Protein–Ligand Interactions in Scarcity: The Stringent Response from Bacteria to Metazoa, and the Unanswered Questions
Abstract
1. Introduction
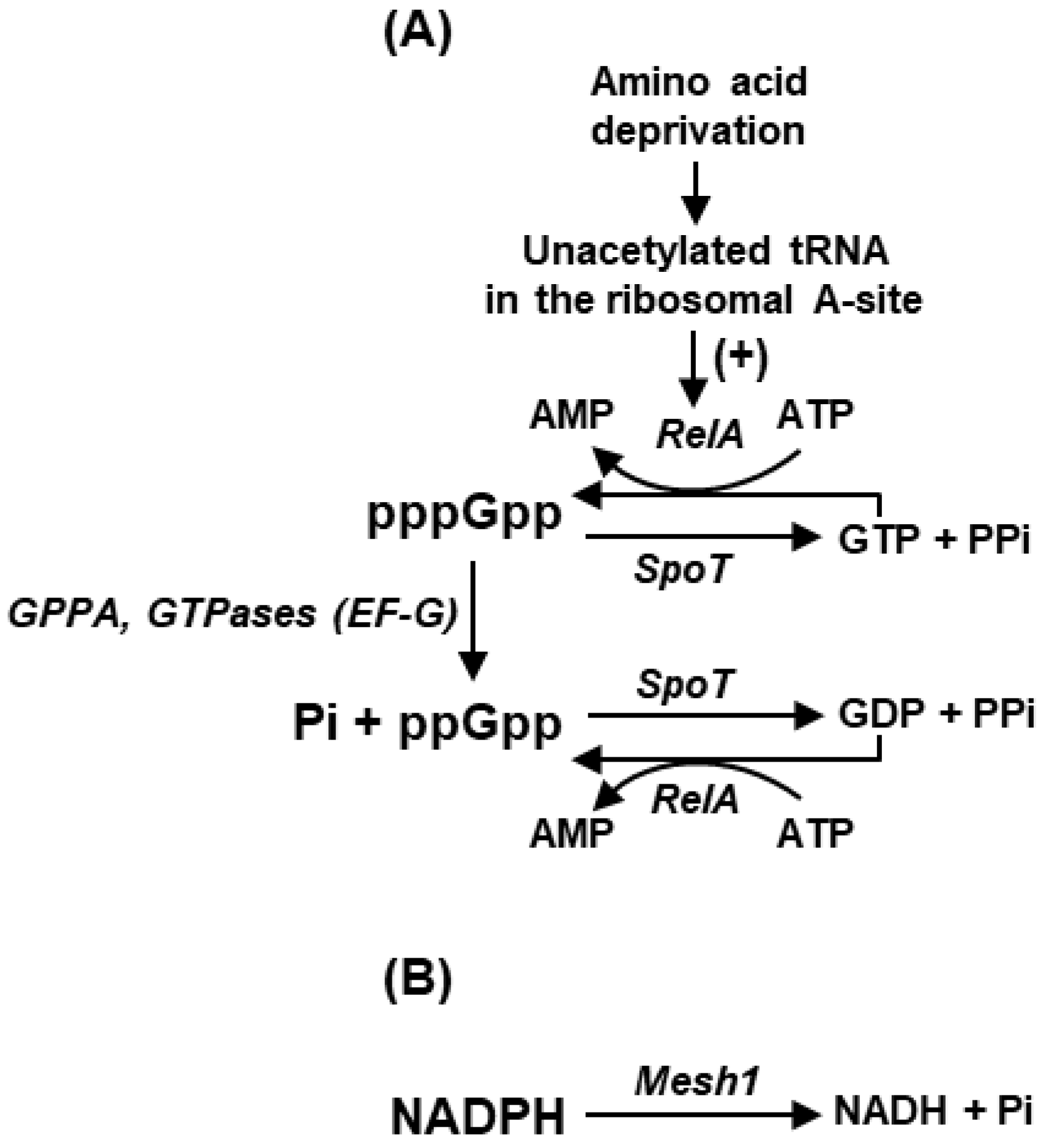
2. Plurality of ppGpp Synthetic and Homeostatic Pathways
3. The Complex Network of (p)ppGpp Signaling
3.1. Transcriptional Regulation
DksA and GreA/B: An Unexpected Similarity
3.2. Regulation of Translation by (p)ppGpp
3.3. Regulation of Replication by (p)ppGpp
3.4. The (p)ppGpp Interactome
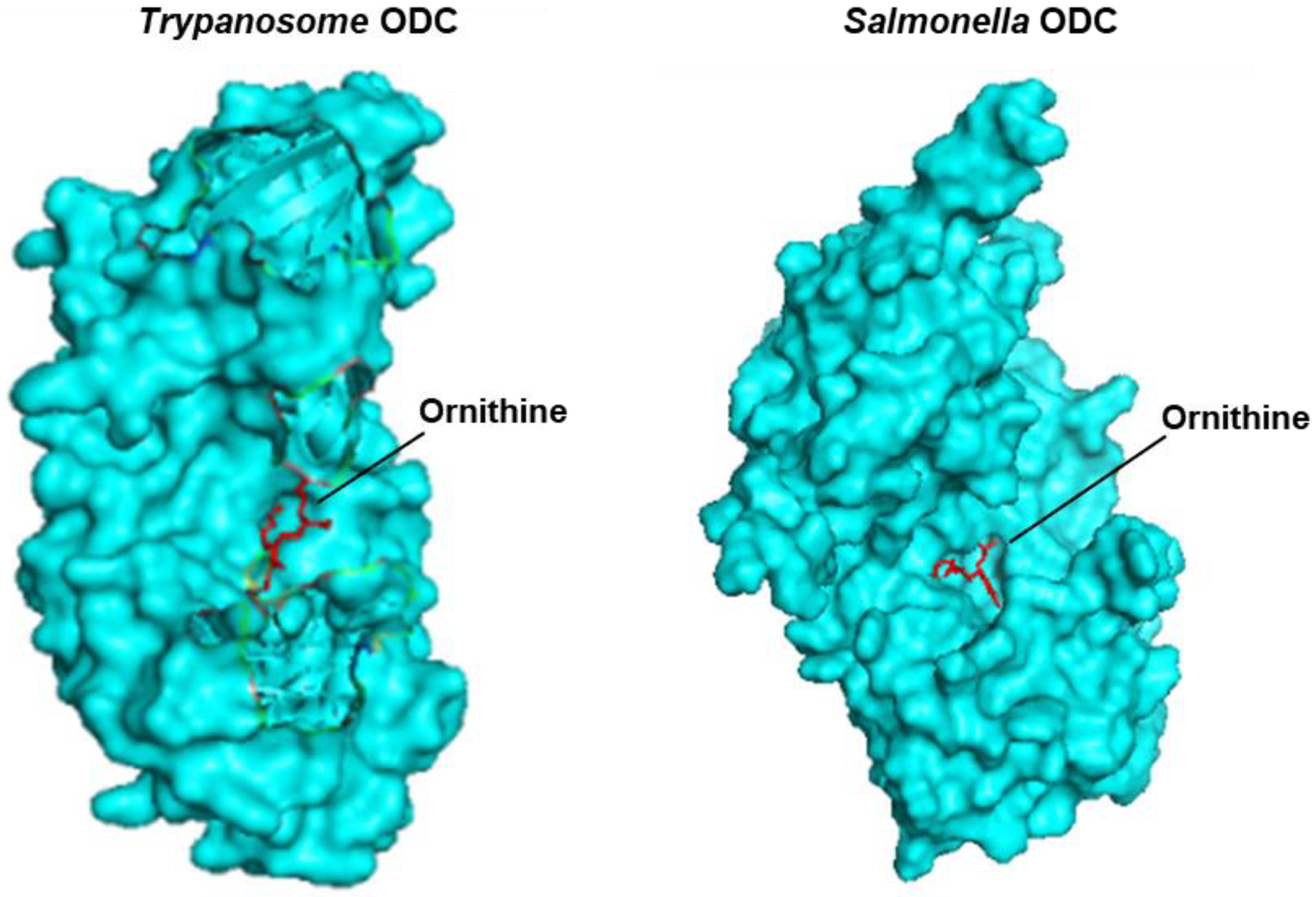
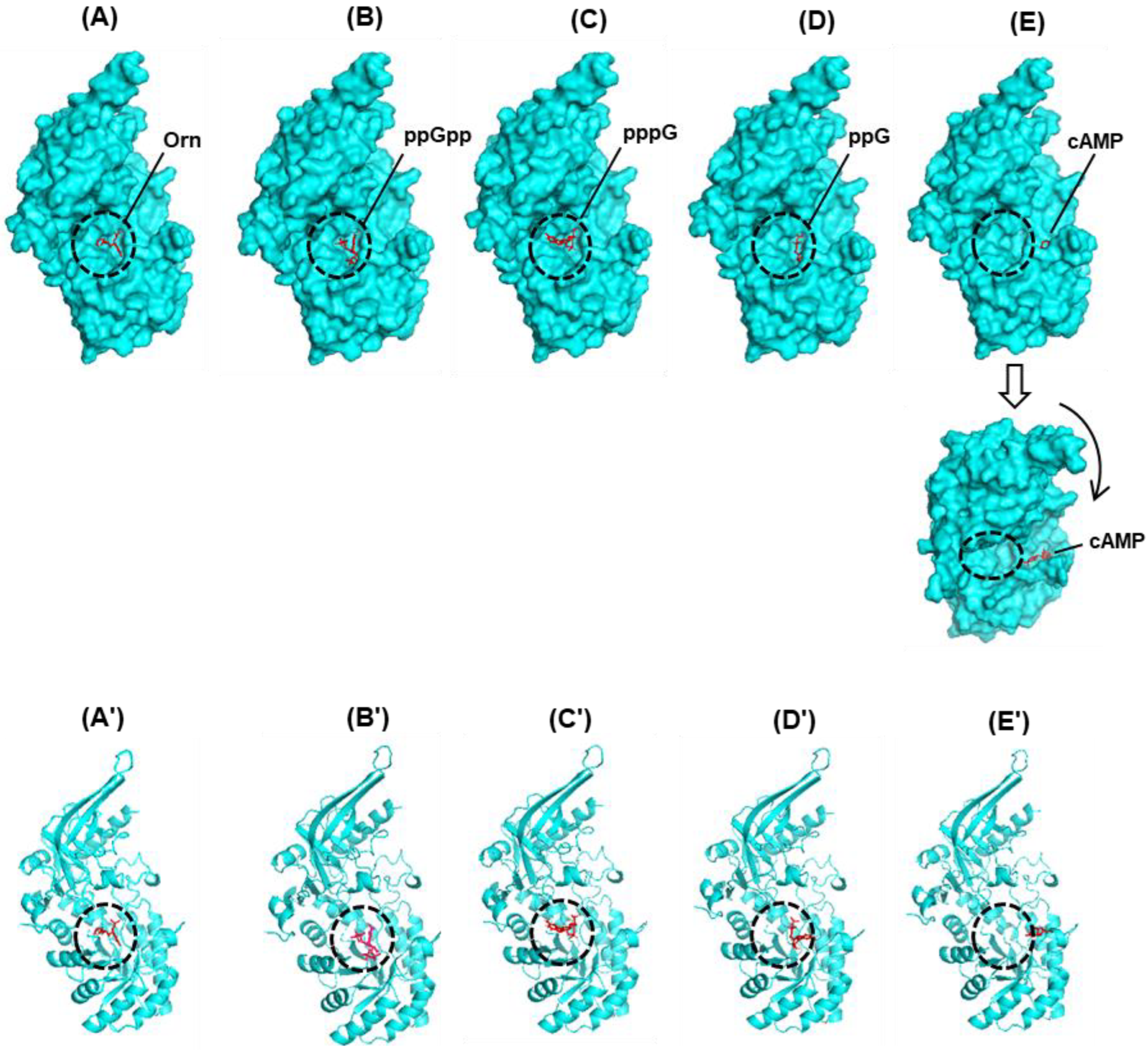
3.4.1. SpeC, a Constitutive Ornithine Decarboxylase
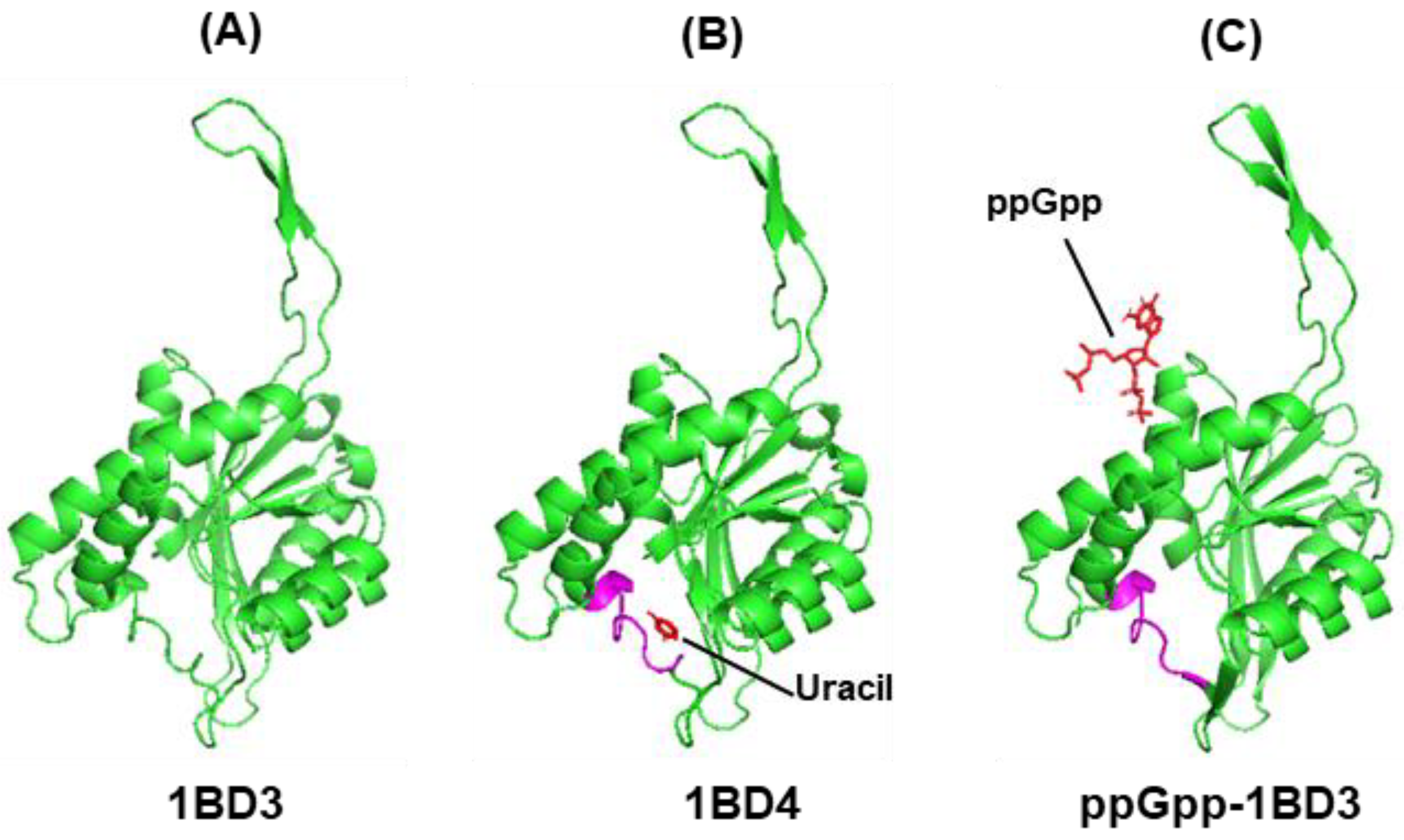
3.4.2. Uracil Phosphoribosyltransferase
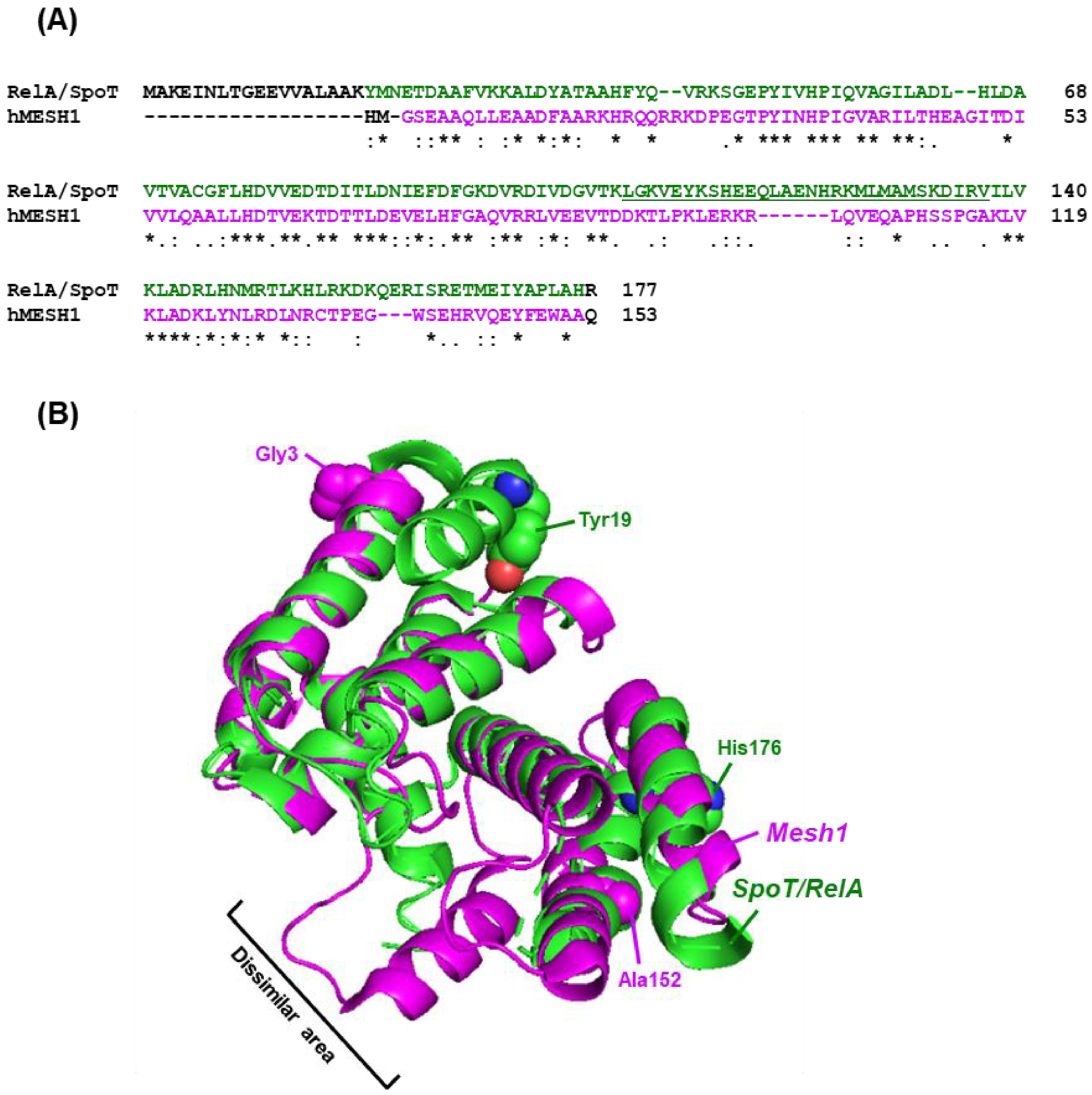
4. Metazoan ‘Stringent-like Response’
5. Unanswered Questions and Future Directions
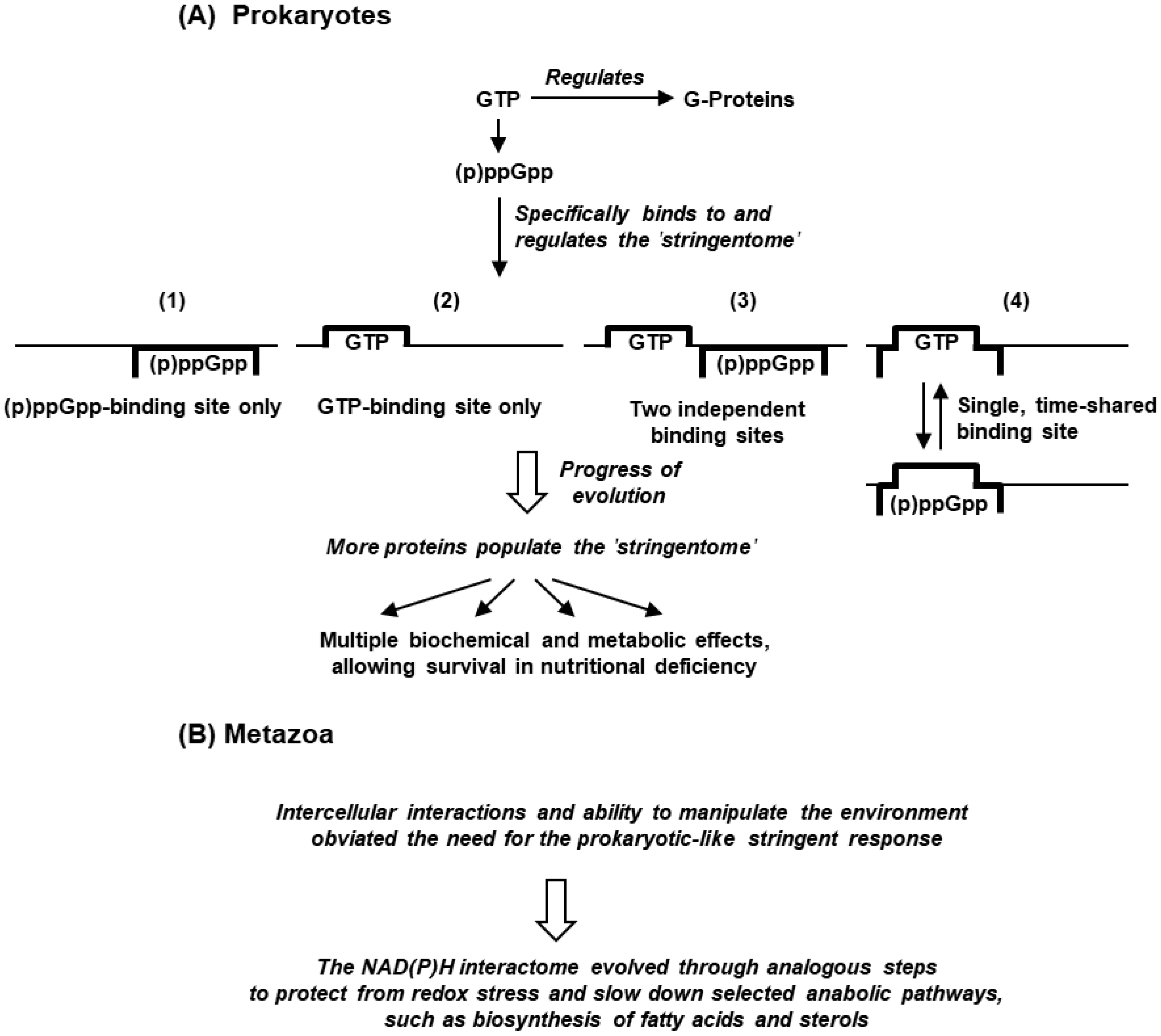
5.1. The ‘Stringentome’
5.2. Evolution of the Stringent Response in Bacteria
5.3. Replacement of Stringent Response by Stringent-like Response
6. Conclusions
Supplementary Materials
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magnusson, L.U.; Farewell, A.; Nyström, T. ppGpp: A global regulator in Escherichia coli. Trends Microbiol. 2005, 13, 236–242. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ascano, M.; Wu, Y.; Barchet, W.; Gaffney, B.L.; Zillinger, T.; Serganov, A.A.; Liu, Y.; Jones, R.A.; Hartmann, G.; et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 2013, 153, 1094–1107. [Google Scholar] [CrossRef]
- Wu, J.; Sun, L.; Chen, X.; Du, F.; Shi, H.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signalling by cytosolic DNA. Science 2013, 339, 826–830. [Google Scholar] [CrossRef]
- Cashel, M.; Gallant, J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature 1969, 221, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Cashel, M.; Kalbacher, B. The control of ribonucleic acid synthesis in Escherichia coli. Characterization of a nucleotide associated with the stringent response. J. Biol. Chem. 1970, 245, 2309–2318. [Google Scholar] [CrossRef] [PubMed]
- Haseltine, W.A.; Block, R.; Gilbert, W.; Weber, K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature 1972, 238, 381–384. [Google Scholar] [CrossRef]
- Sy, J.; Lipmann, F. Identification of the synthesis of guanosine tetraphosphate (MS I) as insertion of a pyrophosphoryl group into the 3′-position in guanosine 5′-diphosphate. Proc. Natl. Acad. Sci. USA 1973, 70, 306–309. [Google Scholar] [CrossRef]
- van der Biezen, E.A.; Sun, J.; Coleman, M.J.; Bibb, M.J.; Jones, J.D. Arabidopsis RelA/SpoT homologs implicate (p)ppGpp in plant signalling. Proc. Natl. Acad. Sci. USA 2000, 97, 3747–3752. [Google Scholar] [CrossRef]
- Tozawa, Y.; Nozawa, A.; Kanno, T.; Narisawa, T.; Masuda, S.; Kasai, K.; Nanamiya, H. Calcium-activated (p)ppGpp synthetase in chloroplasts of land plants. J. Biol. Chem. 2007, 282, 35536–35545. [Google Scholar] [CrossRef]
- Chi, J.T.; Zhou, P. From magic spot ppGpp to MESH1: Stringent response from bacteria to metazoa. PLoS Pathog. 2023, 19, e1011105. [Google Scholar] [CrossRef] [PubMed]
- Ihara, Y.; Ohta, H.; Masuda, S.A. highly sensitive quantification method for the accumulation of alarmone ppGpp in Arabidopsis thaliana using UPLC-ESI-qMS/MS. J. Plant Res. 2015, 128, 511–518. [Google Scholar] [CrossRef]
- Ito, D.; Kawamura, H.; Oikawa, A.; Ihara, Y.; Shibata, T.; Nakamura, N.; Asano, T.; Kawabata, S.-I.; Suzuki, T.; Masuda, S. ppGpp functions as an alarmone in metazoa. Commun. Biol. 2020, 3, 671. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Suzuki, S.; Ito, D.; Tagawa, S.; Shiina, T.; Masuda, S. Plastidial (p)ppGpp synthesis by the Ca2+-dependent RelA-SpoT homolog regulates the adaptation of chloroplast gene expression to darkness in Arabidopsis. Plant Cell Physiol. 2021, 61, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ito, D.; Goto, M.; Suzuki, S.; Masuda, S.; Iba, K.; Kusumi, K. Regulation of ppGpp synthesis and its impact on chloroplast biogenesis during early leaf development in rice. Plant Cell Physiol. 2022, 63, 919–931. [Google Scholar] [CrossRef]
- Cellini, A.; Scoarughi, G.L.; Poggiali, P.; Santino, I.; Sessa, R.; Donini, P.; Cimmino, C. Stringent control in the archaeal genus Sulfolobus. Res. Microbiol. 2004, 155, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.C.; Tenson, T.; Hauryliuk, V. The RelA/SpoT homolog (RSH) superfamily: Distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS ONE 2011, 6, e23479. [Google Scholar] [CrossRef]
- Braun, F.; Recalde, A.; Bähre, H.; Seifert, R.; Albers, S.V. Putative nucleotide-based second messengers in the archaeal model organisms Haloferax volcanii and Sulfolobus acidocaldarius. Front. Microbiol. 2021, 12, 779012. [Google Scholar] [CrossRef]
- Potrykus, K.; Cashel, M. (p)ppGpp: Still magical? Annu. Rev. Microbiol. 2008, 62, 35–51. [Google Scholar] [CrossRef]
- Haseltine, W.A.; Block, R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc. Natl Acad. Sci. USA 1973, 70, 1564–1568. [Google Scholar] [CrossRef]
- Brown, A.; Fernández, I.S.; Gordiyenko, Y.; Ramakrishnan, V. Ribosome-dependent activation of stringent control. Nature 2016, 534, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Winther, K.S.; Roghanian, M.; Gerdes, K. Activation of the stringent response by loading of RelA-tRNA complexes at the ribosomal A-site. Mol. Cell 2018, 70, 95–105.e4. [Google Scholar] [CrossRef] [PubMed]
- Loveland, A.B.; Bah, E.; Madireddy, R.; Zhang, Y.; Brilot, A.F.; Grigorieff, N.; Korostelev, A.A. Ribosome RelA structures reveal the mechanism of stringent response activation. Elife 2016, 5, e17029. [Google Scholar] [CrossRef] [PubMed]
- Mechold, U.; Murphy, H.; Brown, L.; Cashel, M. Intramolecular regulation of the opposing (p)ppGpp catalytic activities of Rel(Seq), the Rel/Spo enzyme from Streptococcus equisimilis. J. Bacteriol. 2002, 184, 2878–2888. [Google Scholar] [CrossRef]
- Payoe, R.; Fahlman, R.P. Dependence of RelA-mediated (p)ppGpp formation on tRNA identity. Biochemistry 2011, 50, 3075–3083. [Google Scholar] [CrossRef] [PubMed]
- Tamman, H.; Van Nerom, K.; Takada, H.; Vandenberk, N.; Scholl, D.; Polikanov, Y.; Hofkens, J.; Talavera, A.; Hauryliuk, V.; Hendrix, J.; et al. A nucleotide-switch mechanism mediates opposing catalytic activities of Rel enzymes. Nat. Chem. Biol. 2020, 16, 834–840. [Google Scholar] [CrossRef]
- Takada, H.; Roghanian, M.; Caballero-Montes, J.; Van Nerom, K.; Jimmy, S.; Kudrin, P.; Trebini, F.; Murayama, R.; Akanuma, G.; Garcia-Pino, A.; et al. Ribosome association primes the stringent factor Rel for tRNA-dependent locking in the A-site and activation of (p)ppGpp synthesis. Nucleic Acids Res. 2021, 49, 444–457. [Google Scholar] [CrossRef]
- Ronneau, S.; Hallez, R. Make and break the alarmone: Regulation of (p)ppGpp synthetase/hydrolase enzymes in bacteria. FEMS Microbiol. Rev. 2019, 43, 389–400. [Google Scholar] [CrossRef]
- Roghanian, M.; Van Nerom, K.; Takada, H.; Caballero-Montes, J.; Tamman, H.; Kudrin, P.; Talavera, A.; Dzhygyr, I.; Ekström, S.; Atkinson, G.C.; et al. (p)ppGpp controls stringent factors by exploiting antagonistic allosteric coupling between catalytic domains. Mol. Cell 2021, 81, 3310–3322.e6. [Google Scholar] [CrossRef]
- Fernández-Coll, L.; Cashel, M. Possible roles for basal levels of (p)ppGpp: Growth efficiency vs. surviving stress. Front. Microbiol. 2020, 11, 592718. [Google Scholar] [CrossRef]
- Kanjee, U.; Ogata, K.; Houry, W.A. Direct binding targets of the stringent response alarmone (p)ppGpp. Mol. Microbiol. 2012, 85, 1029–1043. [Google Scholar] [CrossRef]
- Steinchen, W.; Zegarra, V.; Bange, G. (p)ppGpp: Magic modulators of bacterial physiology and metabolism. Front. Microbiol. 2020, 11, 2072. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Sy, J. Guanosine 5′-triphosphate, 3′-diphosphate 5′-phosphohydrolase. Purification and substrate specificity. J. Biol. Chem. 1983, 258, 1678–1683. [Google Scholar] [CrossRef]
- Battesti, A.; Bouveret, E. Bacteria possessing two RelA/SpoT-like proteins have evolved a specific stringent response involving the acyl carrier protein-SpoT interaction. J. Bacteriol. 2009, 191, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Gallant, J.; Palmer, L.; Pao, C.C. Anomalous synthesis of ppGpp in growing cells. Cell 1977, 11, 181–185. [Google Scholar] [CrossRef]
- Mackow, E.R.; Chang, F.N. Correlation between RNA synthesis and ppGpp content in Escherichia coli during temperature shifts. Mol. Gen. Genet. 1983, 192, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Le, D.; Park, B.R.; Kim, M. Distinct mechanisms coordinate transcription and translation under carbon and nitrogen starvation in Escherichia coli. Nat. Microbiol. 2018, 3, 741–748. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, J.; Wei, S.; Luo, Q.; Li, L.; Li, S.; Tucker, A.; Shao, H.; Zhou, R. The roles of RelA/(p)ppGpp in glucose-starvation induced adaptive response in the zoonotic Streptococcus suis. Sci. Rep. 2016, 6, 27169. [Google Scholar] [CrossRef] [PubMed]
- Bougdour, A.; Gottesman, S. ppGpp regulation of RpoS degradation via anti-adaptor protein IraP. Proc. Natl. Acad. Sci. USA 2007, 104, 12896–12901. [Google Scholar] [CrossRef]
- Vinella, D.; Albrecht, C.; Cashel, M.; D’Ari, R. Iron limitation induces SpoT-dependent accumulation of ppGpp in Escherichia coli. Mol. Microbiol. 2005, 56, 958–970. [Google Scholar] [CrossRef]
- Battesti, A.; Bouveret, E. Acyl carrier protein/SpoT interaction, the switch linking SpoT-dependent stress response to fatty acid metabolism. Mol. Microbiol. 2006, 62, 1048–1063. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, Y.H.; Seok, Y.J. Rsd balances (p)ppGpp level by stimulating the hydrolase activity of SpoT during carbon source downshift in Escherichia coli. Proc. Natl. Acad. Sci. USA 2018, 115, E6845–E6854. [Google Scholar] [CrossRef]
- Fehr, S.; Richter, D. The stringent response to unacylated tRNA, energy-and temperature-downshift in Bacillus stearothermophilus. Arch. Microbiol. 1981, 129, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.K.; Winther, K.S. The RelA hydrolase domain acts as a molecular switch for (p)ppGpp synthesis. Commun. Biol. 2021, 4, 434. [Google Scholar] [CrossRef]
- Srivatsan, A.; Wang, J.D. Control of bacterial transcription, translation and replication by (p)ppGpp. Curr. Opin. Microbiol. 2008, 11, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Bittner, A.N.; Wang, J.D. Diversity in (p)ppGpp metabolism and effectors. Curr. Opin. Microbiol. 2015, 24, 72–79. [Google Scholar] [CrossRef]
- Liu, K.; Myers, A.R.; Pisithkul, T.; Claas, K.R.; Satyshur, K.A.; Amador-Noguez, D.; Keck, J.L.; Wang, J.D. Molecular mechanism and evolution of guanylate kinase regulation by (p)ppGpp. Mol. Cell 2015, 57, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.W.; Fung, D.K.; Wang, J.D. Regulatory themes and variations by the stress-signalling nucleotide alarmones (p)ppGpp in bacteria. Annu. Rev. Genet. 2021, 55, 115–133. [Google Scholar] [CrossRef]
- Bange, G.; Brodersen, D.E.; Liuzzi, A.; Steinchen, W. Two P or Not Two P: Understanding regulation by the bacterial second messengers (p)ppGpp. Annu. Rev. Microbiol. 2021, 75, 383–406. [Google Scholar] [CrossRef]
- Chau, N.Y.E.; Ahmad, S.; Whitney, J.C.; Coombes, B.K. Emerging and divergent roles of pyrophosphorylated nucleotides in bacterial physiology and pathogenesis. PLoS Pathog. 2021, 17, e1009532. [Google Scholar] [CrossRef]
- Irving, S.E.; Choudhury, N.R.; Corrigan, R.M. The stringent response and physiological roles of (pp)pGpp in bacteria. Nat. Rev. Microbiol. 2021, 19, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Dalebroux, Z.D.; Swanson, M.S. ppGpp: Magic beyond RNA polymerase. Nat. Rev. Microbiol. 2012, 10, 203–212. [Google Scholar] [CrossRef]
- Hauryliuk, V.; Atkinson, G.C.; Murakami, K.S.; Tenson, T.; Gerdes, K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol. 2015, 13, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, V.J.; Bremer, H. Characterization of RNA and DNA synthesis in Escherichia coli strains devoid of ppGpp. J. Biol. Chem. 1993, 268, 10851–10862. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.M.; Gaal, T.; Josaitis, C.A.; Gourse, R.L. Mechanism of regulation of transcription initiation by ppGpp. I. Effects of ppGpp on transcription initiation in vivo and in vitro. J. Mol. Biol. 2001, 305, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Potrykus, K.; Murphy, H.; Philippe, N.; Cashel, M. ppGpp is the major source of growth rate control in E. coli. Environ. Microbiol. 2011, 13, 563–575. [Google Scholar] [CrossRef]
- Haugen, S.P.; Ross, W.; Gourse, R.L. Advances in bacterial promoter recognition and its control by factors that do not bind DNA. Nat. Rev. Microbiol. 2008, 6, 507–519. [Google Scholar] [CrossRef]
- Nascimento, M.M.; Lemos, J.A.; Abranches, J.; Lin, V.K.; Burne, R.A. Role of RelA of Streptococcus mutans in global control of gene expression. J. Bacteriol. 2008, 190, 28–36. [Google Scholar] [CrossRef]
- Gaca, A.O.; Abranches, J.; Kajfasz, J.K.; Lemos, J.A. Global transcriptional analysis of the stringent response in Enterococcus faecalis. Microbiology 2012, 158, 1994–2004. [Google Scholar] [CrossRef]
- Geiger, T.; Goerke, C.; Fritz, M.; Schäfer, T.; Ohlsen, K.; Liebeke, M.; Lalk, M.; Wolz, C. Role of the (p)ppGpp synthase RSH, a RelA/SpoT homolog, in stringent response and virulence of Staphylococcus aureus. Infect. Immun. 2010, 78, 1873–1883. [Google Scholar] [CrossRef]
- Durfee, T.; Hansen, A.M.; Zhi, H.; Blattner, F.R.; Ding, J.J. Transcription profiling of the stringent response in Escherichia coli. J. Bacteriol. 2008, 190, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Traxler, M.F.; Summers, S.M.; Nguyen, H.-T.; Zacharia, V.M.; Hightower, G.A.; Smith, J.T.; Conway, T. The global, ppGpp-mediated stringent response to amino acid starvation in Escherichia coli. Mol. Microbiol. 2008, 68, 1128–1148. [Google Scholar] [CrossRef] [PubMed]
- Ross, W.; Vrentas, C.E.; Sanchez-Vazquez, P.; Gaal, T.; Gourse, R.L. The magic spot: A ppGpp binding site on E. coli RNA polymerase responsible for regulation of transcription initiation. Mol. Cell 2013, 50, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Mechold, U.; Potrykus, K.; Murphy, H.; Murakami, K.S.; Cashel, M. Differential regulation by ppGpp versus pppGpp in Escherichia coli. Nucleic Acids Res. 2013, 41, 6175–6189. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Wang, Y.; Steitz, T.A. The mechanism of E. coli RNA polymerase regulation by ppGpp is suggested by the structure of their complex. Mol. Cell 2013, 50, 430–436. [Google Scholar] [CrossRef]
- Perederina, A.; Svetlov, V.; Vassylyeva, M.N.; Tahirov, T.H.; Yokoyama, S.; Artsimovitch, I.; Vassylyev, D.G. Regulation through the secondary channel—Structural framework for ppGpp-DksA synergism during transcription. Cell 2004, 118, 297–309. [Google Scholar] [CrossRef]
- Lennon, C.W.; Ross, W.; Martin-Tumasz, S.; Toulokhonov, I.; Vrentas, C.E.; Rutherford, S.T.; Lee, J.-H.; Butcher, S.E.; Gourse, R.L. Direct interactions between the coiled-coil tip of DksA and the trigger loop of RNA polymerase mediate transcriptional regulation. Genes Dev. 2012, 26, 2634–2646. [Google Scholar] [CrossRef]
- Ross, W.; Sanchez-Vazquez, P.; Chen, A.Y.; Lee, J.H.; Burgos, H.L.; Gourse, R.L. ppGpp Binding to a site at the RNAP-DksA interface accounts for its dramatic effects on transcription initiation during the stringent response. Mol. Cell 2016, 62, 811–823. [Google Scholar] [CrossRef]
- Molodtsov, V.; Sineva, E.; Zhang, L.; Huang, X.; Cashel, M.; Ades, S.E.; Murakami, K.S. Allosteric Effector ppGpp potentiates the inhibition of transcript initiation by DksA, allosteric effector ppGpp potentiates the inhibition of transcript initiation by DksA. Mol. Cell 2018, 69, 828–839.e5. [Google Scholar] [CrossRef]
- Gourse, R.L.; Chen, A.Y.; Gopalkrishnan, S.; Sanchez-Vazquez, P.; Myers, A.; Ross, W. Transcriptional responses to ppGpp and DksA. Annu. Rev. Microbiol. 2018, 72, 163–184. [Google Scholar] [CrossRef]
- Nivedha, A.K.; Lee, S.; Vaidehi, N. Biased agonists differentially modulate the receptor conformation ensembles in Angiotensin II type 1 receptor. J. Mol. Graph. Model. 2022, 118, 108365. [Google Scholar] [CrossRef] [PubMed]
- Egbert, M.; Jones, G.; Collins, M.R.; Kozakov, D.; Vajda, S. FTMove: A web server for detection and analysis of cryptic and allosteric binding sites by mapping multiple protein structures. J. Mol. Biol. 2022, 434, 167587. [Google Scholar] [CrossRef] [PubMed]
- Jishage, M.; Kvint, K.; Shingler, V.; Nyström, T. Regulation of sigma factor competition by the alarmone ppGpp. Genes Dev. 2002, 16, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Kriel, A.; Brinsmade, S.R.; Tse, J.L.; Tehranchi, A.K.; Bittner, A.N.; Sonenshein, A.L.; Wang, J.D. GTP dysregulation in Bacillus subtilis cells lacking (p)ppGpp results in phenotypic amino acid auxotrophy and failure to adapt to nutrient downshift and regulate biosynthesis genes. J. Bacteriol. 2014, 196, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Vrentas, C.E.; Gaal, T.; Berkmen, M.B.; Rutherford, S.T.; Haugen, S.P.; Vassylyev, D.G.; Ross, W.; Gourse, R.L. Still looking for the magic spot: The crystallographically defined binding site for ppGpp on RNA polymerase is unlikely to be responsible for rRNA transcription regulation. J. Mol. Biol. 2008, 377, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Artsimovitch, I.; Patlan, V.; Sekine, S.; Vassylyeva, M.N.; Hosaka, T.; Ochi, K.; Yokoyama, S.; Vassylyev, D.G. Structural basis for transcription regulation by alarmone ppGpp. Cell 2004, 117, 299–310. [Google Scholar] [CrossRef]
- Sanchez-Vazquez, P.; Dewey, C.N.; Kitten, N.; Ross, W.; Gourse, R.L. Genome-wide effects on Escherichia coli transcription from ppGpp binding to its two sites on RNA polymerase. Proc. Natl. Acad. Sci. USA 2019, 116, 8310–8319. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Coll, L.; Potrykus, K.; Cashel, M.; Balsalobre, C. Mutational analysis of Escherichia coli GreA protein reveals new functional activity independent of antipause and lethal when overexpressed. Sci. Rep. 2020, 10, 16074. [Google Scholar] [CrossRef]
- Barik, S.; Das, A. An analysis of the role of host factors in transcription antitermination in vitro by the Q protein of coliphage lambda. Mol. Gen. Genet. 1990, 222, 152–156. [Google Scholar] [CrossRef]
- Feng, G.H.; Lee, D.N.; Wang, D.; Chan, C.L.; Landick, R. GreA-induced transcript cleavage in transcription complexes containing Escherichia coli RNA polymerase is controlled by multiple factors, including nascent transcript location and structure. J. Biol. Chem. 1994, 269, 22282–22294. [Google Scholar] [CrossRef]
- Hsu, L.M.; Vo, N.V.; Chamberlin, M.J. Escherichia coli transcript cleavage factors GreA and GreB stimulate promoter escape and gene expression in vivo and in vitro. Proc. Natl. Acad. Sci. USA 1995, 92, 11588–11592. [Google Scholar] [CrossRef] [PubMed]
- Orlova, M.; Newlands, J.; Das, A.; Goldfarb, A.; Borukhov, S. Intrinsic transcript cleavage activity of RNA polymerase. Proc. Natl. Acad. Sci. USA 1995, 92, 4596–4600. [Google Scholar] [CrossRef]
- Marr, M.T.; Roberts, J.W. Function of transcription cleavage factors GreA and GreB at a regulatory pause site. Mol. Cell 2000, 6, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, C.E.; Borukhov, S.; Orlova, M.; Polyakov, A.; Goldfarb, A.; Darst, S.A. Crystal structure of the GreA transcript cleavage factor from Escherichia coli. Nature 1995, 373, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Vassylyeva, M.N.; Svetlov, V.; Dearborn, A.D.; Klyuyev, S.; Artsimovitch, I.; Vassylyev, D.G. The carboxy-terminal coiled-coil of the RNA polymerase beta’-subunit is the main binding site for Gre factors. EMBO Rep. 2007, 8, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Vinella, D.; Potrykus, K.; Murphy, H.; Cashel, M. Effects on growth by changes of the balance between GreA, GreB, and DksA suggest mutual competition and functional redundancy in Escherichia coli. J. Bacteriol. 2012, 194, 261–273. [Google Scholar] [CrossRef]
- Gralla, J.D. Escherichia coli ribosomal RNA transcription: Regulatory roles for ppGpp, NTPs, architectural proteins and a polymerase-binding protein. Mol. Microbiol. 2005, 55, 973–977. [Google Scholar] [CrossRef]
- DeLano, W.L. PyMOL; DeLano Scientific: San Carlos, CA, USA, 2002. [Google Scholar]
- Milon, P.; Tischenko, E.; Tomsic, J.; Caserta, E.; Folkers, G.; La Teana, A.; Rodnina, M.V.; Pon, C.L.; Boelens, R.; Gualerziet, C.O. The nucleotide-binding site of bacterial translation initiation factor 2 (IF2) as a metabolic sensor. Proc. Natl. Acad. Sci. USA 2006, 103, 13962–13967. [Google Scholar] [CrossRef]
- Diez, S.; Ryu, J.; Caban, K.; Gonzalez, R.L., Jr.; Dworkin, J. The alarmones (p)ppGpp directly regulate translation initiation during entry into quiescence. Proc. Natl. Acad. Sci. USA 2020, 117, 15565–15572. [Google Scholar] [CrossRef]
- Vinogradova, D.S.; Zegarra, V.; Maksimova, E.; Nakamoto, J.A.; Kasatsky, P.; Paleskava, A.; Konevega, A.L.; Milón, P. How the initiating ribosome copes with ppGpp to translate mRNAs. PLoS Biol. 2020, 18, e3000593. [Google Scholar] [CrossRef]
- Rojas, A.M.; Ehrenberg, M.; Andersson, S.G.; Kurland, C.G. ppGpp inhibition of elongation factors Tu, G and Ts during polypeptide synthesis. Mol. Gen. Genet. 1984, 197, 36–45. [Google Scholar] [CrossRef]
- Corrigan, R.M.; Bellows, L.E.; Wood, A.; Gründling, A. ppGpp negatively impacts ribosome assembly affecting growth and antimicrobial tolerance in Gram-positive bacteria. Proc. Natl. Acad. Sci. USA 2016, 113, E1710–E1719. [Google Scholar] [CrossRef] [PubMed]
- Buglino, J.; Shen, V.; Hakimian, P.; Lima, C.D. Structural and biochemical analysis of the Obg GTP binding protein. Structure 2002, 10, 1581–1592. [Google Scholar] [CrossRef]
- Feng, B.; Mandava, C.S.; Guo, Q.; Wang, J.; Cao, W.; Li, N.; Zhang, Y.; Zhang, Y.; Wang, Z.; Wu, J.; et al. Structural and functional insights into the mode of action of a universally conserved Obg GTPase. PLoS Biol. 2014, 12, e1001866. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.-X.; Cao, Y. CB-Dock2: Improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef]
- Schreiber, G.; Ron, E.Z.; Glaser, G. ppGpp-mediated regulation of DNA replication and cell division in Escherichia coli. Curr. Microbiol. 1995, 30, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Ferullo, D.J.; Lovett, S.T. The stringent response and cell cycle arrest in Escherichia coli. PLoS Genet. 2008, 4, e1000300. [Google Scholar] [CrossRef]
- von Freiesleben, U.; Rasmussen, K.V.; Schaechter, M. SeqA limits DnaA activity in replication from oriC in Escherichia coli. Mol. Microbiol. 1994, 14, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Campbell, J.L.; Boye, E.; Kleckner, N. SeqA: A negative modulator of replication initiation in E. coli. Cell 1994, 77, 413–426. [Google Scholar] [CrossRef]
- Nievera, C.; Torgue, J.J.; Grimwade, J.E.; Leonard, A.C. SeqA blocking of DnaA-oriC interactions ensures staged assembly of the E. coli pre-RC. Mol. Cell 2006, 24, 581–592. [Google Scholar] [CrossRef]
- Kraemer, J.A.; Sanderlin, A.G.; Laub, M.T. The stringent response inhibits DNA replication initiation in E. coli by modulating supercoiling of oriC. mBio 2019, 10, e01330-19. [Google Scholar] [CrossRef] [PubMed]
- Blattner, F.R.; Plunkett, G., 3rd; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.D.; Rode, C.K.; Mayhew, G.F.; et al. The complete genome sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.A.; Kornberg, A. Transcriptional activation of initiation of replication from the E. coli chromosomal origin: An RNA-DNA hybrid near oriC. Cell 1988, 55, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Skarstad, K.; Baker, T.A.; Kornberg, A. Strand separation required for initiation of replication at the chromosomal origin of E. coli is facilitated by a distant RNA-DNA hybrid. EMBO J. 1990, 9, 2341–2348. [Google Scholar] [CrossRef]
- Fernández-Coll, L.; Maciag-Dorszynska, M.; Tailor, K.; Vadia, S.; Levin, P.A.; Szalewska-Palasz, A.; Cashel, M. The absence of (p)ppGpp renders initiation of Escherichia coli chromosomal DNA synthesis independent of growth rates. mBio 2020, 11, e03223-19. [Google Scholar] [CrossRef]
- Wang, J.D.; Sanders, G.M.; Grossman, A.D. Nutritional control of elongation of DNA replication by (p)ppGpp. Cell 2007, 128, 865–875. [Google Scholar] [CrossRef]
- Rymer, R.U.; Solorio, F.A.; Tehranchi, A.K.; Chu, C.; Corn, J.E.; Keck, J.L.; Wang, J.D.; Berger, J.M. Binding mechanism of metal NTP substrates and stringent-response alarmones to bacterial DnaG-type primases. Structure 2012, 20, 1478–1489. [Google Scholar] [CrossRef]
- Denapoli, J.; Tehranchi, A.K.; Wang, J.D. Dose-dependent reduction of replication elongation rate by (p)ppGpp in Escherichia coli and Bacillus subtilis. Mol. Microbiol. 2013, 88, 93–104. [Google Scholar] [CrossRef]
- Maciąg-Dorszyńska, M.; Szalewska-Pałasz, A.; Węgrzyn, G. Different effects of ppGpp on Escherichia coli DNA replication in vivo and in vitro. FEBS Open Bio 2013, 3, 161–164. [Google Scholar] [CrossRef]
- Giramma, C.N.; DeFoer, M.B.; Wang, J.D. The alarmone (p)ppGpp regulates primer extension by bacterial primase. J. Mol. Biol. 2021, 433, 167189. [Google Scholar] [CrossRef]
- Keck, J.L.; Roche, D.D.; Lynch, A.S.; Berger, J.M. Structure of the RNA polymerase domain of E. coli primase. Science 2000, 287, 2482–2486. [Google Scholar] [CrossRef] [PubMed]
- Frick, D.N.; Richardson, C.C. DNA primases. Annu. Rev. Biochem. 2001, 70, 39–80. [Google Scholar] [CrossRef]
- Lee, J.B.; Hite, R.K.; Hamdan, S.M.; Xie, X.S.; Richardson, C.C.; van Oijen, A.M. DNA primase acts as a molecular brake in DNA replication. Nature 2006, 439, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zborníková, E.; Rejman, D.; Gerdes, K. Novel (p)ppGpp binding and metabolizing proteins of Escherichia coli. mBio 2018, 9, e02188-17. [Google Scholar] [CrossRef]
- Wang, B.; Dai, P.; Ding, D.; Del Rosario, A.; Grant, R.A.; Pentelute, B.L.; Laub, M.T. Affinity-based capture and identification of protein effectors of the growth regulator ppGpp. Nat. Chem. Biol. 2019, 15, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.M.; Laventie, B.-J.; Lagies, S.; Harter, C.; Prucker, I.; Ritz, D.; Saleem-Batcha, R.; Qiu, D.; Hüttel, W.; Andexer, J.; et al. Photoaffinity capture compounds to profile the magic spot nucleotide interactomes. Angew. Chem. Int. Ed. Engl. 2022, 61, e202201731. [Google Scholar] [CrossRef]
- Berlyn, M.K. Linkage map of Escherichia coli K-12, edition 10: The traditional map. Microbiol. Mol. Biol. Rev. 1998, 62, 814–984. [Google Scholar] [CrossRef]
- Phillips, R.S.; Poteh, P.; Miller, K.A.; Hoover, T.R. STM2360 encodes a d-ornithine/d-lysine decarboxylase in Salmonella enterica serovar typhimurium. Arch. Biochem. Biophys. 2017, 634, 83–87. [Google Scholar] [CrossRef]
- Del Valle, A.H.; Seip, B.; Cervera-Marzal, I.; Sacheau, G.; Seefeldt, A.C.; Innis, C.A. Ornithine capture by a translating ribosome controls bacterial polyamine synthesis. Nat. Microbiol. 2020, 5, 554–561. [Google Scholar] [CrossRef]
- Jackson, L.K.; Goldsmith, E.J.; Phillips, M.A. X-ray structure determination of Trypanosoma brucei ornithine decarboxylase bound to D-ornithine and to G418: Insights into substrate binding and ODC conformational flexibility. J. Biol. Chem. 2003, 278, 22037–22043. [Google Scholar] [CrossRef]
- Hölttä, E.; Jänne, J.; Pispa, J. Ornithine decarboxylase from Escherichia coli: Stimulation of the enzyme activity by nucleotides. Biochem. Biophys. Res. Commun. 1972, 47, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Hölttä, E.; Jänne, J.; Pispa, J. The regulation of polyamine synthesis during the stringent control in Escherichia coli. Biochem. Biophys. Res. Commun. 1974, 59, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.T.; Cohen, S.S. Regulation of ornithine decarboxylase activity by guanine nucleotides: In vivo test in potassium-depleted Escherichia coli. Proc. Natl. Acad. Sci. USA 1976, 73, 3502–3505. [Google Scholar] [CrossRef] [PubMed]
- Canellakis, E.S.; Kyriakidis, D.A.; Heller, J.S.; Pawlak, J.W. The complexity of regulation of ornithine decarboxylase. Med. Biol. 1981, 59, 279–285. [Google Scholar] [PubMed]
- Smithson, D.C.; Lee, J.; Shelat, A.A.; Phillips, M.A.; Guy, R.K. Discovery of potent and selective inhibitors of Trypanosoma brucei ornithine decarboxylase. J. Biol. Chem. 2010, 285, 16771–16781. [Google Scholar] [CrossRef]
- Villela, A.D.; Ducati, R.G.; Rosado, L.A.; Bloch, C.J.; Prates, M.V.; Gonçalves, D.C.; Ramos, C.H.I.; Basso, L.A.; Santos, D.S. Biochemical characterization of uracil phosphoribosyltransferase from Mycobacterium tuberculosis. PLoS ONE 2013, 8, e56445. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Carter, D.; Scott, D.M.; Roos, D.S.; Ullman, B.; Brennan, R.G. Crystal structures of Toxoplasma gondii uracil phosphoribosyltransferase reveal the atomic basis of pyrimidine discrimination and prodrug binding. EMBO J. 1998, 17, 3219–3232. [Google Scholar] [CrossRef]
- Ghode, P.; Jobichen, C.; Ramachandran, S.; Bifani, P.; Sivaraman, J. Structural basis of mapping the spontaneous mutations with 5-flurouracil in uracil phosphoribosyltransferase from Mycobacterium tuberculosis. Biochem. Biophys. Res. Commun. 2015, 467, 577–582. [Google Scholar] [CrossRef]
- Rasmussen, U.B.; Mygind, B.; Nygaard, P. Purification and some properties of uracil phosphoribosyltransferase from Escherichia coli K12. Biochim. Biophys. Acta 1986, 881, 268–275. [Google Scholar] [CrossRef]
- Jaffe, E.K. Morpheeins—A new structural paradigm for allosteric regulation. Trends Biochem. Sci. 2005, 30, 490–497. [Google Scholar] [CrossRef]
- Gupta, K.R.; Arora, G.; Mattoo, A.; Sajid, A. Stringent response in Mycobacteria: From biology to therapeutic potential. Pathogens 2021, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Lee, G.; Lee, J.H.; Kim, H.-Y.; Rhee, H.-W.; Park, S.-Y.; Kim, K.-J.; Kim, Y.; Kim, B.Y.; Hong, J.-I.; et al. A metazoan ortholog of SpoT hydrolyzes ppGpp and functions in starvation responses. Nat. Struct. Mol. Biol. 2010, 17, 1188–1194. [Google Scholar] [CrossRef]
- Mestre, A.A.; Zhou, P.; Chi, J.T. Metazoan stringent-like response mediated by MESH1 phenotypic conservation via distinct mechanisms. Comput. Struct. Biotechnol. J. 2022, 20, 2680–2684. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.C.; Rose, J.; Sun, T.; Wu, J.; Chen, P.H.; Lin, C.C.; Yang, W.H.; Chen, K.Y.; Lee, H.; Xu, E.; et al. MESH1 is a cytosolic NADPH phosphatase that regulates ferroptosis. Nat. Metab. 2020, 2, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Ding, C.C.; Sun, T.; Wu, J.; Chen, K.Y.; Zhou, P.; Chi, J.T. The regulation of ferroptosis by MESH1 through the activation of the integrative stress response. Cell Death Dis. 2021, 12, 727. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kalimuthu, K.; Seok Park, Y.; Makala, H.; Watkins, S.C.; Choudry, M.H.A.; Bartlett, D.L.; Kwon, Y.T.; Lee, Y.J. Ferroptotic agent-induced endoplasmic reticulum stress response plays a pivotal role in the autophagic process outcome. J. Cell. Physiol. 2020, 235, 6767–6778. [Google Scholar] [CrossRef]
- Bitko, V.; Barik, S. An endoplasmic reticulum-specific stress-activated caspase (caspase-12) is implicated in the apoptosis of A549 epithelial cells by respiratory syncytial virus. J. Cell. Biochem. 2001, 80, 441–454. [Google Scholar] [CrossRef]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef]
- Guzow-Krzemińska, B.; Gąsior, T.; Szalewska-Pałasz, A. Phylogenetic relationship of the stringent response-related genes of marine bacteria. Acta Biochim. Pol. 2015, 62, 773–783. [Google Scholar] [CrossRef]
- Barik, S. On the role, ecology, phylogeny, and structure of dual-family immunophilins. Cell Stress Chaperones 2017, 22, 833–845. [Google Scholar] [CrossRef]
- Adams, B.; Musiyenko, A.; Kumar, R.; Barik, S. A novel class of dual-family immunophilins. J. Biol. Chem. 2005, 280, 24308–24314. [Google Scholar] [CrossRef]
- Barik, S. Dual-family peptidylprolyl isomerases (Immunophilins) of select monocellular organisms. Biomolecules 2018, 8, 148. [Google Scholar] [CrossRef]
- Miller, E.B.; Murrett, C.S.; Zhu, K.; Zhao, S.; Goldfeld, D.A.; Bylund, J.H.; Friesner, R.A. Prediction of long loops with embedded secondary structure using the protein local optimization program. J. Chem. Theory Comput. 2013, 9, 1846–4864. [Google Scholar] [CrossRef]
- Jo, S.; Kim, H.Y.; Shin, D.H.; Kim, M.S. Dimerization tendency of 3CLpros of human coronaviruses based on the X-ray crystal structure of the catalytic domain of SARS-CoV-2 3CLpro. Int. J. Mol. Sci. 2022, 23, 5268. [Google Scholar] [CrossRef]
- Giot, L.; Bader, J.S.; Brouwer, C.; Chaudhuri, A.; Kuang, B.; Li, Y.; Hao, Y.L.; Ooi, C.E.; Godwin, B.; Vitols, E.; et al. A protein interaction map of Drosophila melanogaster. Science 2003, 302, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Formstecher, E.; Aresta, S.; Collura, V.; Hamburger, A.; Meil, A.; Trehin, A.; Reverdy, C.; Betin, V.; Maire, S.; Brun, C.; et al. Protein interaction mapping: A Drosophila case study. Genome Res. 2005, 15, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Rual, J.-F.; Venkatesan, K.; Hao, T.; Hirozane-Kishikawa, T.; Dricot, A.; Li, N.; Berriz, G.F.; Gibbons, F.D.; Dreze, M.; Ayivi-Guedehoussou, N.; et al. Towards a proteome-scale map of the human protein-protein interaction network. Nature 2005, 437, 1173–1178. [Google Scholar] [CrossRef]
- Stelzl, U.; Worm, U.; Lalowski, M.; Haenig, C.; Brembeck, F.H.; Goehler, H.; Stroedicke, M.; Zenkner, M.; Schoenherr, A.; Koeppen, S.; et al. A human protein-protein interaction network: A resource for annotating the proteome. Cell 2005, 122, 957–968. [Google Scholar] [CrossRef]
- Das, J.; Vo, T.V.; Wei, X.; Mellor, J.C.; Tong, V.; Degatano, A.G.; Wang, X.; Wang, L.; Cordero, N.A.; Kruer-Zerhusen, N. Cross-species protein interactome mapping reveals species-specific wiring of stress response pathways. Sci. Signal. 2013, 6, ra38. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.W.; Lam, T.; Stanners, C.P. Mammalian cells do not have a stringent response. J. Cell Physiol. 1980, 105, 313–325. [Google Scholar] [CrossRef]
- Gaca, A.O.; Kajfasz, J.K.; Miller, J.H.; Liu, K.; Wang, J.D.; Abranches, J.; Lemos, J.A. Basal levels of (p)ppGpp in Enterococcus faecalis: The magic beyond the stringent response. mBio 2013, 4, e00646-13. [Google Scholar] [CrossRef] [PubMed]
- Barik, S. Evolution of protein structure and stability in global warming. Int. J. Mol. Sci. 2020, 21, 9662. [Google Scholar] [CrossRef] [PubMed]
- Syal, K.; Neethu, R.S.; Janardhan Reddy, M.V.N. The extended (p)ppGpp family: New dimensions in stress response. Curr. Res. Microb. Sci. 2021, 2, 100052. [Google Scholar] [CrossRef] [PubMed]
- Lasunción, M.A.; Martínez-Botas, J.; Martín-Sánchez, C.; Busto, R.; Gómez-Coronado, D. Cell cycle dependence on the mevalonate pathway: Role of cholesterol and non-sterol isoprenoids. Biochem. Pharmacol. 2022, 196, 114623. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barik, S. Protein–Ligand Interactions in Scarcity: The Stringent Response from Bacteria to Metazoa, and the Unanswered Questions. Int. J. Mol. Sci. 2023, 24, 3999. https://doi.org/10.3390/ijms24043999
Barik S. Protein–Ligand Interactions in Scarcity: The Stringent Response from Bacteria to Metazoa, and the Unanswered Questions. International Journal of Molecular Sciences. 2023; 24(4):3999. https://doi.org/10.3390/ijms24043999
Chicago/Turabian StyleBarik, Sailen. 2023. "Protein–Ligand Interactions in Scarcity: The Stringent Response from Bacteria to Metazoa, and the Unanswered Questions" International Journal of Molecular Sciences 24, no. 4: 3999. https://doi.org/10.3390/ijms24043999
APA StyleBarik, S. (2023). Protein–Ligand Interactions in Scarcity: The Stringent Response from Bacteria to Metazoa, and the Unanswered Questions. International Journal of Molecular Sciences, 24(4), 3999. https://doi.org/10.3390/ijms24043999



