution. Am. J. Physiol.-Ren. Physiol. 2007, 292, F796–F803. [Google Scholar] [CrossRef] [PubMed]
- Konrad, F.M.; Mik, E.G.; Bodmer, S.I.A.; Ates, N.B.; Willems, H.F.E.M.; Klingel, K.; de Geus, H.R.H.; Stolker, R.J.; Johannes, T. Acute Normovolemic Hemodilution in the Pig Is Associated with Renal Tissue Edema, Impaired Renal Microvascular Oxygenation, and Functional Loss. Anesthesiology 2013, 119, 256–269. [Google Scholar] [CrossRef]
- Wang, L.; Pei, F.; Wu, J.; Ouyang, B.; Guan, X. Kidney Injury in a Hemodilution Model of Hemorrhagic Shock and Fluid Resuscitation. Am. J. Med. Sci. 2021, 362, 506–511. [Google Scholar] [CrossRef]
- Harrois, A.; Libert, N.; Duranteau, J. Acute kidney injury in trauma patients. Curr. Opin. Crit. Care 2017, 23, 447–456. [Google Scholar] [CrossRef]
- Kirchheim, H.R.; Ehmke, H.; Hackenthal, E.; Löwe, W.; Persson, P. Autoregulation of renal blood flow, glomerular filtration rate and renin release in conscious dogs. Pflügers Arch. 1987, 410, 441–449. [Google Scholar] [CrossRef]
- Finke, R.; Gross, R.; Hackenthal, E.; Huber, J.; Kirchheim, H.R. Threshold pressure for the pressure-dependent renin release in the autoregulating kidney of conscious dogs. Pflügers Arch. 1983, 399, 102–110. [Google Scholar] [CrossRef]
- Kremser, P.C.; Gewertz, B.L. Effect of pentobarbital and hemorrhage on renal autoregulation. Am. J. Physiol.-Ren. Physiol. 1985, 249, F356–F360. [Google Scholar] [CrossRef]
- Johnson, P.C. The Myogenic Response. In The Resistance Vasculature: A Publication of the University of Vermont Center for Vascular Research [Internet]; Bevan, J.A., Halpern, W., Mulvany, M.J., Eds.; Vascular Biomedicine; Humana Press: Totowa, NJ, USA, 1991; pp. 159–168. [Google Scholar] [CrossRef]
- Harder, D.R.; Gilbert, R.; Lombard, J.H. Vascular muscle cell depolarization and activation in renal arteries on elevation of transmural pressure. Am. J. Physiol.-Ren. Physiol. 1987, 253, F778–F781. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Regulation of Renal Blood Flow by Plasma Chloride. J. Clin. Investig. 1983, 71, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Semler, M.W.; Self, W.H.; Wanderer, J.P.; Ehrenfeld, J.M.; Wang, L.; Byrne, D.W.; Stollings, J.L.; Kumar, A.B.; Hughes, C.G.; Hernandez, A.; et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N. Engl. J. Med. 2018, 378, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Schetz, M. Vasopressors and the kidney. Blood Purif. 2002, 20, 243–251. [Google Scholar] [CrossRef]
- Anderson, W.P.; Korner, P.I.; Selig, S.E. Mechanisms involved in the renal responses to intravenous and renal artery infusions of noradrenaline in conscious dogs. J. Physiol. 1981, 321, 21–30. [Google Scholar] [CrossRef]
- Bellomo, R.; Kellum, J.A.; Wisniewski, S.R.; Pinsky, M.R.; Ondulik, B. Effects of Norepinephrine on the Renal Vasculature in Normal and Endotoxemic Dogs. Am. J. Respir. Crit. Care Med. 1999, 159, 1186–1192. [Google Scholar] [CrossRef]
- Schaer, G.L.; Fink, M.P.; Parrillo, J.E. Norepinephrine alone versus norepinephrine plus low-dose dopamine: Enhanced renal blood flow with combination pressor therapy. Crit. Care Med. 1985, 13, 492–496. [Google Scholar] [CrossRef]
- Booke, M.; Hinder, F.; McGuire, R.; Traber, L.D.; Traber, D.L. Noradrenaline and nomega-monomethyl-L-arginine (L-NMMA): Effects on haemodynamics and regional blood flow in healthy and septic sheep. Clin. Sci. 2000, 98, 193–200. [Google Scholar] [CrossRef]
- Mills, L.C.; Moyer, J.H.; Handley, C.A. Effects of various sympathicomimetic drugs on renal hemodynamics in normotensive and hypotensive dogs. Am. J. Physiol.-Leg. Content 1960, 198, 1279–1283. [Google Scholar] [CrossRef]
- Peng, Z.-Y.; Critchley, L.A.H.; Fok, B.S.P. The effects of increasing doses of noradrenaline on systemic and renal circulations in acute bacteraemic dogs. Intensive Care Med. 2005, 31, 1558–1563. [Google Scholar] [CrossRef]
- Maier, M.; Starlinger, M.; Wagner, M.; Meyer, D.; Binder, B.R. The effect of hemorrhagic hypotension on urinary kallikrein excretion, renin activity, and renal cortical blood flow in the pig. Circ. Res. 1981, 48, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Bersten, A.D.; Holt, A.W. Vasoactive drugs and the importance of renal perfusion pressure. New Horiz. 1995, 3, 650–661. [Google Scholar] [PubMed]
- Redfors, B.; Bragadottir, G.; Sellgren, J.; Swärd, K.; Ricksten, S.-E. Effects of norepinephrine on renal perfusion, filtration and oxygenation in vasodilatory shock and acute kidney injury. Intensive Care Med. 2011, 37, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Vatner, S.F. Effects of hemorrhage on regional blood flow distribution in dogs and primates. J. Clin. Investig. 1974, 54, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.L.; Adams, F.F.; Bell, P.D.; Navar, L.G. Impaired renal blood flow autoregulation in ischemic acute renal failure. Kidney Int. 1980, 18, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Pullman, T.N.; Mcclure, W.W. The Response of the Renal Circulation in Man to Constant-Speed Infusions of l -Norepinephrine. Circulation 1954, 9, 600–605. [Google Scholar] [CrossRef]
- Gombos, E.A.; Hulet, W.H.; Bopp, P.; Goldring, W.; Baldwin, D.S.; Chasis, H. Reactivity of renal and systemic circulations to vasoconstrictor agents in normotensive and hypertensive subjects. J. Clin. Investig. 1962, 41, 203–217. [Google Scholar] [CrossRef]
- Lang, C.C.; Rahman, A.R.; Balfour, D.J.K.; Struthers, A.D. Effect of Noradrenaline on Renal Sodium and Water Handling in Euhydrated and Overhydrated Man. Clin. Sci. 1993, 85, 487–494. [Google Scholar] [CrossRef]
- Richer, M.; Robert, S.; Lebel, M. Renal hemodynamics during norepinephrine and low-dose dopamine infusions in man. Crit. Care Med. 1996, 24, 1150–1156. [Google Scholar] [CrossRef]
- Hoogenberg, K.; Smit, A.J.; Girbes, A.R.J. Effects of low-dose dopamine on renal and systemic hemodynamics during incremental norepinephrine infusion in healthy volunteers. Crit. Care Med. 1998, 26, 260–265. [Google Scholar] [CrossRef]
- Myers, B.D.; Deen, W.M.; Brenner, B.M. Effects of norepinephrine and angiotensin II on the determinants of glomerular ultrafiltration and proximal tubule fluid reabsorption in the rat. Circ. Res. 1975, 37, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Hellebrekers, L.J.; Liard, J.F.; Laborde, A.L.; Greene, A.S.; Cowley, A.W. Regional autoregulatory responses during infusion of vasoconstrictor agents in conscious dogs. Am. J. Physiol.-Heart Circ. Physiol. 1990, 259, H1270–H1277. [Google Scholar] [CrossRef] [PubMed]
- Metting, P.J.; Stein, P.M.; Stoos, B.A.; Kostrzewski, K.A.; Britton, S.L. Systemic vascular autoregulation amplifies pressor responses to vasoconstrictor agents. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1989, 256, R98–R105. [Google Scholar] [CrossRef]
- Dunberry-Poissant, S.; Gilbert, K.; Bouchard, C.; Baril, F.; Cardinal, A.-M.; L’Ecuyer, S.; Hylands, M.; Lamontagne, F.; Rousseau, G.; Charbonney, E. Fluid sparing and norepinephrine use in a rat model of resuscitated haemorrhagic shock: End-organ impact. Intensive Care Med. Exp. 2018, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Libert, N.; Laemmel, E.; Harrois, A.; Laitselart, P.; Bergis, B.; Isnard, P.; Terzi, F.; Decante, B.; Mercier, O.; Vicaut, E.; et al. Renal Microcirculation and Function in a Pig Model of Hemorrhagic Shock Resuscitation with Norepinephrine. Am. J. Respir. Crit. Care Med. 2022, 206, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Murakawa, K.; Kobayashi, A. Effects of Vasopressors on Renal Tissue Gas Tensions During Hemorrhagic Shock in Dogs. Crit. Care Med. 1988, 16, 789–792. [Google Scholar] [CrossRef]
- Prunet, B.; Prat, N.; Couret, D.; Cordier, P.-Y.; De Bourmont, S.; Lambert, D.; Asencio, Y.; Meaudre, E.; Michelet, P. Midterm Effects of Fluid Resuscitation Strategies in an Experimental Model of Lung Contusion and Hemorrhagic Shock. Shock 2014, 41, 159–165. [Google Scholar] [CrossRef]
- Harrois, A.; Baudry, N.; Huet, O.; Kato, H.; Dupic, L.; Lohez, M.; Ziol, M.; Vicaut, E.; Duranteau, J. Norepinephrine Decreases Fluid Requirements and Blood Loss While Preserving Intestinal Villi Microcirculation during Fluid Resuscitation of Uncontrolled Hemorrhagic Shock in Mice. Anesthesiology 2015, 122, 1093–1102. [Google Scholar] [CrossRef]
- Prowle, J.R.; Kirwan, C.J.; Bellomo, R. Fluid management for the prevention and attenuation of acute kidney injury. Nat. Rev. Nephrol. 2014, 10, 37–47. [Google Scholar] [CrossRef]
- Demiselle, J.; Fage, N.; Radermacher, P.; Asfar, P. Vasopressin and its analogues in shock states: A review. Ann. Intensive Care 2020, 10, 9. [Google Scholar] [CrossRef]
- Edwards, R.M.; Trizna, W.; Kinter, L.B. Renal microvascular effects of vasopressin and vasopressin antagonists. Am. J. Physiol.-Ren. Physiol. 1989, 256, F274–F278. [Google Scholar] [CrossRef] [PubMed]
- Rudichenko, V.M.; Beierwaltes, W.H. Arginine Vasopressin-lnduced Renal Vasodilation Mediated by Nitric Oxide. J. Vasc. Res. 1995, 32, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Rocco, M.; Conti, G.; Orecchioni, A.; De Gaetano, A.; Cortese, G.; Coluzzi, F.; Vernaglione, E.; Pelaia, P.; Pietropaoli, P. Effects of terlipressin on systemic and regional haemodynamics in catecholamine-treated hyperkinetic septic shock. Intensive Care Med. 2004, 30, 597–604. [Google Scholar] [CrossRef]
- Patel, B.M.; Chittock, D.R.; Russell, J.A.; Walley, K.R. Beneficial Effects of Short-term Vasopressin Infusion during Severe Septic Shock. Anesthesiology 2002, 96, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.C.; Mason, A.J.; Thirunavukkarasu, N.; Perkins, G.D.; Cecconi, M.; Cepkova, M.; Pogson, D.G.; Aya, H.D.; Anjum, A.; Frazier, G.J.; et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. JAMA 2016, 316, 509–518. [Google Scholar] [CrossRef]
- Nagendran, M.; Russell, J.A.; Walley, K.R.; Brett, S.J.; Perkins, G.D.; Hajjar, L.; Mason, A.J.; Ashby, D.; Gordon, A.C. Vasopressin in septic shock: An individual patient data meta-analysis of randomised controlled trials. Intensive Care Med. 2019, 45, 844–855. [Google Scholar] [CrossRef]
- Thiemermann, C.; Szabó, C.; Mitchell, J.A.; Vane, J.R. Vascular hyporeactivity to vasoconstrictor agents and hemodynamic decompensation in hemorrhagic shock is mediated by nitric oxide. Proc. Natl. Acad. Sci. USA 1993, 90, 267–271. [Google Scholar] [CrossRef]
- Sims, C.A.; Guan, Y.; Bergey, M.; Jaffe, R.; Holmes-Maguire, L.; Martin, N.; Reilly, P. Arginine vasopressin, copeptin, and the development of relative AVP deficiency in hemorrhagic shock. Am. J. Surg. 2017, 214, 589–595. [Google Scholar] [CrossRef]
- Raedler, C.; Voelckel, W.G.; Wenzel, V.; Krismer, A.C.; Schmittinger, C.A.; Herff, H.; Mayr, V.D.; Stadlbauer, K.H.; Lindner, K.H.; Königsrainer, A. Treatment of uncontrolled hemorrhagic shock after liver trauma: Fatal effects of fluid resuscitation versus improved outcome after vasopressin. Anesth. Analg. 2004, 98, 1759–1766. [Google Scholar] [CrossRef]
- Stadlbauer, K.H.; Wagner-Berger, H.G.; Krismer, A.C.; Voelckel, W.G.; Konigsrainer, A.; Lindner, K.H.; Wenzel, V. Vasopressin improves survival in a porcine model of abdominal vascular injury. Crit. Care 2007, 11, R81. [Google Scholar] [CrossRef]
- Voelckel, W.G.; Raedler, C.; Wenzel, V.; Lindner, K.H.; Krismer, A.C.; Schmittinger, C.A.; Herff, H.; Rheinberger, K.; Königsrainer, A. Arginine vasopressin, but not epinephrine, improves survival in uncontrolled hemorrhagic shock after liver trauma in pigs. Crit. Care Med. 2003, 31, 1160–1165. [Google Scholar] [CrossRef]
- Sims, C.A.; Yuxia, G.; Singh, K.; Werlin, E.C.; Reilly, P.M.; Baur, J.A. Supplemental arginine vasopressin during the resuscitation of severe hemorrhagic shock preserves renal mitochondrial function. PLoS ONE 2017, 12, e0186339. [Google Scholar] [CrossRef]
- Voelckel, W.G.; Lurie, K.G.; Lindner, K.H.; Zielinski, T.; McKnite, S.; Krismer, A.C.; Wenzel, V. Vasopressin Improves Survival After Cardiac Arrest in Hypovolemic Shock. Anesth. Analg. 2000, 91, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Sims, C.A.; Holena, D.; Kim, P.; Pascual, J.; Smith, B.; Martin, N.; Seamon, M.; Shiroff, A.; Raza, S.; Kaplan, L.; et al. Effect of Low-Dose Supplementation of Arginine Vasopressin on Need for Blood Product Transfusions in Patients with Trauma and Hemorrhagic Shock: A Randomized Clinical Trial. JAMA Surg. 2019, 154, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Spinella, P.C.; Perkins, J.G.; Grathwohl, K.W.; Beekley, A.C.; Niles, S.E.; McLaughlin, D.F.; Wade, C.E.; Holcomb, J.B. Effect of plasma and red blood cell transfusions on survival in patients with combat related traumatic injuries. J. Trauma 2008, 64, S69–S77. [Google Scholar] [CrossRef] [PubMed]
- Blet, A.; McNeil, J.B.; Josse, J.; Cholley, B.; Cinotti, R.; Cotter, G.; Dauvergne, A.; Davison, B.; Duarte, K.; Duranteau, J.; et al. Association between in-ICU red blood cells transfusion and 1-year mortality in ICU survivors. Crit. Care 2022, 26, 307. [Google Scholar] [CrossRef]
- Bickell, W.H.; Wall, M.J.; Pepe, P.E.; Martin, R.R.; Ginger, V.F.; Allen, M.K.; Mattox, K.L. Immediate versus Delayed Fluid Resuscitation for Hypotensive Patients with Penetrating Torso Injuries. N. Engl. J. Med. 1994, 331, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Sampalis, J.S.; Tamim, H.; Denis, R.; Boukas, S.; Ruest, S.-A.; Nikolis, A.; Lavoie, A.; Fleiszer, D.; Brown, R.; Mulder, D.; et al. Ineffectiveness of on-site intravenous lines: Is prehospital time the culprit? J. Trauma 1997, 43, 608–615. [Google Scholar] [CrossRef]
- Schreiber, M.A.; Meier, E.N.; Tisherman, S.A.; Kerby, J.D.; Newgard, C.D.; Brasel, K.; Egan, D.; Witham, W.; Williams, C.; Daya, M.; et al. A Controlled Resuscitation Strategy is Feasible and Safe in Hypotensive Trauma Patients: Results of a Prospective Randomized Pilot Trial. J. Trauma Acute Care Surg. 2015, 78, 687–697. [Google Scholar] [CrossRef]
- Dutton, R.P.; Mackenzie, C.F.; Scalea, T.M. Hypotensive Resuscitation during Active Hemorrhage: Impact on In-Hospital Mortality. J. Trauma Inj. Infect. Crit. Care 2002, 52, 1141–1146. [Google Scholar] [CrossRef]
- Turner, J.; Nicholl, J.; Webber, L.; Cox, H.; Dixon, S.; Yates, D. A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma. Health Technol. Assess. Winch. Engl. 2000, 4, 1–57. [Google Scholar] [CrossRef]
- Madigan, M.C.; Kemp, C.D.; Johnson, J.C.; Cotton, B.A. Secondary Abdominal Compartment Syndrome After Severe Extremity Injury: Are Early, Aggressive Fluid Resuscitation Strategies to Blame? J. Trauma Inj. Infect. Crit. Care 2008, 64, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Maegele, M.; Lefering, R.; Yucel, N.; Tjardes, T.; Rixen, D.; Paffrath, T.; Simanski, C.; Neugebauer, E.; Bouillon, B.; The AG Polytrauma of the German Trauma Society (DGU). Early coagulopathy in multiple injury: An analysis from the German Trauma Registry on 8724 patients. Injury 2007, 38, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Kasotakis, G.; Sideris, A.; Yang, Y.; de Moya, M.; Alam, H.; King, D.R.; Tompkins, R.; Velmahos, G. Aggressive Early Crystalloid Resuscitation adversely affects Outcomes in Adult Blunt Trauma Patients: An Analysis of the Glue Grant Database. J. Trauma Acute Care Surg. 2013, 74, 1215–1222. [Google Scholar] [CrossRef]
- Harada, M.Y.; Ko, A.; Barmparas, G.; Smith, E.J.T.; Patel, B.K.; Dhillon, N.K.; Thomsen, G.M.; Ley, E.J. 10-Year trend in crystalloid resuscitation: Reduced volume and lower mortality. Int. J. Surg. 2017, 38, 78–82. [Google Scholar] [CrossRef]
- Hussmann, B.; Lefering, R.; Waydhas, C.; Touma, A.; Kauther, M.D.; Ruchholtz, S.; Lendemans, S.; Trauma Registry of the German Society for Trauma Surgery. Does increased prehospital replacement volume lead to a poor clinical course and an increased mortality? A matched-pair analysis of 1896 patients of the Trauma Registry of the German Society for Trauma Surgery who were managed by an emergency doctor at the accident site. Injury 2013, 44, 611–617. [Google Scholar] [CrossRef]
- Haut, E.R.; Kalish, B.T.; Cotton, B.A.; Efron, D.T.; Haider, A.H.; Stevens, K.A.; Kieninger, A.N.; Cornwell, E.E.; Chang, D.C. Prehospital intravenous fluid administration is associated with higher mortality in trauma patients: A National Trauma Data Bank analysis. Ann. Surg. 2011, 253, 371–377. [Google Scholar] [CrossRef]
| References | Species | Models of Hemorrhagic Shock | Arterial Pressure Target during Resuscitation | Intervention | Group Compare | Main Results | Limitations |
|---|---|---|---|---|---|---|---|
| Dunberry-Poissant et al. [45] | Anesthetized wistar rats | Blood exsanguination to target MAP 30 mmHg during 60 min, resuscitation after control of bleeding | 55–60 mmHg of MAP target | Resuscitation with 40% of the shed blood withdrawn then used of norepinephrine |
|
|
|
| Libert et al. [46] | Anesthetized pigs | Blood exsanguination to MAP target 30 mmhg–35 mmHg during 90 min, resuscitation after stop of exsanguination | 80–85 mmHg of SAP target | Resuscitation with norepinephrine and fluid |
|
|
|
| Murakawa et al. [47] | Anesthetized dogs | Blood exsanguination to MAP target 50 mmHg during 60 min, | >100 mmHg of MAP target for 90 min | Resuscitation with norepinephrine |
|
|
|
| Prunet et al. [48] | Anesthetized pigs | Chest trauma and blood exsanguination to reach MAP target of 50 mmHg during 90 min | MAP to 70 mmHg | Resuscitation with limited fluid and norepinephrine |
|
| Lower cardiac output in group with use of norepinephrine |
| References | Species | Models of Hemorrhagic Shock | Arterial Pressure Target during Resuscitation | Intervention | Group Compare | Main Results | Limitations |
|---|---|---|---|---|---|---|---|
| Voelckel et al. [62] | Anesthetized pigs | Models of very severe haemorrhagic shock: Dissection of the right liver lobe allowing blood loss, to reach MAP target < 30 mmHg (near fatal hypotension) | Increase MAP without a specific target. | Resuscitation with vasopressin during uncontrolled shock |
| In vasopressin group, renal artery blood flow was restored and remains higher than epinephrine or placebo groups |
No information on the effect on renal function |
| Voelckel et al. [64] | Anesthetized pigs | Models of very severe haemorrhagic shock: Blood exsanguination and ventricular fibrillation was induced with single administration of alternating current. | Return of spontaneous circulation with a MAP ≥ baseline value before exsanguination. | Injection of vasopressin after 4 min of untreated ventricular fibrillation and 4 min of cardiopulmonary resuscitation |
|
In vasopressin group, renal artery blood flow was restored and remains higher than epinephrine or placebo groups | No information on the effect on renal function |
| Sims et al. [65] | Humans | Trauma patients who received at least 6 units of blood products | MAP target ≥ 65 mmHg for 48 h | Randomized study: use of Vasopressin (bolus of 4 U then 0.04 U/min) ± norepinephrine to target ≥ 65 mmHg of MAP |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fage, N.; Asfar, P.; Radermacher, P.; Demiselle, J. Norepinephrine and Vasopressin in Hemorrhagic Shock: A Focus on Renal Hemodynamics. Int. J. Mol. Sci. 2023, 24, 4103. https://doi.org/10.3390/ijms24044103
Fage N, Asfar P, Radermacher P, Demiselle J. Norepinephrine and Vasopressin in Hemorrhagic Shock: A Focus on Renal Hemodynamics. International Journal of Molecular Sciences. 2023; 24(4):4103. https://doi.org/10.3390/ijms24044103
Chicago/Turabian StyleFage, Nicolas, Pierre Asfar, Peter Radermacher, and Julien Demiselle. 2023. "Norepinephrine and Vasopressin in Hemorrhagic Shock: A Focus on Renal Hemodynamics" International Journal of Molecular Sciences 24, no. 4: 4103. https://doi.org/10.3390/ijms24044103
APA StyleFage, N., Asfar, P., Radermacher, P., & Demiselle, J. (2023). Norepinephrine and Vasopressin in Hemorrhagic Shock: A Focus on Renal Hemodynamics. International Journal of Molecular Sciences, 24(4), 4103. https://doi.org/10.3390/ijms24044103






