Genome-Wide Characterization of the PIFs Family in Sweet Potato and Functional Identification of IbPIF3.1 under Drought and Fusarium Wilt Stresses
Abstract
1. Introduction
2. Results
2.1. Identification and Characteristic of PIFs in Sweet Potato and Its Two Diploid Relatives
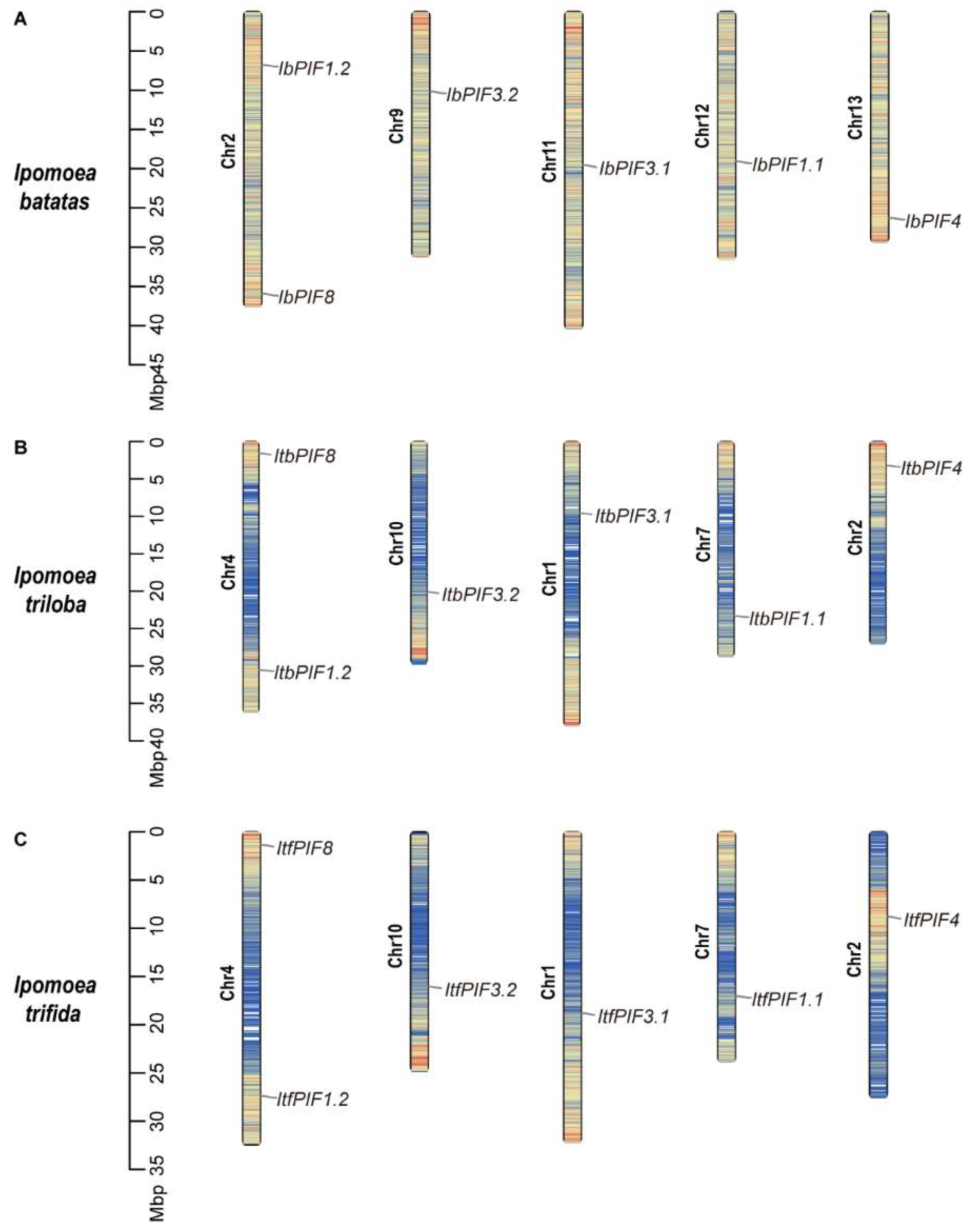
2.2. Chromosomal Location of Sweet Potato and Its Two Diploid Relatives
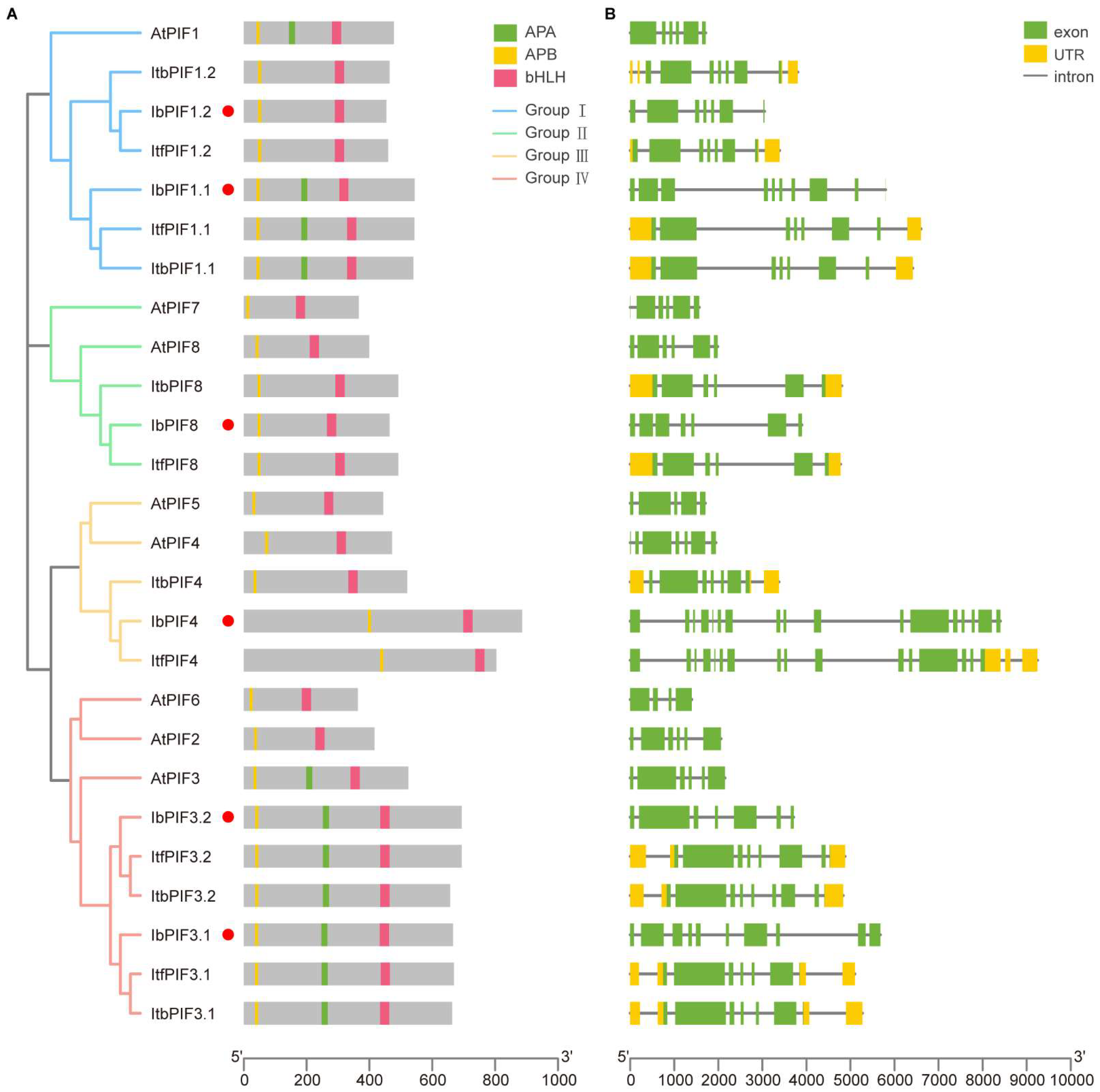
2.3. Phylogenetic Analysis of PIFs in Sweet Potato and Its Two Diploid Relative
2.4. Conserved Domain and Exon–Intron Structure Analysis of PIFs in Sweet Potato and Its Two Diploid Relatives
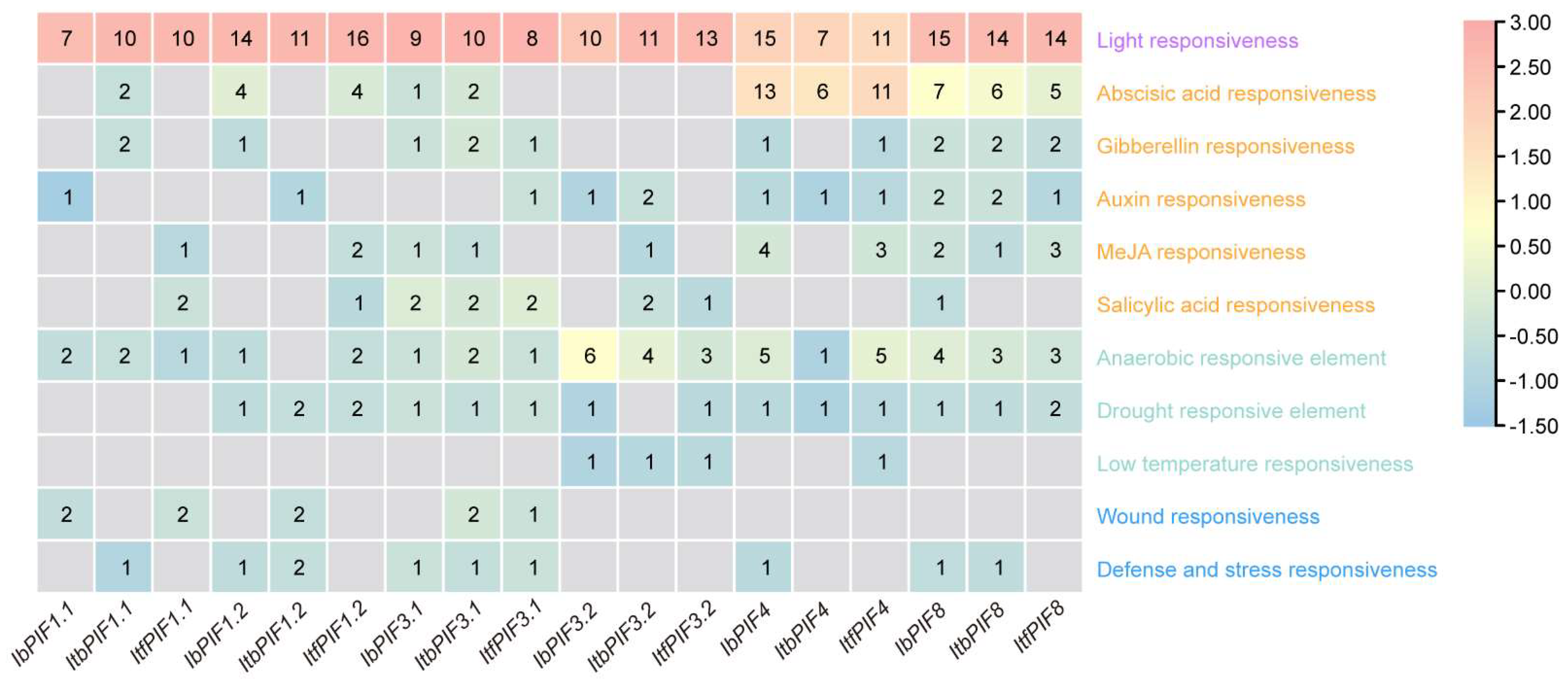
2.5. cis-Element Analysis in the Promoter of PIFs in Sweet Potato and Its Two Diploid Relatives
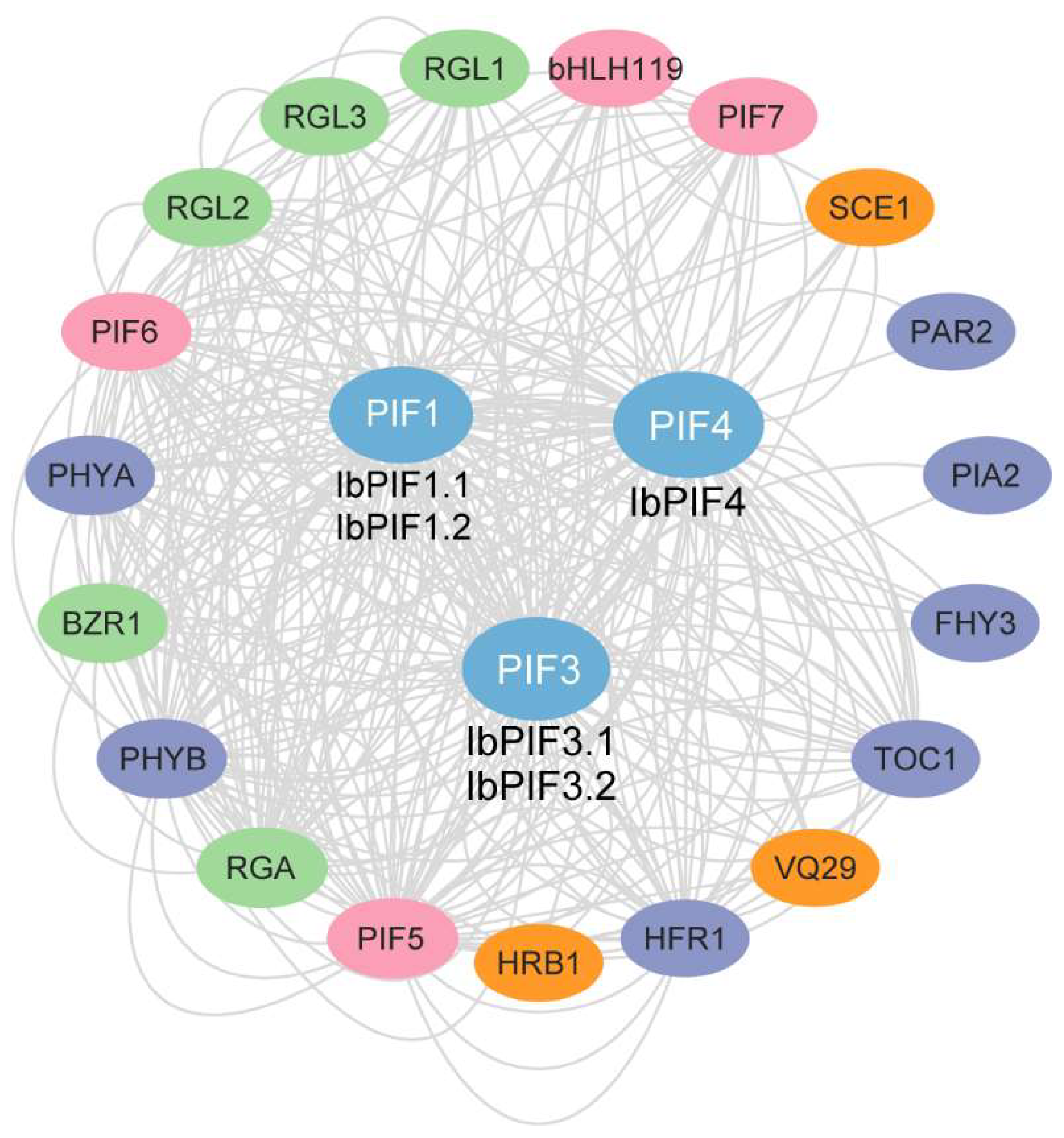
2.6. Protein Interaction Network of IbPIFs in Sweet Potato
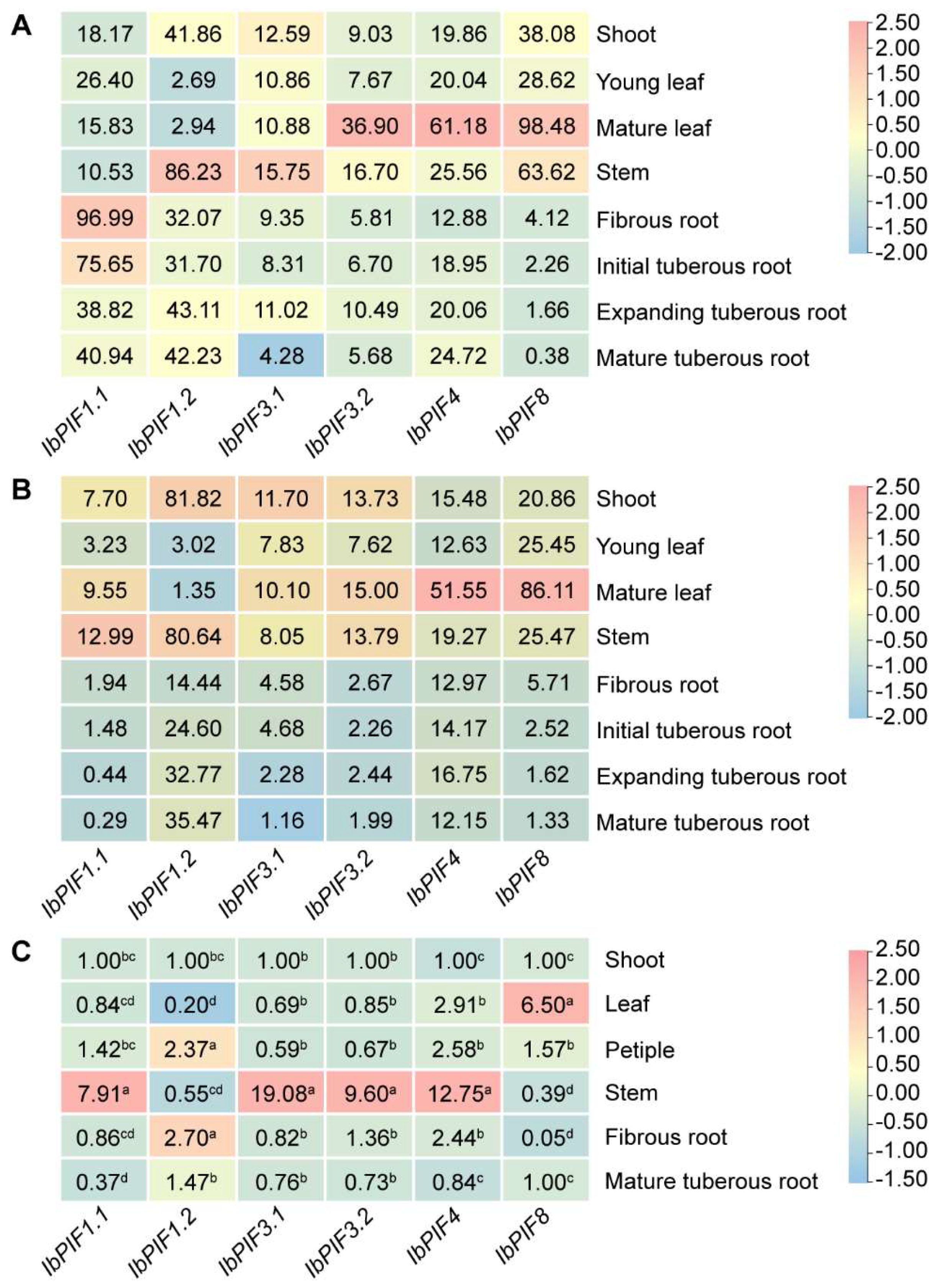
2.7. Expression Analysis of PIFs in Sweet Potato and Its Two Diploid Relatives
2.7.1. Expression Analysis in Various Tissues
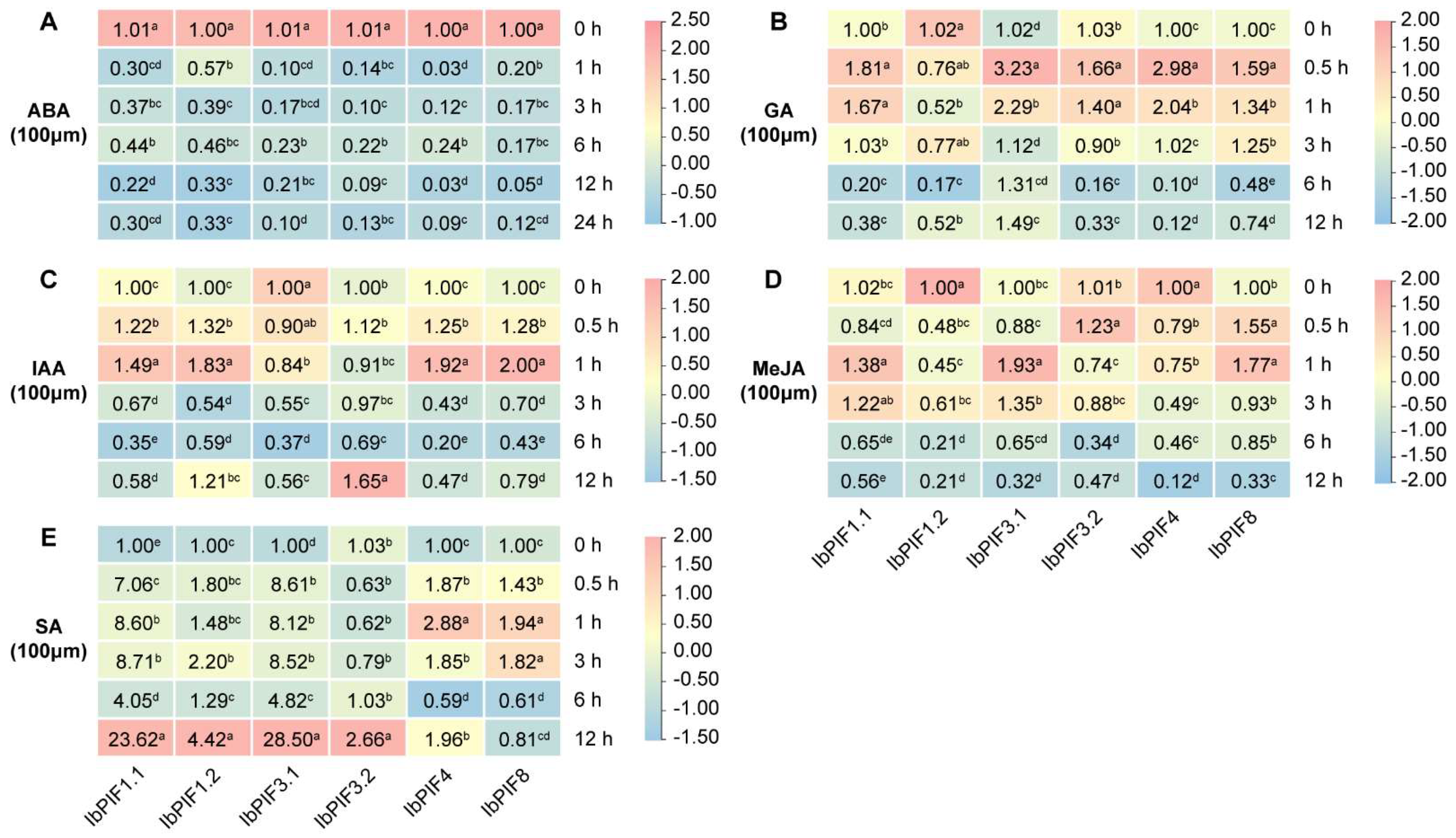
2.7.2. Expression Analysis under Hormone Treatment
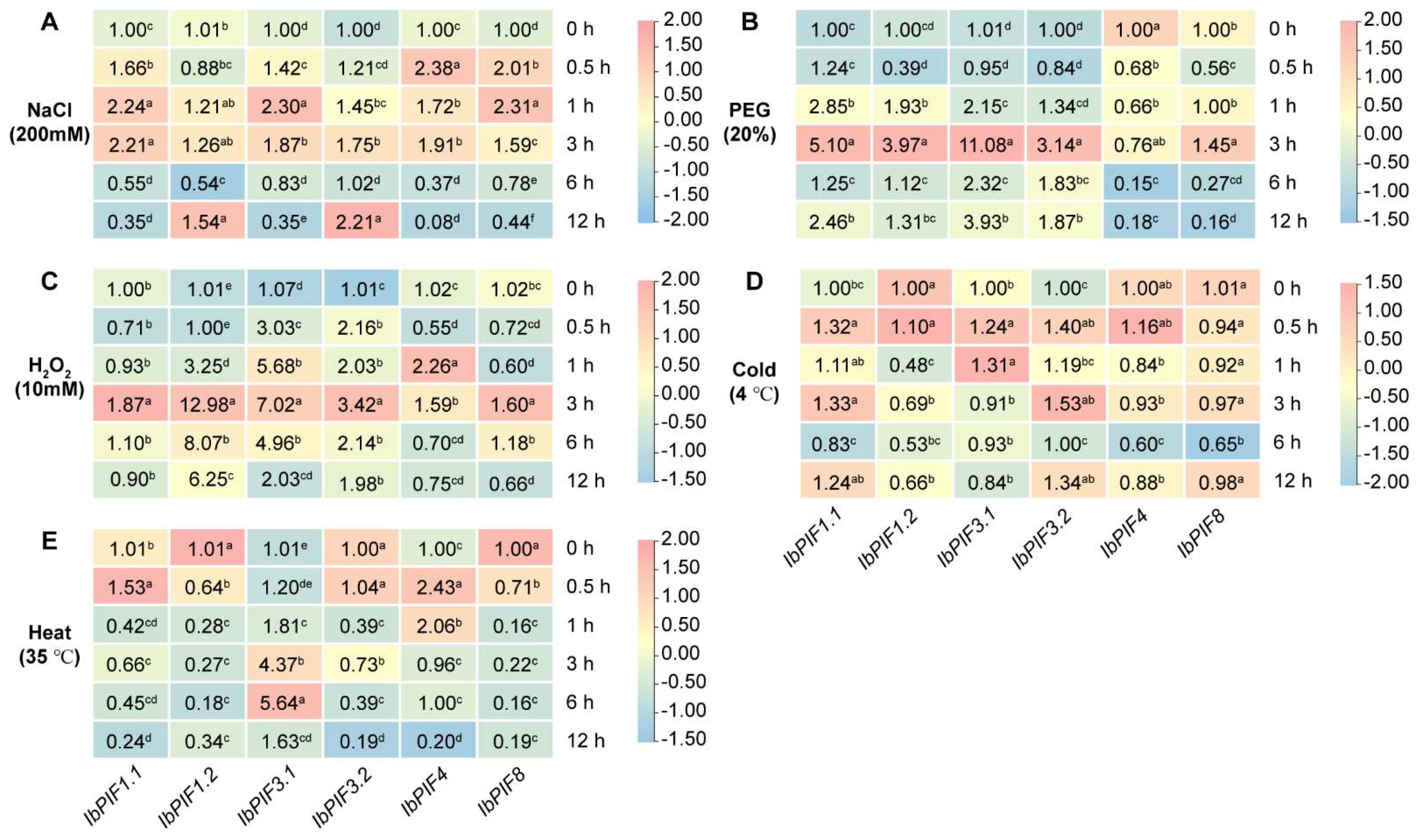
2.7.3. Expression Analysis under Abiotic Stresses
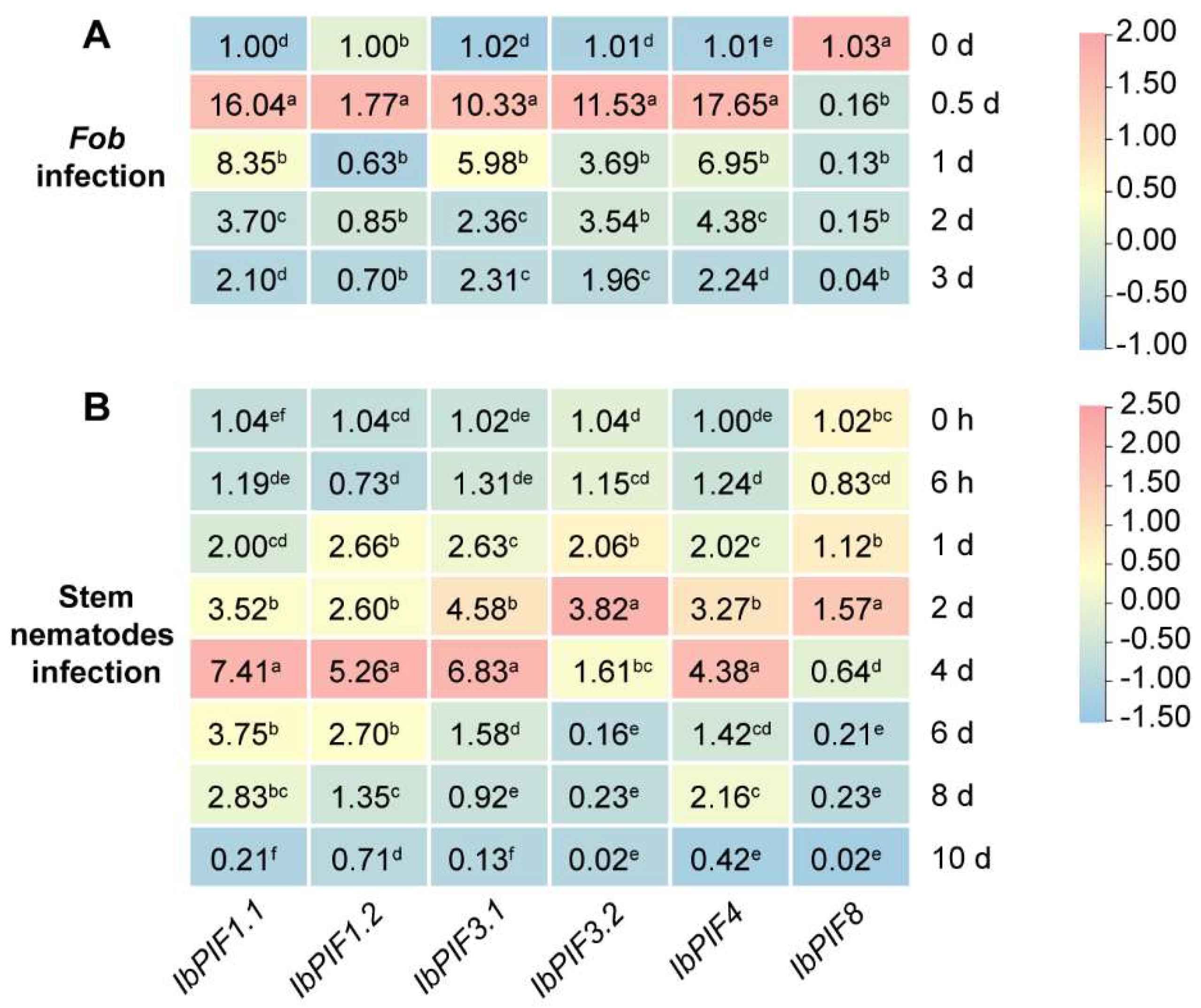
2.7.4. Expression Analysis under Biotic Stresses
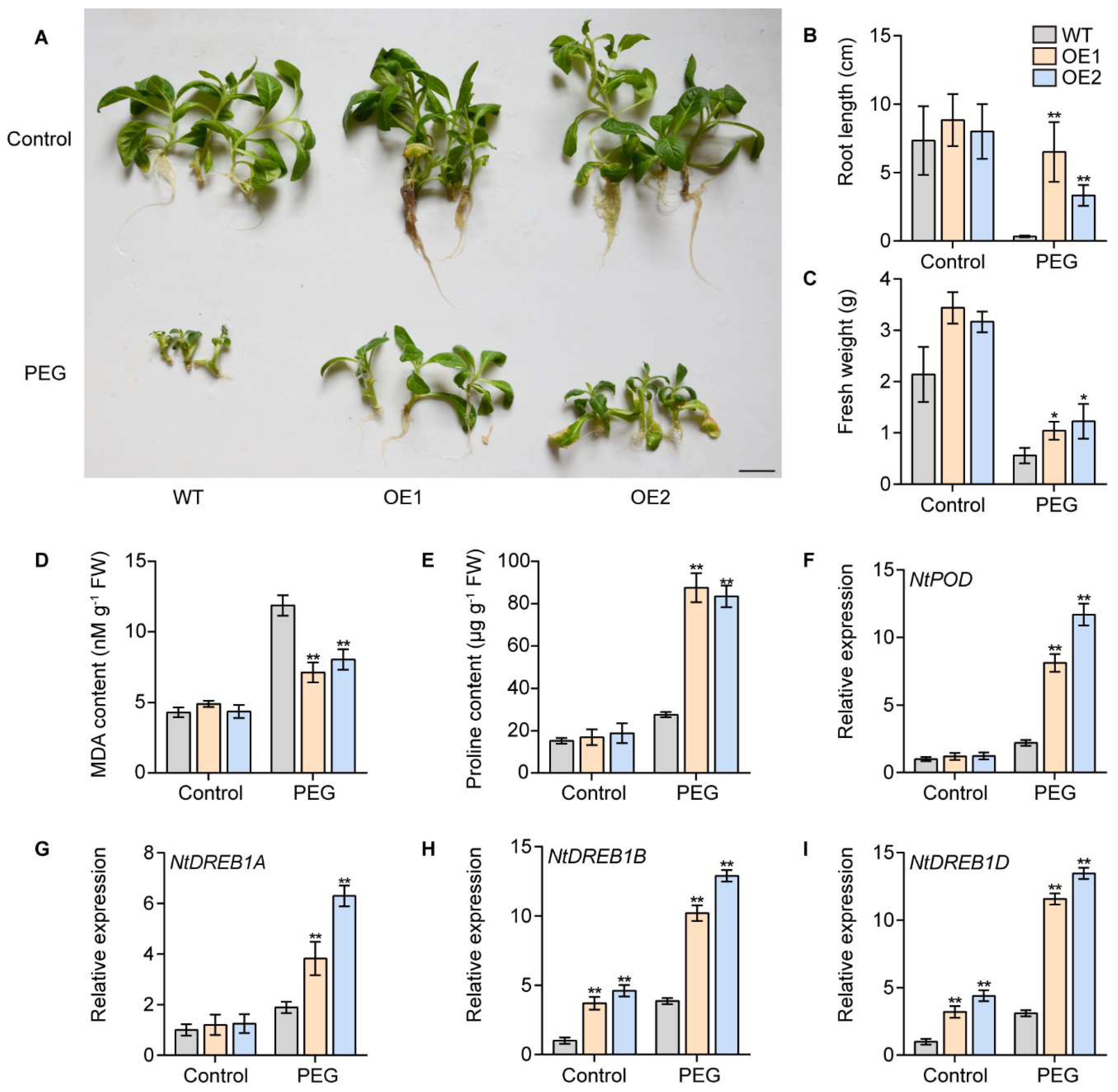
2.8. Overexpression of IbPIF3.1 Enhanced Drought Tolerance of Tobacco
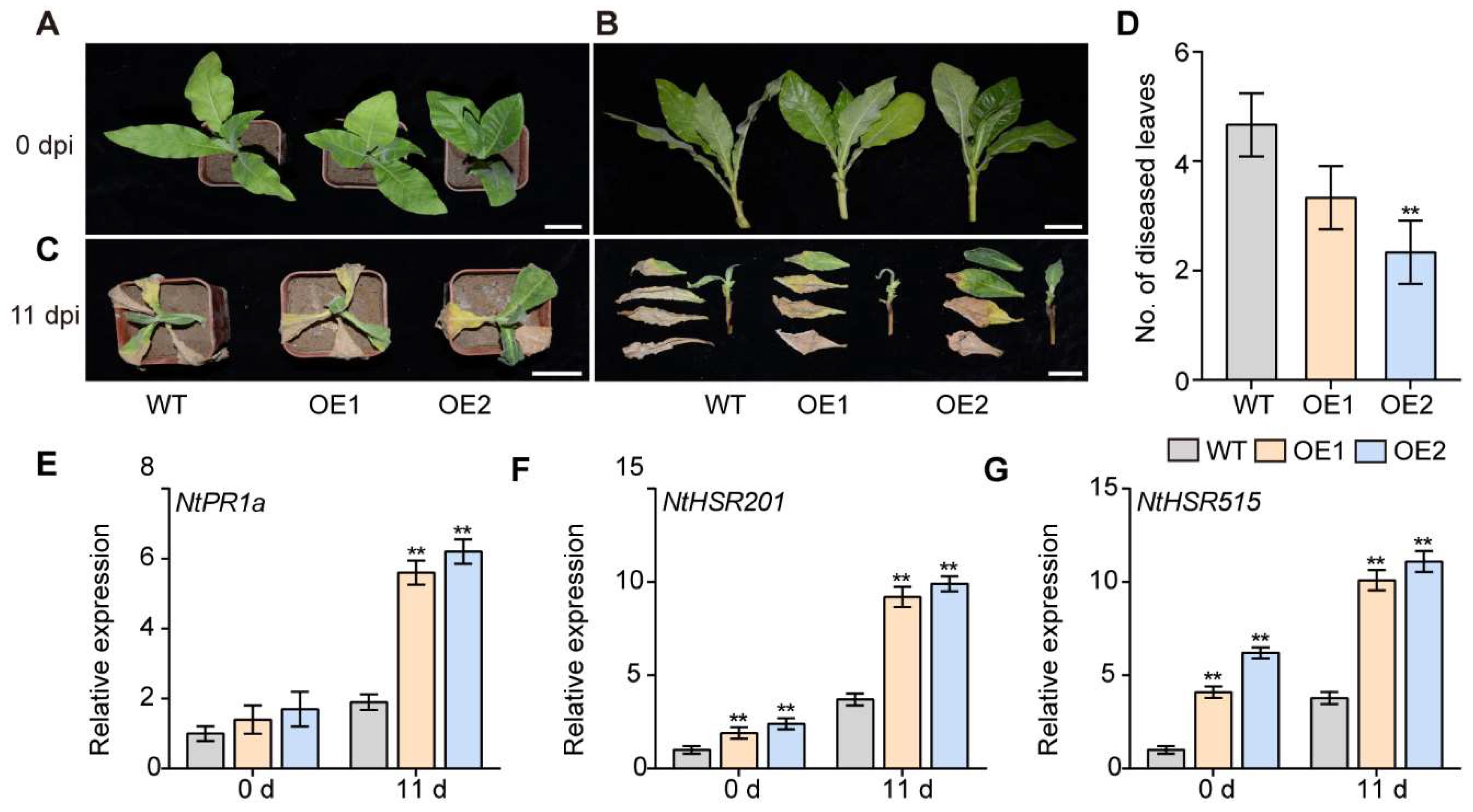
2.9. Overexpression of IbPIF3.1 Enhanced Fob Resistance of Tobacco
3. Discussion
3.1. Identification and Evolution of PIFs Family
3.2. The Expression of PIF Genes Was Tissue-Specific in Sweet Potato
3.3. PIFs Play Important Roles in Hormone Signaling Pathways in Sweet Potato
3.4. PIF Genes Response to Multiple Stresses
3.5. Overexpressing IbPIF3.1 Significantly Enhanced Drought Tolerance and Fob Resistance of Tobacco
4. Materials and Methods
4.1. Identification of PIFs
4.2. Protein Properties Prediction and Chromosomal Distribution of PIFs
4.3. Phylogenetic Analysis of PIFs
4.4. Motifs Identification and Conserved Domain Analysis of PIFs
4.5. Protein Interaction Network of PIFs
4.6. The qRT-PCR Analysis of PIFs
4.7. Transcriptome Analysis
4.8. Production of Transgenic Tobacco Plants
4.9. PEG Stress Treatment
4.10. Fusarium Wilt Resistance Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bae, G.; Choi, G. Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 2008, 59, 281–311. [Google Scholar] [CrossRef] [PubMed]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmulling, T.; Cortleven, A. Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 2021, 44, 645–664. [Google Scholar] [CrossRef] [PubMed]
- Hoang, Q.T.N.; Han, Y.J.; Kim, J.I. Plant Phytochromes and their Phosphorylation. Int. J. Mol. Sci. 2019, 20, 3450. [Google Scholar] [CrossRef] [PubMed]
- Li, F.W.; Mathews, S. Evolutionary aspects of plant photoreceptors. J. Plant Res. 2016, 129, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Quail, P.H. Phytochrome photosensory signalling networks. Nat. Rev. Mol. Cell Biol. 2002, 3, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Cashmore, A.R.; Jarillo, J.A.; Wu, Y.J.; Liu, D.M. Cryptochromes: Blue light receptors for plants and animals. Science 1999, 284, 760–765. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J. Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol. 2011, 21, 664–671. [Google Scholar] [CrossRef]
- Gururani, M.A.; Ganesan, M.; Song, P.S. Photo-biotechnology as a tool to improve agronomic traits in crops. Biotechnol. Adv. 2015, 33, 53–63. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Campos, M.L.; Azevedo, R.A. The Role of Phytochrome in Stress Tolerance. J. Integr. Plant Biol. 2011, 53, 920–929. [Google Scholar] [CrossRef]
- Ballare, C.L. Light Regulation of Plant Defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef]
- Paik, I.; Kathare, P.K.; Kim, J.I.; Huq, E. Expanding Roles of PIFs in Signal Integration from Multiple Processes. Mol. Plant 2017, 10, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Possart, A.; Xu, T.F.; Paik, I.; Hanke, S.; Keim, S.; Hermann, H.M.; Wolf, L.; Hiss, M.; Becker, C.; Huq, E.; et al. Characterization of Phytochrome Interacting Factors from the Moss Physcomitrella patens Illustrates Conservation of Phytochrome Signaling Modules in Land Plants. Plant Cell 2017, 29, 310–330. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.N.; Kathare, P.K.; Huq, E. Phytochromes and Phytochrome Interacting Factors. Plant Physiol. 2018, 176, 1025–1038. [Google Scholar] [CrossRef]
- Leivar, P.; Monte, E. PIFs: Systems Integrators in Plant Development. Plant Cell 2014, 26, 56–78. [Google Scholar] [CrossRef] [PubMed]
- Leivar, P.; Quail, P.H. PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 2011, 16, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Nishihama, R.; Kataoka, H.; Hosaka, M.; Manabe, R.; Nomoto, M.; Tada, Y.; Ishizaki, K.; Kohchi, T. Phytochrome Signaling Is Mediated by Phytochrome Interacting Factor in the Liverwort Marchantia polymorpha. Plant Cell 2016, 28, 1406–1421. [Google Scholar] [CrossRef]
- Rosado, D.; Gramegna, G.; Cruz, A.; Lira, B.S.; Freschi, L.; de Setta, N.; Rossi, M. Phytochrome Interacting Factors (PIFs) in Solanum lycopersicum: Diversity, Evolutionary History and Expression Profiling during Different Developmental Processes. PLoS ONE 2016, 11, 0165929. [Google Scholar] [CrossRef]
- Zhang, K.K.; Zheng, T.; Zhu, X.D.; Jiu, S.T.; Liu, Z.J.; Guan, L.; Jia, H.F.; Fang, J.G. Genome-Wide Identification of PIFs in Grapes (Vitis vinifera L.) and Their Transcriptional Analysis under Lighting/Shading Conditions. Genes 2018, 9, 451. [Google Scholar] [CrossRef]
- Zheng, P.F.; Wang, X.; Yang, Y.Y.; You, C.X.; Zhang, Z.L.; Hao, Y.J. Identification of Phytochrome-Interacting Factor Family Members and Functional Analysis of MdPIF4 in Malus domestica. Int. J. Mol. Sci. 2020, 21, 7350. [Google Scholar] [CrossRef]
- Zhang, X.N.; Xiong, L.G.; Luo, Y.; Wen, B.B.; Wang, K.B.; Liu, Z.H.; Huang, J.A.; Li, J. Identification, Molecular Characteristic, and Expression Analysis of PIFs Related to Chlorophyll Metabolism in Tea Plant (Camellia sinensis). Int. J. Mol. Sci. 2021, 22, 10949. [Google Scholar] [CrossRef]
- Yang, Y.X.; Guang, Y.L.; Wang, F.; Chen, Y.; Yang, W.T.; Xiao, X.F.; Luo, S.; Zhou, Y. Characterization of Phytochrome-Interacting Factor Genes in Pepper and Functional Analysis of CaPIF8 in Cold and Salt Stress. Front. Plant Sci. 2021, 12, 746517. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Huai, D.X.; Chen, Y.N.; Jiang, Y.F.; Ding, Y.B.; Kang, Y.P.; Wang, Z.H.; Yan, L.Y.; Jiang, H.F.; et al. Genome-wide identification of peanut PIF family genes and their potential roles in early pod development. Gene 2021, 781, 145539. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Shang, L.; Jin, Z.; Ding, Y.; Li, Y.; Wang, J.; Hu, B.; Vadim, K.; Lyu, D. Genome-wide identification and characterization of PIF genes and their response to high temperature stress in potato. Zuo Wu Xue Bao 2022, 48, 86–98. [Google Scholar] [CrossRef]
- Li, W.Q.; Liu, Y.; Wang, W.P.; Liu, J.C.; Yao, M.Y.; Guan, M.; Guan, C.Y.; He, X. Phytochrome-interacting factor (PIF) in rapeseed (Brassica napus L.): Genome-wide identification, evolution and expression analyses during abiotic stress, light quality and vernalization. Int. J. Biol. Macromol. 2021, 180, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.R.; Wang, Y.H.; Jia, M.; Zhang, R.R.; Liu, H.; Xu, Z.S.; Xiong, A.S. The phytochrome-interacting factor DcPIF3 of carrot plays a positive role in drought stress by increasing endogenous ABA level in Arabidopsis. Plant Sci. 2022, 322, 111367. [Google Scholar] [CrossRef]
- Leivar, P.; Monte, E.; Al-Sady, B.; Carle, C.; Storer, A.; Alonso, J.M.; Ecker, J.R.; Quail, P.H. The Arabidopsis Phytochrome-Interacting Factor PIF7, Together with PIF3 and PIF4, Regulates Responses to Prolonged Red Light by Modulating phyB Levels. Plant Cell 2008, 20, 337–352. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.C.; Feng, M.; Chen, J.W.; Qin, M.Z.; Wang, W.R.; Bao, Y.; Xu, Q.; Ye, Y.; Ma, C.; et al. The circadian-controlled PIF8-BBX28 module regulates petal senescence in rose flowers by governing mitochondrial ROS homeostasis at night. Plant Cell 2021, 33, 2716–2735. [Google Scholar] [CrossRef]
- Todaka, D.; Nakashima, K.; Maruyama, K.; Kidokoro, S.; Osakabe, Y.; Ito, Y.; Matsukura, S.; Fujita, Y.; Yoshiwara, K.; Ohme-Takagi, M.; et al. Rice phytochrome-interacting factor-like protein OsPIL1 functions as a key regulator of internode elongation and induces a morphological response to drought stress. Proc. Natl. Acad. Sci. USA 2012, 109, 15947–15952. [Google Scholar] [CrossRef]
- Gramegna, G.; Rosado, D.; Carranza, A.P.S.; Cruz, A.B.; Simon-Moya, M.; Llorente, B.; Rodriguez-Concepcion, M.; Freschi, L.; Rossi, M. Phytochrome-Interacting Factor 3 mediates light-dependent induction of tocopherol biosynthesis during tomato fruit ripening. Plant Cell Environ. 2019, 42, 1328–1339. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, M.Q.; Zhang, M.J.; Jiang, W.; Ren, X.Y.; Liang, E.X.; Zhang, D.P.; Zhang, C.Q.; Xiao, N.; Li, Y.; et al. A maize phytochrome-interacting factors protein ZmPIF1 enhances drought tolerance by inducing stomatal closure and improves grain yield in Oryza sativa. Plant Biotechnol. J. 2018, 16, 1375–1387. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, W.; Dai, Y.; Xiao, N.; Zhang, C.Q.; Li, H.; Lu, Y.; Wu, M.Q.; Tao, X.Y.; Deng, D.X.; et al. A maize phytochrome-interacting factor 3 improves drought and salt stress tolerance in rice. Plant Mol. Biol. 2015, 87, 413–428. [Google Scholar] [CrossRef]
- Lee, C.M.; Thomashow, M.F. Photoperiodic regulation of the C-repeat binding factor (CBF) cold acclimation pathway and freezing tolerance in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2012, 109, 15054–15059. [Google Scholar] [CrossRef] [PubMed]
- Kidokoro, S.; Maruyama, K.; Nakashima, K.; Imura, Y.; Narusaka, Y.; Shinwari, Z.K.; Osakabe, Y.; Fujita, Y.; Mizoi, J.; Shinozaki, K.; et al. The Phytochrome-Interacting Factor PIF7 Negatively Regulates DREB1 Expression under Circadian Control in Arabidopsis. Plant Physiol. 2009, 151, 2046–2057. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.K.; Dang, P.Y.; Liu, L.X.; He, C.Z. Cold acclimation by the CBF-COR pathway in a changing climate: Lessons from Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Bao, Y. PIF4: Integrator of light and temperature cues in plant growth. Plant Sci. 2021, 313, 111086. [Google Scholar] [CrossRef] [PubMed]
- Gangappa, S.N.; Berriri, S.; Kumar, S.V. PIF4 Coordinates Thermosensory Growth and Immunity in Arabidopsis. Curr. Biol. 2017, 27, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.Y.; Wu, S.G.; Zhang, H.Y.; Mou, M.H.; Chen, Y.L.; Li, D.B.; Wang, H.P.; Chen, L.G.; Yu, D.Q. The PIFs Redundantly Control Plant Defense Response against Botrytis cinerea in Arabidopsis. Plants 2020, 9, 1246. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Du, Y.X.; Li, F.; Sun, H.Z.; Zhang, J.; Li, J.Z.; Peng, T.; Xin, Z.Y.; Zhao, Q.Z. The basic helix-loop-helix transcription factor, OsPIL15, regulates grain size via directly targeting a purine permease gene OsPUP7 in rice. Plant Biotechnol. J. 2019, 17, 1527–1537. [Google Scholar] [CrossRef]
- Bovell-Benjamin, A.C. Sweet Potato: Sweet potato: A review of its past, present, and future role in human nutrition. Adv. Food Nutr. Res. 2007, 52, 1–59. [Google Scholar] [CrossRef]
- Behera, S.; Chauhan, V.B.S.; Pati, K.; Bansode, V.; Nedunchezhiyan, M.; Verma, A.K.; Monalisa, K.; Naik, P.K.; Naik, S.K. Biology and biotechnological aspect of sweet potato (Ipomoea batatas L.): A commercially important tuber crop. Planta 2022, 256, 40. [Google Scholar] [CrossRef]
- Zhai, H.; Wang, F.B.; Si, Z.Z.; Huo, J.X.; Xing, L.; An, Y.Y.; He, S.Z.; Liu, Q.C. A myo-inositol-1-phosphate synthase gene, IbMIPS1, enhances salt and drought tolerance and stem nematode resistance in transgenic sweet potato. Plant Biotechnol. J. 2016, 14, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, X.R.; Zhi, Y.H.; Li, X.; Zhang, Q.; Niu, J.B.; Wang, J.; Zhai, H.; Zhao, N.; Li, J.G.; et al. A non-tandem CCCH-type zinc-finger protein, IbC3H18, functions as a nuclear transcriptional activator and enhances abiotic stress tolerance in sweet potato. New Phytol. 2019, 223, 1918–1936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Q.; Zhai, H.; Gao, S.P.; Yang, L.; Wang, Z.; Xu, Y.T.; Huo, J.X.; Ren, Z.T.; Zhao, N.; et al. IbBBX24 Promotes the Jasmonic Acid Pathway and Enhances Fusarium Wilt Resistance in Sweet Potato. Plant Cell 2020, 32, 1102–1123. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.F.; Li, X.; Gao, S.P.; Nie, N.; Zhang, H.; Yang, Y.F.; He, S.Z.; Liu, Q.C.; Zhai, H. A Novel WRKY Transcription Factor from Ipomoea trifida, ItfWRKY70, Confers Drought Tolerance in Sweet Potato. Int. J. Mol. Sci. 2022, 23, 686. [Google Scholar] [CrossRef]
- Xue, L.Y.; Wei, Z.H.; Zhai, H.; Xing, S.H.; Wang, Y.X.; He, S.Z.; Gao, S.P.; Zhao, N.; Zhang, H.; Liu, Q.C. The IbPYL8-IbbHLH66-IbbHLH118 complex mediates the abscisic acid-dependent drought response in sweet potato. New Phytol. 2022, 236, 2151–2171. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Zhao, H.Q.; Hu, Y.F.; Zhang, S.S.; He, S.Z.; Zhang, H.; Zhao, N.; Liu, Q.C.; Gao, S.P.; Zhai, H. Expression of the Sweet Potato MYB Transcription Factor IbMYB48 Confers Salt and Drought Tolerance in Arabidopsis. Genes 2022, 13, 1883. [Google Scholar] [CrossRef]
- Gordon, T.R. Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef]
- Lin, S.Q.; Yang, Z.J.; Huang, B.F.; Bi, C.Y.; Huang, X.F.; Chen, G.T.; Nijiati, N.; Chen, X.Y. Comparative proteomic analysis of the sweetpotato provides insights into response mechanisms to Fusarium oxysporum f. sp. batatas. Sci. Rep. 2020, 10, 21368. [Google Scholar] [CrossRef]
- Engler, J.D.; Favery, B.; Engler, G.; Abad, P. Loss of susceptibility as an alternative for nematode resistance. Curr. Opin. Biotechnol. 2005, 16, 112–117. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.N.; Zhang, H.; Zhang, Q.; Zhai, H.; Liu, Q.C.; He, S.Z. The Plasma Membrane-Localized Sucrose Transporter IbSWEET10 Contributes to the Resistance of Sweet Potato to Fusarium oxysporum. Front. Plant Sci. 2017, 8, 197. [Google Scholar] [CrossRef]
- Lyu, R.Q.; Ahmed, S.; Fan, W.J.; Yang, J.; Wu, X.Y.; Zhou, W.Z.; Zhang, P.; Yuan, L.; Wang, H.X. Engineering Properties of Sweet Potato Starch for Industrial Applications by Biotechnological Techniques including Genome Editing. Int. J. Mol. Sci. 2021, 22, 9533. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Moeinzadeh, M.H.; Kuhl, H.; Helmuth, J.; Xiao, P.; Haas, S.; Liu, G.L.; Zheng, J.L.; Sun, Z.; Fan, W.J.; et al. Haplotype-resolved sweet potato genome traces back its hexaploidization history. Nat. Plants 2017, 3, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lau, K.H.; Cao, Q.H.; Hamilton, J.P.; Sun, H.H.; Zhou, C.X.; Eserman, L.; Gemenet, D.C.; Olukolu, B.A.; Wang, H.Y.; et al. Genome sequences of two diploid wild relatives of cultivated sweetpotato reveal targets for genetic improvement. Nat. Commun. 2018, 9, 4580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Z.F.; Jian, S.G. Functional Characterization of Heat Shock Factor (CrHsf) Families Provide Comprehensive Insight into the Adaptive Mechanisms of Canavalia rosea (Sw.) DC. to Tropical Coral Islands. Int. J. Mol. Sci. 2022, 23, 12357. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.H.; Dassanayake, M.; Haas, J.S.; Kropornika, A.; Wright, C.; d’Urzo, M.P.; Hong, H.; Ali, S.; Hernandez, A.; Lambert, G.M.; et al. Genome structures and halophyte-specific gene expression of the extremophile Thellungiella parvula in comparison with Thellungiella salsuginea (Thellungiella halophila) and Arabidopsis. Plant Physiol. 2010, 154, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.N.; Wang, X.; Li, Y.; Yu, H.Y.; Li, J.H.; Lu, Y.G.; Li, H.X.; Ouyang, B. Genomic Organization, Phylogenetic and Expression Analysis of the B-BOX Gene Family in Tomato. Front. Plant Sci. 2016, 7, 1552. [Google Scholar] [CrossRef] [PubMed]
- Koen, E.; Trapet, P.; Brule, D.; Kulik, A.; Klinguer, A.; Atauri-Miranda, L.; Meunier-Prest, R.; Boni, G.; Glauser, G.; Mauch-Mani, B.; et al. beta-Aminobutyric acid (BABA)-induced resistance in Arabidopsis thaliana: Link with iron homeostasis. Mol. Plant Microbe. Interact. 2014, 27, 1226–1240. [Google Scholar] [CrossRef]
- Meier, S.; Bastian, R.; Donaldson, L.; Murray, S.; Bajic, V.; Gehring, C. Co-expression and promoter content analyses assign a role in biotic and abiotic stress responses to plant natriuretic peptides. BMC Plant Biol. 2008, 8, 24. [Google Scholar] [CrossRef]
- Wagner, A. Distributed robustness versus redundancy as causes of mutational robustness. Bioessays 2005, 27, 176–188. [Google Scholar] [CrossRef]
- Fernandez-Pozo, N.; Menda, N.; Edwards, J.D.; Saha, S.; Tecle, I.Y.; Strickler, S.R.; Bombarely, A.; Fisher-York, T.; Pujar, A.; Foerster, H.; et al. The Sol Genomics Network (SGN)-from genotype to phenotype to breeding. Nucleic. Acids Res. 2015, 43, D1036–D1041. [Google Scholar] [CrossRef]
- Kaltenegger, E.; Eich, E.; Ober, D. Evolution of Homospermidine Synthase in the Convolvulaceae: A Story of Gene Duplication, Gene Loss, and Periods of Various Selection Pressures. Plant Cell 2013, 25, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, M.Q.; Zhang, M.J.; Jiang, W.; Liang, E.X.; Zhang, D.P.; Zhang, C.Q.; Xiao, N.; Chen, J.M. Roles of a maize phytochrome-interacting factors protein ZmPIF3 in regulation of drought stress responses by controlling stomatal closure in transgenic rice without yield penalty. Plant Mol. Biol. 2018, 97, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Rosado, D.; Trench, B.; Bianchetti, R.; Zuccarelli, R.; Alves, F.R.R.; Purgatto, E.; Floh, E.I.S.; Nogueira, F.T.S.; Freschi, L.; Rossi, M. Downregulation of Phytochrome-Interacting Factor 4 Influences Plant Development and Fruit Production. Plant Physiol. 2019, 181, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Yao, J.; Mei, C.S.; Tong, X.H.; Zeng, L.J.; Li, Q.; Xiao, L.T.; Sun, T.P.; Li, J.G.; Deng, X.W.; et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 2012, 109, E1192–E1200. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Gabriele, S.; Rizza, A.; Martone, J.; Circelli, P.; Costantino, P.; Vittorioso, P. The Dof protein DAG1 mediates PIL5 activity on seed germination by negatively regulating GA biosynthetic gene AtGA3ox1. Plant J. 2010, 61, 312–323. [Google Scholar] [CrossRef]
- Kim, J.; Kang, H.; Park, J.; Kim, W.; Yoo, J.; Lee, N.; Kim, J.; Yoon, T.Y.; Choi, G. PIF1-Interacting Transcription Factors and Their Binding Sequence Elements Determine the in Vivo Targeting Sites of PIF1. Plant Cell 2016, 28, 1388–1405. [Google Scholar] [CrossRef] [PubMed]
- Nozue, K.; Harmer, S.L.; Maloof, J.N. Genomic Analysis of Circadian Clock-, Light-, and Growth-Correlated Genes Reveals Phytochrome-Interacting Factor5 as a Modulator of Auxin Signaling in Arabidopsis. Plant Physiol. 2011, 156, 357–372. [Google Scholar] [CrossRef]
- Mo, W.; Tang, W.; Du, Y.; Jing, Y.; Bu, Q.; Lin, R. Phytochrome-Interacting Factor-Like14 and Slender Rice1 Interaction Controls Seedling Growth under Salt Stress. Plant Physiol. 2020, 184, 506–517. [Google Scholar] [CrossRef]
- Fiorucci, A.S.; Galvao, V.C.; Ince, Y.C.; Boccaccini, A.; Goyal, A.; Petrolati, L.A.; Trevisan, M.; Fankhauser, C. Phytochrome Interacting Factor 7 is important for early responses to elevated temperature in Arabidopsis seedlings. New Phytol. 2020, 226, 50–58. [Google Scholar] [CrossRef]
- Bertioli, D.J.; Cannon, S.B.; Froenicke, L.; Huang, G.D.; Farmer, A.D.; Cannon, E.K.S.; Liu, X.; Gao, D.Y.; Clevenger, J.; Dash, S.; et al. The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat. Genet. 2016, 48, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Praz, C.R.; Bourras, S.; Zeng, F.; Sanchez-Martin, J.; Menardo, F.; Xue, M.; Yang, L.; Roffler, S.; Boni, R.; Herren, G.; et al. AvrPm2 encodes an RNase-like avirulence effector which is conserved in the two different specialized forms of wheat and rye powdery mildew fungus (vol 213, pg 1301, 2017). New Phytol. 2019, 222, 2038. [Google Scholar] [CrossRef]
- Cao, A.H.; Xing, L.P.; Wang, X.Y.; Yang, X.M.; Wang, W.; Sun, Y.L.; Qian, C.; Ni, J.L.; Chen, Y.P.; Liu, D.J.; et al. Serine/threonine kinase gene Stpk-V, a key member of powdery mildew resistance gene Pm21, confers powdery mildew resistance in wheat. Proc. Natl. Acad. Sci. USA 2011, 108, 7727–7732. [Google Scholar] [CrossRef] [PubMed]
- Reuscher, S.; Furuta, T.; Bessho-Uehara, K.; Cosi, M.; Jena, K.K.; Toyoda, A.; Fujiyama, A.; Kurata, N.; Ashikari, M. Assembling the genome of the African wild rice Oryza longistaminata by exploiting synteny in closely related Oryza species. Commun. Biol. 2018, 1, 162. [Google Scholar] [CrossRef]
- Li, J.H.; Chen, C.R.; Wei, J.J.; Pan, Y.; Su, C.G.; Zhang, X.G. SpPKE1, a Multiple Stress-Responsive Gene Confers Salt Tolerance in Tomato and Tobacco. Int. J. Mol. Sci. 2019, 20, 2478. [Google Scholar] [CrossRef]
- Si, Z.Z.; Wang, L.J.; Qiao, Y.K.; Roychowdhury, R.; Ji, Z.X.; Zhang, K.; Han, J.L. Genome-wide comparative analysis of the nucleotide-binding site-encoding genes in four Ipomoea species. Front. Plant Sci. 2022, 13, 723. [Google Scholar] [CrossRef]
- Zhou, J.M.; Zhang, Y.L. Plant Immunity: Danger Perception and Signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, L.Y.; Chen, Y.N.; Xiao, N.; Zhang, D.P.; Zhang, M.J.; Wang, W.G.; Zhang, C.Q.; Zhang, A.N.; Li, H.; et al. Phytochrome interacting factor regulates stomatal aperture by coordinating red light and abscisic acid. Plant Cell 2022, 34, 4293–4312. [Google Scholar] [CrossRef]
- Jiang, B.C.; Shi, Y.T.; Peng, Y.; Jia, Y.X.; Yan, Y.; Dong, X.J.; Li, H.; Dong, J.; Li, J.G.; Gong, Z.Z.; et al. Cold-Induced CBF-PIF3 Interaction Enhances Freezing Tolerance by Stabilizing the phyB Thermosensor in Arabidopsis. Mol. Plant 2020, 13, 894–906. [Google Scholar] [CrossRef]
- Thines, B.C.; Youn, Y.W.; Duarte, M.I.; Harmon, F.G. The time of day effects of warm temperature on flowering time involve PIF4 and PIF5. J. Exp. Bot. 2014, 65, 1141–1151. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Roberts, L.J. Measurement of lipid peroxidation. Curr. Protoc. Toxicol. 1998, 28, 659–671. [Google Scholar] [CrossRef]
- Farmer, E.E.; Mueller, M.J. ROS-Mediated Lipid Peroxidation and RES-Activated Signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.L.; Bianucci, E.; Giordano, W.; Castro, S.; Becker, D.F. Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol. Biochem. 2020, 151, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Qin, F.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 2006, 18, 1292–1309. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Chen, M.; Guo, J.K.; Wang, Y.X.; Min, D.H.; Jiang, Q.Y.; Ji, H.T.; Huang, C.Y.; Wei, W.; Xu, H.J.; et al. Overexpression of soybean DREB1 enhances drought stress tolerance of transgenic wheat in the field. J. Exp. Bot. 2020, 71, 1842–1857. [Google Scholar] [CrossRef]
- Sudisha, J.; Sharathchandra, R.G.; Amruthesh, K.N.; Kumar, A.; Shetty, H.S. Pathogenesis Related Proteins in Plant Defense Response. In Plant Defence: Biological Control; Progress in Biological Control; Springer: Dordrecht, The Netherlands, 2012; Volume 12, pp. 379–403. [Google Scholar] [CrossRef]
- Ghorbel, M.; Zribi, I.; Missaoui, K.; Drira-Fakhfekh, M.; Azzouzi, B.; Brini, F. Differential regulation of the durum wheat Pathogenesis-related protein (PR1) by Calmodulin TdCaM1.3 protein. Mol. Biol. Rep. 2021, 48, 347–362. [Google Scholar] [CrossRef]
- Coll, N.S.; Epple, P.; Dangl, J.L. Programmed cell death in the plant immune system. Cell Death Differ. 2011, 18, 1247–1256. [Google Scholar] [CrossRef]
- Czernic, P.; Huang, H.C.; Marco, Y. Characterization of hsr201 and hsr515, two tobacco genes preferentially expressed during the hypersensitive reaction provoked by phytopathogenic bacteria. Plant Mol. Biol. 1996, 31, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for Visualization and Analysis of Biological Networks. Methods Mol. Biol. 2011, 696, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Mei, J.Z.; Zhao, W.W.; Hou, W.Q.; Zhang, Y.; Xu, T.; Wu, S.Y.; Zhang, L. Phylogenetic Analysis of the SQUAMOSA Promoter-Binding Protein-Like Genes in Four Ipomoea Species and Expression Profiling of the IbSPLs during Storage Root Development in Sweet Potato (Ipomoea batatas). Front. Plant Sci. 2022, 12, 3291. [Google Scholar] [CrossRef] [PubMed]

| Gene ID | Gene | CDS | Protein | Genomic | MW | pI | Instability | Gravy | Subcellular | Best Hits | Arabidopsis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | (bp) | (aa) | (bp) | (kDa) | Gene ID | ||||||
| Ib12g49455 | IbPIF1.1 | 1635 | 544 | 8025 | 59.534 | 5.79 | 62.27 | −0.583 | nucleus | AtPIF1 | At2g20180 |
| Ib02g5235 | IbPIF1.2 | 1365 | 454 | 3275 | 48.728 | 5.29 | 55.99 | −0.508 | nucleus | AtPIF1 | At2g20180 |
| Ib11g44153 | IbPIF3.1 | 2097 | 698 | 6758 | 663 | 5.98 | 54.32 | −0.58 | nucleus | AtPIF3 | At1g09530 |
| Ib09g35474 | IbPIF3.2 | 2085 | 694 | 4825 | 657 | 7.17 | 56.32 | −0.581 | nucleus | AtPIF3 | At1g09530 |
| Ib13g54841 | IbPIF4 | 2661 | 886 | 8671 | 97.031 | 6.58 | 43.27 | −0.518 | nucleus | AtPIF4 | At2g43010 |
| Ib02g9143 | IbPIF8 | 1395 | 464 | 4500 | 49.17 | 6.55 | 54.59 | −0.545 | nucleus | AtPIF8 | At4g00050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, N.; Huo, J.; Sun, S.; Zuo, Z.; Chen, Y.; Liu, Q.; He, S.; Gao, S.; Zhang, H.; Zhao, N.; et al. Genome-Wide Characterization of the PIFs Family in Sweet Potato and Functional Identification of IbPIF3.1 under Drought and Fusarium Wilt Stresses. Int. J. Mol. Sci. 2023, 24, 4092. https://doi.org/10.3390/ijms24044092
Nie N, Huo J, Sun S, Zuo Z, Chen Y, Liu Q, He S, Gao S, Zhang H, Zhao N, et al. Genome-Wide Characterization of the PIFs Family in Sweet Potato and Functional Identification of IbPIF3.1 under Drought and Fusarium Wilt Stresses. International Journal of Molecular Sciences. 2023; 24(4):4092. https://doi.org/10.3390/ijms24044092
Chicago/Turabian StyleNie, Nan, Jinxi Huo, Sifan Sun, Zhidan Zuo, Yanqi Chen, Qingchang Liu, Shaozhen He, Shaopei Gao, Huan Zhang, Ning Zhao, and et al. 2023. "Genome-Wide Characterization of the PIFs Family in Sweet Potato and Functional Identification of IbPIF3.1 under Drought and Fusarium Wilt Stresses" International Journal of Molecular Sciences 24, no. 4: 4092. https://doi.org/10.3390/ijms24044092
APA StyleNie, N., Huo, J., Sun, S., Zuo, Z., Chen, Y., Liu, Q., He, S., Gao, S., Zhang, H., Zhao, N., & Zhai, H. (2023). Genome-Wide Characterization of the PIFs Family in Sweet Potato and Functional Identification of IbPIF3.1 under Drought and Fusarium Wilt Stresses. International Journal of Molecular Sciences, 24(4), 4092. https://doi.org/10.3390/ijms24044092






