Tissue-Oxygen-Adaptation of Bone Marrow-Derived Mesenchymal Stromal Cells Enhances Their Immunomodulatory and Pro-Angiogenic Capacity, Resulting in Accelerated Healing of Chemical Burns
Abstract
1. Introduction
2. Results
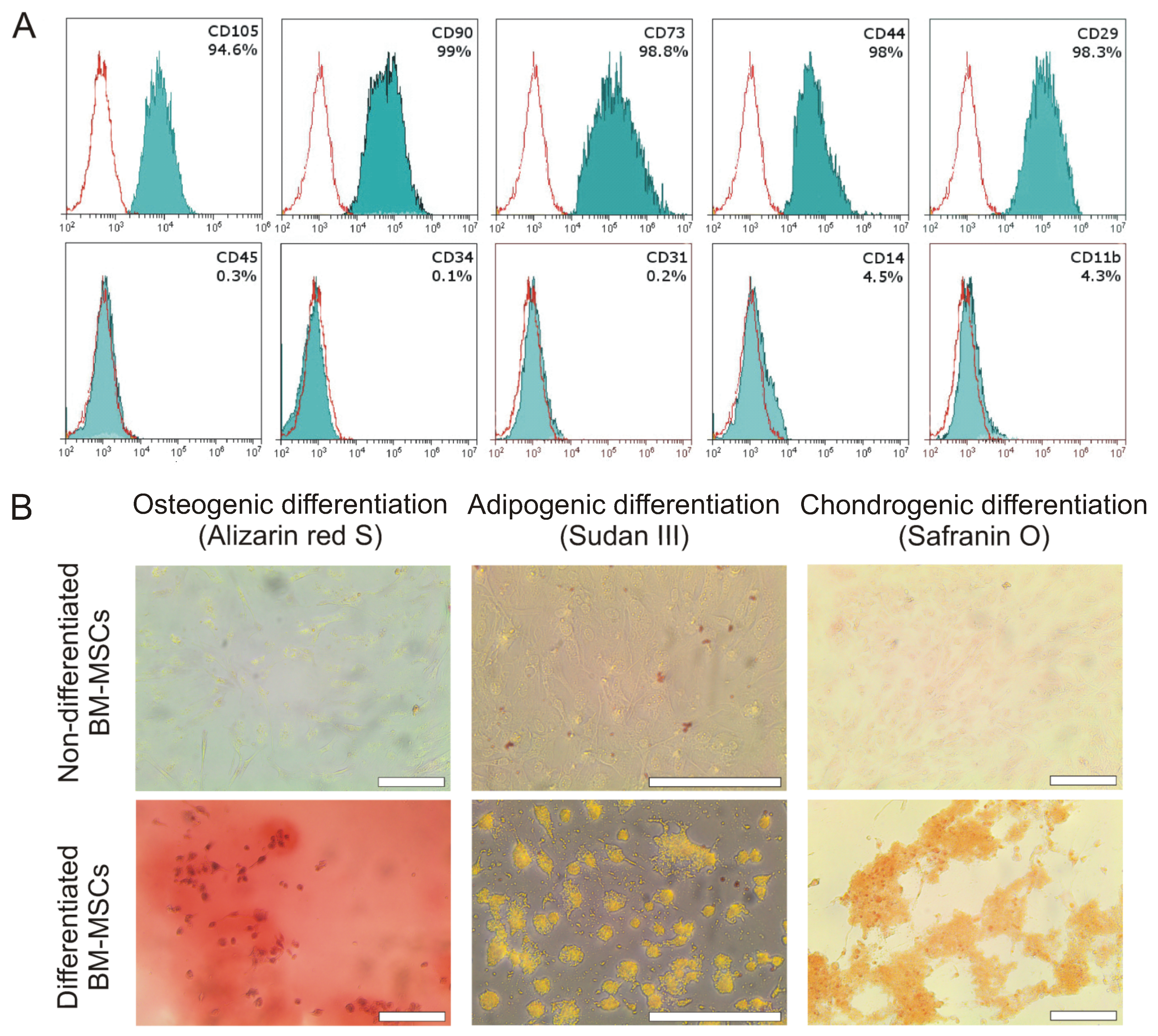
2.1. Characterization of Bone Marrow MSCs
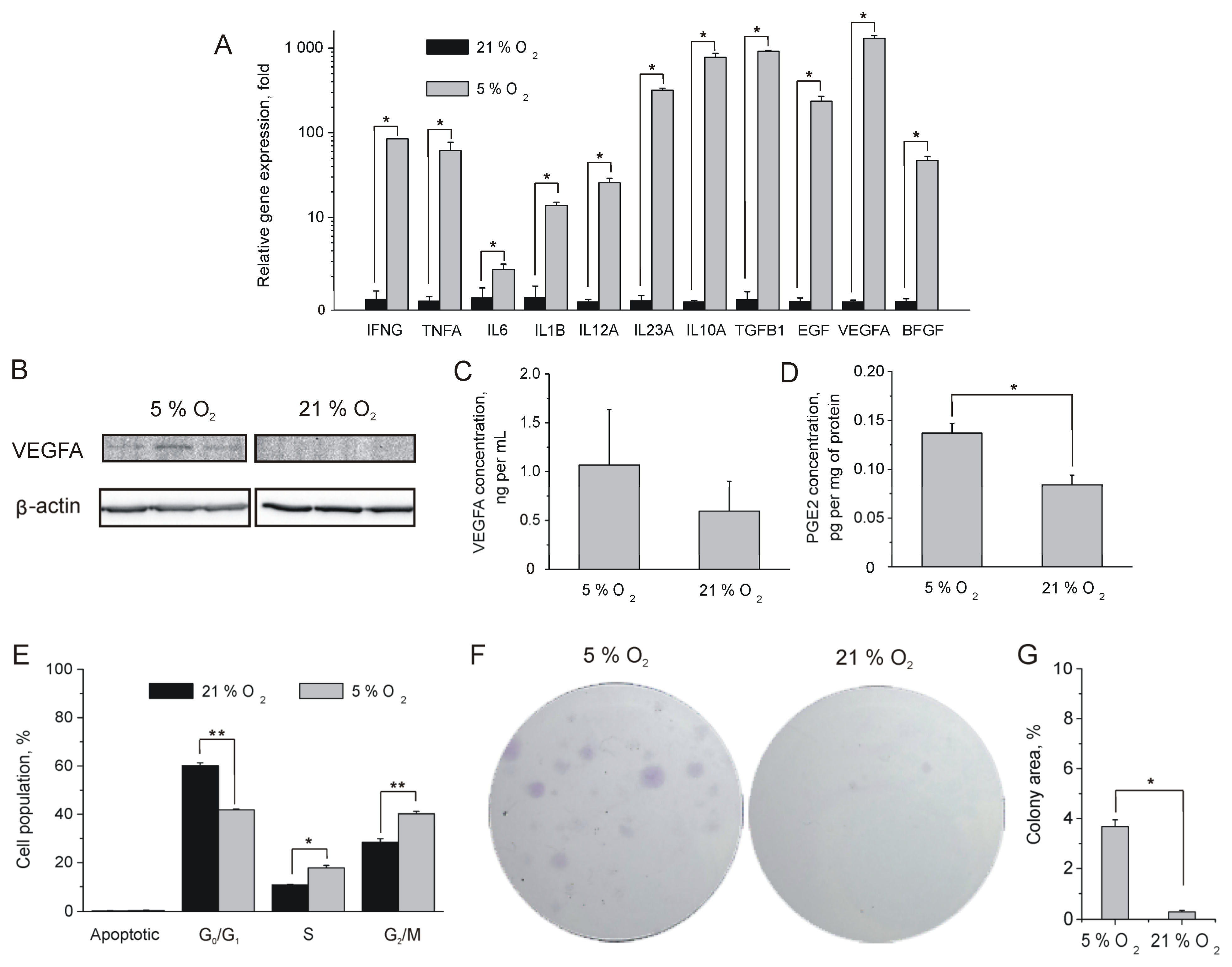
2.2. Influence of Oxygen Content on MSC Proliferation and Paracrine Activity
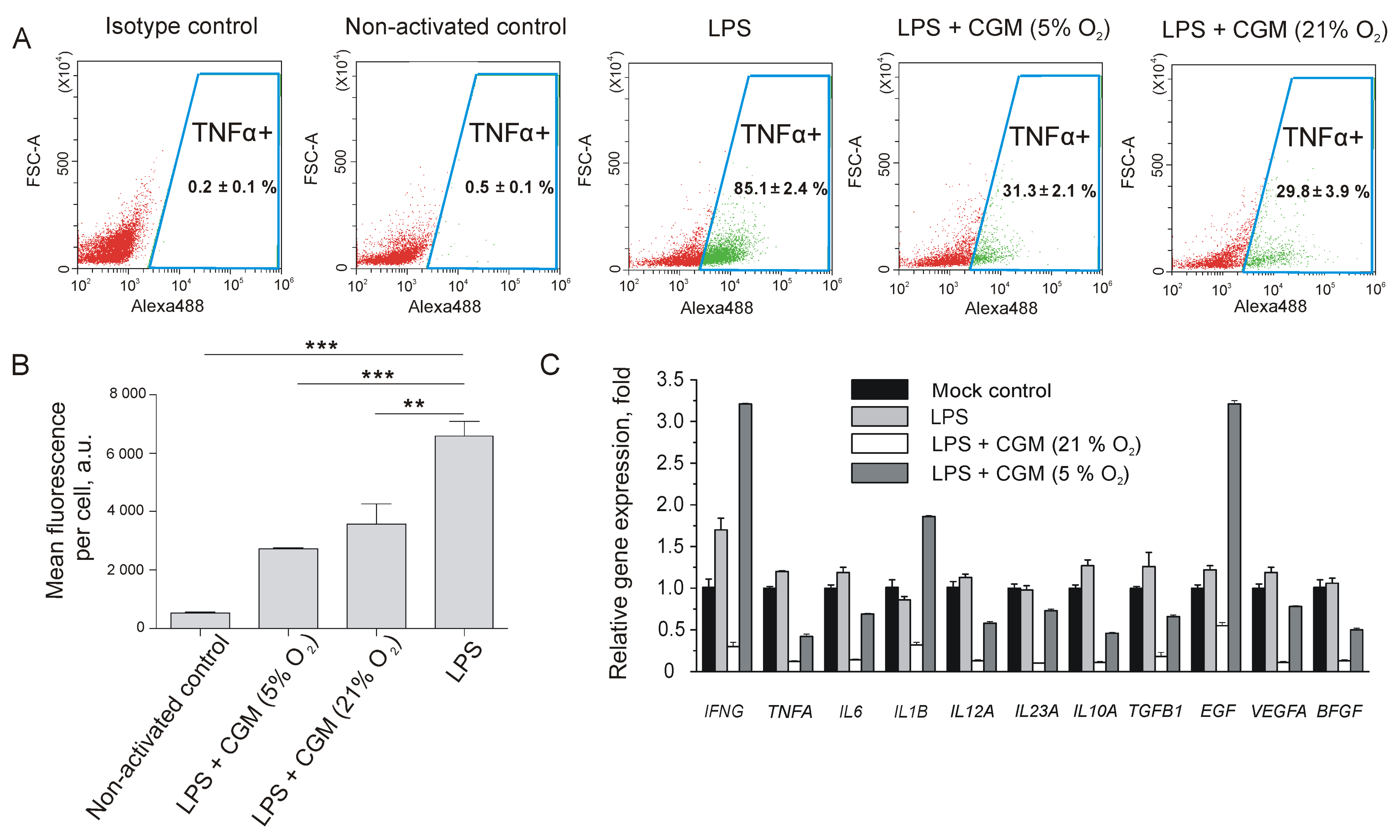
2.3. Impact of MSC-Conditioned Medium on LPS-Activated Macrophages, Fibroblasts, and Endotheliocytes
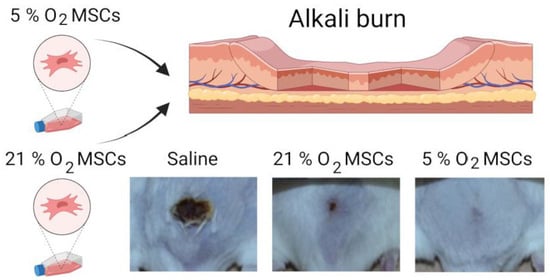
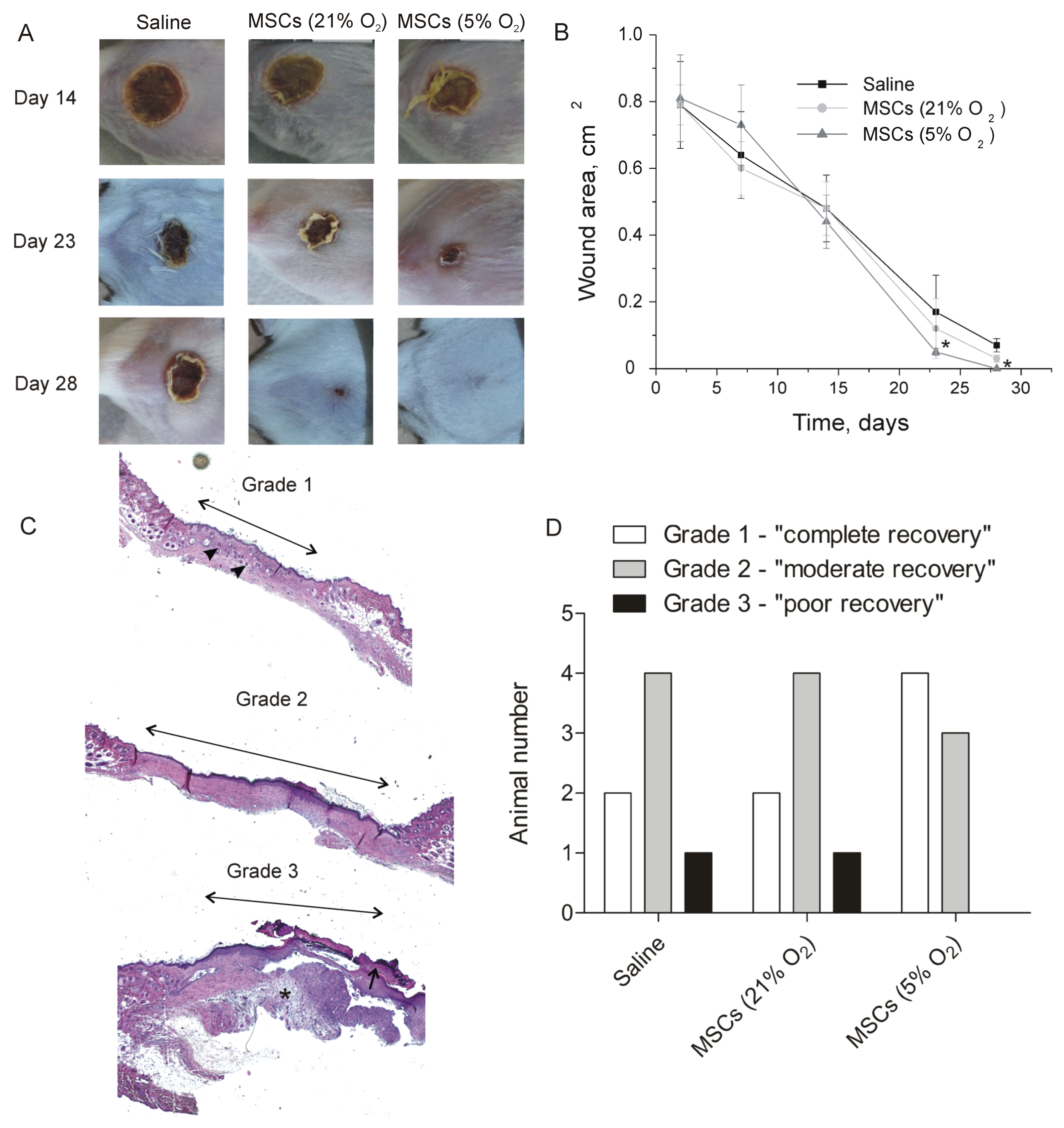
2.4. Therapeutic Effect of Tissue-Oxygen-Adapted MSCs in an Alkali Burn Injury Model
3. Discussion
4. Materials and Methods
4.1. Isolation of Primary MSCs from Bone Marrow and Collection of MSC CGMs
4.2. Immunophenotyping of the Isolated MSCs
4.3. Differentiation of the Isolated MSCs
4.4. DNA Profile Analysis
4.5. Colony Formation Assay
4.6. Western Blot
4.7. Immunofluorescent Analysis
4.8. BLItz Analysis of VEGFA Content
4.9. Flow Cytometry Analysis of TNFα Expression in LPS-Activated Macrophages after the Treatment with MSC CGM
4.10. RT-PCR
4.11. Tube Formation Assay
4.12. Scratch Assay
4.13. In Vivo Study
4.14. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sagaradze, G.D.; Basalova, N.A.; Efimenko, A.Y.; Tkachuk, V.A. Mesenchymal Stromal Cells as Critical Contributors to Tissue Regeneration. Front. Cell Dev. Biol. 2020, 8, 576176. [Google Scholar] [CrossRef] [PubMed]
- Durymanov, M.O.; Boyarintsev, V.V.; Biryukov, S.A.; Gorina, E.V.; Filkov, G.I. Methodological Approaches to Development of Cell-Based Medicinal Product for Treatment of Patients with Cold Injury in the Arctic. Hum. Physiol. 2020, 46, 798–805. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Wang, W.E.; Zeng, C. How to Improve the Survival of Transplanted Mesenchymal Stem Cell in Ischemic Heart? Available online: https://www.hindawi.com/journals/sci/2016/9682757/abs/ (accessed on 26 September 2019).
- Sagrinati, C.; Ronconi, E.; Lazzeri, E.; Lasagni, L.; Romagnani, P. Stem-Cell Approaches for Kidney Repair: Choosing the Right Cells. Trends Mol. Med. 2008, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human Mesenchymal Stem Cells Differentiate to a Cardiomyocyte Phenotype in the Adult Murine Heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Jung, S.; Kim, J.-H.; Kim, J.-H.; Yim, C.; Yim, C.; Lee, M.; Lee, M.; Kang, H.J.; Kang, H.J.; et al. Therapeutic Effects of a Mesenchymal Stem Cell-based Insulin-like Growth Factor-1/Enhanced Green Fluorescent Protein Dual Gene Sorting System in a Myocardial Infarction Rat Model. Mol. Med. Rep. 2018, 18, 5563–5571. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Nauta, A.C.; Morrison, S.D.; Hu, M.S.; Zimmermann, A.S.; Chung, M.T.; Glotzbach, J.P.; Wong, V.W.; Walmsley, G.G.; Peter, H.L.; et al. PHD-2 Suppression in Mesenchymal Stromal Cells Enhances Wound Healing. Plast. Reconstr. Surg. 2018, 141, 55e–67e. [Google Scholar] [CrossRef]
- Noiseux, N.; Gnecchi, M.; Lopez-Ilasaca, M.; Zhang, L.; Solomon, S.D.; Deb, A.; Dzau, V.J.; Pratt, R.E. Mesenchymal Stem Cells Overexpressing Akt Dramatically Repair Infarcted Myocardium and Improve Cardiac Function despite Infrequent Cellular Fusion or Differentiation. Mol. Ther. 2006, 14, 840–850. [Google Scholar] [CrossRef]
- Xu, B.; Luo, Y.; Liu, Y.; Li, B.-Y.; Wang, Y. Platelet-Derived Growth Factor-BB Enhances MSC-Mediated Cardioprotection via Suppression of MiR-320 Expression. Am. J. Physiol.-Heart Circ. Physiol. 2015, 308, H980–H989. [Google Scholar] [CrossRef]
- Maffioli, E.; Nonnis, S.; Angioni, R.; Santagata, F.; Calì, B.; Zanotti, L.; Negri, A.; Viola, A.; Tedeschi, G. Proteomic Analysis of the Secretome of Human Bone Marrow-Derived Mesenchymal Stem Cells Primed by pro-Inflammatory Cytokines. J. Proteom. 2017, 166, 115–126. [Google Scholar] [CrossRef]
- Hahn, J.-Y.; Cho, H.-J.; Kang, H.-J.; Kim, T.-S.; Kim, M.-H.; Chung, J.-H.; Bae, J.-W.; Oh, B.-H.; Park, Y.-B.; Kim, H.-S. Pre-Treatment of Mesenchymal Stem Cells with a Combination of Growth Factors Enhances Gap Junction Formation, Cytoprotective Effect on Cardiomyocytes, and Therapeutic Efficacy for Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 51, 933–943. [Google Scholar] [CrossRef]
- Yates, C.C.; Nuschke, A.; Rodrigues, M.; Whaley, D.; Dechant, J.J.; Taylor, D.P.; Wells, A. Improved Transplanted Stem Cell Survival in a Polymer Gel Supplemented with Tenascin C Accelerates Healing and Reduces Scarring of Murine Skin Wounds. Cell Transpl. 2017, 26, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Jeong, S.I.; Shin, Y.M.; Lim, K.S.; Shin, H.S.; Lee, Y.M.; Koh, H.C.; Kim, K.-S. Transplantation of Mesenchymal Stem Cells within a Poly (Lactide-Co-ɛ-Caprolactone) Scaffold Improves Cardiac Function in a Rat Myocardial Infarction Model. Eur. J. Heart Fail. 2009, 11, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Leroux, L.; Descamps, B.; Tojais, N.F.; Séguy, B.; Oses, P.; Moreau, C.; Daret, D.; Ivanovic, Z.; Boiron, J.-M.; Lamazière, J.-M.D. Hypoxia Preconditioned Mesenchymal Stem Cells Improve Vascular and Skeletal Muscle Fiber Regeneration after Ischemia through a Wnt4-Dependent Pathway. Mol. Ther. 2010, 18, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Imura, T.; Tomiyasu, M.; Otsuru, N.; Nakagawa, K.; Otsuka, T.; Takahashi, S.; Takeda, M. Hypoxic Preconditioning Increases the Neuroprotective Effects of Mesenchymal Stem Cells in a Rat Model of Spinal Cord Injury. J. Stem Cell Res. 2017, 7, 1953–1960. [Google Scholar] [CrossRef]
- Cruz, F.F.; Rocco, P.R.M. Hypoxic Preconditioning Enhances Mesenchymal Stromal Cell Lung Repair Capacity. Stem Cell Res. Ther. 2015, 6, 1–2. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Huo, Y.; Yang, Y.; Wang, Y. Hypoxia-Pretreated Human MSCs Attenuate Acute Kidney Injury through Enhanced Angiogenic and Antioxidative Capacities. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-P.; Chio, C.-C.; Cheong, C.-U.; Chao, C.-M.; Cheng, B.-C.; Lin, M.-T. Hypoxic Preconditioning Enhances the Therapeutic Potential of the Secretome from Cultured Human Mesenchymal Stem Cells in Experimental Traumatic Brain Injury. Clin. Sci. 2013, 124, 165–176. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Li, X.; Su, X.; Xiao, H.; Yang, J. Hypoxic Preconditioning of Human Umbilical Cord Mesenchymal Stem Cells Is an Effective Strategy for Treating Acute Lung Injury. Stem Cells Dev. 2021, 30, 128–134. [Google Scholar] [CrossRef]
- Wang, X.; Shen, K.; Wang, J.; Liu, K.; Wu, G.; Li, Y.; Luo, L.; Zheng, Z.; Hu, D. Hypoxic Preconditioning Combined with Curcumin Promotes Cell Survival and Mitochondrial Quality of Bone Marrow Mesenchymal Stem Cells, and Accelerates Cutaneous Wound Healing via PGC-1α/SIRT3/HIF-1α Signaling. Free Radic. Biol. Med. 2020, 159, 164–176. [Google Scholar] [CrossRef]
- Yang, H.; Fierro, F.; So, M.; Yoon, D.J.; Nguyen, A.V.; Gallegos, A.; Bagood, M.D.; Rojo-Castro, T.; Alex, A.; Stewart, H.; et al. Combination Product of Dermal Matrix, Human Mesenchymal Stem Cells, and Timolol Promotes Diabetic Wound Healing in Mice. Stem Cells Transl. Med. 2020, 9, 1353–1364. [Google Scholar] [CrossRef]
- Muhammad, G.; Xu, J.; Bulte, J.W.; Jablonska, A.; Walczak, P.; Janowski, M. Transplanted Adipose-Derived Stem Cells Can Be Short-Lived yet Accelerate Healing of Acid-Burn Skin Wounds: A Multimodal Imaging Study. Sci. Rep. 2017, 7, 4644. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, Y.M.; Lee, S.H. Hypoxic Preconditioning Promotes the Bioactivities of Mesenchymal Stem Cells via the HIF-1α-GRP78-Akt Axis. Int. J. Mol. Sci. 2017, 18, 1320. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Li, F.; Fang, J.; Xu, L.; Sun, C.; Han, J.; Hua, T.; Zhang, Z.; Feng, Z.; Jiang, X. Hypoxia Inducible Factor 1α Promotes Survival of Mesenchymal Stem Cells under Hypoxia. Am. J. Transl. Res. 2017, 9, 1521–1529. [Google Scholar] [PubMed]
- Ishiuchi, N.; Nakashima, A.; Doi, S.; Yoshida, K.; Maeda, S.; Kanai, R.; Yamada, Y.; Ike, T.; Doi, T.; Kato, Y.; et al. Hypoxia-Preconditioned Mesenchymal Stem Cells Prevent Renal Fibrosis and Inflammation in Ischemia-Reperfusion Rats. Stem Cell Res. Ther. 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, H.; Deng, L.; Cong, X.; Chen, X. Expression and Secretion of Interleukin-1β, Tumour Necrosis Factor-α and Interleukin-10 by Hypoxia- and Serum-Deprivation-Stimulated Mesenchymal Stem Cells. FEBS J 2010, 277, 3688–3698. [Google Scholar] [CrossRef]
- Volkova, M.V.; Boyarintsev, V.V.; Trofimenko, A.V.; Biryukov, S.A.; Gorina, E.V.; Filkov, G.I.; Durymanov, M.O. Adaptation of Bio-Layer Interferometry for Quantitative Assessment of the Vascular Endothelial Growth Factor Content in Cell-Conditioned Culture Medium. Biophysics 2020, 65, 935–941. [Google Scholar] [CrossRef]
- Sylakowski, K.; Bradshaw, A.; Wells, A. Mesenchymal Stem Cell/Multipotent Stromal Cell Augmentation of Wound Healing: Lessons from the Physiology of Matrix and Hypoxia Support. Am. J. Pathol. 2020, 190, 1370–1381. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, C.; Shen, M.; Yang, M.; Jin, Z.; Ding, L.; Jiang, W.; Yang, J.; Chen, H.; Cao, F.; et al. Autophagy Mediates the Beneficial Effect of Hypoxic Preconditioning on Bone Marrow Mesenchymal Stem Cells for the Therapy of Myocardial Infarction. Stem Cell Res. 2017, 8, 89. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Rodriguez-Vita, J.; Lawrence, T. The Resolution of Inflammation and Cancer. Cytokine Growth Factor Rev. 2010, 21, 61–65. [Google Scholar] [CrossRef]
- El Sadik, A.O.; El Ghamrawy, T.A.; Abd El-Galil, T.I. The Effect of Mesenchymal Stem Cells and Chitosan Gel on Full Thickness Skin Wound Healing in Albino Rats: Histological, Immunohistochemical and Fluorescent Study. PLoS ONE 2015, 10, e0137544. [Google Scholar] [CrossRef] [PubMed]
- Imbarak, N.; Abdel-Aziz, H.I.; Farghaly, L.M.; Hosny, S. Effect of Mesenchymal Stem Cells versus Aloe Vera on Healing of Deep Second-Degree Burn. Stem Cell Investig. 2021, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Volkova, M.V.; Boyarintsev, V.V.; Trofimenko, A.V.; Kovaleva, E.V.; Al Othman, A.; Melerzanov, A.V.; Filkov, G.I.; Rybalkin, S.P.; Durymanov, M.O. Local Injection of Bone-Marrow Derived Mesenchymal Stromal Cells Alters a Molecular Expression Profile of a Contact Frostbite Injury Wound and Improves Healing in a Rat Model. Burns 2022, in press. [CrossRef] [PubMed]
- Volkova, M.; Durymanov, M. Therapeutic Potential of Mesenchymal Stromal Cells for the Treatment of Frostbite Injury. Burns 2022, 48, 1277–1278. [Google Scholar] [CrossRef]
- Huang, C.-K.; Tsai, M.-Y.; Luo, J.; Kang, H.-Y.; Lee, S.O.; Chang, C. Suppression of Androgen Receptor Enhances the Self-Renewal of Mesenchymal Stem Cells Through Elevated Expression of EGFR. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 1222–1234. [Google Scholar] [CrossRef]
- De Luca, A.; Gallo, M.; Aldinucci, D.; Ribatti, D.; Lamura, L.; D’Alessio, A.; De Filippi, R.; Pinto, A.; Normanno, N. Role of the EGFR Ligand/Receptor System in the Secretion of Angiogenic Factors in Mesenchymal Stem Cells. J. Cell. Physiol. 2011, 226, 2131–2138. [Google Scholar] [CrossRef]
- Walter, M.N.M.; Wright, K.T.; Fuller, H.R.; MacNeil, S.; Johnson, W.E.B. Mesenchymal Stem Cell-Conditioned Medium Accelerates Skin Wound Healing: An in Vitro Study of Fibroblast and Keratinocyte Scratch Assays. Exp. Cell Res. 2010, 316, 1271–1281. [Google Scholar] [CrossRef]
- Palao, R.; Monge, I.; Ruiz, M.; Barret, J.P. Chemical Burns: Pathophysiology and Treatment. Burns 2010, 36, 295–304. [Google Scholar] [CrossRef]
- Xiao, D.Z.T.; Wu, S.Y.Q.; Mak, A.F.T. Accumulation of Loading Damage and Unloading Reperfusion Injury—Modeling of the Propagation of Deep Tissue Ulcers. J. Biomech. 2014, 47, 1658–1664. [Google Scholar] [CrossRef]
- Toldo, S.; Mauro, A.G.; Cutter, Z.; Abbate, A. Inflammasome, Pyroptosis, and Cytokines in Myocardial Ischemia-Reperfusion Injury. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H1553–H1568. [Google Scholar] [CrossRef]
- Awad, A.S.; Rouse, M.; Huang, L.; Vergis, A.L.; Reutershan, J.; Cathro, H.P.; Linden, J.; Okusa, M.D. Compartmentalization of Neutrophils in the Kidney and Lung Following Acute Ischemic Kidney Injury. Kidney Int. 2009, 75, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lu, Z.; Lyu, X.; Liu, Q.; Pan, S. A Longitudinal Study of T2 Mapping Combined With Diffusion Tensor Imaging to Quantitatively Evaluate Tissue Repair of Rat Skeletal Muscle After Frostbite. Front. Physiol. 2021, 11, 597638. [Google Scholar] [CrossRef] [PubMed]
- Cade, F.; Paschalis, E.I.; Regatieri, C.V.; Vavvas, D.G.; Dana, R.; Dohlman, C.H. Alkali Burn to the Eye: Protection Using TNF-α Inhibition. Cornea 2014, 33, 382. [Google Scholar] [CrossRef] [PubMed]
- Sotozono, C.; He, J.; Matsumoto, Y.; Kita, M.; Imanishi, J.; Kinoshita, S. Cytokine Expression in the Alkali-Burned Cornea. Curr. Eye Res. 1997, 16, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.D.; Hammerberg, C.; Baadsgaard, O.; Elder, J.T.; Chan, L.S.; Sauder, D.N.; Voorhees, J.J.; Fisher, G. IL-1 Activity Is Reduced in Psoriatic Skin. Decreased IL-1 Alpha and Increased Nonfunctional IL-1 Beta. J. Immunol. 1990, 144, 4593–4603. [Google Scholar] [CrossRef]
- Hu, P.; Yang, Q.; Wang, Q.; Shi, C.; Wang, D.; Armato, U.; Prà, I.D.; Chiarini, A. Mesenchymal Stromal Cells-Exosomes: A Promising Cell-Free Therapeutic Tool for Wound Healing and Cutaneous Regeneration. Burn. Trauma 2019, 7, 38. [Google Scholar] [CrossRef]
- Gupta, S.; Rawat, S.; Krishnakumar, V.; Rao, E.P.; Mohanty, S. Hypoxia Preconditioning Elicit Differential Response in Tissue-Specific MSCs via Immunomodulation and Exosomal Secretion. Cell Tissue Res. 2022, 388, 535–548. [Google Scholar] [CrossRef]
- Shen, N.; Qi, X.; Bagrov, D.V.; Krechetov, S.P.; Sharapov, M.G.; Durymanov, M.O. Surface Modification of Fibroblasts with Peroxiredoxin-1-Loaded Polymeric Microparticles Increases Cell Mobility, Resistance to Oxidative Stress and Collagen I Production. Colloids Surf. B Biointerfaces 2022, 219, 112834. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkova, M.V.; Shen, N.; Polyanskaya, A.; Qi, X.; Boyarintsev, V.V.; Kovaleva, E.V.; Trofimenko, A.V.; Filkov, G.I.; Mezentsev, A.V.; Rybalkin, S.P.; et al. Tissue-Oxygen-Adaptation of Bone Marrow-Derived Mesenchymal Stromal Cells Enhances Their Immunomodulatory and Pro-Angiogenic Capacity, Resulting in Accelerated Healing of Chemical Burns. Int. J. Mol. Sci. 2023, 24, 4102. https://doi.org/10.3390/ijms24044102
Volkova MV, Shen N, Polyanskaya A, Qi X, Boyarintsev VV, Kovaleva EV, Trofimenko AV, Filkov GI, Mezentsev AV, Rybalkin SP, et al. Tissue-Oxygen-Adaptation of Bone Marrow-Derived Mesenchymal Stromal Cells Enhances Their Immunomodulatory and Pro-Angiogenic Capacity, Resulting in Accelerated Healing of Chemical Burns. International Journal of Molecular Sciences. 2023; 24(4):4102. https://doi.org/10.3390/ijms24044102
Chicago/Turabian StyleVolkova, Marina V., Ningfei Shen, Anna Polyanskaya, Xiaoli Qi, Valery V. Boyarintsev, Elena V. Kovaleva, Alexander V. Trofimenko, Gleb I. Filkov, Alexandre V. Mezentsev, Sergey P. Rybalkin, and et al. 2023. "Tissue-Oxygen-Adaptation of Bone Marrow-Derived Mesenchymal Stromal Cells Enhances Their Immunomodulatory and Pro-Angiogenic Capacity, Resulting in Accelerated Healing of Chemical Burns" International Journal of Molecular Sciences 24, no. 4: 4102. https://doi.org/10.3390/ijms24044102
APA StyleVolkova, M. V., Shen, N., Polyanskaya, A., Qi, X., Boyarintsev, V. V., Kovaleva, E. V., Trofimenko, A. V., Filkov, G. I., Mezentsev, A. V., Rybalkin, S. P., & Durymanov, M. O. (2023). Tissue-Oxygen-Adaptation of Bone Marrow-Derived Mesenchymal Stromal Cells Enhances Their Immunomodulatory and Pro-Angiogenic Capacity, Resulting in Accelerated Healing of Chemical Burns. International Journal of Molecular Sciences, 24(4), 4102. https://doi.org/10.3390/ijms24044102