Role of Nasal Fibroblasts in Airway Remodeling of Chronic Rhinosinusitis: The Modulating Functions Reexamined
Abstract
1. Introduction
2. Normal Wound-Healing Process in Nasal Fibroblasts
3. Remodeling Modulation of Nasal Fibroblasts in CRS
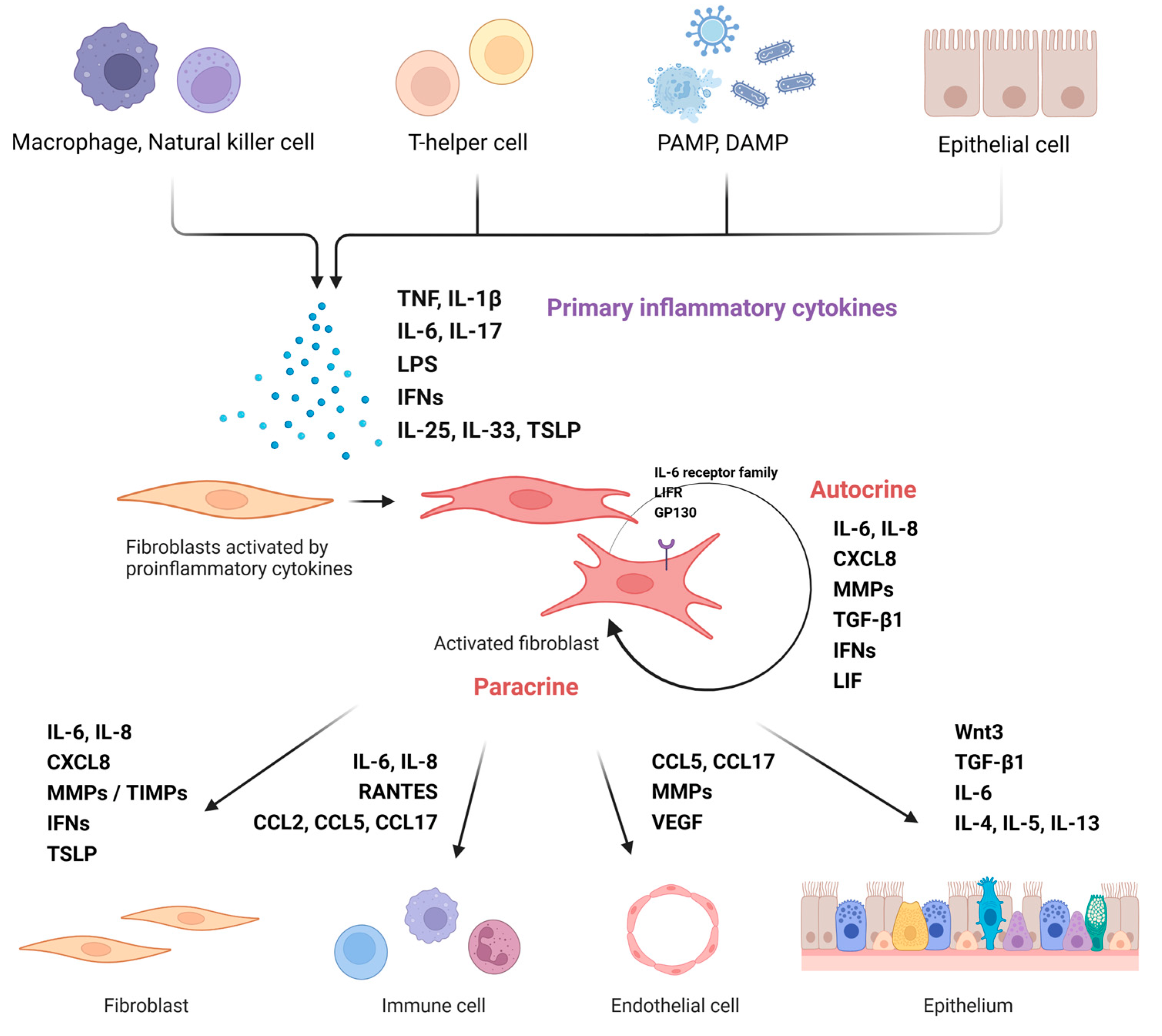
3.1. Nasal Fibroblast Activation
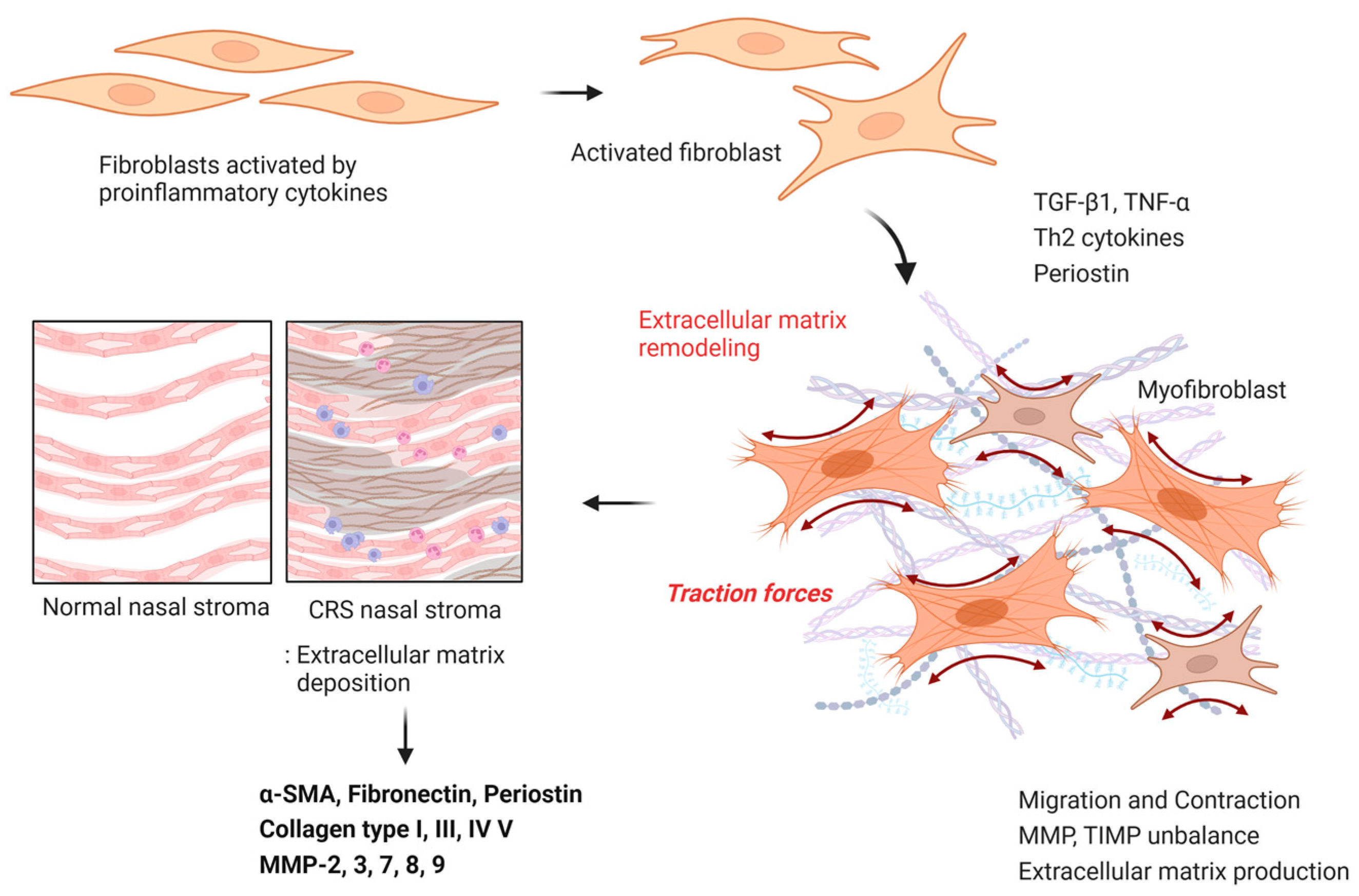
3.2. ECM Accumulation
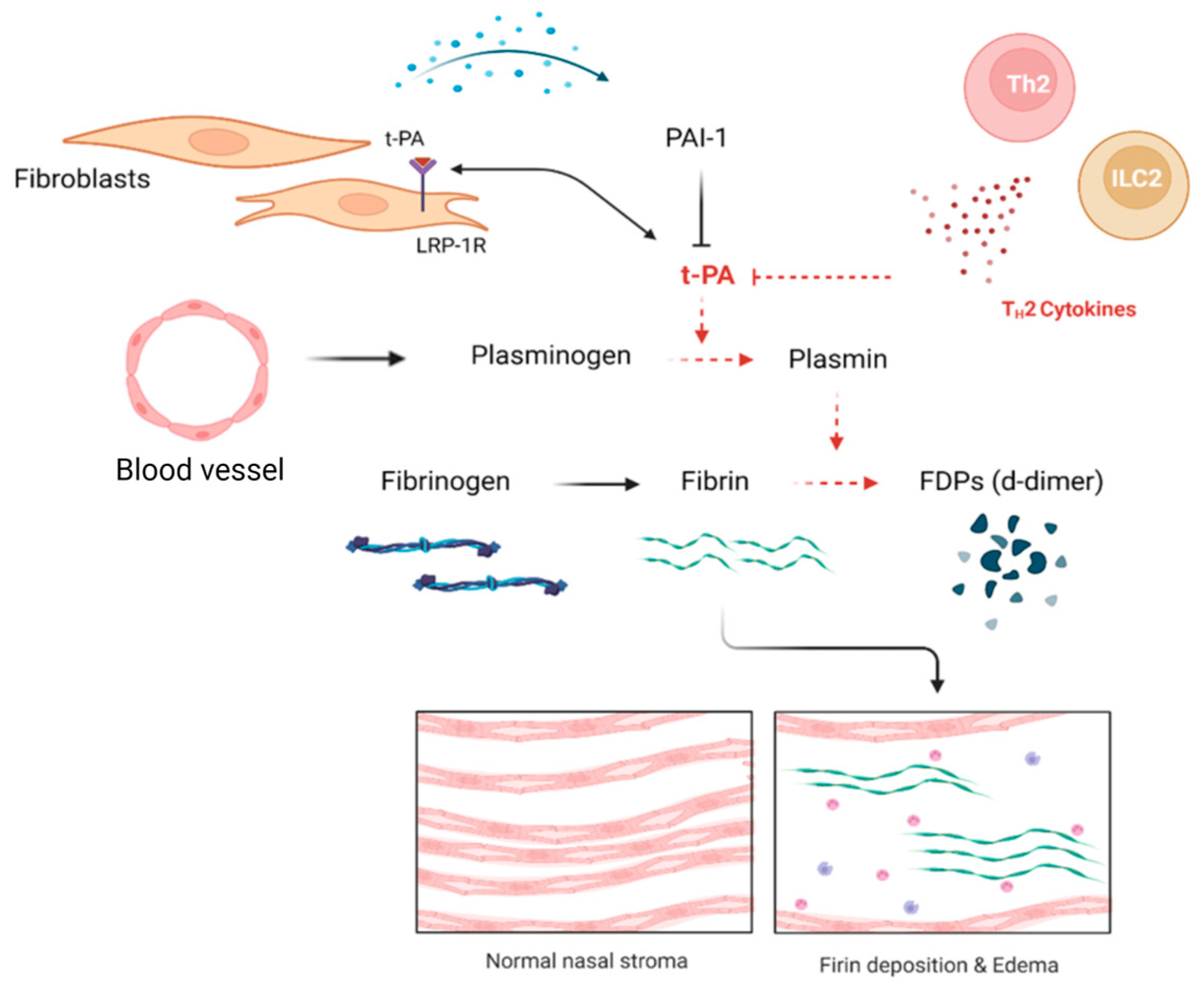
3.3. Fibriolytic System Modulation; Fibrin Deposition
3.4. Epithelial Remodeling
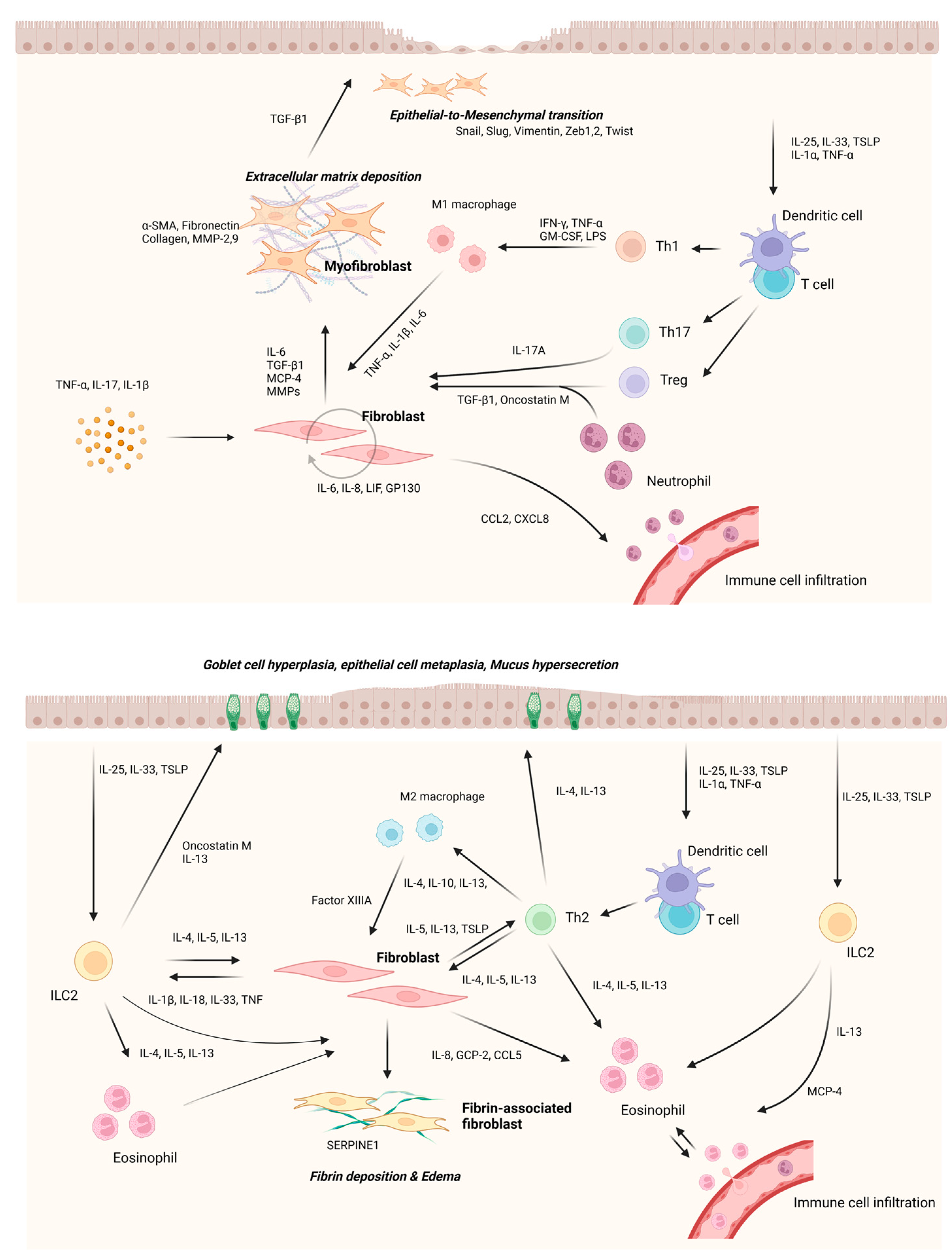
4. Cross-Talk between Nasal Fibroblasts and Immune Cells
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Dietz de Loos, D.; Lourijsen, E.S.; Wildeman, M.A.M.; Freling, N.J.M.; Wolvers, M.D.J.; Reitsma, S.; Fokkens, W.J. Prevalence of chronic rhinosinusitis in the general population based on sinus radiology and symptomatology. J. Allergy Clin. Immunol. 2019, 143, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, R.R.; Kingdom, T.T.; Smith, T.L.; Bleier, B.; DeConde, A.; Luong, A.U.; Poetker, D.M.; Soler, Z.; Welch, K.C.; Wise, S.K.; et al. International consensus statement on allergy and rhinology: Rhinosinusitis 2021. Int. Forum Allergy Rhinol. 2021, 11, 213–739. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Asaka, D.; Yoshikawa, M.; Okushi, T.; Matsuwaki, Y.; Moriyama, H.; Otori, N. Identification of chronic rhinosinusitis phenotypes using cluster analysis. Am. J. Rhinol. Allergy 2012, 26, 172–176. [Google Scholar] [CrossRef]
- Van Zele, T.; Claeys, S.; Gevaert, P.; Van Maele, G.; Holtappels, G.; Van Cauwenberge, P.; Bachert, C. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 2006, 61, 1280–1289. [Google Scholar] [CrossRef]
- Brescia, G.; Zanotti, C.; Parrino, D.; Barion, U.; Marioni, G. Nasal polyposis pathophysiology: Endotype and phenotype open issues. Am. J. Otolaryngol. 2018, 39, 441–444. [Google Scholar] [CrossRef]
- Cho, S.-W.; Kim, D.W.; Kim, J.-W.; Lee, C.H.; Rhee, C.-S. Classification of chronic rhinosinusitis according to a nasal polyp and tissue eosinophilia: Limitation of current classification system for Asian population. Asia Pac. Allergy 2017, 7, 121–130. [Google Scholar] [CrossRef]
- Lee, K.; Tai, J.; Lee, S.H.; Kim, T.H. Advances in the Knowledge of the Underlying Airway Remodeling Mechanisms in Chronic Rhinosinusitis Based on the Endotypes: A Review. Int. J. Mol. Sci. 2021, 22, 910. [Google Scholar] [CrossRef]
- Pinet, K.; McLaughlin, K.A. Mechanisms of physiological tissue remodeling in animals: Manipulating tissue, organ, and organism morphology. Dev. Biol. 2019, 451, 134–145. [Google Scholar] [CrossRef]
- Boulet, L.P. Airway remodeling in asthma: Update on mechanisms and therapeutic approaches. Curr. Opin. Pulm. Med. 2018, 24, 56–62. [Google Scholar] [CrossRef]
- Samitas, K.; Carter, A.; Kariyawasam, H.H.; Xanthou, G. Upper and lower airway remodelling mechanisms in asthma, allergic rhinitis and chronic rhinosinusitis: The one airway concept revisited. Allergy 2018, 73, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Van Bruaene, N.; Bachert, C. Tissue remodeling in chronic rhinosinusitis. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–408; 410–412. [Google Scholar] [CrossRef] [PubMed]
- Halloran, C.M.; Slavin, J.P. Pathophysiology of Wound Healing. Surgery (Oxford) 2002, 20, i–v. [Google Scholar] [CrossRef]
- Selvarajah, J.; Saim, A.B.; Bt Hj Idrus, R.; Lokanathan, Y. Current and Alternative Therapies for Nasal Mucosa Injury: A Review. Int. J. Mol. Sci. 2020, 21, 480. [Google Scholar] [CrossRef]
- Hong, S.M.; Park, I.H.; Um, J.Y.; Shin, J.M.; Lee, H.M. Stimulatory effects of histamine on migration of nasal fibroblasts. Int. Forum Allergy Rhinol. 2015, 5, 923–928. [Google Scholar] [CrossRef]
- Shin, J.-M.; Park, I.-H.; Moon, Y.-M.; Hong, S.-M.; Cho, J.-S.; Um, J.-Y.; Lee, H.-M. Inhibitory Effect of Prostaglandin E2 on the Migration of Nasal Fibroblasts. Am. J. Rhinol. Allergy 2014, 28, e120–e124. [Google Scholar] [CrossRef]
- Shin, J.M.; Park, J.H.; Yang, H.W.; Lee, H.M.; Park, I.H. Cigarette smoke extract inhibits cell migration and contraction via the reactive oxygen species/adenosine monophosphate-activated protein kinase pathway in nasal fibroblasts. Int. Forum Allergy Rhinol. 2020, 10, 356–363. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.H. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef]
- Yeo, N.K.; Eom, D.W.; Oh, M.Y.; Lim, H.W.; Song, Y.J. Expression of matrix metalloproteinase 2 and 9 and tissue inhibitor of metalloproteinase 1 in nonrecurrent vs recurrent nasal polyps. Ann. Allergy Asthma Immunol. 2013, 111, 205–210. [Google Scholar] [CrossRef]
- Kim, H.-J.; Park, J.-H.; Shin, J.-M.; Yang, H.-W.; Lee, H.-M.; Park, I.-H. TGF-β1-induced HSP47 regulates extracellular matrix accumulation via Smad2/3 signaling pathways in nasal fibroblasts. Sci. Rep. 2019, 9, 15563. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Pawankar, R.; Fukumoto, A.; Yagi, T. Heterogeneous response of nasal and lung fibroblasts to transforming growth factor-beta 1. Clin. Exp. Allergy 2008, 38, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Kang, J.H.; Han, I.H.; Um, J.Y.; Lee, H.M. Activation of TLR4 induces VEGF expression via Akt pathway in nasal polyps. Clin. Exp. Allergy 2013, 43, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Tojima, I.; Nakamura, K.; Arai, H.; Kouzaki, H.; Shimizu, T. Nasal polyp fibroblasts (NPFs)-derived exosomes are important for the release of vascular endothelial growth factor from cocultured eosinophils and NPFs. Auris Nasus Larynx 2022, 49, 407–414. [Google Scholar] [CrossRef]
- Uchida, J.; Kanai, K.; Asano, K.; Watanabe, S.; Hisamitsu, T.; Suzaki, H. Influence of fluticasone propionate on the production of vascular endothelial growth factor and basic fibroblast growth factor from nasal fibroblasts in vitro. In Vivo 2004, 18, 767–770. [Google Scholar]
- Cho, J.S.; Kang, J.H.; Park, I.H.; Lee, H.M. Steroids inhibit vascular endothelial growth factor expression via TLR4/Akt/NF-κB pathway in chronic rhinosinusitis with nasal polyp. Exp. Biol. Med. (Maywood) 2014, 239, 913–921. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Ball, S.L.; Mann, D.A.; Wilson, J.A.; Fisher, A.J. The Role of the Fibroblast in Inflammatory Upper Airway Conditions. Am. J. Pathol. 2016, 186, 225–233. [Google Scholar] [CrossRef]
- Saji, F.; Nonaka, M.; Pawankar, R. Expression of RANTES by IL-1 beta and TNF-alpha stimulated nasal polyp fibroblasts. Auris Nasus Larynx 2000, 27, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Kang, J.H.; Um, J.Y.; Han, I.H.; Park, I.H.; Lee, H.M. Lipopolysaccharide induces pro-inflammatory cytokines and MMP production via TLR4 in nasal polyp-derived fibroblast and organ culture. PLoS ONE 2014, 9, e90683. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Yang, H.W.; Park, J.H.; Shin, J.M.; Kim, T.H.; Lee, S.H.; Lee, H.M.; Park, I.H. Lipopolysaccharide regulates thymic stromal lymphopoietin expression via TLR4/MAPK/Akt/NF-κB-signaling pathways in nasal fibroblasts: Differential inhibitory effects of macrolide and corticosteroid. Int. Forum Allergy Rhinol. 2021, 11, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef]
- Jin, Y.W.; Cho, J.S.; Kim, H.; Kim, C.D.; Jin, S.Y.; Cha, C.I. Effects of Dexamethasone on RANTES Expression of Nasal Fibroblast. Korean J. Otorhinolaryngol.-Head Neck Surg. 2001, 44, 930–935. [Google Scholar]
- Park, S.-K.; Jin, Y.-D.; Park, Y.-K.; Yeon, S.-H.; Xu, J.; Han, R.-N.; Rha, K.-S.; Kim, Y.-M. IL-25-induced activation of nasal fibroblast and its association with the remodeling of chronic rhinosinusitis with nasal polyposis. PLoS ONE 2017, 12, e0181806. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.-P. Interleukin-33 (IL-33): A critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine 2022, 156, 155891. [Google Scholar] [CrossRef]
- Cao, L.; Liu, F.; Liu, Y.; Liu, T.; Wu, J.; Zhao, J.; Wang, J.; Li, S.; Xu, J.; Dong, L. TSLP promotes asthmatic airway remodeling via p38-STAT3 signaling pathway in human lung fibroblast. Exp. Lung Res. 2018, 44, 288–301. [Google Scholar] [CrossRef]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorganic Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Noss, E.H.; Mizoguchi, F.; Huppertz, C.; Wei, K.S.; Watts, G.F.M.; Brenner, M.B. Autocrine Loop Involving IL-6 Family Member LIF, LIF Receptor, and STAT4 Drives Sustained Fibroblast Production of Inflammatory Mediators. Immunity 2017, 46, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Nguyen, H.N.; Brenner, M.B. Fibroblast pathology in inflammatory diseases. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Zhang, H. Extracellular matrix: An important regulator of cell functions and skeletal muscle development. Cell Biosci. 2021, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Schleimer, R.P. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 331–357. [Google Scholar] [CrossRef]
- Lee, S.-A.; Yang, H.-W.; Um, J.-Y.; Shin, J.-M.; Park, I.-H.; Lee, H.-M. Vitamin D attenuates myofibroblast differentiation and extracellular matrix accumulation in nasal polyp-derived fibroblasts through smad2/3 signaling pathway. Sci. Rep. 2017, 7, 7299. [Google Scholar] [CrossRef]
- Wang, Q.P.; Jiang, M.J.; Li, Z.Q. Effect of myofibroblast accumulation on the formation and development of nasal polyps. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2006, 41, 381–383. [Google Scholar]
- Meng, J.; Zhou, P.; Liu, Y.; Liu, F.; Yi, X.; Liu, S.; Holtappels, G.; Bachert, C.; Zhang, N. The development of nasal polyp disease involves early nasal mucosal inflammation and remodelling. PLoS ONE 2013, 8, e82373. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Eyibilen, A.; Cayli, S.; Aladag, I.; Koç, S.; Gurbuzler, L.; Atay, G.A. Distribution of matrix metalloproteinases MMP-1, MMP-2, MMP-8 and tissue inhibitor of matrix metalloproteinases-2 in nasal polyposis and chronic rhinosinusitis. Histol. Histopathol. 2011, 26, 615–621. [Google Scholar] [CrossRef]
- Kostamo, K.; Tervahartiala, T.; Sorsa, T.; Richardson, M.; Toskala, E. Metalloproteinase function in chronic rhinosinusitis with nasal polyposis. Laryngoscope 2007, 117, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Luo, D.; Wang, S.; Huang, R.; Dong, P. Salvianolic acid B inhibits myofibroblast differentiation and extracellular matrix accumulation in nasal polyp fibroblasts via the TGF-β1 signaling pathway. Mol. Med. Rep. 2021, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Park, J.H.; Kang, B.; Lee, S.A.; Park, I.H.; Lee, H.M. Effect of doxycycline on transforming growth factor-beta-1-induced matrix metalloproteinase 2 expression, migration, and collagen contraction in nasal polyp-derived fibroblasts. Am. J. Rhinol. Allergy 2016, 30, 385–390. [Google Scholar] [CrossRef]
- Shimizu, T.; Kanai, K.; Asano, K.; Hisamitsu, T.; Suzaki, H. Suppression of matrix metalloproteinase production in nasal fibroblasts by tranilast, an antiallergic agent, in vitro. Mediat. Inflamm 2005, 2005, 150–159. [Google Scholar] [CrossRef]
- Du, K.; Wang, M.; Zhang, N.; Yu, P.; Wang, P.; Li, Y.; Wang, X.; Zhang, L.; Bachert, C. Involvement of the extracellular matrix proteins periostin and tenascin C in nasal polyp remodeling by regulating the expression of MMPs. Clin. Transl. Allergy 2021, 11, e12059. [Google Scholar] [CrossRef] [PubMed]
- Mehra, D.; Sternberg, D.I.; Jia, Y.; Canfield, S.; Lemaitre, V.; Nkyimbeng, T.; Wilder, J.; Sonett, J.; D’Armiento, J. Altered lymphocyte trafficking and diminished airway reactivity in transgenic mice expressing human MMP-9 in a mouse model of asthma. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2010, 298, L189–L196. [Google Scholar] [CrossRef]
- Li, X.; Meng, J.; Qiao, X.; Liu, Y.; Liu, F.; Zhang, N.; Zhang, J.; Holtappels, G.; Luo, B.; Zhou, P.; et al. Expression of TGF, matrix metalloproteinases, and tissue inhibitors in Chinese chronic rhinosinusitis. J. Allergy Clin. Immunol. 2010, 125, 1061–1068. [Google Scholar] [CrossRef]
- Malinsky, R.R.; Valera, F.C.; Cavallari, F.E.; Küpper, D.S.; Milaneze, C.; Silva, J.S.; Tamashiro, E.; Anselmo-Lima, W.T. Matrix metalloproteinases and their impact on sinusal extension in chronic rhinosinusitis with nasal polyps. Eur. Arch. Otorhinolaryngol. 2013, 270, 1345–1348. [Google Scholar] [CrossRef]
- Can, I.H.; Ceylan, K.; Caydere, M.; Samim, E.E.; Ustun, H.; Karasoy, D.S. The expression of MMP-2, MMP-7, MMP-9, and TIMP-1 in chronic rhinosinusitis and nasal polyposis. Otolaryngol.–Head Neck Surg. 2008, 139, 211–215. [Google Scholar] [CrossRef]
- Kim, D.Y.; Cho, S.H.; Takabayashi, T.; Schleimer, R.P. Chronic Rhinosinusitis and the Coagulation System. Allergy Asthma Immunol. Res. 2015, 7, 421–430. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care (New Rochelle) 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Kim, D.Y. Lessons From Localized Chronic Rhinosinusitis With Nasal Polyps. Allergy Asthma Immunol. Res. 2021, 13, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, T.; Kato, A.; Peters, A.T.; Hulse, K.E.; Suh, L.A.; Carter, R.; Norton, J.; Grammer, L.C.; Cho, S.H.; Tan, B.K.; et al. Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression. Am. J. Respir. Crit. Care Med. 2013, 187, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Akkawi, S.; Nassar, T.; Tarshis, M.; Cines, D.B.; Higazi, A.A. LRP and alphavbeta3 mediate tPA activation of smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1351–H1359. [Google Scholar] [CrossRef]
- Kato, A.; Schleimer, R.P.; Bleier, B.S. Mechanisms and pathogenesis of chronic rhinosinusitis. J. Allergy Clin. Immunol. 2022, 149, 1491–1503. [Google Scholar] [CrossRef]
- Brims, F.J.; Chauhan, A.J.; Higgins, B.; Shute, J.K. Coagulation factors in the airways in moderate and severe asthma and the effect of inhaled steroids. Thorax 2009, 64, 1037–1043. [Google Scholar] [CrossRef]
- Pawliczak, R.; Lewandowska-Polak, A.; Kowalski, M.L. Pathogenesis of nasal polyps: An update. Curr. Allergy Asthma Rep. 2005, 5, 463–471. [Google Scholar] [CrossRef]
- Huang, W.T.; Akhter, H.; Jiang, C.; MacEwen, M.; Ding, Q.; Antony, V.; Thannickal, V.J.; Liu, R.M. Plasminogen activator inhibitor 1, fibroblast apoptosis resistance, and aging-related susceptibility to lung fibrosis. Exp. Gerontol. 2015, 61, 62–75. [Google Scholar] [CrossRef]
- Sejima, T.; Madoiwa, S.; Mimuro, J.; Sugo, T.; Ishida, T.; Ichimura, K.; Sakata, Y. Expression profiles of fibrinolytic components in nasal mucosa. Histochem. Cell Biol. 2004, 122, 61–73. [Google Scholar] [CrossRef]
- Samarakoon, R.; Higgins, S.P.; Higgins, C.E.; Higgins, P.J. TGF-beta1-induced plasminogen activator inhibitor-1 expression in vascular smooth muscle cells requires pp60(c-src)/EGFR(Y845) and Rho/ROCK signaling. J. Mol. Cell Cardiol. 2008, 44, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Böscke, R.; Vladar, E.K.; Könnecke, M.; Hüsing, B.; Linke, R.; Pries, R.; Reiling, N.; Axelrod, J.D.; Nayak, J.V.; Wollenberg, B. Wnt Signaling in Chronic Rhinosinusitis with Nasal Polyps. Am. J. Respir. Cell Mol. Biol. 2017, 56, 575–584. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, H.; Yin, J. The Role of Epithelial-Mesenchymal Transition in Chronic Rhinosinusitis. Int. Arch. Allergy Immunol. 2022, 183, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Dobzanski, A.; Khalil, S.M.; Lane, A.P. Nasal polyp fibroblasts modulate epithelial characteristics via Wnt signaling. Int. Forum Allergy Rhinol. 2018, 8, 1412–1420. [Google Scholar] [CrossRef]
- Nonaka, M.; Fukumoto, A.; Ogihara, N.; Pawankar, R.; Sakanushi, A.; Yagi, T. Expression of MCP-4 by TLR ligand-stimulated nasal polyp fibroblasts. Acta Oto-Laryngol. 2007, 127, 1304–1309. [Google Scholar] [CrossRef]
- Kondo, M.; Tamaoki, J.; Takeyama, K.; Nakata, J.; Nagai, A. Interleukin-13 induces goblet cell differentiation in primary cell culture from Guinea pig tracheal epithelium. Am. J. Respir. Cell Mol. Biol. 2002, 27, 536–541. [Google Scholar] [CrossRef]
- Kanoh, S.; Tanabe, T.; Rubin, B.K. IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin. Exp. Allergy 2011, 41, 1747–1756. [Google Scholar] [CrossRef]
- Czerwaty, K.; Piszczatowska, K.; Brzost, J.; Ludwig, N.; Szczepański, M.J.; Dżaman, K. Immunological Aspects of Chronic Rhinosinusitis. Diagnostics 2022, 12, 2361. [Google Scholar] [CrossRef]
- Carsuzaa, F.; Béquignon, É.; Dufour, X.; de Bonnecaze, G.; Lecron, J.C.; Favot, L. Cytokine Signature and Involvement in Chronic Rhinosinusitis with Nasal Polyps. Int. J. Mol. Sci. 2021, 23, 417. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Francou, A.; Anderson, K.V. The Epithelial-to-Mesenchymal Transition in Development and Cancer. Annu. Rev. Cancer Biol. 2020, 4, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tao, P.; Zhou, Q.; Li, J.; Yu, Z.; Wang, X.; Li, J.; Li, C.; Yan, M.; Zhu, Z.; et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget 2017, 8, 20741–20750. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 218. [Google Scholar] [CrossRef]
- Davidson, S.; Coles, M.; Thomas, T.; Kollias, G.; Ludewig, B.; Turley, S.; Brenner, M.; Buckley, C.D. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 2021, 21, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, H. Mucosal epithelial cells: The initial sentinels and responders controlling and regulating immune responses to viral infections. Cell Mol. Immunol. 2021, 18, 1628–1630. [Google Scholar] [CrossRef]
- Schleimer, R.P.; Kato, A.; Kern, R.; Kuperman, D.; Avila, P.C. Epithelium: At the interface of innate and adaptive immune responses. J. Allergy Clin. Immunol. 2007, 120, 1279–1284. [Google Scholar] [CrossRef]
- Cohn, L.; Homer, R.J.; Marinov, A.; Rankin, J.; Bottomly, K. Induction of airway mucus production By T helper 2 (Th2) cells: A critical role for interleukin 4 in cell recruitment but not mucus production. J. Exp. Med. 1997, 186, 1737–1747. [Google Scholar] [CrossRef]
- Cohn, L.; Whittaker, L.; Niu, N.; Homer, R.J. Cytokine regulation of mucus production in a model of allergic asthma. Novartis Found. Symp. 2002, 248, 201–213; discussion 213–220; 277–282. [Google Scholar]
- Gieseck, R.L.; Wilson, M.S.; Wynn, T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018, 18, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Kojima, T.; Fuchimoto, J.; Obata, K.; Keira, T.; Himi, T.; Sawada, N. Regulation of interleukin-33 and thymic stromal lymphopoietin in human nasal fibroblasts by proinflammatory cytokines. Laryngoscope 2012, 122, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, P.J.; Jimenez, S.A. The significance of macrophage polarization subtypes for animal models of tissue fibrosis and human fibrotic diseases. Clin. Transl. Med. 2015, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, M.; Ericsson, A.C. Function of Macrophages in Disease: Current Understanding on Molecular Mechanisms. Front. Immunol. 2021, 12, 620510. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Van Linthout, S.; Miteva, K.; Tschöpe, C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 2014, 102, 258–269. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, X.; Tan, S.; Luo, C.; Zhou, J.; Zhang, S.; Li, Z.; Lin, H.; Zhang, W. M2 macrophage-related gene signature in chronic rhinosinusitis with nasal polyps. Front. Immunol. 2022, 13, 1047930. [Google Scholar] [CrossRef]
- Takabayashi, T.; Kato, A.; Peters, A.T.; Hulse, K.E.; Suh, L.A.; Carter, R.; Norton, J.; Grammer, L.C.; Tan, B.K.; Chandra, R.K.; et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2013, 132, 584–592.e584. [Google Scholar] [CrossRef]
- Travers, J.; Rothenberg, M.E. Eosinophils in mucosal immune responses. Mucosal Immunol. 2015, 8, 464–475. [Google Scholar] [CrossRef]
- Wirnsberger, G.; Hebenstreit, D.; Posselt, G.; Horejs-Hoeck, J.; Duschl, A. IL-4 induces expression of TARC/CCL17 via two STAT6 binding sites. Eur. J. Immunol. 2006, 36, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Z.; Yap, H.M.; Shaari, K.; Tham, C.L.; Sulaiman, M.R.; Israf, D.A. Blockade of Eosinophil-Induced Bronchial Epithelial-Mesenchymal Transition with a Geranyl Acetophenone in a Coculture Model. Front. Pharmacol. 2017, 8, 837. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, A.; Hosoki, K.; Toda, M.; Miyake, Y.; Matsushima, Y.; Matsumoto, T.; Boveda-Ruiz, D.; Gil-Bernabe, P.; Nagao, M.; Sugimoto, M.; et al. Eosinophils promote epithelial to mesenchymal transition of bronchial epithelial cells. PLoS ONE 2013, 8, e64281. [Google Scholar] [CrossRef]
- Yang, H.W.; Park, J.H.; Jo, M.S.; Shin, J.M.; Kim, D.W.; Park, I.H. Eosinophil-Derived Osteopontin Induces the Expression of Pro-Inflammatory Mediators and Stimulates Extracellular Matrix Production in Nasal Fibroblasts: The Role of Osteopontin in Eosinophilic Chronic Rhinosinusitis. Front. Immunol. 2022, 13, 777928. [Google Scholar] [CrossRef] [PubMed]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Pothoven, K.L.; Norton, J.E.; Suh, L.A.; Carter, R.G.; Harris, K.E.; Biyasheva, A.; Welch, K.; Shintani-Smith, S.; Conley, D.B.; Liu, M.C.; et al. Neutrophils are a major source of the epithelial barrier disrupting cytokine oncostatin M in patients with mucosal airways disease. J. Allergy Clin. Immunol. 2017, 139, 1966–1978.e1969. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, A.; Miwa, M.; Ono, N.; Homma, H.; Hirotsu, M.; Ikeda, K. Comparative analysis of cytokine release from epithelial cell cultures of the upper airway. Rhinology 2015, 53, 135–141. [Google Scholar] [CrossRef]
- Higashino, N.; Koma, Y.-i.; Hosono, M.; Takase, N.; Okamoto, M.; Kodaira, H.; Nishio, M.; Shigeoka, M.; Kakeji, Y.; Yokozaki, H. Fibroblast activation protein-positive fibroblasts promote tumor progression through secretion of CCL2 and interleukin-6 in esophageal squamous cell carcinoma. Lab. Investig. 2019, 99, 777–792. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, N.; Bo, M.; Holtappels, G.; Zheng, M.; Lou, H.; Wang, H.; Zhang, L.; Bachert, C. Diversity of T(H) cytokine profiles in patients with chronic rhinosinusitis: Abmulticenter study in Europe, Asia, and Oceania. J. Allergy Clin. Immunol. 2016, 138, 1344–1353. [Google Scholar] [CrossRef]
- Lee, M.; Kim, D.W.; Khalmuratova, R.; Shin, S.H.; Kim, Y.M.; Han, D.H.; Kim, H.J.; Kim, D.Y.; Rhee, C.S.; Park, J.W.; et al. The IFN-γ-p38, ERK kinase axis exacerbates neutrophilic chronic rhinosinusitis by inducing the epithelial-to-mesenchymal transition. Mucosal Immunol. 2019, 12, 601–611. [Google Scholar] [CrossRef]
- Mincham, K.T.; Bruno, N.; Singanayagam, A.; Snelgrove, R.J. Our evolving view of neutrophils in defining the pathology of chronic lung disease. Immunology 2021, 164, 701–721. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.M.; Yang, H.W.; Park, J.H.; Kim, T.H. Role of Nasal Fibroblasts in Airway Remodeling of Chronic Rhinosinusitis: The Modulating Functions Reexamined. Int. J. Mol. Sci. 2023, 24, 4017. https://doi.org/10.3390/ijms24044017
Shin JM, Yang HW, Park JH, Kim TH. Role of Nasal Fibroblasts in Airway Remodeling of Chronic Rhinosinusitis: The Modulating Functions Reexamined. International Journal of Molecular Sciences. 2023; 24(4):4017. https://doi.org/10.3390/ijms24044017
Chicago/Turabian StyleShin, Jae Min, Hyun Woo Yang, Jae Hyung Park, and Tae Hoon Kim. 2023. "Role of Nasal Fibroblasts in Airway Remodeling of Chronic Rhinosinusitis: The Modulating Functions Reexamined" International Journal of Molecular Sciences 24, no. 4: 4017. https://doi.org/10.3390/ijms24044017
APA StyleShin, J. M., Yang, H. W., Park, J. H., & Kim, T. H. (2023). Role of Nasal Fibroblasts in Airway Remodeling of Chronic Rhinosinusitis: The Modulating Functions Reexamined. International Journal of Molecular Sciences, 24(4), 4017. https://doi.org/10.3390/ijms24044017





